Summary
Wall, JS, Solomon A, Kennel, SJ. Development and evaluation of agents for targeting visceral amyloid. Visceral amyloidosis is a rare disease characterized by the deposition in organs and tissues of protein fibrils, heparan sulfate proteoglycan as well as serum amyloid P component and other serum proteins. Imaging these pathologic deposits aids in the clinical management of patients with amyloidosis. Whole body scintigraphic imaging of amyloid load as well as organ specific anatomic imaging provides information that can inform prognosis and can be used to monitor disease progression or response to therapy. These capabilities are limited in the USA, which has led to our development and evaluation of two new reagents that specifically target amyloid in vivo and have been used to image visceral deposits in mice and patients with AL amyloidosis. The fibril-reactive mAb 11-1F4, when labeled with iodine-124 was shown to bind AL amyloid in patients by using PET/CT imaging. These studies were performed to support the evaluation of this reagent as a novel immunotherapy for AL patients. In addition, we have identified a heparin-binding peptide that co-localizes with murine AA amyloid in vivo and can be used to image the deposits. The interaction of this peptide, designated p5, with amyloid is dependent on the net positive charge and truncated forms that would be more desirable as clinical imaging agents were found to be significantly less efficient for amyloid imaging. The development and positive preclinical validation of these two reagents offers potential new therapeutic and diagnostic tools for patients with these devastating diseases.
Introduction
Amyloidosis is a fatal protein-folding disorder characterised by the formation of well-structured protein aggregates that deposit in organs and tissues in conjunction with serum amyloid P component (SAP) and heparan sulfate proteoglycans (HSPG) (1-7). The unrelenting accumulation of amyloid invariably leads to organ dysfunction and severe morbidity or death. Amyloid can affect any organ or tissue but the kidneys, pancreas, liver, spleen, nervous tissue and heart constitute the major sites of deposition in patients with familial or sporadic forms of amyloid disease. Although varying in aetiology and site of deposition, the amyloid deposits share remarkable structural homogeneity consisting of unbranched fibrils of ~ 10 nm in diameter and comprising proteins that adopt a cross-β pleated sheet secondary structure as evidenced by x-ray diffraction and NMR (8, 9). The deposits can be cerebral, as in patients with Alzheimer’s, Huntington’s or prion diseases, or they can involve visceral organs as seen in patients with light chain (AL) amyloidosis, reactive (AA) amyloidosis and senile systemic amyloidosis (transthyretin amyloid; ATTR). Furthermore, deposits may be localised or systemic, in which the precursor protein is produced locally (at the site of deposition) or circulates in the blood stream, respectively (10). The peripheral amyloidoses are orphan disorders but account for more than 5,000 new patients annually in the USA alone.
Currently, in the USA, there are no reliable methods to document the extent, the progression, or the resolution of visceral amyloid deposits in patients. To this end, we have, over the last decade, developed amyloid-reactive antibody and peptide agents for imaging visceral amyloid deposits that we anticipate will provide a non-invasive, quantitative, objective method to assist in diagnosis and prognostication. These quantitative, non-invasive measurements can also provide valuable insight into the patient response to anti-amyloid therapies. Before discussing our progress in this field we will briefly describe the important role that imaging can play and many of the techniques that are currently employed to study patients with visceral amyloidosis.
Amyloidosis is an “anatomic” pathology that, unlike the solid tumours associated with cancer that can be detected by functional imaging such as positron emission tomography (PET), has no metabolic or “functional” features, other than expansion of the deposits. Thus, standard clinical anatomical imaging modalities, magnetic resonance imaging (MRI), x-ray computed tomography (CT) and ultrasound (US), can be used to visualise relatively large amyloid deposits in organs and tissues (11, 12). However, more often than not, it is the malformation of organ architecture, caused by the amyloid mass, that is visualised e.g. thickening of the intraventricular septum of the heart, rather than the actual amyloid deposits. Therefore, although anatomic imaging of patients with peripheral amyloidosis is generally not diagnostic, it can assist in guiding biopsies, visualising rare tumour-like amyloid masses, and providing prognostic indicators in patients with cardiac amyloidosis. With the advent of dual-modality imaging capabilities such as PET/CT, SPECT/CT and now PET/MR, radiolabelled, amyloid-specific imaging agents can provide molecular insight to compliment the high resolution anatomical imaging thereby providing unparalleled visualisation of the amyloid as well as its pathologic sequelae.
Whole body planar scintigraphy or single photon emission tomography (SPECT) using iodine-123 (123I)-labelled SAP has been used for this purpose in Europe for more than two decades (13-16). SAP scintigraphy provides images of amyloid load that can be used to compliment routine diagnosis by histochemical analysis of tissue biopsies, to aid in prognostication and diagnosis, and to document response to therapy (17, 18). Although 123I-SAP imaging is the most common imaging technique for the detection of amyloid in the peripheral organs, radiotracers such as technetium-99m (99mTc)-aprotinin and 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) have also been used and have proven particularly effective for imaging cardiac amyloid deposits (19-23). Radiolabelled SAP is the gold standard tracer for clinical imaging of visceral amyloidosis; however, it has not been approved by the US Food and Drug Administration due to the human plasma source of this protein. Therefore, alternative techniques are required to document, non-invasively and longitudinally, the whole body burden and organ distribution of peripheral amyloid.
The Important Role of Imaging in the Development of the Therapeutic Monoclonal Antibody 11-1F4
The monoclonal antibody (mAb) 11-1F4 was generated using heat and acid denatured κ4 immunoglobulin light chain protein LEN as an immunogen (24). This antibody bound to both ALκ and λ amyloid fibrils but did not react with free light chain proteins in solution as shown by enzyme-linked immunoassay. MAb 11-1F4 was later shown to react selectively with a neo-epitope formed at the N-terminus of immunoglobulin light chain proteins that adopt a non-native folding pattern when in amyloid fibrils (25-27). Light chains folded properly as in circulation or associated with heavy chains in the form of antibodies do not bind to mAb 11-1F4. Since 11-1F4 mAb binds to amyloid fibrils, and therefore may have opsonising activity, it presented potential as an immunotherapeutic agent for patients with ALκ or ALλ amyloidosis. Due to the lack of an experimental animal model of AL amyloidosis and the scant reports of this disease occurring naturally in non-laboratory animals, we developed a murine model of human “amyloidoma” in which to test the therapeutic potential of 11-1F4 (28, 29). In this model human AL amyloid, extracted from autopsy-derived liver or spleen tissues, is injected subcutaneously (sc) between the scapulae of Balb/c mice, where it becomes vascularised within 7 days post injection (Fig. 1 A-C). Remarkably, when these mice were administered 11-1F4 mAb iv, the rate of dissolution of the “amyloidoma” was dramatically increased. Further data indicated that the removal of the amyloid was dependent on an antibody-mediated cellular process, involving opsonisation of the amyloid by the 11-1F4 mAb (28). To test whether the 11-1F4 mAb was indeed localising with the sc amyloidoma, we used small animal imaging techniques to visualise the distribution of radiolabelled, 125I-11-1F4 in amyloidoma-bearing mice by using dual modality SPECT and x-ray computed tomographic (CT) imaging (30-32). The 125I-11-1F4 mAb was readily observed to be associated with the human amyloid mass in SPECT images at 72 h post injection (Fig. 1 D & E). This indicated that 11-1F4 mAb was indeed capable of binding to amyloid in vivo and, perhaps equally importantly, was shown not to bind to amyloid-free organs or tissues in the mouse – an important requirement for an immunotherapeutic reagent. These findings were confirmed by quantifying the radioactivity associated with tissues and organs as well as by micro-autoradiography (30).
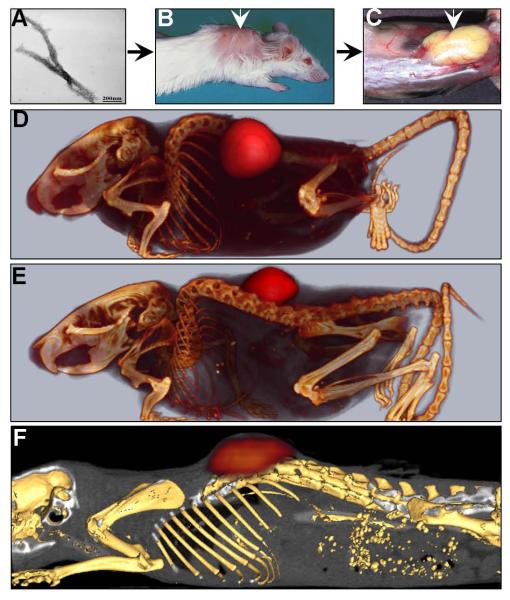
Figure 1.
The 11-1F4 mAb localizes with human AL amyloid in a mouse model of amyloidoma. Human AL amyloid extract, shown by using electron microscopy to contain fibrillar material (A), was injected sub-cutaneously between the scapulae of Balb/c mice (B) and became vascularized within 7 days (C). 125I-labelled 11-1F4 mAb administered intravenously via the lateral tail vein, localised with both ALκ (D) and ALλ (E) in SPECT images. Similarly, GMP-grade 124I-11-1F4 bound ALκ amyloid as evidenced in PET images (F).
Although encouraging, preclinical imaging data could not predict whether the 11-1F4 mAb would bind amyloid efficiently in a patient with AL in whom there was significant (>1 mg/mL) circulating free light chain precursor protein. This could only effectively be addressed by performing imaging studies in human patients with AL. To translate the preclinical 11-1F4 mAb imaging procedure into humans, it was necessary to use a higher energy radioisotope than 125I, which cannot be imaged in large subjects due to attenuation and scatter of the low energy photons emitted by this nuclide. Furthermore, to generate rapid, whole body, quantitative, high-resolution tomographic images of the mAb biodistribution in vivo, we chose to use PET/CT imaging. For this, we used I-124 to radiolabel 11-1F4 since I-124 is a positron emitter and has a convenient 4 day half-life. In preliminary mouse studies using 124I-labelled 11-1F4 mAb prepared for human use, we again demonstrated, using small animal PET/CT imaging, the interaction of the mAb with human amyloidoma (30) (Fig. 1 F).
Based upon our positive preclinical imaging findings that demonstrated the reactivity of mAb 11-1F4 with human AL amyloid xenografts in mice, we began an investigation into the biodistribution of this reagent in patients with AL by using PET/CT imaging of AL patients using the 124I-11-1F4 (33). These studies were performed to demonstrate the specific binding of 11-1F4 to tissue amyloid in vivo in the presence of circulating light chain proteins and thus, support the clinical evaluation of 11-1F4 as an immunotherapeutic reagent. Visual inspection of the PET images revealed specific co-localisation of the antibody with AL amyloid in ~ 60% of the patients imaged to date (n = 38). Predominantly, amyloid in the liver and spleen imaged well, as did deposits in the adrenal glands, lymph nodes, and bone marrow (see. Fig. 2). However, the 124I-11-1F4 failed to image amyloid in all patients. Notably, amyloid in heart and kidneys (2 organs most prone to fatal failure due to amyloid burden) was rarely discerned by visual inspection of the PET images.
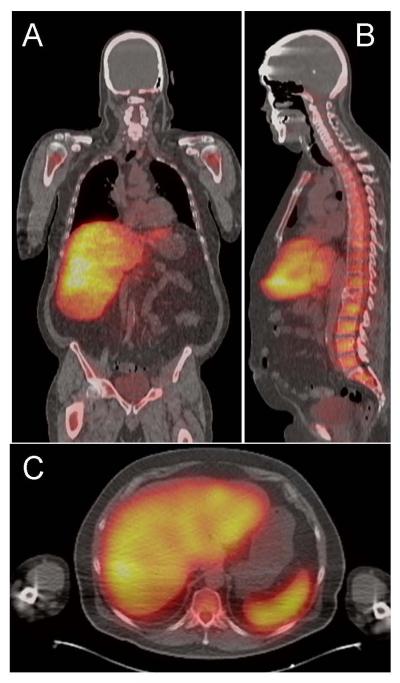
Figure 2.
The 124I-labelled 11-1F4 mAb specifically bound AL amyloid in patients. (A) Coronal, (B) sagital, and (C) transaxial PET/CT images demonstrated the localisation of the 124I-11-1F4 mAb in the liver, spleen, and bone marrow of a patient with AL amyloidosis.
Our demonstration of the specific uptake of 11-1F4 mAb in visceral amyloid deposits provides impetus for its evaluation as an immunotherapeutic agent in patients with AL. In this regard, PET/CT imaging of patients using 124I-11-1F4 will be used to stratify the patient population and define those subjects more likely to benefit from the immunotherapy, based on specific organ uptake in PET images. Additionally, the ability to non-invasively and longitudinally imaged tissue amyloid load using 124I-11-1F4 will provide an additional tool for monitoring response to the therapy in those patients enrolled in an 11-1F4 therapy trial.
Although using imaging to demonstrate the targeting of a fibril-specific mAb to amyloid in man is a significant achievement, this antibody will likely not be an effective first-line diagnostic and disease-monitoring imaging agent for all patients. Thus, with the continuing need to determine the presence and biodistribution of amyloid in the major target organs of patients and in those subjects entered in therapeutic clinical trials, we have evaluated the use of amyloid-associated HSPG as a target for imaging due to its ubiquitous presence in high concentrations of all amyloid deposits, irrespective of the nature of the fibril protein (34-36). We have used small peptide probes that bind amyloid-associated HSPG. These peptides can be chemically synthesised, have a better likelihood of extravasation (as compared to larger proteins such as SAP or mAb 11-1F4) and thus may provide superior imaging of extra-vascular amyloid deposits.
Amyloid imaging using heparin-binding peptide p5
The presence of HSPG in all amyloid deposits, regardless of the type of protein fibril, has been well established, and its importance in the etiology of the disease has been demonstrated in vivo and in vitro (34, 37, 38). Indeed, organs that overexpressed the heparanase enzyme in a transgenic mouse model, and therefore lacked significant sulfated proteoglycans, did not support AA amyloidogenesis (37). Furthermore, soluble HS mimetics, such as polyvinyl sulfonate, have been used successfully to inhibit AA amyloidosis in mice (39, 40). Amyloid-associated HSPG has been shown by chromatographic analyses (41-44) and by reaction with antibodies (45, 46) to be biochemically distinct from that found in the extra-cellular matrices and plasma membranes of healthy tissues. Notably, amyloid-associated HSPGs appear to be more heavily sulfated than those in normal tissue (41, 43, 44, 46). The high concentration and unique chemical structure makes it a potential biomarker that may be targeted with suitable imaging reagents for the purpose of diagnosis, prognostication, and monitoring response to therapy.
Our preliminary studies to test this hypothesis used HS-reactive scFvs that exhibited differential reactivity to the glycosaminoglycan. We have demonstrated using small animal SPECT imaging, biodistribution studies and micro-autoradiography that the scFv NS4F5, that binds hyper-sulfated (heparin-like) HS, was singularly capable of specific amyloid binding in vivo in mice using these techniques (46). Based on these observations, made using our murine model of AA (46), we next evaluated a series of 7 heparin-binding peptides as potential imaging agents for amyloid in vivo by using small animal SPECT imaging, tissue biodistribution measurements, and micro-autoradiography. For these studies, we used a transgenic mouse, designated H2-Ld-huIL-6 Tg Balb/c (C) (H2/IL-6) that constitutively expresses human interleukin 6 protein resulting in the high circulating concentrations of acute phase proteins including serum amyloid protein A (sAA), the precursor of AA amyloid (47, 48). These mice develop spontaneous AA in the spleen, liver, kidney, heart, pancreas, and eventually all organs (Fig. 3 A-D). This process can be initiated and expedited by iv injection of AA amyloid extract that acts as an amyloid enhancing factor (AEF) by seeding the pathology (47).
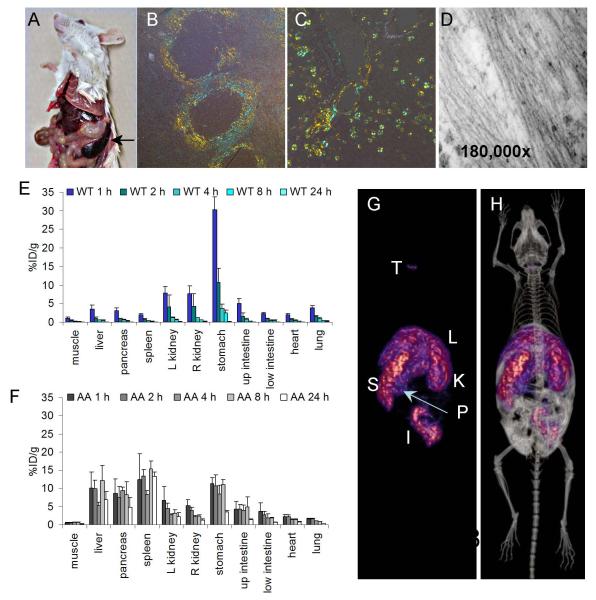
Figure 3.
The heparin-binding peptide p5 binds to amyloid in a murine model of AA amyloidosis. (A) AA amyloid develops in the H2/IL-6 transgenic mouse resulting in splenomegaly (arrow) as well as hepatomegaly and ultimately renal failure due to amyloid burden in the papilla. Significant AA appears as perifollicular deposits in the spleen and within the hepatic sinusoids as evidence by blue-gold birefringence in Congo red-stained tissue sections (B & C). The amyloid contains characteristic fibrils when viewed by electron microscopy (D). 125I-p5 peptide was cleared from the tissues of healthy wild type (WT) mice over 24 h (E), but was retained in amyloid-laden liver, spleen, pancreas, and intestines of mice with AA (F). SPECT (G) and SPECT fused with ip contrast-enhanced CT (H) imaging showed 125I-p5 peptide uptake in the spleen (S), liver (L), kidney (K), pancreas (P), and intestines (I). Traces of free iodide, liberated during catabolism were seen in the thyroid (T).
From studies on the 7 candidate peptides, we identified one, designated p5 (a 31-amino acid, polybasic, heparin-binding peptide), that bound rapidly and specifically with amyloid in vivo and in sufficient concentrations as to be imaged by SPECT as early as 1 h and as late as 24 h post injection (pi) (49). The peptide was rapidly cleared from the circulation and amyloid free organs (Fig. 3E) but was retained in the H2/IL-6 mice for more than 24 h post injection in amyloid-laden organs, such as the liver, pancreas, and spleen (Fig 3F). High resolution SPECT/CT imaging of 125I-p5 peptide in H2/IL-6 mice with AA revealed uptake of the tracer in the liver, spleen, kidney, pancreas and intestines (Fig. 3 G). These sites of uptake were more readily identified, particularly the pancreas, when contrast-enhanced CT image data were co-registered with the SPECT data (Fig. 3H). The specific binding of the p5 peptide with amyloid within these organs was confirmed by using micro-autoradiography (49). This technique provides a means to observe the microscopic distribution of the 125I-labelled peptide within formalin-fixed paraffin embedded tissue sections that are exposed to photographic emulsion. The distribution of radiotracer, indicated by the black silver granules in amyloid laden tissues (Fig.4), correlated precisely with the presence of amyloid based on Congo red staining of consecutive tissue sections and was consistently more easily visualised.
Figure 4.
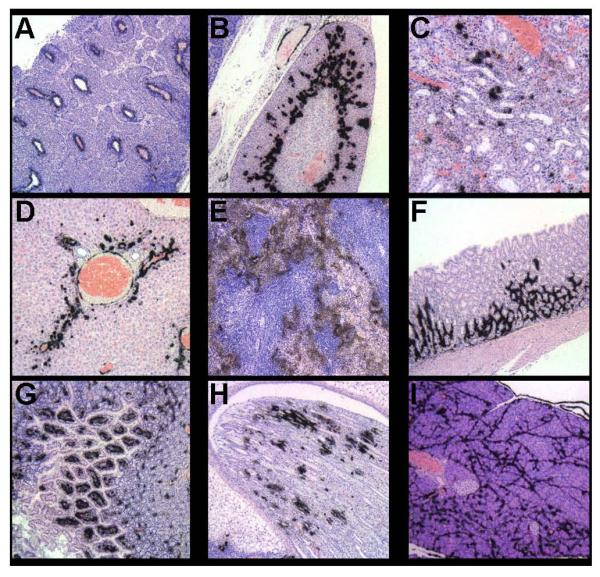
Peptide p5 specifically binds to AA amyloid when injected iv into AA mice as evidenced in micro-autoradiographs. Black deposits indicate the presence of 125I-p5 at sites confirmed to contain amyloid by Congo red staining of consecutive tissue sections. (A) lymph node, (B) adrenal gland, (C) renal cortex, (D) liver, (E) spleen, (F) gastric wall, (G) small intestine, (H) renal papilla, and (I) pancreas.
The retention of this 125I peptide in amyloid-laden organs in vivo was shown to increase linearly with the amyloid load as evidenced by a comparison with qualitative Congo red scoring of stained tissue sections (49). In addition, we have observed scant radioactivity within healthy organs. Finally, a biotinylated form of peptide p5 was shown to stain human AA, AL, ATTR and Aβ amyloid in formalin-fixed paraffin-embedded tissue sections (49). These data support the evaluation of this basic peptide as a novel agent for the clinical detection of peripheral organ amyloidosis in man by using molecular imaging.
In considering the translation of peptide p5 into the clinic, we tested several variants of the original sequence to identify the optimal peptide. The cost of producing good manufacturing practice (GMP)-grade peptide p5 is based on the number of amino acids, therefore, we have evaluated the amyloid imaging efficacy of 2 truncated forms of the peptide, designated p5(1-24) and p5(1-17). To directly compare the efficacy of each peptide with the parental p5 reagent, we performed in vivo dual energy SPECT imaging studies in which the truncated p5 peptides were radiolabelled with 125I, and the p5 was labelled with 99mTc. Both forms of the peptide were co-injected into the same mouse, and the biodistribution of each one simultaneously assessed by SPECT imaging using both high and low energy window acquisitions as well as dual isotope tissue biodistribution measurements (Fig. 5). Both p5(1-24) and p5(1-17) were found in lower concentrations in the liver, spleen, and pancreas of mice with AA as compared to 99mTc-p5 (Figs. 5A & B). This quantitative analysis was confirmed in the SPECT images. The 99mTc-p5 peptide was seen in the liver, spleen, and intestines (Fig. 5C & E) whereas the p5(1-24) was seen only in the spleen, with some free 125I-iodide (liberated during catabolism) observed in the stomach (Fig. 5D &E). Similar results were observed with 125I-p5(1-17) in that there was little binding to any amyloid deposits in vivo, and only 125I-iodide was seen in the stomach (Fig. 5G & H), as compared to the dramatic uptake of 125I-p5 in the amyloid in visceral organs (Fig. 5F).
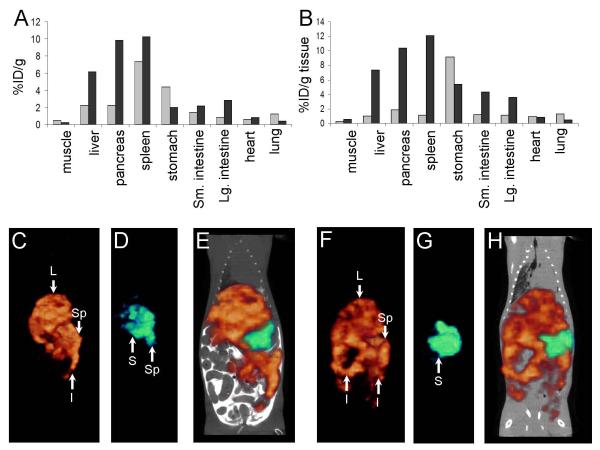
Figure 5.
Truncated forms of peptide p5 do not effectively bind or image murine AA amyloid in vivo. Dual energy biodistribution of 125I-p5(1-24) (A, grey) and 99mTc-p5(A, black), or 125I-p5(1-17) (B, grey) and 99mTc-p5(B, black) demonstrated that truncation of p5 peptide decreased the reactivity with AA amyloid in vivo. This was confirmed in SPECT and CT contrast-enhanced SPECT/CT imaging. SPECT images of (C) 99mTc-p5, (D) 125I-p5(1-24), and the fused image (E) as well as (F) 99mTc-p5, (G) 125I-p5(1-17), and the fused image (H). L, liver; Sp, spleen; I, intestine, and; S, stomach.
We had initially hypothesized that truncation of the p5 peptide, and therefore reduction in the net positive charge, would result in a slight decrease in the amyloid reactivity of the radiolabelled product in vivo but possibly result in a faster whole body clearance giving it an advantage over the full length p5 peptide. However, the reduction in peptide size and charge resulted in a dramatic decrease in amyloid reactivity. The truncated peptides could not be used to detect amyloid deposits in vivo based on SPECT imaging. With the result that shorter peptides were inferior, we also tested if a longer peptide might actually bind amyloid better. A peptide with 14 more amino acids including three extra AQK motifs was tested. The longer peptide p5(+14) was able to bind to and image amyloid in the AA mice, but gave significant background binding in WT mice and thus was an inferior imaging agent (data not shown). Based on these findings, we continue to develop the full-length p5 peptide (31-mer) as the prime candidate for imaging visceral amyloid in patients.
Conclusion
Imaging visceral amyloid deposits either by 123I-SAP scintigraphy, MRI, US or CT is common practice in these patients and provides the physician with a more complete picture of the disease and a means to monitor progression or regression of the pathology in response to therapy. To further assist in the management of patients with visceral amyloidosis, we have developed a mAb that specifically targets both ALλ and ALκ amyloid deposits and may prove to be therapeutically efficacious. Furthermore, we have demonstrated that the mAb 11-1F4, when radiolabelled, can be used to image AL amyloid-laden organs and tissues. This will allow the physician to identify patients who may benefit from 11-1F4 treatment as well as non-invasively monitor response to therapy and change in amyloid burden in patients.
However, due to certain limitations in 11-1F4 imaging we have also identified a novel polybasic heparin-binding peptide that, due to its structural properties, binds amyloid specifically and can be used to image amyloid in vivo. When radioiodinated, the p5 peptide can be used to visualise the presence of amyloid by using small animal SPECT or PET imaging up to 24 h pi. The ability to bind many extra-cellular amyloid deposits, the rapid loss of signal from unbound 125I-p5, and the lack of binding to healthy tissues support the evaluation of this peptide as a potential amyloid imaging agent for the non-invasive, quantitative detection and monitoring of disease in patients with amyloidosis.
References
- 1.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48(2):241–53. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 2.Kisilevsky R, Ancsin JB, Szarek WA, Petanceska S. Heparan sulfate as a therapeutic target in amyloidogenesis: prospects and possible complications. Amyloid. 2007;14(1):21–32. doi: 10.1080/13506120601116419. [DOI] [PubMed] [Google Scholar]
- 3.Ohashi K. Pathogenesis of beta2-microglobulin amyloidosis. Pathology international. 2001;51(1):1–10. doi: 10.1046/j.1440-1827.2001.01156.x. [DOI] [PubMed] [Google Scholar]
- 4.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349(6):583–96. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 5.Merlini G, Westermark P. The systemic amyloidoses: clearer understanding of the molecular mechanisms offers hope for more effective therapies. J Intern Med. 2004;255(2):159–78. doi: 10.1046/j.1365-2796.2003.01262.x. [DOI] [PubMed] [Google Scholar]
- 6.De Lorenzi E, Giorgetti S, Grossi S, Merlini G, Caccialanza G, Bellotti V. Pharmaceutical strategies against amyloidosis: old and new drugs in targeting a “protein misfolding disease”. Curr Med Chem. 2004;11(8):1065–84. doi: 10.2174/0929867043455549. [DOI] [PubMed] [Google Scholar]
- 7.Merlini G. Systemic amyloidosis: are we moving ahead? Neth J Med. 2004;62(4):104–5. [PubMed] [Google Scholar]
- 8.Goldsbury C, Baxa U, Simon MN, Steven AC, Engel A, Wall JS, et al. Amyloid structure and assembly: insights from scanning transmission electron microscopy. Journal of structural biology. 2010;173(1):1–13. doi: 10.1016/j.jsb.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson R, Eisenberg D. Recent atomic models of amyloid fibril structure. Current opinion in structural biology. 2006;16(2):260–5. doi: 10.1016/j.sbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, et al. A primer of amyloid nomenclature. Amyloid. 2007;14(3):179–83. doi: 10.1080/13506120701460923. [DOI] [PubMed] [Google Scholar]
- 11.Ketteler M, Koch KM, Floege J. Imaging techniques in the diagnosis of dialysis-related amyloidosis. Semin Dial. 2001;14(2):90–3. doi: 10.1046/j.1525-139x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 12.Sueyoshi E, Sakamoto I, Okimoto T, Hayashi K, Tanaka K, Toda G. Cardiac amyloidosis: typical imaging findings and diffuse myocardial damage demonstrated by delayed contrast-enhanced MRI. Cardiovasc Intervent Radiol. 2006;29(4):710–2. doi: 10.1007/s00270-004-0162-x. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins PN, Pepys MB. Imaging amyloidosis with radiolabelled SAP. European journal of nuclear medicine. 1995;22(7):595–9. doi: 10.1007/BF01254559. [DOI] [PubMed] [Google Scholar]
- 14.Hazenberg BP, van Rijswijk MH, Piers DA, Lub-de Hooge MN, Vellenga E, Haagsma EB, et al. Diagnostic performance of 123I-labeled serum amyloid P component scintigraphy in patients with amyloidosis. The American journal of medicine. 2006;119(4):355, e15–24. doi: 10.1016/j.amjmed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Jager PL, Hazenberg BP, Franssen EJ, Limburg PC, van Rijswijk MH, Piers DA. Kinetic studies with iodine-123-labeled serum amyloid P component in patients with systemic AA and AL amyloidosis and assessment of clinical value. J Nucl Med. 1998;39(4):699–706. [PubMed] [Google Scholar]
- 16.Hawkins PN, Myers MJ, Epenetos AA, Caspi D, Pepys MB. Specific localization and imaging of amyloid deposits in vivo using 123I-labeled serum amyloid P component. The Journal of experimental medicine. 1988;167(3):903–13. doi: 10.1084/jem.167.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rydh A, Suhr O, Hietala SO, Ahlstrom KR, Pepys MB, Hawkins PN. Serum amyloid P component scintigraphy in familial amyloid polyneuropathy: regression of visceral amyloid following liver transplantation. European journal of nuclear medicine. 1998;25(7):709–13. doi: 10.1007/s002590050273. [DOI] [PubMed] [Google Scholar]
- 18.Tan SY, Irish A, Winearls CG, Brown EA, Gower PE, Clutterbuck EJ, et al. Long term effect of renal transplantation on dialysis-related amyloid deposits and symptomatology. Kidney international. 1996;50(1):282–9. doi: 10.1038/ki.1996.313. [DOI] [PubMed] [Google Scholar]
- 19.Glaudemans AW, Slart RH, Zeebregts CJ, Veltman NC, Tio RA, Hazenberg BP, et al. Nuclear imaging in cardiac amyloidosis. European journal of nuclear medicine and molecular imaging. 2009;36(4):702–14. doi: 10.1007/s00259-008-1037-1. [DOI] [PubMed] [Google Scholar]
- 20.Han S, Chong V, Murray T, McDonagh T, Hunter J, Poon FW, et al. Preliminary experience of 99mTc-Aprotinin scintigraphy in amyloidosis. European journal of haematology. 2007;79(6):494–500. doi: 10.1111/j.1600-0609.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 21.Schaadt BK, Hendel HW, Gimsing P, Jonsson V, Pedersen H, Hesse B. 99mTc-aprotinin scintigraphy in amyloidosis. J Nucl Med. 2003;44(2):177–83. [PubMed] [Google Scholar]
- 22.Puille M, Altland K, Linke RP, Steen-Muller MK, Kiett R, Steiner D, et al. 99mTc-DPD scintigraphy in transthyretin-related familial amyloidotic polyneuropathy. European journal of nuclear medicine and molecular imaging. 2002;29(3):376–9. doi: 10.1007/s00259-001-0730-0. [DOI] [PubMed] [Google Scholar]
- 23.Rapezzi C, Guidalotti P, Salvi F, Riva L, Perugini E. Usefulness of 99mTc-DPD scintigraphy in cardiac amyloidosis. Journal of the American College of Cardiology. 2008;51(15):1509–10. doi: 10.1016/j.jacc.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 24.Solomon A, Weiss DT, Macy SD, Antonucci RA. Immunocytochemical detection of kappa and lambda light chain V region subgroups in human B-cell malignancies. Am J Pathol. 1990;137(4):855–62. [PMC free article] [PubMed] [Google Scholar]
- 25.O’Nuallain B, Allen A, Ataman D, Weiss DT, Solomon A, Wall JS. Phage display and peptide mapping of an immunoglobulin light chain fibril-related conformational epitope. Biochemistry. 2007;46(45):13049–58. doi: 10.1021/bi701255m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Nuallain B, Allen A, Kennel SJ, Weiss DT, Solomon A, Wall JS. Localization of a conformational epitope common to non-native and fibrillar immunoglobulin light chains. Biochemistry. 2007;46(5):1240–7. doi: 10.1021/bi0616605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Nuallain B, Murphy CL, Wolfenbarger DA, Kennel SJ, Solomon A, Wall JS, editors. Amyloid and Amyloidosis: Proceedings of the Xth International Symposium on Amyloidosis. CRC Press; Tours, France: 2005. The Amyloid-Reactive Monoclonal Antibody 11-1F4 Binds a Cryptic Epitope on Fibrils and Partially Denatured Immunoglobulin Light Chains and Inhibits Fibrillogenesis. [Google Scholar]
- 28.Hrncic R, Wall J, Wolfenbarger DA, Murphy CL, Schell M, Weiss DT, et al. Antibody-mediated resolution of light chain-associated amyloid deposits [In Process Citation] Am J Pathol. 2000;157(4):1239–46. doi: 10.1016/S0002-9440(10)64639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hrncic R, Wall JS, Murphy C, Solomon A, editors. International symposium on amyloidosis; Amyloid and amyloidosis. Parthenon Pub. Group; Rochester, MN: New York, N.Y.; London: 1998. Dissolution of human amyloid extract in the mouse. [Google Scholar]
- 30.Wall JS, Kennel SJ, Paulus M, Gregor J, Richey T, Avenell J, et al. Radioimaging of Light Chain Amyloid with a Fibril-Reactive Monoclonal Antibody. J Nucl Med. 2006;47(12):2016–24. [PMC free article] [PubMed] [Google Scholar]
- 31.Wall JS, Paulus MJ, GLeason S, Gregor J, Solomon A, Kennel SJ. Micro-Imaging of Amyloid in Mice. Methods in Enzymology. 2006;412:161–82. doi: 10.1016/S0076-6879(06)12011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall JS, Pedlus MJ, Kennel SJ, GLeason S, Baba J, Gregor J, et al., editors. Amyloid and Amyloidosis: Proceedings of the Xth International Symposium on Amyloidosis. CRC Press; Tours, France: 2005. Radioimaging of Primary (AL) Amyloidosis with an Amyloid-Reactive Monoclonal Antibody. [Google Scholar]
- 33.Wall JS, Kennel SJ, Stuckey AC, Long MJ, Townsend DW, Smith GT, et al. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood. 2010;116(13):2241–4. doi: 10.1182/blood-2010-03-273797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ancsin JB. Amyloidogenesis: historical and modern observations point to heparan sulfate proteoglycans as a major culprit. Amyloid. 2003;10(2):67–79. doi: 10.3109/13506120309041728. [DOI] [PubMed] [Google Scholar]
- 35.Kisilevsky R. Review: amyloidogenesis-unquestioned answers and unanswered questions. Journal of structural biology. 2000;130(2-3):99–108. doi: 10.1006/jsbi.2000.4222. [DOI] [PubMed] [Google Scholar]
- 36.O’Callaghan P, Sandwall E, Li JP, Yu H, Ravid R, Guan ZZ, et al. Heparan sulfate accumulation with Abeta deposits in Alzheimer’s disease and Tg2576 mice is contributed by glial cells. Brain pathology (Zurich, Switzerland) 2008;18(4):548–61. doi: 10.1111/j.1750-3639.2008.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JP, Galvis ML, Gong F, Zhang X, Zcharia E, Metzger S, et al. In vivo fragmentation of heparan sulfate by heparanase overexpression renders mice resistant to amyloid protein A amyloidosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(18):6473–7. doi: 10.1073/pnas.0502287102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snow AD, Bramson R, Mar H, Wight TN, Kisilevsky R. A temporal and ultrastructural relationship between heparan sulfate proteoglycans and AA amyloid in experimental amyloidosis. J Histochem Cytochem. 1991;39(10):1321–30. doi: 10.1177/39.10.1940305. [DOI] [PubMed] [Google Scholar]
- 39.Inoue S, Hultin PG, Szarek WA, Kisilevsky R. Effect of poly(vinylsulfonate) on murine AA amyloid: a high-resolution ultrastructural study. Laboratory investigation; a journal of technical methods and pathology. 1996;74(6):1081–90. [PubMed] [Google Scholar]
- 40.Dember LM, Hawkins PN, Hazenberg BP, Gorevic PD, Merlini G, Butrimiene I, et al. Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med. 2007;356(23):2349–60. doi: 10.1056/NEJMoa065644. [DOI] [PubMed] [Google Scholar]
- 41.Lindahl B, Lindahl U. Amyloid-specific heparan sulfate from human liver and spleen. The Journal of biological chemistry. 1997;272(42):26091–4. doi: 10.1074/jbc.272.42.26091. [DOI] [PubMed] [Google Scholar]
- 42.Snow AD, Kisilevsky R, Stephens C, Anastassiades T. Characterization of tissue and plasma glycosaminoglycans during experimental AA amyloidosis and acute inflammation. Qualitative and quantitative analysis. Laboratory investigation; a journal of technical methods and pathology. 1987;56(6):665–75. [PubMed] [Google Scholar]
- 43.Stenstad T, Magnus JH, Husby G. Characterization of proteoglycans associated with mouse splenic AA amyloidosis. The Biochemical journal. 1994;303(Pt 2):663–70. doi: 10.1042/bj3030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugumaran G, Elliott-Bryant R, Phung N, Vitseva O, Kuberan B, Lech M. Characterization of splenic glycosaminoglycans accumulated in vivo in experimentally induced amyloid-susceptible and amyloid-resistant mice. Scandinavian journal of immunology. 2004;60(6):574–83. doi: 10.1111/j.0300-9475.2004.01516.x. [DOI] [PubMed] [Google Scholar]
- 45.Bruinsma IB, te Riet L, Gevers T, ten Dam GB, van Kuppevelt TH, David G, et al. Sulfation of heparan sulfate associated with amyloid-beta plaques in patients with Alzheimer’s disease. Acta neuropathologica. 2010;119(2):211–20. doi: 10.1007/s00401-009-0577-1. [DOI] [PubMed] [Google Scholar]
- 46.Smits NC, Kurup S, Rops AL, ten Dam GB, Massuger LF, Hafmans T, et al. The heparan sulfate motif (GlcNS6S-IdoA2S)3, common in heparin, has a strict topography and is involved in cell behavior and disease. The Journal of biological chemistry. 2010;285(52):41143–51. doi: 10.1074/jbc.M110.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon A, Weiss DT, Schell M, Hrncic R, Murphy CL, Wall J, et al. Transgenic mouse model of AA amyloidosis. Am J Pathol. 1999;154(4):1267–72. doi: 10.1016/S0002-9440(10)65378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wall JS, Richey T, Allen A, Donnell R, Kennel SJ, Solomon A. Quantitative tomography of early-onset spontaneous AA amyloidosis in interleukin 6 transgenic mice. Comparative medicine. 2008;58(6):542–50. [PMC free article] [PubMed] [Google Scholar]
- 49.Wall JS, Richey T, Stuckey A, Donnell R, Macy S, Martin EB, et al. In vivo molecular imaging of peripheral amyloidosis using heparin-binding peptides. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1103247108. [DOI] [PMC free article] [PubMed] [Google Scholar]