Abstract
Background
We sought to determine the prognostic value of pathologic response to neoadjuvant chemotherapy with concurrent trastuzumab.
Patients and methods
Two hundred and twenty-nine women with HER2/neu (HER2)-overexpressing breast cancer were treated with neoadjuvant chemotherapy plus trastuzumab between 2001 and 2008. Patients were grouped based on pathologic complete response (pCR, n = 114) or less than pCR (<pCR, n = 115); as well as by pathologic stage. Locoregional recurrence-free (LRFS), distant metastasis-free (DMFS), recurrence-free (RFS), and overall survival (OS) rates were compared.
Results
The median follow-up was 63 (range 53–77) months. There was no difference in clinical stage between patients with pCR or <pCR. Compared with patients achieving <pCR, those with the pCR had higher 5-year rates of LRFS (100% versus 95%, P = 0.011), DMFS (96% versus 80%, P < 0.001), RFS (96% versus 79%, P < 0.001), and OS (95% versus 84%, P = 0.006). Improvements in RFS and OS were seen with decreasing post-treatment stage. Failure to achieve a pCR was the strongest independent predictor of recurrence (hazard ratio [HR] = 4.09, 95% confidence interval [CI]: 1.67–10.04, P = 0.002) and death (HR = 4.15, 95% CI: 1.39–12.38, P = 0.011).
Conclusions
pCR and lower pathologic stage after neoadjuvant chemotherapy with trastuzumab are the strongest predictors of recurrence and survival and are surrogates of the long-term outcome in patients with HER2-overexpressing disease.
Keywords: breast cancer, HER2, neoadjuvant chemotherapy, pathologic complete response, trastuzumab
introduction
Potential benefits of neoadjuvant chemotherapy include tumor downsizing allowing appropriately selected patients to undergo breast-conserving therapy (BCT), assessment of response to therapy, and early treatment of micrometastatic disease [1, 2]. Response to neoadjuvant chemotherapy is an early surrogate of long-term prognosis, as pathologic complete response (pCR) has been shown to correlate with an improved outcome [3–5].
With the routine use of trastuzumab, improvements in survival among women with HER2/neu (HER2)-overexpressing tumors have been demonstrated in the metastatic and adjuvant settings [6–10]. More recently, the efficacy of trastuzumab delivered in the neoadjuvant setting has been evaluated. In the first randomized trial to evaluate the addition of trastuzumab to chemotherapy in the neoadjuvant setting, Buzdar et al. [11] reported a 66.7% pCR rate among patients receiving chemotherapy plus trastuzumab versus 25% among patients receiving chemotherapy alone (P = 0.02). After this, the GeparQuattro [12] and NOAH [13] (NeOAdjuvant Herceptin) trials have shown improved pCR rates among patients receiving trastuzumab, with an event-free survival benefit seen at 3 years among patients treated with trastuzumab in the NOAH trial. Long-term clinical outcomes as a function of achieving a pCR have not been reported from these studies.
In this study, we evaluated the prognostic value of achieving a pCR following neoadjuvant chemotherapy with trastuzumab. Locoregional recurrence-free, distant metastasis-free (DMFS), recurrence-free (RFS), and overall survival (OS) outcomes were evaluated to characterize the long-term benefit of a pCR.
materials and methods
patient population and treatment
Patients with non-metastatic, non-inflammatory HER2-overexpressing breast cancer treated with neoadjuvant chemotherapy plus trastuzumab between 2001 and 2008 were identified. HER2-overexpressing disease was defined as 3+ on immunohistochemistry or gene amplification on fluorescence in situ hybridization. HER2 status was confirmed by a dedicated breast pathologist. Patients received a variety of neoadjuvant regimens (Table 1). Following the completion of neoadjuvant chemotherapy, all patients underwent surgery; either total mastectomy with axillary lymph node evaluation or BCT (segmental mastectomy, axillary node evaluation, and whole breast irradiation). After a favorable clinical response to neoadjuvant therapy, most of the patients were offered BCT. Mastectomy was carried out according to patient preference, diffuse residual calcifications, or when clinical response assessment inaccurately predicted more extensive residual disease. Excised specimens were routinely subjected to intraoperative assessment. Specimens were oriented, marked, and sectioned into 3- to 5-mm sections examined grossly by a pathologist, and then subjected to specimen radiograph. If these evaluations suggested a close margin, re-excision was carried out at the initial operation. All patients undergoing BCT received external-beam whole-breast irradiation with tangential fields. Regional nodal radiation in the case of BCT or post-mastectomy radiation for patients undergoing mastectomy was generally recommended for patients presenting with clinical stage III disease and for those with residual positive lymph nodes identified pathologically. Adjuvant endocrine therapy was generally administered for hormone receptor-positive disease.
Table 1.
Chemotherapy regimens used in patients with HER2-overexpressing breast cancer
| Regimen | Number of patients receiving |
|---|---|
| Paclitaxel 225 mg/m2 q3w × 4 | 54 |
| FEC75a q3w × 4 | |
| Trastuzumab administered weeklyb | |
| Paclitaxel 80 mg/m2 weekly × 12 | 94 |
| FEC75a q3w × 4 | |
| Trastuzumab administered weeklyb | |
| TCHc 4–8 cycles | 28 |
| Paclitaxel 175 mg/m2 q3w × 4 or 80 mg/m2 weekly × 12 with weekly trastuzumab | 13 |
| FEC100d or FACe q3w × 4 without trastuzumab | |
| FEC100 or FAC without trastuzumab | 14 |
| Paclitaxel 80 mg/m2 weekly × 12 + weekly trastuzumab or q3w docetaxel + trastuzumab | |
| ACf q3w × 4 | 8 |
| Paclitaxel 80 mg/m2 weekly + trastuzumab | |
| ACf q3w × 4 | 5 |
| Paclitaxel 175 mg/m2 q3w + trastuzumab | |
| ACf q3w × 4 | 3 |
| Docetaxel 100 mg/m2 q3w + trastuzumab | |
| Paclitaxel 80 mg/m2 weekly + trastuzumab | 2 |
| FEC75 q3w × 4 + trastuzumab | 2 |
| Other | 6 |
aFEC75 = fluorouracil 500 mg/m2, epirubicin 75 mg/m2, and cyclophosphamide 500 mg/m2.
bTrastuzumab given as a 4-mg/kg loading dose, followed by 2 mg/kg weekly during the 24 weeks of chemotherapy.
cTCH = docetaxel 75 mg/m2, carboplatin area under the curve of 6, and trastuzumab 8 mg/kg loading dose, followed by 6 mg/kg every 3 weeks.
dFEC100 = fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2.
eFAC = fluorouracil 500 mg/m2, doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2.
fAC = doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2.
end points and statistical methods
A pCR was defined as no residual invasive disease in the breast or axillary lymph nodes, regardless of non-invasive disease status. For additional comparison, patients were categorized by the extent of remaining disease according to pathologic stage as defined by the 7th edition of the American Joint Committee on Cancer staging system.
Locoregional recurrence was defined as any relapse within the breast or ipsilateral chest wall, and/or ipsilateral axillary, internal mammary, infraclavicular, or supraclavicular nodal regions, regardless of systemic disease status. Distant relapse included all non-locoregional recurrences and was determined independent of locoregional disease status. All survival outcomes were calculated from the date of diagnosis to the date of relapse, date when the patient was last known to be relapse free, or death.
Statistical analysis was carried out using SPSS (SPSS, Inc., Chicago, IL) and Stata (StataCorp LP, College Station, TX). The chi-square statistic was used to evaluate differences in prognostic factors between groups. Kaplan–Meier analysis was used to calculate survival outcomes and subgroups compared using log-rank statistic. Cox proportional hazards regression was used to estimate relapse and survival risk between subgroups. Covariates used in the model included estrogen and/or progesterone receptor (ER/PR) status, age, menopausal status, T-stage, N-stage, stage group, grade, presence of lymphovascular invasion (LVI), surgery type, radiation therapy, surgical margin status, treatment with hormone therapy, pathologic response to neoadjuvant therapy, and length of trastuzumab treatment. The proportional hazards assumption was tested and upheld. All tests were two-sided with an alpha = 0.05. This study was approved by the MD Anderson Institutional Review Board.
results
patient characteristics and clinical outcomes
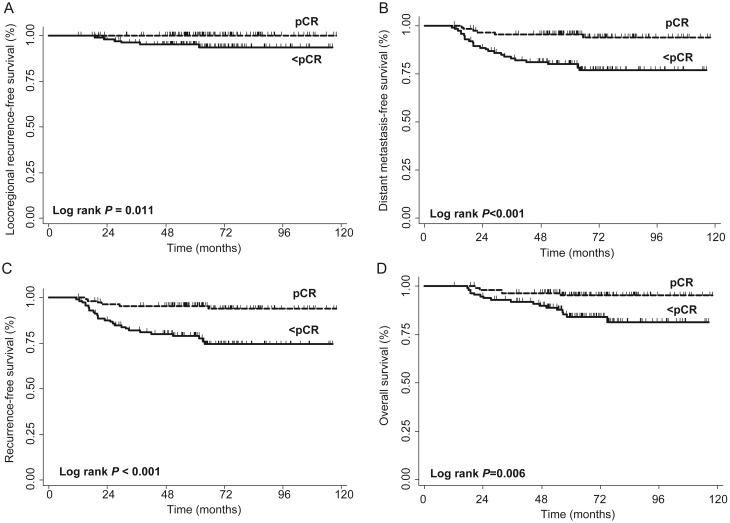
A pCR was achieved in 114 (49.8%) patients. Disease and treatment characteristics according to pathologic response are listed in Table 2. The median follow-up was 63 (interquartile range, 53–77) months. None of the patients achieving a pCR had a locoregional failure. Locoregional failures occurred in six patients with less than a pCR (Figure 1A), DMFS was higher among patients achieving a pCR compared with less than a pCR (5-year DMFS, 96% versus 80%, P < 0.001, Figure 1B). Five-year RFS (96% versus 79%, P < 0.001) and OS (95% versus 84%, P = 0.006) rates were also significantly higher among patients achieving a pCR (Figure 1C and D).
Table 2.
Patient, disease, and treatment characteristics according to pathologic complete response or less than pathologic complete response
| Characteristic | pCR (N = 114), n (%) | Less than pCR (N = 115), n (%) | P-value |
|---|---|---|---|
| Median age in years (range) | 50 (21–81) | 47 (25–77) | 0.041 |
| Menopausal status | |||
| Pre-menopausal | 48 (42) | 66 (58) | 0.039 |
| Post-menopausal | 54 (47) | 36 (31) | |
| Unknown | 12 (11) | 13 (11) | |
| Clinical T-stage | |||
| T1 | 20 (18) | 13 (11) | 0.347 |
| T2 | 60 (53) | 57 (50) | |
| T3 | 20 (17) | 26 (23) | |
| T4 | 13 (11) | 19 (16) | |
| Unknown | 1 (1) | 0 (0) | |
| Clinical N-stage | |||
| N0 | 33 (29) | 34 (29) | 0.708 |
| N1 | 53 (47) | 54 (47) | |
| N2 | 5 (4) | 2 (2) | |
| N3 | 23 (20) | 25 (22) | |
| Stage group | |||
| Stage I | 4 (3) | 3 (3) | 0.638 |
| Stage II | 59 (52) | 66 (57) | |
| Stage III | 50 (44) | 46 (40) | |
| Unknown | 1 (1) | 0 (0) | |
| ER/PR status | |||
| Negative | 70 (61) | 46 (40) | 0.004 |
| Positive | 44 (39) | 68 (59) | |
| Unknown | 0 (0) | 1 (1) | |
| Nuclear grade | |||
| Grade 1 | 0 (0) | 0 (0) | 0.097 |
| Grade 2 | 22 (19) | 35 (30) | |
| Grade 3 | 91 (80) | 80 (70) | |
| Unknown | 1 (1) | 0 (0) | |
| LVI | |||
| Absent | 100 (88) | 82 (71) | 0.007 |
| Present | 14 (12) | 32 (28) | |
| Unknown | 0 (0) | 1 (1) | |
| Local therapy | |||
| BCS + XRT | 47 (41) | 31 (27) | 0.003 |
| Mastectomy + XRT | 39 (34) | 65 (57) | |
| Mastectomy alone | 28 (25) | 19 (16) | |
| Surgical margin status | |||
| Negative | 114 (100) | 100 (87) | <0.001 |
| Close or positivea | 0 (0) | 13 (11) | |
| Unknown | 0 (0) | 2 (2) | |
| Full year trastuzumab | |||
| No | 65 (57) | 54 (47) | 0.128 |
| Yes | 49 (43) | 61 (53) | |
aClose or positive margins = tumor cells <2 mm from margin. Only one patient had a positive margin.
pCR, pathologic complete response; ER/PR, estrogen receptor/progesterone receptor; LVI, lymphovascular invasion; BCS, breast conserving surgery; XRT, radiation therapy.
Figure 1.
Kaplan–Meier curves of (A) locoregional recurrence-free, (B) distant metastasis-free, (C) recurrence-free, and (D) overall survival outcomes among patients achieving a pathologic complete response (pCR) versus less than a pCR.
To determine the significance of residual ductal carcinoma in situ (DCIS), patients categorized as a pCR with residual DCIS were identified (n = 35) and compared with those that had no residual invasive disease or DCIS in the breast and axillary lymph nodes (n = 79). No locoregional failures were seen in either group. At 5 years, DMFS was 95% versus 97% (P = 0.428), and OS was 96% versus 94% (P = 0.655) for those with no residual invasive disease or DCIS versus those with residual DCIS.
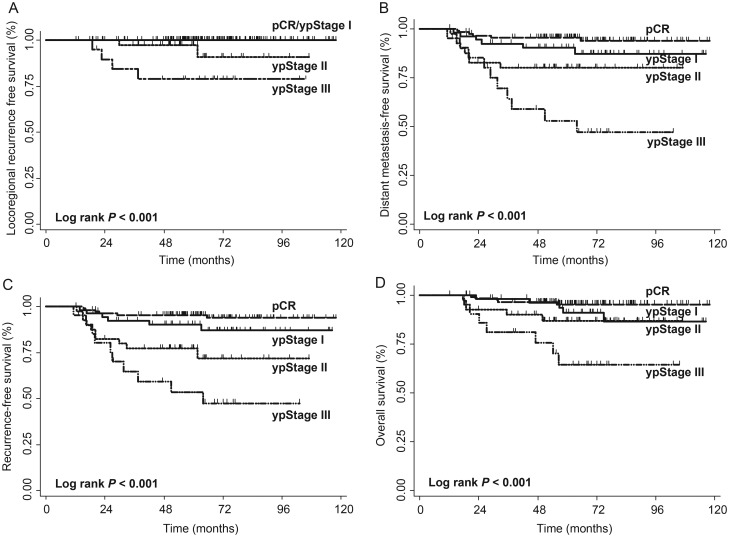
Following neoadjuvant therapy, 54 (23%) patients had pathologic stage I disease, 40 (18%) stage II, and 21 (9%) stage III disease. Survival outcomes were inversely correlated with increasing burden of remaining disease (Figure 2A–D).
Figure 2.
Kaplan–Meier curves of (A) locoregional recurrence-free, (B) distant metastasis-free, (C) recurrence-free, and (D) overall survival outcomes among pathologic stage groups (pCR, yp stage I–III).
Cox regression analysis for factors associated with distant relapse
The most significant predictor for distant metastasis on multivariate analysis was failure to achieve a pCR (adjusted hazard ration [HR] 3.75, 95% confidence interval [CI] 1.52–9.26, P = 0.004). Patients with clinical T3–4 compared with T1–2 disease, and patients with LVI, were more likely to have systemic relapse (adjusted HR 2.61, 95% CI 1.27–5.39, P = 0.009 and adjusted HR 2.13, 95% CI 1.02–4.46, P = 0.045, respectively). Assessment for interaction between pCR and ER/PR status on distant metastasis risk was not significant (P = 0.099). Whether trastuzumab was given for a full-year or less did not influence distant metastasis risk (HR 0.88, 95% CI 0.43–1.82, P = 0.727).
Cox regression analysis for recurrence-free and overall survival
Predictors of RFS and OS are listed in Table 3. Of evaluated factors, failure to achieve a pCR was the strongest predictor of disease recurrence and death. To a lesser degree, advanced clinical T-stage (T3–4) increased the hazard of recurrence and death compared with patients with stage T1–2 tumors, after controlling for other factors. LVI was a significant predictor of disease recurrence. No interaction was seen between ER/PR status and pCR on RFS and OS (P = 0.103 and 0.147, respectively).
Table 3.
Adjusted hazard ratios of factors predicting for any recurrence and all-cause mortality among all patients
| Characteristics | Adjusted hazard ratio (95% confidence interval) | P-value |
|---|---|---|
| Any recurrence | ||
| Pathologic response | ||
| pCR | 1.0 | – |
| <pCR | 4.09 (1.67–10.04) | 0.002 |
| Clinical T-stage | ||
| T1–2 | 1.0 | – |
| T3–4 | 2.23 (1.11–4.48) | 0.024 |
| LVI | ||
| No | 1.0 | – |
| Yes | 2.21 (1.08–4.51) | 0.029 |
| All-cause mortality | ||
| Pathologic response | ||
| pCR | 1.0 | – |
| <pCR | 4.15 (1.39–12.38) | 0.011 |
| Clinical T-stage | ||
| T1–2 | 1.0 | – |
| T3–4 | 2.89 (1.28–6.99) | 0.018 |
pCR, pathologic complete response; LVI, lymphovascular invasion.
Among pathologic stage groups, an increased risk of recurrence and death was seen among patients with increasing pathologic stage (Table 4). Compared with patients achieving a pCR, patients with pathologic stage II or III disease had a significantly higher risk of recurrence and death. No interaction between pCR and locoregional treatment on recurrence and survival outcomes was seen.
Table 4.
Adjusted hazard ratios of any recurrence and all-cause mortality by pathologic stage groupa
| Stage group | Any recurrence |
All-cause mortality |
||
|---|---|---|---|---|
| Adjusted hazard ratio (95% confidence interval) | P-value | Adjusted hazard ratio (95% confidence interval) | P-value | |
| pCR | 1.0 | – | 1.0 | – |
| yp Stage I | 2.18 (0.70–6.77) | 0.177 | 2.60 (0.70–9.70) | 0.154 |
| yp Stage II | 4.85 (1.74–13.51) | 0.003 | 3.84 (1.03–14.36) | 0.046 |
| yp Stage III | 7.83 (2.72–22.58) | <0.001 | 8.66 (2.48–30.26) | 0.001 |
aOther evaluated covariates did not reach statistical significance in the Cox regression model.
pCR, pathologic complete response.
discussion
The addition of trastuzumab to anthracycline and taxane-based chemotherapy in the neoadjuvant setting among women with HER2-positive breast cancer has resulted in pCR rates ranging from 32% to 66% and improvements in event-free survival [11–14]. In this study, we report the long-term benefit of achieving a pCR. Specifically, pCR was a significant predictor of DMFS, RFS, and OS. Moreover, an increased risk of recurrence and death was seen among patients with increasing burden of remaining disease after neoadjuvant therapy. After controlling for disease- and treatment-related factors, achieving a pCR was the strongest predictor of long-term outcome.
Our results are consistent with data from the TECHNO trial, a phase II non-randomized study [15] of 217 patients with HER2-overexpressing disease who received 6 months of neoadjuvant epirubicin and paclitaxel-based (Bristol-Myers Squibb, New York) chemotherapy and trastuzumab. In this trial, pCR was achieved in 39%. Disease-free and OS outcomes were superior among patients achieving a pCR compared with those with less than pCR, and pCR was the strongest prognostic factor for relapse and death. Locoregional and distant patterns of relapse were not described in that study. Similarly, in our study, we found that achieving a pCR was the strongest predictor of long-term disease control and survival, even after controlling for various disease and treatment-related factors. In addition, locoregional and distant relapse rates were encouragingly low after a median follow-up of 5 years.
Our study highlights the complex interplay between tumor biology, treatment response, and long-term clinical outcome. In our series, neoadjuvant trastuzumab-containing chemotherapy was effective in eradicating invasive disease in nearly 50% of patients and in our multivariate model, pCR retained a strong association with survival, even after controlling for standard clinical and pathologic prognostic factors. For patients not achieving a pCR, we found an inverse relationship between residual disease, as indicated by pathologic stage, and long-term disease control and survival. In the subset of patients with residual DCIS after neoadjuvant therapy, no differences in distant metastasis and OS outcomes were noted compared with those achieving eradication of invasive and non-invasive disease in the breast and axilla. This is consistent with a recent meta-analysis of 12 randomized neoadjuvant chemotherapy trials [16]. This analysis found that 13% of patients had a pCR defined as no residual invasive disease in the breast or axilla and 18% had a pCR defined as no residual invasive or in situ disease in the breast. A pCR by either definition predicted for improved event-free survival and OS. These data and our results are in contrast to a study by von Minckwitz et al. [17] that evaluated over 6000 patients enrolled on seven prospective trials of neoadjuvant chemotherapy and showed that patients with residual DCIS in the breast did worse with respect to disease-free survival and trended towards worse OS. In subset analyses evaluating 622 patients with HER2-positive disease that received trastuzumab as part of their neoadjuvant chemotherapy, a pCR defined as no residual invasive disease or DCIS in the breast and axillary lymph nodes was associated with improved disease-free and OS [18]. Although a higher percentage of patients with hormone receptor-negative tumors in our study achieved a pCR than those with hormone receptor-positive disease as seen in other studies [5, 19, 20], no interaction between hormone receptor status and pCR on distant metastasis, disease relapse, or OS was seen, highlighting the prognostic significance of pCR on long-term outcome independent of hormone receptor status. In addition, whether trastuzumab was delivered in the neoadjuvant setting alone or continued for 1 year did not impact relapse and survival outcomes in our study.
Limitations of this study include its retrospective design that did not permit the assessment of independent causality between pathologic response and outcome. Among the strengths of this study include specialized pathology review of all cases, consistent multidisciplinary care and median follow-up greater than 5 years during which most of the recurrences have been shown to occur in patients with HER2-overexpressing breast cancer.
In conclusion, achievement of pCR among patients with HER2-overexpressing breast cancer was the strongest predictor of disease control and survival. Given recent success of combined HER2-directed therapies in the neoadjuvant setting achieving even higher pCR rates [21], a greater benefit on long-term outcome would be predicted with this strategy. These findings underscore the potential value of pathologic response as an early surrogate of long-term outcome, and an important metric for future studies evaluating new HER2-targeted therapies. Consistent with this, the Food and Drug Administration has proposed [22] using pCR as a surrogate end point, allowing for accelerated approval of therapeutics. Such an approach would allow for the most promising investigational drugs to be rapidly incorporated into treatment algorithms maximizing benefit for breast cancer patients with high-risk disease.
funding
This work was supported in part by National Cancer Institute at the National Institutes of Health through the MD Anderson Institutional Core Training Grant (T32CA77050) and Institutional Cancer Center support grant (CA016672).
disclosure
Dr Hortoabgyi reports serving as a consultant for Allergen, Genentech, Novartis, and Sanofi. All remaining authors have declared no conflicts of interest.
references
- 1.Bonadonna G, Valagussa P. Primary chemotherapy in operable breast cancer. Semin Oncol. 1996;23:464–474. [PubMed] [Google Scholar]
- 2.Booser DJ, Hortobagyi GN. Treatment of locally advanced breast cancer. Semin Oncol. 1992;19:278–285. [PubMed] [Google Scholar]
- 3.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 4.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 5.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 9.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 11.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 14.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer. Clin Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 15.Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 16.Cortazar P, Zhang L, Untch M, et al. Meta-analysis Results from the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) Cancer Res. 2012;72(24 Suppl):93s. ; Abstract S1–11. [Google Scholar]
- 17.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 18.Loibl S, von Minckwitz G, Blohmer JU, et al. pCR as a surrogate in HER2-positive patients treated with trastuzumab. Cancer Res. 2011;71(24 Suppl):111s. ; Abstract S5–4. [Google Scholar]
- 19.Ring AE, Smith IE, Ashley S, et al. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004;91:2012–2017. doi: 10.1038/sj.bjc.6602235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 21.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]