Abstract
Background
Aromatase inhibitors (AIs) may cause a rise in estrogen levels due to ovarian function recovery in women with clinical chemotherapy-induced ovarian failure (CIOF). We carried out a prospective registry trial to identify predictors of ovarian function recovery during AI therapy.
Patients and methods
Women with hormone receptor (HR)-positive breast cancer who remained amenorrheic and had hormonal levels consistent with ovarian failure after adjuvant chemotherapy were enrolled in a multi-institutional clinical trial of anastrozole. Subjects underwent frequent assessment using an ultrasensitive estradiol assay. Multivariable analysis was used to evaluate clinical and biochemical predictors of ovarian function recovery within 48 weeks.
Results
Recovery of ovarian function during AI therapy was observed in 13 of 45 (28.9%) assessable subjects after a median 2.1 months (range 0.6–11.9). Median age at chemotherapy initiation was statistically significantly different between those who regained ovarian function (43 years, range 40–51) and those who remained postmenopausal (49 years, range 44–52; P < 0.0001).
Conclusions
A significant proportion of women with CIOF recover ovarian function during AI therapy, including a woman over age 50 at initiation of chemotherapy. Tamoxifen remains the standard of care for women with CIOF. If an AI is used, patients should be monitored frequently with high-quality estradiol assays.
Clinicaltrials.gov: NCT00555477.
Keywords: aromatase inhibitor, breast cancer, chemotherapy-induced ovarian failure, estradiol, ovarian function
introduction
Mortality from hormone receptor (HR)-positive breast cancer has been declining, in part because of adjuvant endocrine therapy [1]. Tamoxifen was the standard adjuvant endocrine therapy for decades in all women with HR-positive disease [1]. More recently, data from large, prospective, randomized, controlled trials have demonstrated superior progression-free and overall survival of aromatase inhibitor (AI) therapy compared with tamoxifen in postmenopausal women [2]. Despite this superiority, the standard of care for premenopausal women remains tamoxifen, because the AIs are ineffective in women with functional ovaries.
Patients with HR-positive disease at higher risk of disease recurrence are often treated with adjuvant chemotherapy before adjuvant endocrine therapy. The majority of premenopausal women treated with adjuvant chemotherapy will develop transient amenorrhea, and a subset will develop permanent chemotherapy-induced ovarian failure (CIOF) [3, 13–15]. These women may be appropriate candidates for therapy with AIs, but were not included in the large clinical trials evaluating upfront adjuvant AI therapy, and there are concerns about the potential safety of AI use in this population [4, 16, 17].
In 2006, a retrospective analysis of women age ≥40 with chemotherapy-induced amenorrhea revealed a 27% incidence of ovarian function recovery during AI therapy [4]. However, reliable predictors of ovarian function recovery have not been identified. In addition, assessment of ovarian function can be challenging, because many commercially available estradiol assays lack sensitivity and accuracy at very low serum estradiol concentrations [5].
Other methods for assessment of ovarian reserve have been reported, including evaluation of serum biomarkers, such as anti-Müllerian hormone (AMH), inhibin B and follicle-stimulating hormone (FSH) [6, 18]. These tests are routinely used for infertility evaluation of premenopausal women [19]. However, it is unknown whether these assessments may be useful for prediction of ovarian function recovery or for monitoring of ovarian function recovery during AI therapy in women with CIOF.
We initiated a clinical trial of anastrozole (Arimidex®, AstraZeneca, Wilmington, DE) therapy in pre- and perimenopausal women with biochemically confirmed CIOF in order to prospectively evaluate ovarian function recovery, as determined by a commercially available ultrasensitive estradiol assay. The primary objective was to prospectively estimate the incidence of ovarian function recovery during AI therapy, and to evaluate clinical and biochemical predictors of ovarian function recovery during treatment.
methods
patients
Women with HR-positive stage I–III breast cancer who were pre- or perimenopausal at the time of cyclophosphamide-based chemotherapy initiation, became amenorrheic during or within 8 weeks of completing chemotherapy, and remained amenorrheic since chemotherapy were eligible. Patients must have been at the age of ≤57 at the time of chemotherapy initiation and age ≤60 at the time of enrollment. Pre- or peri-menopausal was defined as the occurrence of menses within 6 months before starting chemotherapy. For patients who had undergone hysterectomy without bilateral oophorectomy, serum estradiol and FSH had to be consistent with premenopausal status according to the institutional standards within 6 months before chemotherapy initiation.
All indicated surgery was completed before enrollment; concurrent radiation therapy and trastuzumab were permitted. Chemotherapy was completed at least 8 weeks before enrollment. Prior adjuvant tamoxifen was allowed; prior AI therapy was not permitted. No supplemental oral, topical, or vaginal estrogenic medications were permitted within 4 weeks of enrollment or during study participation. Patients were excluded if they had received cytotoxic chemotherapy before breast cancer diagnosis; prior bilateral oophorectomy or pelvis radiation; known hepatic failure; ECOG performance status >2; or if pregnant or nursing. The clinical trial was approved by the Institutional Review Board at all three participating institutions, and all enrolled subjects provided written informed consent.
study design, part 1
In the initial version of the clinical trial (designated part 1), subjects were required to have serum estradiol and FSH concentrations within the postmenopausal range according to local institutional guidelines within 28 days of enrollment. Subjects taking tamoxifen at the time of screening were only required to have postmenopausal serum estradiol concentrations.
Enrolled subjects underwent baseline evaluation using a high-throughput liquid chromatography (HTLC)-tandem mass spectroscopy (MS/MS) ultrasensitive estradiol assay (Quest Diagnostics, #30289, level of quantification 2 pg/ml), which can provide clinical results in 4–5 days to permit treatment-decision making [7]. Subjects initiated anastrozole 1 mg orally daily if baseline serum estradiol was ≤20 pg/ml. Estradiol assessment was carried out 2, 4, 6, 8, 10, 12, 16, 20, 24, 28, 36, 48, 60, and 72 weeks after anastrozole initiation, or if the patient experienced vaginal bleeding. Anastrozole was continued if serum estradiol remained <10 pg/ml, and discontinued if serum estradiol increased >20 pg/ml. If serum estradiol was 10–20 pg/ml, the level was rechecked within 1 week. If the repeat serum estradiol was ≥10 pg/ml, the subject discontinued study participation.
study design, part 2
Analysis of the first 18 subjects enrolled revealed a greater than expected discontinuation of AI therapy because of elevated serum estradiol concentrations. Discontinuation was believed to be primarily due to greater than expected variability in the assay as opposed to true recovery of ovarian function. Therefore, the protocol was amended.
Serum ultrasensitive estradiol concentrations were assessed in duplicate. Subjects with an average baseline estradiol concentration of ≤20 pg/ml were considered eligible and initiated treatment with anastrozole 1 mg orally daily. On day 14 after anastrozole initiation, if average estradiol was >30 pg/ml, subjects discontinued study participation; otherwise, anastrozole was continued (see supplementary Methods, available at Annals of Oncology online).
laboratory assessments
Serum samples for the correlative biomarker studies were collected at baseline and at weeks 2, 4, 12, 24, and 48. See supplementary Methods, available at Annals of Oncology online, for details about correlative assay methods and additional evaluation of the Quest estradiol assay.
statistical analysis
The primary end point was ovarian function recovery, defined as average estradiol concentration ≥30 pg/ml or return of menses, within 48 weeks of AI initiation. Isolated vaginal spotting was not included in the primary end point. Because of poor accrual, the trial was closed to enrollment after 69 of a planned 150 patients were enrolled. Univariate analysis was used to study associations between ovarian function recovery and clinical and biochemical factors. For continuous covariates (age at AI or chemotherapy initiation, time since chemotherapy, and baseline serum FSH and average estradiol concentrations), t-tests were used to compare the difference between the groups with and without ovarian function recovery. For categorical covariates [body mass index (BMI; <25, 25–30, >30), prior tamoxifen (yes versus no), and regularity of menses at chemotherapy initiation (regular versus irregular)), Fisher's exact tests were used to examine the differences. Multivariate logistic regression was used to model the probability of recovering ovarian function using the same covariates listed above.
The analyses were carried out using SAS (9.2) and R (2.12.2). P < 0.05 was considered statistically significant.
results
patient characteristics
This clinical trial was conducted in two parts, as described in the Methods section (supplementary Figure S1, available at Annals of Oncology online). Sixty-nine subjects enrolled; 59 initiated treatment with anastrozole (part 1: 14 patients; part 2: 45 patients; Table 1). Of the subset treated in part 2, the median age at chemotherapy initiation was 48.7 (range 40.3–55.3) and the median age at study enrollment was 50.3 years (range 40.7–56.8).
Table 1.
Patient characteristics
| Characteristic | Part 1 |
Part 2 |
||||
|---|---|---|---|---|---|---|
| All patients (n = 14) | Ovarian function recoverya (n = 8) | No ovarian function recoveryb (n = 4) | All patients (n = 45) | Ovarian function recovery (n = 13) | No ovarian function recovery (n = 19) | |
| Median age at chemotherapy, years (range) | 45 (37–51) | 44 (37–51) | 47 (44–49) | 48 (40–55) | 43 (40–51) | 49 (44–52) |
| Median age at AI therapy, years (range) | 46 (44–52) | 47 (44–52) | 48 (44–50) | 50 (40–56) | 45 (40–56) | 50 (46–55) |
| Mean BMI (SD) | 27.6 (5.7) | 28.1 (6.1) | 27.0 (3.7) | 27.6 (7.8) | 29.2 (12.1) | 26.7 (4.8) |
| Prior chemotherapy regimen | ||||||
| No taxane | 3 (21%) | 3 (38%) | 0 | 9 (20%) | 2 (15%) | 3 (16%) |
| Taxane-based | 11 (78.6%) | 5 (62%) | 4 (100%) | 36 (80%) | 13 (85%) | 16 (84%) |
| Median time since chemotherapy initiation, years (range) | 0.8 (0.4–6.4) | 2.7 (0.5–6.4) | 0.7 (0.6–1.0) | 1.4 (0.3–5.9) | 0.6 (0.4–4.8) | 2.0 (0.4–5.9) |
| Prior tamoxifen, no (%) | 7 (50%) | 6 (75%) | 1 (25%) | 28 (62%) | 7 (54%) | 14 (74%) |
| Mean baseline estradiol, pg/ml (SD) | 5.7 (4.9) | 4.4 (4.6) | 5.3 (4.3) | 8.4 (7.8) | 9.5 (8.5) | 8.4 (8.4) |
| Mean baseline FSH (SD) | 49.7 (18.0) | 45.1 (16.8) | 58.8 (19.0) | 56.4 (31.1) | 55.8 (28.4) | 54.2 (28.2) |
| prior tamoxifen | 38.3 (11.6) | 39.6 (12.2) | 30.8 | 43.3 (23.1) | 51.7 (30.7) | 44.9 (24.5) |
| no prior tamoxifen | 65.5 (12.0) | 61.6 (22.2) | 68.1 (4.0) | 78.5 (29.4) | 80.3 (14.8) | 78.4 (24.1) |
| Mean baseline AMH | <0.17 | <0.17 | <0.17 | <0.17 | <0.17 | <0.17 |
| Mean baseline Inhibin B | <10 | <10 | <10 | 10.1 (0.4) | 10.3 (0.8) | <10 |
| Time to primary end point, months (range) | 1.8 (0.7–3.8) | NA | 2.1 (0.6–11.9) | NA | ||
aOvarian function recovery defined as elevated estradiol or recurrent menses.
bOnly includes subjects who remained on protocol-directed therapy for at least 48 weeks without evidence of ovarian function recovery.
AI, aromatase inhibitor; AMH, anti-Mullerian hormone; BMI, body mass index; FSH, follicle-stimulating hormone; SD, standard deviation; NA, not applicable.
incidence of recovery of ovarian function
In part 1, we used a conservative definition of ovarian function recovery. Eight of the 14 treated subjects (57%) met the definition of ovarian function recovery; estradiol concentrations at the time of treatment discontinuation ranged from 10 to 158 pg/ml, and 37.5% were <20 pg/ml (supplementary Figure S2A, available at Annals of Oncology online). None discontinued therapy because of recurrent menses. The median time to ovarian function recovery was 1.8 months (range 0.7–3.8; Table 1).
Because of the high frequency of subjects who met the criteria for ovarian function recovery at low serum estradiol concentrations in part 1, the definition was revised. Of the 45 treated subjects in part 2, 13 (29%) met the new definition of the primary end point, with average estradiol concentrations ranging from <2 to 368 pg/ml at treatment discontinuation (supplementary Figure S2B, available at Annals of Oncology online; supplementary Table S1, available at Annals of Oncology online). Three subjects had simultaneous bleeding and increased estradiol levels. One subject discontinued therapy because of persistent vaginal bleeding of uncertain etiology, despite no concomitant increase in the estradiol level. The median-time to ovarian function recovery in part 2 was 2.1 months (range 0.6–11.9; Table 1).
Nineteen (42.2%) women remained on anastrozole for at least 48 weeks without ovarian function recovery. The other 13 (28.9%) women withdrew from study participation before the 48 week time point for the reasons listed in supplementary Figure S1, available at Annals of Oncology online, after a median 5.6 months (range 1.0–10.1); none experienced increased estradiol concentrations during study participation.
vaginal bleeding during study participation
Of the 59 treated subjects, 10 (17%) reported vaginal bleeding during study participation (supplementary Table S2, available at Annals of Oncology online). As mentioned above, three subjects reported bleeding concurrent with elevated estradiol concentration. The other seven subjects had postmenopausal estradiol concentrations at the time the bleeding occurred. As described above, one discontinued study participation because of persistent menses; the other six subjects reported isolated episodes of spotting and continued study participation.
change in biochemical markers of ovarian reserve during study participation
Serum AMH concentrations were undetectable in all subjects at all time points. At baseline, serum inhibin B concentrations were undetectable in all but two subjects. During AI treatment, three subjects had intermittently detectable inhibin B levels, including two who discontinued therapy because of elevated estradiol levels and one who completed protocol-directed therapy.
Baseline serum FSH concentrations were not statistically significantly different between those subjects who recovered ovarian function and those who remained postmenopausal, even after accounting for tamoxifen therapy (Table 1). When analyzed over time, there were no significant differences in FSH levels between those who recovered ovarian function and those who remained postmenopausal (data not shown).
predictors of ovarian function recovery
Analysis of predictors of ovarian function recovery was limited to the subjects enrolled in part 2. Women with younger age at initiation of either chemotherapy or AI therapy were more likely to experience recovery of ovarian function (P < 0.0001 and P = 0.0001, respectively). BMI, prior tamoxifen, regularity of menses and baseline estradiol, AMH, FSH and inhibin B concentrations were not predictive of recovery of ovarian function.
Multivariate analysis was carried out using the same potential predictors. When age at chemotherapy initiation was included in the model, only younger age was significantly predictive of increased likelihood of recovery of ovarian function [adjusted odds ratio 0.48 (95% CI 0.30–0.77), P = 0.0022; Table 2]. Almost identical findings were obtained when the age at AI therapy initiation was included in the model (supplementary Table S3, available at Annals of Oncology online).
Table 2.
Multivariable analysis of predictors of recurrence of ovarian function during aromatase inhibitor (AI) therapy in women with chemotherapy-induced ovarian failure (CIOF)
| Predictor | Odds ratio | P value |
|---|---|---|
| Age at chemotherapy | 0.48 (0.30–0.77) | 0.0022 |
| Time since chemotherapy | 0.90 (0.35–2.28) | 0.81 |
| BMI 25–30 versus <25 | 1.91 (0.16–23.22) | 0.61 |
| >30 versus <25 | 1.50 (0.13–17.87) | 0.75 |
| Menses at chemotherapy (irregular versus regular) | 0.21 (0.01–5.60) | 0.35 |
| Prior tamoxifen (yes versus no) | 0.29 (0.02–5.62) | 0.41 |
| Baseline estradiol | 0.91 (0.74–1.11) | 0.34 |
BMI, body mass index.
concordance between estradiol assessments
In part 2, serum estradiol concentrations were assessed in duplicate using the ultrasensitive estradiol assay (HTLC-MS/MS) at each time point (n = 465 duplicate samples) to evaluate concordance for samples analyzed using the same methodology. The concordance correlation coefficient was 0.96 (r2 = 0.92, P < 0.0001). There were a few significantly discordant values, with one measurement in the postmenopausal range and the other in the premenopausal range (supplementary Figure S3, available at Annals of Oncology online).
In a separate patient cohort of postmenopausal women (see supplementary Methods, available at Annals of Oncology online), concordance between the ultrasensitive estradiol assay (HTLC-MS/MS) and both a standard estradiol assay (MLabs) and a ‘gold standard’ research estradiol assay (GC-MS/MS, PharmaNet) was assessed [8]. Higher concordance rates were noted for the comparison between the two MS-based assays (supplementary Table S4, available at Annals of Oncology online). The majority of values with both the MS-based assays were within the postmenopausal range (Pearson's r = 0.77; P < 0.0001), as expected, although there were numerically more outliers with the HTLC-MS/MS assay than with the GC-MS/MS assay (supplementary Figure S4, available at Annals of Oncology online).
discussion
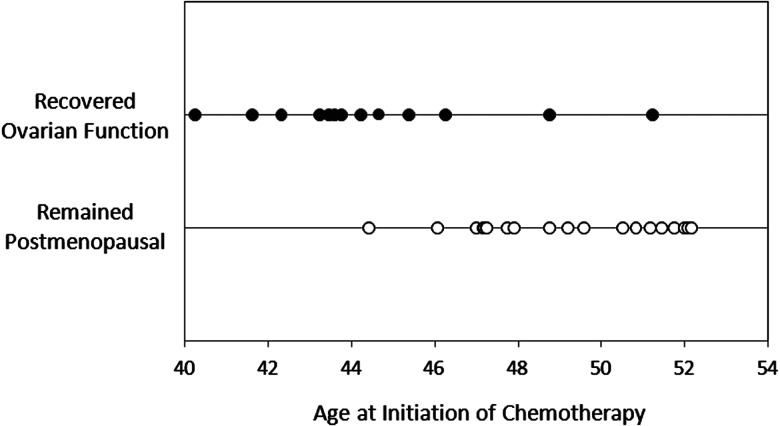
In this prospective clinical trial of older pre- and perimenopausal women with biochemically confirmed CIOF, about one-quarter of women recovered ovarian function during AI therapy as defined in the protocol. Most ovarian function recovery occurred in younger women, although an elevated estradiol concentration was detected in 1 of 16 (6.3%) AI-treated women age >50 at the time of chemotherapy initiation (Figure 1), and in 2 of 12 (16.7%) with irregular menses (and presumed to be perimenopausal) at the time of chemotherapy initiation. In addition, although most ovarian function recovery occurred within the first 3 months of AI initiation, one patient developed increased estradiol 48 weeks following AI initiation. Of the 13 subjects who discontinued AI therapy because of ovarian function recovery, 10 (77%) were detected through laboratory assessment alone and had not experienced recurrent vaginal bleeding.
Figure 1.
Age at chemotherapy initiation for each subject with (solid circle) and without (open circle) recovery of ovarian function. Each circle represents an individual subject.
Our reported findings of an ∼25% incidence of ovarian function recovery during AI therapy are similar to the retrospective results from Smith et al. [4] as well as a prospective Spanish study by Guerrero et al. [9]. Our study differs substantially from the latter study, however, since the majority of our patients were incidentally found to have increased estradiol levels during monitoring, whereas most of the patients on the Spanish study reported recurrent menses. This likely explains the shorter time to ovarian function recovery demonstrated in our study (2.1 months) compared with the Spanish study (5.7 months). In our study, menstrual bleeding was neither sensitive nor specific for assessing ovarian function.
Our findings highlight the need for close monitoring of serum estradiol levels in patients at risk of ovarian function recovery, rather than following patients clinically for return of menstrual bleeding, in order to minimize the duration of treatment with a potentially ineffective therapy. Unfortunately, monitoring patients at risk of ovarian function recovery is compounded by the poor performance of standard estradiol assays, the methodology available at most laboratories, for quantification of very low estradiol concentrations in AI-treated women [5, 10]. Ultrasensitive assays that incorporate tandem mass spectroscopy have been shown to be more sensitive than standard estradiol assays at very low estradiol concentrations [7, 8]. A few ultrasensitive assays, including the one used in this clinical trial, are available commercially for the clinical care of patients with a relatively short turnaround time. However, discordant results can still be obtained (supplementary Figures S3 and S4, available at Annals of Oncology online).
To address the challenge of accurate ovarian function assessment in AI-treated patients, we evaluated other potential baseline predictors of ovarian function recovery. Serum estradiol concentration at the time of AI initiation is not predictive of ovarian function recovery. Based on our findings, it also appears that serum AMH, FSH, and inhibin B concentrations are not by themselves predictive, and do not increase the predictive value of estradiol. In fact, serum AMH concentration appears to be particularly uninformative in this patient population, since all values were below the level of detection, consistent with the findings reported by others [17]. In addition, AMH levels decline over time and can be difficult to detect in women over age 40, even in the absence of chemotherapy. However, since pre-chemotherapy AMH has been shown to predict long-term ovarian function [11], it remains possible that assessment of these markers before chemotherapy may be useful for predicting post-chemotherapy ovarian function recovery during AI therapy.
For women receiving tamoxifen, serum FSH levels are frequently suppressed, which makes interpretation of the value difficult. Our results suggest that FSH is not predictive of ovarian function recovery. In fact, since multiple subjects who ultimately recovered ovarian function had baseline FSH values between 60 and 100 mIU/mL, well within the postmenopausal range, it does not appear that a high baseline FSH measurement reliably indicates that a patient is permanently postmenopausal.
A limitation of the study is small sample size, which is partially a reflection of the inconvenience associated with obtaining frequent blood draws in patients on an oral therapy. Furthermore, due to the high incidence of discontinuation early in the study (part 1), we changed the definition of the primary end point mid-way through the trial. Therefore, we limited analysis of potential clinical and biochemical predictive factors to patients enrolled in part 2, further limiting the evaluable sample size.
In summary, tamoxifen remains the standard of care for women with HR-positive breast cancer who were pre- or perimenopausal at the time of chemotherapy initiation. If up-front AI therapy is preferable, for example in women with a history of venous thromboembolic disease, or if women are considering a switch from tamoxifen to AI therapy [12], considerable caution should be exercised. Patients should be monitored frequently with ultrasensitive estradiol assays, especially within the first few months of AI initiation, although the optimal frequency and duration of monitoring remain unclear.
funding
This work was supported by the National Cancer Institute at the National Institutes of Health [grant number 5K07CA134747] to NLH; AstraZeneca; the Fashion Footwear Association of New York/QVC Presents Shoes on Sale™ to DFH and the University of Michigan Comprehensive Cancer Center to NLH. NLH received research funding from AstraZeneca, Lilly Pharmaceuticals and sanofi aventis. DFH received research funding from AstraZeneca. DFH and VS received research funding from Novartis and Pfizer.
disclosure
AstraZeneca provided the study medication. VS received honoraria from AstraZeneca. All the remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank, and in memoriam of, the late Dr MaryFran Sowers for her input and thoughtful comments at the time of study design. The authors also appreciate the insightful comments of Dr Mitch Dowsett during the design, conduct, and analysis of this trial. They also thank the participating patients and research study staff at each of the clinical trial sites.
references
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. doi:10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. doi:10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 3.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. doi:10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 4.Smith IE, Dowsett M, Yap Y-S, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. JCO. 2006;24:2444–2447. doi: 10.1200/JCO.2005.05.3694. doi:10.1200/JCO.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 5.Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev. 2007;16:1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. doi:10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 6.Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–3483. doi: 10.1210/jc.2008-0567. doi:10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–384. doi: 10.1373/clinchem.2003.025478. doi:10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 8.Santen RJ, Demers L, Ohorodnik S, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. doi:10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Guerrero A, Gavila J, Folkerd E, et al. Incidence and predictors of ovarian function recovery (OFR) in breast cancer (BC) patients with chemotherapy-induced amenorrhea (CIA) who switched from tamoxifen to exemestane. Ann Oncol. 2013;24:674–679. doi: 10.1093/annonc/mds464. [DOI] [PubMed] [Google Scholar]
- 10.Dowsett M, Folkerd E. Deficits in plasma oestradiol measurement in studies and management of breast cancer. Breast Cancer Res. 2005;7:1–4. doi: 10.1186/bcr960. doi:10.1186/bcr960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RA, Cameron DA. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. doi:10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 12.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. doi:10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- References 13–19 are listed in the supplemental references list available at Annals of Oncology online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.