Abstract
Background
There is currently no standard of care for the second-line treatment of advanced pancreatic cancer. The aim of this analysis was to compare the different therapeutic approaches in this setting.
Methods
We carried out a systematic analysis of second-line studies in advanced pancreatic cancer that have progressed on or following gemcitabine and published or presented from 2000 to 2012.
Results
Forty-four clinical trials (t) were identified; of which 34 met the inclusion criteria treating an aggregate total of 1503 patients (n). Patients who received treatments (t: 33; n: 1269) had a median overall survival (OS) of 6 months compared with 2.8 months for patients who received best supportive care only (t: 2; n: 234) (P = 0.013). The gemcitabine and platinum-based combination (t: 5; n: 154) provided a median progression-free survival and OS of 4 and 6 months compared with 1.6 and 5.3 for the rest of the regimens (t: 29; n: 1349) (P = 0.059 and 0.10, respectively) and 2.9 and 5.7 for the combination of 5-fluorouracil and platinum agents (t: 12; n: 450) (P = 0.60 and 0.22, respectively).
Conclusion(s)
Although not conclusive, these data showed that the advantage of second-line chemotherapy in pancreatic cancer is very limited and there is a need for more studies.
Keywords: analysis, cancer, pancreatic, review, second-line, treatment
introduction
Pancreatic cancer has an estimated 5-year survival rate of 5%–6% and the majority of patients present with unresectable disease [1, 2]. For the past 10–15 years, gemcitabine has been considered the front-line chemotherapy in both locally advanced and metastatic disease due to its positive effect on quality of life and—to a lesser extent—overall survival [3]. While gemcitabine-based combinations have not been shown to be unequivocally more effective compared with gemcitabine alone, several analyses have suggested benefit in defined subpopulations such as patients with good performance status (PS) and metastatic disease [4–6]. Recently, FOLFIRINOX has emerged as an alternative to gemcitabine in the first-line setting after demonstrating superior survival outcome (median OS 11.1 versus 6.8 months, P < 0.001) [7]. However, this regimen is not suitable for patients with poor performance status (PS) and for these patients gemcitabine-based therapy will remain a favorable first-line option [7, 8]. In the second-line setting, there is no consensus on the optimal treatment. This is due, in part, to the paucity of trials in this patient population. In addition, only ≤50% of patients who fail first-line treatment are still physically fit enough to be offered second-line treatment [4, 7]. It has also not been unequivocally established that chemotherapy provides better efficacy compared with best supportive care (BSC), since studies that tried to address this question were underpowered and poorly designed [9, 10]. To further address these questions, we carried out a comprehensive analysis of the second-line trials in locally advanced or metastatic pancreatic cancer.
methods
The primary objectives of this study were to determine whether treatment provides any superior effect over BSC and to determine the regimen that provides the best outcome. Secondary objectives were to compare the outcome of platinum-based compounds in combination with either gemcitabine or 5-fluorouracil (5-FU) and to determine the trend of treatment outcomes over time. We identified the data for this analysis by performing a PubMed search using the term ‘second-line therapy AND advanced pancreatic cancer’. In addition, we reviewed the references of the relevant articles and the abstracts presented in ASCO, GI ASCO, ESMO, ECCO, and WCGC. Searches were limited to human studies published in English from 2000 to 2012. Exclusion criteria were trials that used chemotherapy other than gemcitabine in the first-line setting, novel investigational or targeted agents other than erlotinib in the second-line setting. Targeted agents were excluded since they represent a class of drugs with different mechanisms of action. However, since erlotinib is the only targeted agent that showed a survival benefit in the first-line setting [11], trials that used erlotinb were included. The following details were extracted: study start and completion dates, number of patients, second-line regimen, and outcomes, including the percentage of responders or the response rate (RR), the median progression-free survival (PFS), and overall survival (OS). In the trials that included more than one arm, each arm was analyzed separately.
statistical analysis
For each trial or arm in the analyses, using results as presented in the relevant publications, the percentage who responded (RR), the median PFS and the median OS were obtained and used as the primary data being analyzed. In an exploratory manner, we compared the distributions of those three outcome variables (RR, PFS, and OS) according to the following categorical variables with the Wilcoxon rank sum test: BSC versus all others, 5-FU plus platinum agents versus all others (but excluding BSC), gemcitabine plus platinum agents versus all others (but excluding BSC), taxane-based regimens versus all others (but excluding BSC), erlotinib-based regimens versus all others (but excluding BSC), and gemcitabine plus platinum agents versus 5-FU plus platinum agents. Exact tests were used as appropriate. All reported P-values are two tailed. In view of the number of tests carried out, we considered P < 0.005 as statistically significant, while 0.005 < P < 0.05 indicated a strong statistical trend.
results
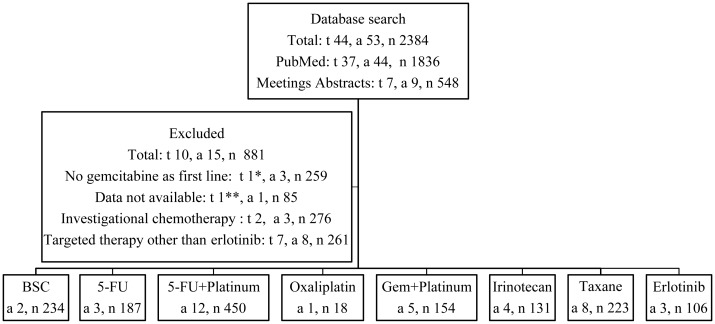
The results of the search identified 38 published trials and 6 abstracts presented at scientific meetings. These 44 trials (t) comprised of 53 arms (a) and treated an aggregate total of 2384 patients (n). Out of these 44 trials, 7 used targeted therapy other than erlotinib [12–18], 2 used novel investigational chemotherapy [19, 20], and the efficacy data were not reported in one trial [7]. Therefore, only 34 trials met the inclusion criteria [9, 10, 21–52] comprising of 38 arms and treating an aggregate total of 1503 patients. The search results are summarized in Figure 1 and supplementary Table S1, available at Annals of Oncology online .
Figure 1.
Study selection. t, number of trials; a, number of arms; n, number of treated patients; BSC, best supportive care; 5-FU, 5-fluorouracil; Gem, gemcitabine. In the trial by Conroy et al., patients were randomized to two arms FOLFIRINOX or gemcitabine then received a second-line of gemcitabine if they progressed on FOLFIRINOX* or 5-FU-based regimen if they progressed on gemcitabine**.
BSC versus treatments
In order to determine whether second-line treatment has any impact on outcome, we reviewed the clinical trials that included a BSC arm in their designs. Two phase III trials compared BSC to ‘active’ treatments [9, 10]. The first study by the German CONKO-study group was a phase III trial that randomized patients in a 1 : 1 ratio to BSC or OFF (oxaliplatin, folinic acid, and 5-FU) [10]. A total of 165 patients were required to demonstrate a statistical difference in survival. However, only 46 patients were accrued and this trial was terminated early. Patients on the OFF arm (n: 23) had median OS of 4.82 months compared with 2.30 months in the BSC arm (n: 23) (P = 0.008). In the second study by Jacobs et al., the physician's best choice (BC) including BSC (n: 211) was compared with rubitecan, an oral topoisomerase I inhibitor that showed promising activity in previous studies (n: 198) [9]. The majority of patients on the BC arm (89%) received alternative chemotherapy leaving only 11% of patients (n: 23) to receive BSC only. In addition, 49% of patients on the BC arm crossed over to the rubitecan arm at time of progression. This trial reported no significant difference in median OS between BC and rubitecan (3.3 versus 3.8 months, P = 0.62). Patients who crossed over to the rubitecan arm had a longer median survival compared with patients who did not (5.2 versus 2 months, P < 0.0001). In our analysis, we compared the outcomes of BSC in these two trials (a: 2; n: 234) to the outcomes of all treatments administered in the remaining 36 analyzed arms (a: 36; n: 1269). We found a trend toward an improved OS with treatments compared with BSC only (P = 0.013). However, there was no statistical difference in RR or PFS (P = 0.20 and 0.26, respectively) (Figures 2–4).
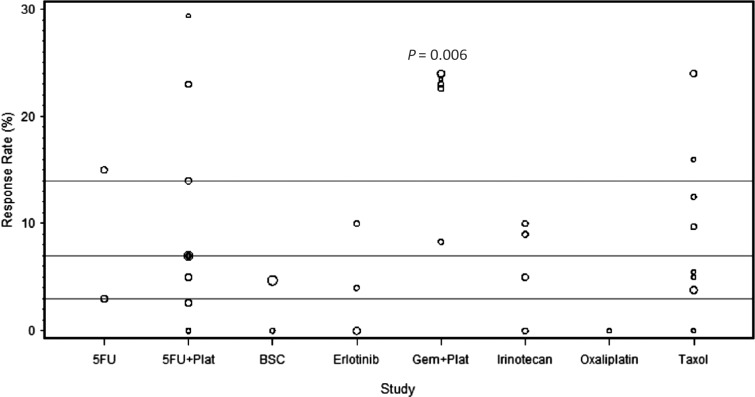
Figure 2.
The response rate (RR) of each of the analyzed studies presented as dot plots. The three horizontal lines in each figure represent the quartiles of all the data combined. Circle size is proportional to the number of patients on each trial. The combination of gemcitabine (Gem) and platinum agents (Plat) provided a trend toward an improved RR (P = 0.006) compared with the other regimens.
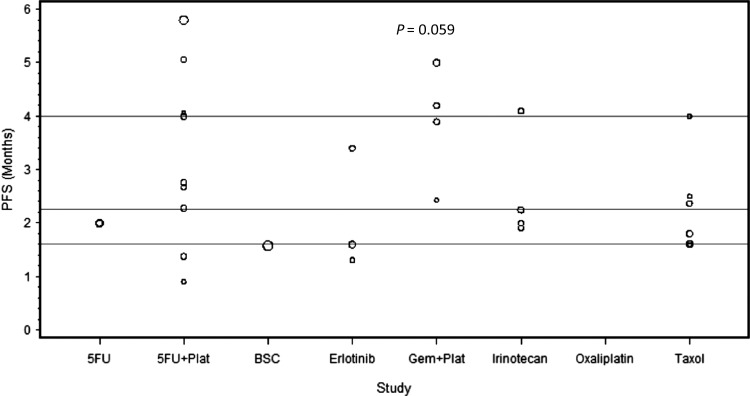
Figure 3.
The median progression-free survival (PFS) of each of the analyzed studies presented as dot plots. The three horizontal lines in each figure represent the quartiles of all the data combined. Circle size is proportional to the number of patients on each trial. The combination of gemcitabine (Gem) and platinum agents (Plat) provided a trend toward an improved PFS (P = 0.059) compared with the other regimens.
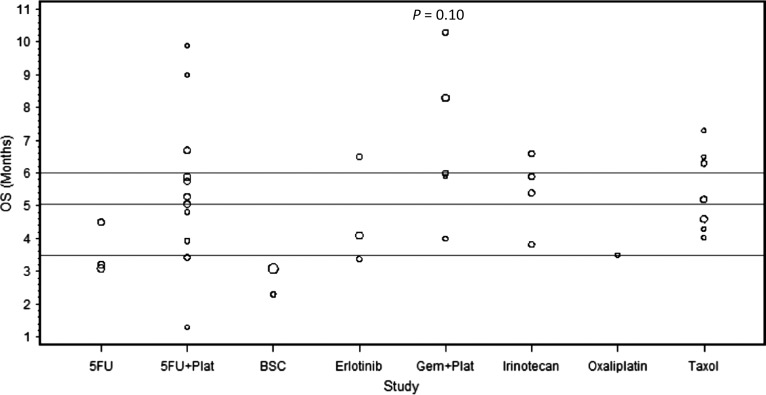
Figure 4.
The median overall survival (OS) of each of the analyzed studies presented as dot plots. The three horizontal lines in each figure represent the quartiles of all the data combined. Circle size is proportional to the number of patients on each trial. The combination of gemcitabine (Gem) and platinum agents (Plat) did not provide a significant improvement in OS (P = 0.10) compared with the rest of the regimens.
5-FU in combination with platinum agents versus other treatments
The combination of platinum agents and 5-FU has shown activity in several GI malignancies including esophageal, gastric, and colorectal cancers [53, 54]. We examined the activity of this combination in the second-line setting in pancreatic cancer. Twelve trials evaluated the efficacy of 5-FU in combination with either oxaliplatin or cisplatin, treating a total of 450 patients. Oxaliplatin was combined with either 5-FU—in 8 trials (n: 279) [10, 21, 22, 26–28, 31, 33]—or capecitabine—in 2 trials (n: 54) [29, 34]. Two trials used cisplatin in combination with either 5-FU (n: 100) [32] or S-1 (n: 17) [30]. The median number of treated patients per trial was 30 with a range of (15–100). Of these 12 trials, the CONKO-003 trial was the only phase III randomized study comparing OFF (oxaliplatin, folinic acid, and 5-FU) to FF (folinic acid and 5-FU) [22]. The CONKO-003 trial showed a survival benefit of adding oxaliplatin to 5-FU (5.89 versus 3.09 months, P = 0.01). In our analysis, the combination of 5-FU and platinum agents provided a median RR of 7% with a range of (0–29.4). The median PFS and OS were 2.9 and 5.7 months with a range of (0.9–5.8) and (1.3–10.7), respectively. The combination of 5-FU and platinum agents (a: 12, n: 450) did not show superior outcomes compared with the rest of the treatments (a: 26, n: 1053) in terms of RR, PFS or OS (P = 0.50, 0.27, 0.76, respectively) (Figures 2–4).
gemcitabine in combination with platinum agents versus other treatments
Combined analyses have suggested a potential survival benefit from adding platinum agents to gemcitabine compared with gemcitabine alone in the first-line setting in advanced pancreatic cancer [4–6]. We sought to determine the efficacy of this combination in the second-line setting. Five trials investigated the effect of adding platinum agents to gemcitabine after disease progression on gemcitabine, treating a total of 154 patients. Gemcitabine was combined with oxaliplatin in 2 trials (n: 50) [45, 49]; while the remaining three trials investigated the combination of gemcitabine with liposomal cisplatin (n: 24) [46], cisplatin plus 5-FU and epirubicin (n: 46) [47], or cisplatin plus 5-FU and irinotecan (n: 34) [48]. Gemcitabine was administered as a fixed dose rate (FDR) of 10 mg/m2/min in four trials [45, 47–49] and as a standard infusion rate over 30-min in one trial [46] (supplementary Table S2, available at Annals of Oncology online). The median number of treated patients per trial was 33 with a range of (17–46). The RR ranged from 8.3 to 24% with a median of 23%. The median PFS and OS were 4 and 6 months with a range of (2.4–5) and (4–10.3), respectively. When compared with other treatments (a: 33, n: 1349) the combination of gemcitabine and platinum agents (a: 5, n: 154) provided a trend toward an improved RR and PFS (P = 0.006 and 0.059, respectively) with no significant improvement in OS (P = 0.10). When compared with 5-FU in combination with platinum agents (a: 12, n: 450), the combination of gemcitabine and platinum agents (a: 5, n: 154) showed a strong trend toward an improved RR (P = 0.03) with no difference in PFS or OS (P = 0.60, 0.22, respectively) (Figures 2–4).
taxane-based regimens versus other treatments
Taxane-based chemotherapy is considered the standard of care in many malignancies including breast and lung cancers [55, 56]. We analyzed the activity of this treatment in the second-line setting in pancreatic cancer. Seven trials used taxane-based regimens treating a total of 223 patients. Of these seven trials, only one treated patients on two arms, irinotecan plus raltitrexed (n: 19) versus raltitrexed alone (n: 19) [38]. Taxane was used as a single agent in four trials (n: 108) [38–40, 44] and in combination with either capecitabine—in two trials (n: 55) [41, 43]—irinotecan (n: 19) [38] or oxaliplatin (n: 41) [42]—in 2 trials. The median number of treated patients per trial was 21 with a range of (18–52). The RR ranged from 0 to 24% with a median of 8.7%. The median PFS and OS were 2 and 5.2 months with a range of (1.6–4) and (4.3–7.3), respectively. Our analysis showed no superior outcomes for taxane-based therapy (a: 8, n: 223) in comparison with other regimens (a: 30, n: 1280) in terms of RR, PFS, or OS (P = 0.81, 0.33, 0.59, respectively) (Figures 2–4).
erlotinib versus other treatments
Erlotinib is the only targeted agent that showed a survival benefit when combined with gemcitabine in the first-line setting [11]. In an attempt to identify the activity of this agent in the second line, we analyzed the three trials that used erlotinib in this setting and treated a total of 106 patients. One trial used erlotinib as a single agent (n: 50) [51], while two trials used erlotinib in combination with capecitabine (n: 30) [50] or bevacizumab (n: 26) [52]. The median number of treated patients per trial was 30 with a range of (26–50). The RR ranged from 0 to 10% with a median of 4%. The median PFS and OS were 1.6 and 4.1 months with a range of (1.4–3.4) and (3.7–6.5), respectively. Our analysis demonstrated that erlotinib-based regimens (a: 3, n: 106) failed to show any statistical significant improvement in RR, PFS, or OS when compared with the other regimens (a: 35, n: 1397) (P = 0.39, 0.21, 0.52, respectively) (Figures 2–4).
treatment effect trend over time
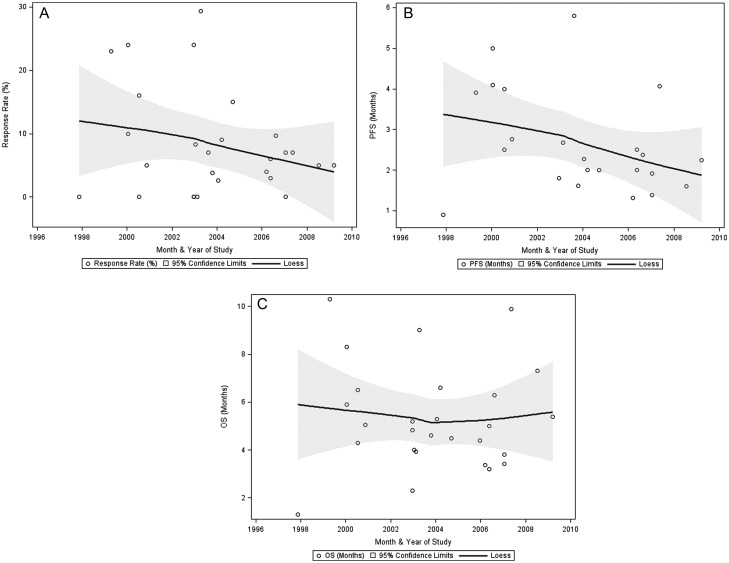
Given the lack of progress in pancreatic cancer treatment, we analyzed the outcome trends over time in the second-line setting. We plotted the RR, PFS, and OS of each of the analyzed regimens over the last 13 years as shown in Figure 5. The earliest starting date of the studies was November 1997 and the latest ending date was August 2010. The median RR was 8.3%. The median PFS and OS were 2.9 and 6 months, respectively. Unexpectedly, there was a negative trend for RR and PFS over time while there was no change in OS.
Figure 5.
The treatment outcome trends over time plotted against the month and the year of the studies including (A) the response rate (RR), (B) the progression-free survival (PFS), and (C) the overall survival (OS).
discussion
There is currently no standard of care for locally advanced or metastatic pancreatic cancer that has progressed following either FOLFIRINOX [7] or gemcitabine-based regimen [6, 11]. While there are potential options, there is no proven benefit for any regimen and treatment choice is generally an extrapolation from front-line studies. This comprehensive analysis indicates a benefit of treatment, mainly with the combination of gemcitabine and platinum agents, in patients who have progressed on gemcitabine in the first-line setting.
Given the modest impact of chemotherapy in pancreatic cancer, the first question is whether there is a proven benefit associated with any therapy compared with BSC. In contrast to other GI malignancies such as colorectal and gastric cancers where the evidence of chemotherapy benefit over BSC in the second-line setting is established [57, 58], such evidence is lacking in pancreatic cancer. The German CONKO Group trial was stopped early due to insufficient accrual [10]. Likewise in the study by Jacobs et al., only 11% of the patients on the BC arm received BSC only (n: 23) with almost 50% crossover rate to the treatment arm [9]. In our analysis, the treatments provided a trend toward an improved OS compared with BSC only (median OS of 6 versus 2.8 months, P = 0.013). However, these results are limited by the small patient samples on the BSC arms and the lack of quality-of-life assessment on both of these trials. Indeed, randomizing patients to BSC will remain a challenge given this patient population's poor prognosis.
Owing to the improvement in OS provided by the addition of oxaliplatin to 5-FU (n: 76) compared with 5-FU (n: 84) in the CONKO 3 study (5.89 versus 3.09 months, P = 0.010), this regimen has been widely used in the second-line setting [22]. In the CONKO 3 study, patients on the combination arm received more cycles of chemotherapy and had lower pain level assessment, which could be attributed to a better disease control. As expected, patients with good PS derived the most survival benefit. Although our analysis demonstrated no statistical significant improvement in outcomes of the 5-FU and platinum agents combination (a: 12; n: 450) compared with the rest of the regimens (a: 26, n: 1053), it did show a similar efficacy compared with gemcitabine and platinum agents combination (a: 5; n: 154) in terms of PFS and OS. Of note, these analyzed regimens used different platinum agents, 5-FU doses, and schedules.
Indeed gemcitabine remains the first-line treatment option for patients who are not candidates for FOLFIRINOX. However, the majority of patients develop resistance to gemcitabine in a short period of time suggesting a pre-existence of resistant cell subpopulations or stromal alterations [59, 60]. The combination of gemcitabine and platinum agents (a: 5, n: 154) was the only regimen that provided superior outcomes compared with the rest of the regimens (a: 33, n: 1349) in terms of RR and PFS (P = 0.006 and 0.059, respectively). However, the improvement in RR and PFS did not translate into a survival benefit (P = 0.10). This may have been influenced by subsequent treatments, the method of gemcitabine administration (FDR of 10 mg/m2/min versus 30-min infusion standard rate), and the amount of cycles the patients were able to receive based on the regimen's tolerability (supplementary Table S2, available at Annals of Oncology online).
Despite many efforts to improve the outcomes of the second-line treatments in advanced pancreatic cancer, these outcomes remain dismal. We demonstrated a worsening trend over the last decade in RR (median 8.3%) and PFS (median 2.9 months) with no change in OS (median 6 months) (Figure 5). One possibility to explain these trends is the incorporation of the RECIST criteria ‘Response Evaluation Criteria in Solid Tumors (RECIST)’ in the assessment of tumor response and time to progression in trials conducted after the year of 2000, resulting in a strict standardized evaluation of outcomes [61]. Noteworthy, neither PFS nor RR was found to be validated surrogate of OS in pancreatic cancer. It has been established that performance status and disease stage, locally advanced versus metastatic, have a major impact on outcome over any treatment effect in pancreatic cancer [62]. However, here we found no evidence for correlation between any of these variables and PFS or OS (data not shown).
To our knowledge, this is the first analysis to compare systematically the efficacy of the most widely used regimens in the second-line setting in pancreatic cancer. Our analysis is limited by the small sample size, the lack of randomization, the heterogeneity of the patients' characteristics and regimens, and the exploratory nature of our statistical design. In addition, our data should be interpreted carefully due to the large selection bias since only ≤50% of patients who received first-line treatment qualified for a second line.
Furthermore, these second-line regimens have been used in patients who were not gemcitabine-naïve. This practice is likely to change since FOLFIRINOX became the standard first line in patients with good performance status. As a result, gemcitabine would become, by default, the standard second-line agent. Whether gemcitabine is the appropriate choice and whether it should be used as a single agent or in combination with other agents after FOLFIRINOX failure remains to be determined.
Novel approaches in pancreatic cancer treatment are desperately needed. There have been some advances in the recent years in the molecular and biological understanding of this disease. These advances include the discovery of the important role of the stroma in the drug delivery to the cancer cells [63], the diverse genetic alteration especially in metastatic disease [64], and the impact of stem cells on disease resistance to chemo and radiation therapy [65]. These discoveries may provide the future landscape of pancreatic cancer treatment.
In conclusion, our data support the use of chemotherapy over best supportive care in the second-line setting in pancreatic cancer. The combination of platinum agents with either gemcitabine or 5-FU is preferred in comparison with other regimens. However, the survival benefit provided by these combinations is limited and should be interpreted with caution given the selection bias in this patient population. There is a clear need for well-designed, randomized, and adequately powered clinical trials in the second-line setting after FOLFIRNOX failure. Indeed, future efforts must focus on individual therapy strategies including identifying genetic mutations and new biomarkers predictive of response, in addition to studying the molecular biology of these chemotherapy agents (i.e. ERCC-1, methylation of the MLH1 gene, RRM1). Nevertheless, exploiting recent understanding of the pancreatic tumor and stroma microenvironments in order to improve the therapeutic outcome in this disease is needed.
funding
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. doi:10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. doi:10.1016/S1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33(Suppl 1):S18–S22. doi: 10.1016/s0959-8049(96)00324-3. doi:10.1016/S0959-8049(96)00324-3. [DOI] [PubMed] [Google Scholar]
- 4.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. doi:10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann V, Labianca R, Hinke A, et al. Increased survival using platinum analog combined with gemcitabine as compared to single-agent gemcitabine in advanced pancreatic cancer: pooled analysis of two randomized trials, the GERCOR/GISCAD intergroup study and a German multicenter study. Ann Oncol. 2007;18:1652–1659. doi: 10.1093/annonc/mdm283. doi:10.1093/annonc/mdm283. [DOI] [PubMed] [Google Scholar]
- 6.Sultana A, Tudur Smith C, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer. 2008;99:6–13. doi: 10.1038/sj.bjc.6604436. doi:10.1038/sj.bjc.6604436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. doi:10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann V, Haas M, Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev. 2012;38:843–853. doi: 10.1016/j.ctrv.2011.12.004. doi:10.1016/j.ctrv.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs AD, Burris HA, Rivkin S, et al. A randomized phase III study of rubitecan (ORA) vs. best choice (BC) in 409 patients with refractory pancreatic cancer report from a North-American multi-center study. J Clin Oncol 2004; 22(Suppl); Abstr 4013. [Google Scholar]
- 10.Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676–1681. doi: 10.1016/j.ejca.2011.04.011. doi:10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. doi:10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 12.Milella M, Gelibter A, Di Cosimo S, et al. Pilot study of celecoxib and infusional 5-fluorouracil as second-line treatment for advanced pancreatic carcinoma. Cancer. 2004;101:133–138. doi: 10.1002/cncr.20338. doi:10.1002/cncr.20338. [DOI] [PubMed] [Google Scholar]
- 13.Pino MS, Milella M, Gelibter A, et al. Capecitabine and celecoxib as second-line treatment of advanced pancreatic and biliary tract cancers. Oncology. 2009;76:254–261. doi: 10.1159/000205388. doi:10.1159/000205388. [DOI] [PubMed] [Google Scholar]
- 14.Brell JM, Matin K, Evans T, et al. Phase II study of docetaxel and gefitinib as second-line therapy in gemcitabine pretreated patients with advanced pancreatic cancer. Oncology. 2009;76:270–274. doi: 10.1159/000206141. doi:10.1159/000206141. [DOI] [PubMed] [Google Scholar]
- 15.Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–198. doi: 10.1200/JCO.2008.18.9514. doi:10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astsaturov IA, Meropol NJ, Alpaugh RK, et al. Phase II and coagulation cascade biomarker study of bevacizumab with or without docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2011;34:70–75. doi: 10.1097/COC.0b013e3181d2734a. doi:10.1097/COC.0b013e3181d2734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ignatiadis M, Polyzos A, Stathopoulos GP, et al. A multicenter phase II study of docetaxel in combination with gefitinib in gemcitabine-pretreated patients with advanced/metastatic pancreatic cancer. Oncology. 2006;71:159–163. doi: 10.1159/000106064. doi:10.1159/000106064. [DOI] [PubMed] [Google Scholar]
- 18.O'Reilly EM. A phase II trial of sunitinib (S) in previously-treated pancreas adenocarcinoma (PAC), CALGB 80603. J Clin Oncol 2008; 26(Suppl); Abstr 4515. [Google Scholar]
- 19.Burris HA, III, Rivkin S, Reynolds R, et al. Phase II trial of oral rubitecan in previously treated pancreatic cancer patients. Oncologist. 2005;10:183–190. doi: 10.1634/theoncologist.10-3-183. doi:10.1634/theoncologist.10-3-183. [DOI] [PubMed] [Google Scholar]
- 20.Cereda S, Reni M, Rognone A, et al. Salvage therapy with mitomycin and ifosfamide in patients with gemcitabine-resistant metastatic pancreatic cancer: a phase II trial. Chemotherapy. 2011;57:156–161. doi: 10.1159/000324865. doi:10.1159/000324865. [DOI] [PubMed] [Google Scholar]
- 21.Pelzer U, Stieler J, Roll L, et al. Second-line therapy in refractory pancreatic cancer. results of a phase II study. Onkologie. 2009;32:99–102. doi: 10.1159/000197769. doi:10.1159/000197769. [DOI] [PubMed] [Google Scholar]
- 22.Pelzer U, Kubica K, Stieler J, et al. A randomized trial in patients with gemcitabine refractory pancreatic cancer. Final results of the CONKO 003 study. J Clin Oncol; 2008. 26(Suppl); Abstr 4508. [Google Scholar]
- 23.Morizane C, Okusaka T, Furuse J, et al. A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2009;63:313–319. doi: 10.1007/s00280-008-0741-7. doi:10.1007/s00280-008-0741-7. [DOI] [PubMed] [Google Scholar]
- 24.Heinemann V, Vehling-Kaiser U, Waldschmidt D, et al. Gemcitabine plus erlotinib followed by capecitabine versus capecitabine plus erlotinib followed by gemcitabine in advanced pancreatic cancer: final results of a randomised phase 3 trial of the ‘Arbeitsgemeinschaft Internistische Onkologie’ (AIO-PK0104) Gut. 2012;62:751–759. doi: 10.1136/gutjnl-2012-302759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Androulakis N, Syrigos K, Polyzos A, et al. Oxaliplatin for pretreated patients with advanced or metastatic pancreatic cancer: a multicenter phase II study. Cancer Invest. 2005;23:9–12. doi:10.1081/CNV-46502. [PubMed] [Google Scholar]
- 26.Tsavaris N, Kosmas C, Skopelitis H, et al. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: a phase II study. Invest New Drugs. 2005;23:369–375. doi: 10.1007/s10637-005-1446-y. doi:10.1007/s10637-005-1446-y. [DOI] [PubMed] [Google Scholar]
- 27.Novarino A, Satolli MA, Chiappino I, et al. Oxaliplatin, 5-fluorouracil, and leucovorin as second-line treatment for advanced pancreatic cancer. Am J Clin Oncol. 2009;32:44–48. doi: 10.1097/COC.0b013e31817be5a9. doi:10.1097/COC.0b013e31817be5a9. [DOI] [PubMed] [Google Scholar]
- 28.Gebbia V, Maiello E, Giuliani F, et al. Second-line chemotherapy in advanced pancreatic carcinoma: a multicenter survey of the Gruppo Oncologico Italia Meridionale on the activity and safety of the FOLFOX4 regimen in clinical practice. Ann Oncol. 2007;18(Suppl 6):vi124–vi127. doi: 10.1093/annonc/mdm240. doi:10.1093/annonc/mdm240. [DOI] [PubMed] [Google Scholar]
- 29.Xiong HQ, Varadhachary GR, Blais JC, et al. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008;113:2046–2052. doi: 10.1002/cncr.23810. doi:10.1002/cncr.23810. [DOI] [PubMed] [Google Scholar]
- 30.Togawa A, Yoshitomi H, Ito H, et al. Treatment with an oral fluoropyrimidine, S-1, plus cisplatin in patients who failed postoperative gemcitabine treatment for pancreatic cancer: a pilot study. Int J Clin Oncol. 2007;12:268–273. doi: 10.1007/s10147-007-0674-x. doi:10.1007/s10147-007-0674-x. [DOI] [PubMed] [Google Scholar]
- 31.Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101:1658–1663. doi: 10.1038/sj.bjc.6605374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahan L, Bonnetain F, Ychou M, et al. Combination 5-fluorouracil, folinic acid and cisplatin (LV5FU2-CDDP) followed by gemcitabine or the reverse sequence in metastatic pancreatic cancer: final results of a randomised strategic phase III trial (FFCD 0301) Gut. 2010;59:1527–1534. doi: 10.1136/gut.2010.216135. doi:10.1136/gut.2010.216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitry E, Ducreux M, Ould-Kaci M, et al. Oxaliplatin combined with 5-FU in second line treatment of advanced pancreatic adenocarcinoma. Results of a phase II trial. Gastroenterol Clin Biol. 2006;30:357–363. doi: 10.1016/s0399-8320(06)73188-8. doi:10.1016/S0399-8320(06)73188-8. [DOI] [PubMed] [Google Scholar]
- 34.Gasent Blesa J, Alberola-Candel V, Giner Marco V, et al. Phase II trial of second-line chemotherapy in metastatic pancreas cancer with the combination of oxaliplatin (Ox) and capecitabine (Cp). J Clin Oncol; 2009. 27(Suppl); Abstr 15561. [Google Scholar]
- 35.Yi SY, Park YS, Kim HS, et al. Irinotecan monotherapy as second-line treatment in advanced pancreatic cancer. Cancer Chemother Pharmacol. 2009;63:1141–1145. doi: 10.1007/s00280-008-0839-y. doi:10.1007/s00280-008-0839-y. [DOI] [PubMed] [Google Scholar]
- 36.Cantore M, Rabbi C, Fiorentini G, et al. Combined irinotecan and oxaliplatin in patients with advanced pre-treated pancreatic cancer. Oncology. 2004;67:93–97. doi: 10.1159/000080993. doi:10.1159/000080993. [DOI] [PubMed] [Google Scholar]
- 37.Ko AH, Tempero MA, Shan Y, et al. A multinational phase II study of liposome irinotecan (PEP02) for patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol; 2011. 29(Suppl 4); Abst 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrich-Pur H, Raderer M, Verena Kornek G, et al. Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br J Cancer. 2003;88:1180–1184. doi: 10.1038/sj.bjc.6600883. doi:10.1038/sj.bjc.6600883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeck S, Weigang-Kohler K, Fuchs M, et al. Second-line chemotherapy with pemetrexed after gemcitabine failure in patients with advanced pancreatic cancer: a multicenter phase II trial. Ann Oncol. 2007;18:745–751. doi: 10.1093/annonc/mdl463. doi:10.1093/annonc/mdl463. [DOI] [PubMed] [Google Scholar]
- 40.Oettle H, Arnold D, Esser M, et al. Paclitaxel as weekly second-line therapy in patients with advanced pancreatic carcinoma. Anticancer Drugs. 2000;11:635–638. doi: 10.1097/00001813-200009000-00006. doi:10.1097/00001813-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Katopodis O, Polyzos A, Kentepozidis N, et al. Second-line chemotherapy with capecitabine (Xeloda) and docetaxel (Taxotere) in previously treated, unresectable adenocarcinoma of pancreas: the final results of a phase II trial. Cancer Chemother Pharmacol. 2011;67:361–368. doi: 10.1007/s00280-010-1329-6. doi:10.1007/s00280-010-1329-6. [DOI] [PubMed] [Google Scholar]
- 42.Reni M, Pasetto L, Aprile G, et al. Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant metastatic pancreatic cancer. Br J Cancer. 2006;94:785–791. doi: 10.1038/sj.bjc.6603026. doi:10.1038/sj.bjc.6603026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaya M, Lopes GL, Roman E, Jr, et al. Phase II trial of capecitabine and docetaxel as second line therapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007; 25(Suppl); Abstr 15029. [Google Scholar]
- 44.Hosein PJ, Pastorini VH, Gomez CM, et al. A phase II trial of nab-paclitaxel (NP) in patients with advanced pancreatic cancer (PC) who have progressed on gemcitabine-based therapy. Gastrointestinal Cancers Symposium; 2010. Abstr 214. [Google Scholar]
- 45.Demols A, Peeters M, Polus M, et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer. 2006;94:481–485. doi: 10.1038/sj.bjc.6602966. doi:10.1038/sj.bjc.6602966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stathopoulos GP, Boulikas T, Vougiouka M, et al. Liposomal cisplatin combined with gemcitabine in pretreated advanced pancreatic cancer patients: a phase I-II study. Oncol Rep. 2006;15:1201–1204. [PubMed] [Google Scholar]
- 47.Reni M, Cereda S, Mazza E, et al. PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) regimen as second-line therapy in patients with progressive or recurrent pancreatic cancer after gemcitabine-containing chemotherapy. Am J Clin Oncol. 2008;31:145–150. doi: 10.1097/COC.0b013e31814688f7. doi:10.1097/COC.0b013e31814688f7. [DOI] [PubMed] [Google Scholar]
- 48.Kozuch P, Grossbard ML, Barzdins A, et al. Irinotecan combined with gemcitabine, 5-fluorouracil, leucovorin, and cisplatin (G-FLIP) is an effective and noncrossresistant treatment for chemotherapy refractory metastatic pancreatic cancer. Oncologist. 2001;6:488–495. doi: 10.1634/theoncologist.6-6-488. doi:10.1634/theoncologist.6-6-488. [DOI] [PubMed] [Google Scholar]
- 49.Fortune BE, Li X, Kosuri KV, et al. Fixed-dose-rate gemcitabine in combination with oxaliplatin in patients with metastatic pancreatic cancer refractory to standard-dose-rate gemcitabine: a single-institute study. Oncology. 2009;76:333–337. doi: 10.1159/000209962. doi:10.1159/000209962. [DOI] [PubMed] [Google Scholar]
- 50.Kulke MH, Blaszkowsky LS, Ryan DP, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787–4792. doi: 10.1200/JCO.2007.11.8521. doi:10.1200/JCO.2007.11.8521. [DOI] [PubMed] [Google Scholar]
- 51.Tang P, Gill S, Au HJ, et al. Phase II trial of erlotinib in advanced pancreatic cancer (PC). J Clin Oncol; 2009. 27(Suppl); Abstr 4609. [Google Scholar]
- 52.Ko AH, Dito E, Schillinger B, et al. A phase II study of bevacizumab (BEV) plus erlotinib (ERL) in patients with gemcitabine (GEM)-refractory metastatic pancreatic cancer (MPC). J Clin Oncol; 2008. 26(Suppl); Abstr 4516. [DOI] [PubMed] [Google Scholar]
- 53.Group MRCOCW. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. doi:10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg RM. N9741: a phase III study comparing irinotecan to oxaliplatin-containing regimens in advanced colorectal cancer. Clin Colorectal Cancer. 2002;2:81. doi: 10.1016/S1533-0028(11)70509-6. doi:10.1016/S1533-0028(11)70509-6. [DOI] [PubMed] [Google Scholar]
- 55.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. doi:10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 56.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. doi:10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 57.Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer–a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. doi: 10.1016/j.ejca.2011.06.002. doi:10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham D, Pyrhonen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352:1413–1418. doi: 10.1016/S0140-6736(98)02309-5. doi:10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 59.Kim MP, Gallick GE. Gemcitabine resistance in pancreatic cancer: picking the key players. Clin Cancer Res. 2008;14:1284–1285. doi: 10.1158/1078-0432.CCR-07-2247. doi:10.1158/1078-0432.CCR-07-2247. [DOI] [PubMed] [Google Scholar]
- 60.Andersson R, Aho U, Nilsson BI, et al. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782–786. doi: 10.1080/00365520902745039. doi:10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 61.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 62.Tas F, Sen F, Odabas H, et al. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol. 2012 doi: 10.1007/s10147-012-0474-9. September 21 [epub ahead of print], doi: 10.1007/s10147-012-0474-9. [DOI] [PubMed] [Google Scholar]
- 63.Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. doi:10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iacobuzio-Donahue CA, Velculescu VE, Wolfgang CL, et al. Genetic basis of pancreas cancer development and progression: insights from whole-exome and whole-genome sequencing. Clin Cancer Res. 2012;18:4257–4265. doi: 10.1158/1078-0432.CCR-12-0315. doi:10.1158/1078-0432.CCR-12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. doi:10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.