Abstract
Increasing numbers of elderly transplant recipients and a growing demand for organs from older donors impose pressing challenges on transplantation medicine. Continuous and complex modifications of the immune system in parallel to aging have a major impact on transplant outcome and organ quality. Both, altered alloimmune responses and increased immunogenicity of organs present risk factors for inferior patient and graft survival. Moreover, a growing body of knowledge on age-dependent modifications of allorecognition and alloimmune responses may require age-adapted immunosuppression and organ allocation.
Here, we summarize relevant aspects of immunosenescence and their possible clinical impact on organ transplantation.
Keywords: immunosenescence, aging, innate immunity, adaptive immunity, organ transplantation, transplant outcome, immunosuppression, organ allocation
Introduction
Improved longevity linked to medical progress and demographic changes have dramatically increased the amount of older patients developing end-stage organ disease. While only 7.6 percent of new patients with end-stage renal disease (ESRD) were older than 75 years in 1980, more than 20% of patients with ESRD were ≥ 75 years in 2004 [1]. Consequently, the majority of those in need for organ transplantation are currently older than 50 years [2]. In an attempt to meet the rapidly increasing demand, organs from so-called expanded criteria donors (ECD) have been used more frequently. In fact, more than half of all currently transplanted kidneys are from donors >50 years [2]. Clearly, changing demographics and an increasing longevity are likely to contribute to a further increase of the elderly in need for medical care including organ transplantation.
Immunosenescence can affect all immune compartments and does not necessarily represent a uniform compromise of immunological efficacy but rather individual shifts in function and regulation. Clinical implications of immunosenescence include increased risks of infections, malignancies, autoimmune disorders, atherosclerosis and neuro-degenerative changes. Consequences of immunosenescence have broad implications in organ transplantation and may require an adapted immunosuppression when treating older recipients or when utilizing organs from older donors.
Of note, age criteria are only inconsistently applied in studies on immunosenescence, thus contributing to conflicting data in aging research [3].
Clinical implications of advanced recipient age
Elderly individuals with end-stage renal disease undergoing transplantation have shown better long-term survival than matched controls staying on dialysis [4]. Improved life expectancies were observed although older recipients are more likely to receive older and functionally compromised organs [5]. At the same time it has to be noted that older recipients represent a highly selected patient population [4,6] Graft survival censored for death with a functioning graft improves with age. Of note, older recipients have an overall higher mortality [7,8] and almost 50% of graft losses in old recipients are related to death with a functioning graft [9]. Moreover, more than 50% of all mortalities in older recipients have been linked to cardiovascular disease, infections or malignancies – complications that are all exacerbated by immunosuppressive therapy and age [10].
Clinical trials have confirmed reduced rates of acute rejections in older recipients receiving corneal, kidney, cardiac, liver and lung transplantation [5,11–16]. In renal transplantation, <25% of graft failures in old recipients have been attributed to rejections compared with 50% in recipients <45 years [17]. Acute rejections occurring in older recipients seem more detrimental and have a more pronounced impact on patient and graft survival [18]. Both, organ quality and age may be of importance in this context as old recipients are more likely to receive organs from older donors [5].
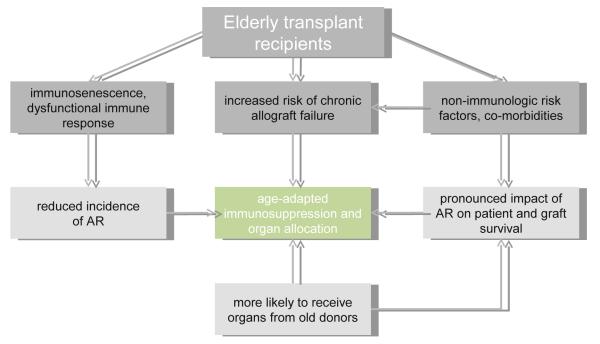
Furthermore, recipient age has been identified as an independent risk factor for chronic allograft failure in clinical studies and experimental models [7,19,20]. Both, organ age and an increased susceptibility to calcineurin inhibitor (CNI) -related nephrotoxicity may be of importance in this context (Fig. 1).
Figure 1. Clinical implications of immunosenescence for the elderly transplant recipient.
Modified immune responses subsequent to immunosenescence are clinically characterized by higher rates of chronic allograft failure and less frequent, however more detrimental acute rejections, implicating age-adapted immunosuppression and organ allocation. AR, acute rejection.
Principles of immunosenescence and alloimmune responses
Hematopoietic stem cells
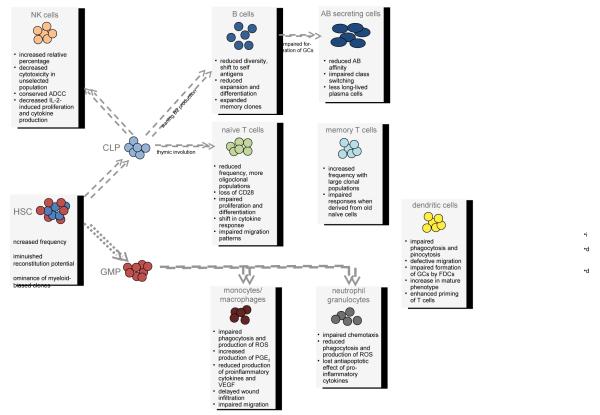
Cellular components of the immune system are mostly short-lived and require continuous replenishment. Hematopoietic stem cells (HSC) are long-lived and comprise only 0.01% of the bone marrow population. At any given cell division, HSC have the potential to give rise to all blood cell types of the myeloid and lymphoid lineages. Alternatively, they may self-renew to produce more HSCs. Despite their extensive proliferative and regenerative capacity, a growing body of evidence suggests that these cells show signs of aging with important functional implications [21,22] (Fig. 2).
Figure 2. Cellular substrates of the aging immune system.
Immunosenescence impacts innate and adaptive immune responses on all levels. HSC, hematopoietic stem cell; CLP, common lymphoid progenitor; ADCC, antibody-dependent cell-mediated cytotoxicity; GC, germinal center; AB, antibody; FDC, follicular dendritic cell; GMP, granulocyte-macrophage progenitor; ROS, reactive oxygen species; PGE2, Prostaglandin E2; VEGF, vascular endothelial growth factor.
Reconstitution potential
Both, clinical and experimental data support a measurable and successive functional decline of the reconstitution capacity of old purified HSCs [23–25].
An age-dependent accumulation of damaged genomic and mitochondrial DNA subsequent to oxidative stress might be the most important factor driving the functional decline [26,27]. Old HSCs seem to have compromised abilities to repair DNA damages, impacting cell cycle regulation. Of note, the cell-cycle inhibitor p16INK4a increases with age in HSCs [28].
Stem cell niches formed by stromal cells in the bone marrow maintain regulatory interactions with HSCs. Since stromal cells are exposed to comparable environmental damages and stress, a modified support provided by these niches might also be of relevance for the observed age-related changes [29].
Quantitative changes
The functional compromise of the immune response with aging is in part compensated by increased frequencies and an enhanced expansion of old HSCs [30,31]. Despite the prevailing view that the frequency of HSCs diminishes in parallel to a compromised cellularity in the bone marrow of the elderly [32], more recent data have suggested an increase in the frequency of human HSCs with aging [22,33].
Lineage potentials
Murine HSCs have shown changes in lineage potential with aging resulting in an attenuated lymphoid and preserved or even increased myeloid lineage output [34]. A more robust self-renewal potential [35] and a differential response to the aging cytokine milieu [36] have been proposed for the progressive predominance of myeloid-biased HSC clones. These changes are accompanied by the down-regulation of genes mediating lymphoid specification and function. At the same time, an up-regulation of genes involved in myeloid specification and function is observed [37]. Interestingly, pediatric leukemias tend to involve lymphoid lineages while leukemias in the adult population tend to involve myeloid leukemias [38,39].
Modifications of T cell compartments during immunosenescence
T cell generation
After entering the thymus, T-cell progenitors originating from the bone marrow interact with stromal cells, macrophages and dendritic cells to undergo a number of proliferative and differentiation events that lead to the emigration of mature and functional T cells into the peripheral T cell pool.
Clinically, thymic involution starts at the age of one year and advances rapidly with puberty [40]. Thymic involution has been shown to correlate with an enlargement of the perivascular space possibly as a consequence to the loss of thymocytes, thymic stroma and thymic epithelial space, all areas in which thymopoiesis takes place [41].
The aging thymus retains its capacity to produce naïve T cells despite a significant atrophy. More reliable measurements of changes in thymic output with the so called signal joint T cell receptor excision circle (sjTREC) assay have recently revealed that the output of T cells declines as a function of thymopoietic tissue [42].
Several hypotheses have been brought forward to explain thymic involution, including a decline in supply of bone marrow progenitors, alterations in the productive rearrangement of TCRs, loss of cells in the thymic microenvironment and alterations in circulating (GH, GnRH) or intrathymic (IL-7, neurotrophins, thymic hormones) hormones, cytokines, and growth factors [43]. The infiltrating adipose tissue replacing the thymus may also be a contributing factor, either directly or through the release of soluble factors [44].
T cell receptor repertoire
The loss in thymic output with age does not result in significant modifications of the total number of peripheral T cells [45]. Indeed, the total number of peripheral T-cells seems to be regulated via a compensatory process of thymus-independent expansion of mature T cells following low-affinity interactions with self-peptide/MHC complexes [46,47]. Moreover, aging is linked to a significantly limited TCR repertoire with T-cell diversity dropping 1000-fold in individuals >70 years [48,49]. Changes in the TCR repertoire are also expected to impact mechanisms of allorecognition [50].
CD28− T cells
CD28 is a co-stimulatory receptor that plays a pivotal role in antigen-dependent activation, proliferation and survival of T cells. By age 80, 10-15% of peripheral blood CD4+ T cells and 50-60% of CD8+ T cells lack the expression of CD28. In contrast, at birth, virtually all human T cells express CD28 [51]. As these cells are frequently expanding oligoclonally, their T cell receptors display reduced diversity [52]. Of note, CD28− T cells show an altered expression of additional co-stimulatory receptors [53] and a gain in cytolytic functions [54]. CD28− T cells also acquire expression of NK cell receptors such as killer immunoglobulin-like receptors (KIRs) [55] which fundamentally influences signal recognition as ligands for these receptors are not limited to APCs.
Loss of CD28 expression in T cells with age has been attributed to repeated antigenic stimulation, a process that can also be observed in-vitro [56]. CD28− T cells have shorter telomeres than their CD28+ counterparts within the same clonal population and might have already reached their limit of proliferative potential [57]. In addition, the presence of type I interferons during TCR activation increases the proportion of CD28− T cells in culture [58], suggesting an important role for a pro-inflammatory environment during immunosenescence. Some evidence is also suggesting that the generation of CD28− T cells might be driven or accelerated by chronic viral stimulation, most prominently linked to herpes viridae such as CMV [59].
Of note, an efficient CMV immunosurveillance can be maintained with increased age. In immunocompromised patients such as transplant recipients this balance, however, can be disturbed, accelerating the clonality of CD8+ T cells which may potentially contribute to a higher level of chronic subclinical inflammation [60]. Of interest, transfer of these T cells from old into young mice led to reduced resistance to viral challenge [61].
Memory T cells
The proportion of memory T cells increases with age, possibly as a consequence to a cumulative exposure to pathogens and environmental antigens [62]. Selected changes in lymphocyte turnover, particularly in the memory CD8+ T cell compartment, have been reported in mice. Human and murine CD8+ memory T cells were also found to have a longer half-life than other T cell subsets [63,64]. Furthermore, memory cells derived from old naïve cells seem to proliferate less well, produce reduced levels of cytokines and provide less cognate helper function [65]. Although old mice displayed a larger number of memory T cells prior to transplantation, these cells did not exhibit enhanced alloreactivity compared with young memory T cells [66].
Proliferative Response
T cells enter a state of reduced proliferative capacity when telomeres are reduced to a critical length after a certain number of cell divisions known as the ‘Hayflick’ limit [67,68]. The loss of CD28 expression has been associated with loss of proliferative capacity of T cells during repeated cycles of replication, a process termed ‘replicative senescence’ [69]. CD28− T cells show irreversible cell-cycle arrest in addition to apoptosis resistance and reduced proliferative responses [70]. Previous studies had established limited proliferative responses of old T cells to antigenic and mitotic stimuli [71,72].
In line with these observations, adoptively transferred old T-cells proliferated less well in response to their specific antigen [73] and young T cell-deficient mice reconstituted with old T cells demonstrated a delayed rejection of allogeneic skin allografts, illustrating an overall decline in T cell-mediated alloresponses with increasing age [74].
T cell signaling
Old CD4+ T cells stimulated ex vivo with irradiated donor spleen cells manifested impaired allospecific IL-2 and IFN-γ responses [74]. Those observations are in line with previous reports showing limited capacities of old T cells to produce and respond to IL-2 upon stimulation with antigen [75]. This process has also been linked to an age-dependent and limited activation of the transcription factors AP-1 and NF-AT [76].
The two classical signals required for T cell activation (TCR ligation and co-stimulation) are affected by aging. Old murine CD4+ T cells are less efficient in forming TCR synapses with APCs [77] and show a limited expression of several activation and differentiation markers such as CD40L/CD154, CD25 and CD28 [78,79]. Additional changes in the signaling cascades of old T cells include impairments in calcium metabolism, tyrosine kinase phosphorylation and protein kinase C translocation [80] as wells as alterations in cell membrane lipid rafts [81].
Cytokine responses
A number of studies suggest an imbalance between Th1 and Th2 responses in aging and some, but not all reports have linked aging to a decreased Th1/Th2 ratio [82–84]. The overall frequency of type 1 and type 2 cytokine-producing cells seems to increase with age, potentially influenced by higher frequencies of memory T cells which have less strict requirements for stimulation while producing a broader set of cytokines [85,86]. High levels of lymphocyte function-associated antigen 1 (LFA-1) on CD28− T cells also reduce the activation threshold of these cells [87].
The role of Th17 immune responses in aging is still unclear, although some have reported on a shift in cytokine expression towards augmented IL-17 alloimmune responses [88,89].
Migration
Recent observations indicate that the expression of selected pro-inflammatory chemokines and receptors are modified in old human and murine T cells, possibly influencing T cell migration patterns. Changes in the expression of CCR7 and CD62L were linked to a defective homing of T-cells to secondary lymphoid organs [90].
Regulatory T cells
Regulatory T cells (Tregs) mediate suppressive functions through various mechanisms [91]. Autoimmune diseases, chronic inflammation and cancer have been linked to quantitative and qualitative defects of Tregs. As some of these disorders have a higher incidence in older individuals, age-related changes in this subset have been of interest [92].
As an alternative to the declining thymic output, Tregs can be generated through a peripheral mechanism [93]. Although most studies have not seen a correlation between numbers of Tregs and aging, few selected studies have shown an increase in the frequency of Tregs with age [94]. These differences might in part be due to different phenotypic definitions, since some studies used only CD25 and other studies used CD25 and Foxp3 as markers for Tregs. In a recent experimental study, we were able to show that Treg functions in old recipient mice remained intact with age [73]. Those findings have also been confirmed clinically [95,96].
Apoptosis and T cell survival
Aging affects major signaling pathways of T cell apoptosis [97]. Clinical studies have shown that both, old naïve and memory T cells have an increased CD95/Fas expression [98] and an decreased expression of Bcl-2, both correlating with enhanced apoptosis [99]. The functional relevance of these findings is still being debated [100]. Successive shortening of telomeric DNA, as described for T cells with advancing age, is an additional independent factor for increased apoptosis [101].
B cell compartment
B cell generation
The production of B cells wanes with increasing age [102]. Both, early B cell progenitors and the expression of critical transcriptional regulators including E2A gene products such as E47 are reduced with aging [103,104]. In line with these changes, the expression of recombination activating gene (RAG) enzymes, which are crucial for the passage through the pro- and pre-B cell stages, is diminished in old individuals [105]. A limited expression of downstream products of E2A has also been demonstrated in peripheral B cells from old mice [106]. Moreover, in-vivo labeling has revealed that production rates in pro-, pre- and immature bone marrow B cell pools diminish with age [107]. Thus, maintaining the number of peripheral B cells despite decreased output seems to be facilitated trough a decreased turnover of mature B cells [108].
Aging may also impact the balance between B1 and B2 cells: As B2 production wanes with age, the proportional contribution of B1 cells may increase [109]. B1 cells characterized by polyspecificity and low-affinity self-reactivity are a self-renewing pool predominantly found in peritoneal and pleural cavities [110].
B cell receptor diversity and specificity
A significant and age-dependent loss in diversity of the B cell receptor (BCR) has been correlated with poor health and compromised survival [111]. In addition to a reduced output of naïve B cells and intrinsic repertoire differences in B cells generated from old HSCs, some truncation of the repertoire might reflect expanded clones of memory B cells [112].
These changes may also lead to a shift in antibody specificity. In most inbred mouse strains, the spontaneous appearance of autoreactive antibodies is linked to increasing age. Moreover, a compromised selection of newly formed B cells may increase the likelihood of autoreactive B cells to survive. BLyS/BAFF, a B lymphocyte stimulator regulating survival pathways via BLyS receptor 3 [113] provides a limited survival resource during aging for which newly formed and mature B cells compete [114]. Expanded clones of memory B cells might also lead to increased autoantibody titers since some of these were initially expanded and selected by cross-reactive antigens or self-antigens.
B cell responses
Germinal center (GC) formation and kinetics are impaired in older mice [115]. Correspondingly, B cell expansion, antibody affinity maturation and memory B cell differentiation are compromised while amounts of long-lived plasma cells in the bone marrow decline [116,117]. Reduced CD40L/CD154 expression by T cells and a modified cytokine environment [78] may explain the age-dependent limited formation of GCs [112]. Furthermore, follicular dendritic cells as organizers of the lymphoid microarchitecture in GCs have been found to be less effective in trapping and dispersing antigen, correlating with fewer and smaller GCs [118]. The generation of high affinity antibodies is also compromised by intrinsic class switching defects secondary to decreased induction of E47 and activation-induced cytidine deaminase [119].
Aging affects innate immune responses and graft immunogenicity
Innate immune cells express a variety of pattern recognition receptors (PRR) that recognize conserved pathogen-associated patterns (PAMP) and damage-associated molecular patterns (DAMPs) [120]. They also express additional receptors for complement factors, antibodies, and receptors that can sense “self” and “missing self” [121]. This wide array of receptors allows them to critically impact transplant outcome by influencing the initiation, duration and the overall character of alloimmune responses [122].
Surgery, tissue trauma, consequences of ischemia/reperfusion injury, vascular dysfunctions, and graft preservation are all inevitably linked to graft damage. In response to inflammatory cytokines/chemokines and complement products, IRI mobilizes intragraft DCs and causes a rapid and massive cellular infiltration of various immune cells including monocytes/macrophages, neutrophils, NK cells, DCs, T and B cells [123]. Stimulation of PRRs by DAMPS on damaged graft cells initiate the induction of additional inflammatory cytokines. APCs mature in response to these signals to induce adaptive immune responses [124] and graft cells expressing markers of cellular stress become the target of NK-mediated killing [125].
Dendritic cells
In remains debated if numbers and phenotype of DCs remain unchanged during aging [126–128], or if peripheral human myeloid DCs (mDCs) decline with age [129]. A higher frequency of mature phenotypes with an increased expression of co-stimulatory molecules CD86 and CD83 has been observed in old DCs [129]. Various numerical and phenotypic age-dependent changes in DCs have been described for skin [130], mucosal immune system [131], thymus [132] and brain [133], implying that the impact of aging on DCs might vary depending on specific subsets and the tissue of residence.
Age-dependent effects on antigen sensing and activation of DCs are discussed controversial. Several clinical studies have reported comparable levels of TLR-induced activation and cytokine secretion by monocyte-derived DCs [127,128] while others found a compromised cytokine secretion upon TLR-dependent stimulation [126]. Impaired migration of DCs to draining lymph nodes has been observed both, experimentally and clinically[134,135]. Intrinsic defects of DCs and the aging microenvironment may be of relevance in this context. Data on the capacity of old DCs to prime and activate T cells have been inconsistent [136–138].
Macrophages
A significant decrease in macrophage precursors and macrophages was found in the bone marrow of old individuals [32]. Both, aging human and rodent macrophages seem to express less MHC class II molecules [139,140].
Several reports using murine models indicate a decline in phagocytosis, opsonization, and tumor cell killing by old murine peritoneal macrophages [141,142]. Macrophages from old rats also demonstrated a decrease in the ability to produce superoxide anion upon incubation with IFN-γ or opsonized zymosan [143]. An abrogation in the mitogen-activated protein kinase (MAPK) pathway may be of additional importance in this context [144]. Both, decreased [145] as well as increased [146] amounts of inducible nitric oxide synthase (iNOS) mRNA have been reported in old murine macrophages. Recently, age-specific nitrite-production patterns based on the dose of IFN-γ used for stimulation have been demonstrated [147].
Age has also been associated with an increased production of PGE2 by macrophages [148]. PGE2 has been linked to DC functions by altering the secretion of IL-12, IL-10, IL-2 and by decreasing the expression of MHC class II, all impacting proliferative responses in T cells and the Th1/Th2 cytokine balance [149–151].
It has been discussed whether macrophages are the source of elevated levels of pro-inflammatory cytokines found in the elderly [152]. Several recent reports suggested a decrease in the production of pro-inflammatory cytokines by both, human and murine macrophages [153,154].
In wound healing, macrophages promote angiogenesis and help clearing the wound bed from infections. Clinical wound healing studies demonstrated a delay in monocyte and macrophage infiltration with age associated with a decreased expression of adhesion molecules [155]. Peritoneal macrophages from old mice also produced less VEGF upon stimulation [156].
Natural killer cells
NK cells of old individuals in the SENIEUR protocol demonstrated preserved [157] or even enhanced [158] cytotoxicity. In other studies, age-specific compromises in NK cell cytotoxicity have been correlated with an impaired turnover of inositol triphosphate [159–161]. Interestingly, antibody-dependent cell-mediated cytotoxicity does seem to be preserved with aging [162] and changes in intracellular signaling were not observed in this pathway [161].
An age-related relative increase of human NK cells has been reported [163] and may represent a compensatory mechanism during immunosenescence [164]. These changes were also associated with a phenotypic and functional shift towards highly cytotoxic CD56dim populations [165]. Both, human and murine NK cells have shown a decreased proliferative response following IL-2 stimulation, associated with a decrease in Ca2+ mobilization [165]. Moreover, IL2-induced production of IFN-γ and other chemokines was decreased in NK cells from old individuals [165,166], possibly compromising adaptive immune responses driven by NK cells.
Neutrophil granulocytes
Numbers of circulating neutrophils and their capacity to migrate to the site of inflammation do not seem to be compromised with age [167–170]. In-vitro studies have confirmed an unimpaired adhesion of neutrophils to vascular endothelial cells [171]; chemotaxis, however, was found to be impaired [172,173] in the elderly.
There seems to be an age-dependent loss of microbiocidal capacity of neutrophils [174]. Impaired phagocytosis of opsonized bacteria or yeast by neutrophils in the elderly have been observed [175,176] and Fc receptor-mediated production of reactive oxygen species (ROS) was found to be significantly decreased in elderly individuals [177,178].
Mechanistically, decreased intracellular Ca2+ levels in stimulated neutrophils [179] and diminished actin polymerization [180] seem to be playing a role. Old neutrophils also showed impaired anti-apoptotic responses to pro-inflammatory signals like IL-2, LPS or GM-CSF, associated with compromised lipid raft function [181,182].
The impact of aging on organ quality and immunogenicity
A large retrospective study demonstrated that kidneys from old donors failed earlier: the projected graft half-life was reduced to 5 years if the donor was older than 60 years, compared with 10.2 years when kidneys from young donors were transplanted [183]. Of note, an adverse effect of donor age was not observed in living donor transplants, indicating that unspecific injuries have a more pronounced affect in older organs [184,185].
Intrinsic functional impairments of old organs may play a role. In fact, autopsy studies showed a decline in kidney weight, number of glomeruli and mean glomerular volume with increasing age [186]. Moreover, longitudinal studies have shown a diminished renal reserve with aging, accompanied by functional deficits [187], potentially leading to more detrimental injuries subsequent to unspecific injuries and cellular distress. It is unclear whether those effects are related to aging itself or if they represent an accumulation of injuries subsequent to undetected or minimal renal disease.
A retrospective clinical analysis showed an increased need for postoperative dialysis when transplanting old kidneys [183] and donor age has been identified as an independent risk factor for DGF [18]. DGF, in turn, has been linked to increased rates of acute rejection episodes [188].
Several studies have demonstrated an increased susceptibility for IRI with increasing age. In heart transplantation, an increased release of mitochondrial reactive oxygen species has been linked to the observed age-related differences [189]. Augmented age-related IR injuries have also been shown in experimental and clinical liver [190], kidney [191,192] and musculoskeletal studies [193,194].
Tissue injury induces a stereotypic injury response that promotes immune recognition and subsequent injury [195]. This pattern can then, at least in theory, initiate a vicious cycle of repeated injury and injury responses [196] leading to higher immunogenicity of older grafts [197].
An increased immunogenicity of old donor organs may also be mediated by intragraft DCs. Enhanced antigen-presenting capacities of DCs have been reported previously [198–200]. In our own experimental work, we observed that old murine DCs induced more potent alloimmune responses in-vitro (unpublished observations). Clinically, older monocyte-derived DCs (MDDCs) have shown impaired capacities of phagocytosis and pinocytosis [201] in addition to an impaired phagocytosis of apoptotic cells. The latter might potentially lead to an accumulation of necrotic cells which subsequently activate DCs and enhance antigen presentation and secretion of pro-inflammatory cytokines [202]. Inflamm-aging presents a more integrated concept of how donor age may impact graft immunogenicity. Aging is associated with a compromised competence and integrity of epithelial barriers [203]. Subclinical infections may thus increase the accumulation of antigenic burden and represent a persisting challenge to the innate immune system, which – together with deficiencies in adaptive immunity and compromised HSCs – may gain importance in preserving immunologic protection [204]. This shift may lead to the reported elevated levels of pro-inflammatory cytokines in the elderly [205] and may also increase the overall pro-inflammatory state of organs for transplantation. In keeping with this concept, hearts from old mice contained significantly elevated frequencies of donor-derived leukocytes prior to transplantation [73].
The augmented immunogenicity of older organs may also lead to a more vigorous immune response. In fact, an increased incidence of acute rejection episodes after transplantation of old kidneys has been noted [18,206,207]. The Eurotransplant Senior Program reported higher rejection rates. Of note, this program aims for brief ischemic times regardless of HLA matching [208]. In a recent large retrospective analysis of the UNOS database, we found that increased donor age was associated with higher frequencies of acute rejection episodes [5,16].
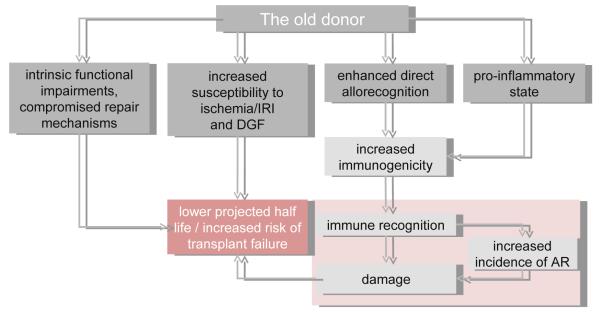
Experimentally, transplantation of old organs was associated with more potent early immune responses [209,210] and recipients of old grafts demonstrated higher frequencies of effector/memory T-cells and increased in-vitro alloreactivity [211]. Following an acute rejection, old organs may have a compromised capacity to repair. Clinically, increased rates of graft losses were observed for kidneys from old donors after DGF [212]. Fewer functioning nephrons may accelerate the consequences of specific and unspecific injuries in old kidneys and consequences of multiple injuries may also contribute to premature senescence limiting efficient repair mechanisms in older organs [213] (Fig. 3).
Figure 3. Increasing donor age as a risk factor for inferior transplant outcome.
Intrinsic functional impairments, susceptibility to IRI and DGF, enhanced direct allorecognition, and a more pro-inflammatory state of organs from old donors contribute to a cycle of damage, modified immune recognition and compromised repair that ultimately translates into increased risk of transplant failure and inferior transplant outcome. AR, acute rejection.
Clinical implications
Organ allocation
The transplantation of older kidneys into older recipients has been proposed in an effort to translate consequences of organ and recipient age into optimized allocation systems [17]. This approach is based, at least in part, on the principle that a kidney from an older donor might be sufficient to meet the metabolic demands of older recipients while allowing an optimized utilization of older organs [214]. The less vigorous alloresponse of old recipients may also counterbalance the increased immunogenicity of old organs.
The Eurotransplant Senior Program (ESP) has implemented these concepts by allocating kidneys from donors >65 years of age regardless of HLA matching to non-sensitized local recipients ≥65 years of age [215]. In a 5-year follow-up study, waiting times had decreased significantly and allocation to local recipients led to reduced cold ischemic times and less frequent DGF [208]. While a slightly higher rate of acute rejection episodes occurred, patient and graft survival were comparable to the standard allocation scheme. A detailed risk-benefit analysis for particular age cohorts with respect to comorbidities, however, has not been established and seems of great clinical relevance.
Age-adapted immunosuppression
Age-related changes in the immune system may be of particular relevance for the management of immunosuppression in the elderly. Lower doses or different drug combinations may be able to provide an appropriate level of immunosuppression while minimizing side effects. In parallel to recipient age, risks for infections increase and mortality rates related to infections in renal allograft recipients >65 years approach that of wait-listed patients [216,217]. Recipient age has also been identified as a strong predictor of post-transplant malignancies [218] and a fivefold increase has been identified in recipients ≥65 years compared with recipients 18-34 years of age [219].
Many age-related factors may influence the pharmacology of immunosuppressive drugs in the elderly. In addition to age-intrinsic effect on pharmacodyamics, pharmacokinetics may be also altered by reduced gastric emptying, decreased splanchnic blood flow, in addition to changes in cytochrome isoenyzmes, P-glycoprotein and compromised protein binding [220]. Decreased hepatic blood flow and decreased renal clearance may also augment organ-specific toxicities [221]. Cyclosporine is highly lipophilic and as fat content increases with age its volume of distribution may increase [220]. Numerous co-morbid conditions in the elderly and drug-drug interactions caused by medication may augment side-effects of immunosuppressive drugs furthermore.
Prospective randomized trials evaluating adapted immunosuppressive protocols for old transplant recipients are so far not available. The elderly are in fact often excluded from clinical trials, possibly because of co-morbid conditions, altered drug pharmacokinetics, and higher rates of adverse effects.
The augmented immunogenicity of older organs may require a more potent early immunosuppression. The preferred induction immunosuppressive agent in the elderly, however, is unclear. With a reduced risk for infections and malignancies compared with antilymphocytic agents, interleukin 2 receptor antagonists (IL2RA) may be preferable in older recipients.[222,223]
Clinical studies on the utilization of azathioprine over mycophenolate mofetil (MMF) are conflicting. In a retrospective study, elderly renal transplant recipients receiving mycophenolate mofetil (MMF)/cyclosporine/prednisone were compared with those on azathioprine/ cyclosporine/prednisone. Older patients treated with azathioprine had a lower rate of opportunistic infection, reduced mortality and improved graft survival [224]. In contrast, Meier-Kriesche et al found MMF in the elderly to be associated with improved patient and graft survival and lower rates of acute rejection compared with azathioprine [225].
Protocols designed for the minimization of maintenance immunosuppression in the elderly have mainly focused on CNI avoidance or withdrawal. In two studies with MMF and steroid maintenance following induction with basiliximab, patient and allograft survival as well as graft function were comparable to standard protocols [226,227]. Furthermore, a retrospective cohort study recently reported that reduced doses of MMF and tacrolimus in renal transplant recipients over 60 years of age were associated with improved graft and patient survival without increased risks for AR [228].
The role of mammalian target of rapamycin (mTOR) inhibitors in immunosuppressive protocols for the elderly is still controversial. An improvement in renal function [227] and a reduced incidence of post-transplant malignancies [229] have been reported in mTOR based calcineurin inhibitor (CNI) free immunosuppressive protocols. At the same time, abnormal lipid metabolism, pulmonary infections and impaired wound healing have been linked to treatment with mTOR inhibitors [230]. Similarly, the benefit of newly established co-stimulatory blockade approaches in older renal transplant recipients remains unclear, especially with clinical reports on altered expression of CTLA4 on T cells from aged individuals [231,232]. In a recent experimental study, co-stimulatory blockade-based treatment failed to extend allograft survival in older mice to the same extent as in younger recipients [66].
Conclusions
Aging as a non-directional process based on numerous extrinsic and intrinsic factors affects the immune system in a global and complex way. While many functions seem to deteriorate with age, regulatory shifts may eventually lead to misbalanced or overzealous immune responses. Understanding the consequences of immunosenescence and organ age on alloimmune responses will be critical as the age of transplant recipients is continually increasing and there is a growing demand for organs from aged donors.
Advanced donor and advanced recipient age are both risk factors for poorer transplant outcome. Organs from old donors are more immunogenic and have shown impaired repair mechanisms and compromised functional reserves. Elderly organ transplant recipients mount dysfunctional alloimmune responses with an increased risk of chronic allograft failure and a more detrimental impact of acute rejection episodes on transplant outcome. However, immunosenescence may not be necessarily conceptualized as a less vigorous but rather as a fundamentally modified immune response. Changes in organ quality and alloimmune responses with aging demand adapted organ allocation concepts and modified immunosuppressive protocols for which more clinical studies are in need. From an immunological and utilitarian view, older organs may be do best in older recipients.
With much of the experimental data on immunosenescence gained outside of transplantation, further investigations into understanding the impact of age on function and balance of pathways of allospecific and organ age-specific immune responses is warranted.
Clinical implications of immunosenescence are far reaching beyond organ transplantation and it is expected that research in this area will allow us to better understand age-impacted processes such as autoimmunity, tolerance induction, vaccination, and wound healing [233–238].
Acknowledgments
This work was supported by grants from the NIH (AG039449) and the Instituto Carlos Slim de la Salud.
T.H. was supported by Charité Foundation and International Academy of Life Sciences/German Academic Exchange Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation Reviews.
References
- 1.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. ’United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):961–72. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 3.Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, Kennes B, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28(1):47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 5.Tullius SG, Tran H, Guleria I, Malek SK, Tilney NL, Milford E. The combination of donor and recipient age is critical in determining host immunoresponsiveness and renal transplant outcome. Ann Surg. 2010;252(4):662–74. doi: 10.1097/SLA.0b013e3181f65c7d. [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Cangro CB, Hariharan S, Hricik DE, Kerman RH, Roth D, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1(Suppl 2):3–95. [PubMed] [Google Scholar]
- 7.Meier-Kriesche HU, Ojo AO, Cibrik DM, Hanson JA, Leichtman AB, Magee JC, et al. Relationship of recipient age and development of chronic allograft failure. Transplantation. 2000;70(2):306–10. doi: 10.1097/00007890-200007270-00012. [DOI] [PubMed] [Google Scholar]
- 8.Lufft V, Kliem V, Tusch G, Dannenberg B, Brunkhorst R. Renal transplantation in older adults: is graft survival affected by age? A case control study. Transplantation. 2000;69(5):790–4. doi: 10.1097/00007890-200003150-00019. [DOI] [PubMed] [Google Scholar]
- 9.Takemoto S, Terasaki PI. Donor age and recipient age. Clin Transpl. 1988;(3):345–56. [PubMed] [Google Scholar]
- 10.Bradley B a. Rejection and recipient age. Transpl Immunol. 2002;10(2-3):125–32. doi: 10.1016/s0966-3274(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 11.Vail A, Gore SM, Bradley BA, Easty DL, Rogers CA, Armitage WJ. Conclusions of the corneal transplant follow up study. Br J Ophthalmol. 1997;81(8):631–6. doi: 10.1136/bjo.81.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirsch JD, Stratta RJ, Armbrust MJ, D’Alessandro AM, Sollinger HW, Kalayoglu M, et al. Cadaveric renal transplantation with cyclosporine in patients more than 60 years of age. Transplantation. 1989;47(2):259–61. doi: 10.1097/00007890-198902000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Renlund DG, Gilbert EM, O’Connell JB, Gay WA, Jones KW, Burton NA, et al. Age-associated decline in cardiac allograft rejection. Am J Med. 1987;83(3):391–8. doi: 10.1016/0002-9343(87)90746-7. [DOI] [PubMed] [Google Scholar]
- 14.Zetterman RK, Belle SH, Hoofnagle JH, Lawlor S, Wei Y, Everhart J, et al. Age and liver transplantation: a report of the Liver Transplantation Database. Transplantation. 1998;66(4):500–6. doi: 10.1097/00007890-199808270-00015. [DOI] [PubMed] [Google Scholar]
- 15.Snell GI, De Hoyos A, Winton T, Maurer JR. Lung transplantation in patients over the age of 50. Transplantation. 1993;55(3):562–6. doi: 10.1097/00007890-199303000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Tullius SG, Milford E. Kidney allocation and the aging immune response. N Engl J Med. 2011;364(14):1369–70. doi: 10.1056/NEJMc1103007. [DOI] [PubMed] [Google Scholar]
- 17.Cecka JM, Terasaki PI. Optimal use for older donor kidneys: older recipients. Transplant Proc. 1995;27(1):801–2. [PubMed] [Google Scholar]
- 18.De Fijter JW, Mallat MJ, Doxiadis II, Ringers J, Rosendaal FR, Claas FH, et al. Increased immunogenicity and cause of graft loss of old donor kidneys. J Am Soc Nephrol. 2001;12(7):1538–46. doi: 10.1681/ASN.V1271538. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Yao Y, Liu S, Antus B, Zou H, Lutz J, et al. Recipient age affects chronic allograft nephropathy in rats. Transplant Proc. 2001;33(7-8):3341. doi: 10.1016/s0041-1345(01)02438-1. [DOI] [PubMed] [Google Scholar]
- 20.Tullius SG, Reutzel-Selke A, Bachmann U, Jurisch A, Nieminen-Kelhä M, Pratschke J, et al. Influence of recipient and donor age on long-term renal allograft function in an experimental model. Transplant Proc. 2001;33(7-8):3345–6. doi: 10.1016/s0041-1345(01)02440-x. [DOI] [PubMed] [Google Scholar]
- 21.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3(4):329–33. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 22.Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol. 2010;22(4):500–6. doi: 10.1016/j.coi.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2(9):1011–6. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Astle CM, Harrison DE. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28(4):442–50. doi: 10.1016/s0301-472x(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 25.Lansdorp PM, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993;178(3):787–91. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood. 2003;102(5):1626–33. doi: 10.1182/blood-2002-10-3035. [DOI] [PubMed] [Google Scholar]
- 27.Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447(7145):686–90. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 28.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443(7110):421–6. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 29.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98(21):12072–7. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce DJ, Anjos-Afonso F, Ridler CM, Eddaoudi A, Bonnet D. Age-dependent increase in side population distribution within hematopoiesis: implications for our understanding of the mechanism of aging. Stem Cells. 2007;25(4):828–35. doi: 10.1634/stemcells.2006-0405. [DOI] [PubMed] [Google Scholar]
- 31.De Haan G, Nijhof W, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89(5):1543–50. [PubMed] [Google Scholar]
- 32.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117(1-3):57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 33.Taraldsrud E, Grøgaard HK, Solheim S, Lunde K, Fløisand Y, Arnesen H, et al. Age and stress related phenotypical changes in bone marrow CD34+ cells. Scand J Clin Lab Invest. 2009;69(1):79–84. doi: 10.1080/00365510802419447. [DOI] [PubMed] [Google Scholar]
- 34.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111(12):5553–61. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107(12):5465–70. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6(3):265–78. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102(26):9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Signer RAJ, Montecino-Rodriguez E, Witte ON, McLaughlin J, Dorshkind K. Age-related defects in B lymphopoiesis underlie the myeloid dominance of adult leukemia. Blood. 2007;110(6):1831–9. doi: 10.1182/blood-2007-01-069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards BK, Howe HL, Ries LAG, Thun MJ, Rosenberg HM, Yancik R, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–92. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 40.Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20(4):250–6. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- 41.Mackall CL, Gress RE. Thymic aging and T-cell regeneration. Immunol Rev. 1997;160:91–102. doi: 10.1111/j.1600-065x.1997.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 42.Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, et al. Generation of functional thymocytes in the human adult. Immunity. 1999;10(5):569–75. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 43.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 44.Pond CM. Paracrine relationships between adipose and lymphoid tissues: implications for the mechanism of HIV-associated adipose redistribution syndrome. Trends Immunol. 2003;24(1):13–8. doi: 10.1016/s1471-4906(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 45.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187(11):1839–48. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156(12):4609–16. [PubMed] [Google Scholar]
- 47.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naïve T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci U S A. 1999;96(23):13306–11. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Möwes B, et al. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35(6):1987–94. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- 49.Naylor K, Li G, Vallejo AN, Lee W-W, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174(11):7446–52. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 50.Asano Y, Komuro T, Kubo M, Sano K, Tada T. Age-related degeneracy of T cell repertoire: influence of the aged environment on T cell allorecognition. Gerontology. 1990;36(Suppl 1):3–9. doi: 10.1159/000213226. [DOI] [PubMed] [Google Scholar]
- 51.Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, et al. Expansion of cytotoxic CD8+ CD28− T cells in healthy ageing people, including centenarians. Immunology. 1996;88(4):501–7. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batliwalla F, Monteiro J, Serrano D, Gregersen PK. Oligoclonality of CD8+ T cells in health and disease: aging, infection, or immune regulation? Hum Immunol. 1996;48(1-2):68–76. doi: 10.1016/0198-8859(96)00077-8. [DOI] [PubMed] [Google Scholar]
- 53.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 54.Azuma M, Phillips JH, Lanier LL. CD28− T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150(4):1147–59. [PubMed] [Google Scholar]
- 55.Li G, Weyand CM, Goronzy JJ. Epigenetic mechanisms of age-dependent KIR2DL4 expression in T cells. J Leukoc Biol. 2008;84(3):824–34. doi: 10.1189/jlb.0807583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–69. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 57.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol. 1996;156(10):3587–90. [PubMed] [Google Scholar]
- 58.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12(7):1005–13. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 59.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175(12):8218–25. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 60.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169(4):1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 61.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200(10):1347–58. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint J-P, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127(3):274–81. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh CD, Sprent J. Aging leads to disturbed homeostasis of memory phenotype CD8(+) cells. J Exp Med. 2002;195(3):283–93. doi: 10.1084/jem.20011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallace DL, Zhang Y, Ghattas H, Worth A, Irvine A, Bennett AR, et al. Direct measurement of T cell subset kinetics in vivo in elderly men and women. J Immunol. 2004;173(3):1787–94. doi: 10.4049/jimmunol.173.3.1787. [DOI] [PubMed] [Google Scholar]
- 65.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100(25):15053–8. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du W, Shen H, Galan A, Goldstein DR. An age-specific CD8+ T cell pathway that impairs the effectiveness of strategies to prolong allograft survival. J Immunol. 2011;187(7):3631–40. doi: 10.4049/jimmunol.1100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Effros RB. From Hayflick to Walford: the role of T cell replicative senescence in human aging. Exp Gerontol. 2004;39(6):885–90. doi: 10.1016/j.exger.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Kaszubowska L. Telomere shortening and ageing of the immune system. J Physiol Pharmacol. 2008;59(Suppl 9):169–86. [PubMed] [Google Scholar]
- 69.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29(6):601–9. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 70.Brzezińska A, Magalska A, Szybińska A, Sikora E. Proliferation and apoptosis of human CD8(+)CD28(+) and CD8(+)CD28(−) lymphocytes during aging. Exp Gerontol. 2004;39(4):539–44. doi: 10.1016/j.exger.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 71.Hobbs MV, Ernst DN, Torbett BE, Glasebrook AL, Rehse MA, McQuitty DN, et al. Cell proliferation and cytokine production by CD4+ cells from old mice. J Cell Biochem. 1991;46(4):312–20. doi: 10.1002/jcb.240460406. [DOI] [PubMed] [Google Scholar]
- 72.Murasko DM, Weiner P, Kaye D. Decline in mitogen induced proliferation of lymphocytes with increasing age. Clin Exp Immunol. 1987;70(2):440–8. [PMC free article] [PubMed] [Google Scholar]
- 73.Denecke C, Bedi DS, Ge X, Kim IK-E, Jurisch A, Weiland A, et al. Prolonged graft survival in older recipient mice is determined by impaired effector T-cell but intact regulatory T-cell responses. PLoS One. 2010;5(2):e9232. doi: 10.1371/journal.pone.0009232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen H, Tesar BM, Du W, Goldstein DR. Aging impairs recipient T cell intrinsic and extrinsic factors in response to transplantation. PLoS One. 2009;4(1):e4097. doi: 10.1371/journal.pone.0004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190(7):1013–24. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whisler RL, Beiqing L, Chen M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol. 1996;169(2):185–95. doi: 10.1006/cimm.1996.0109. [DOI] [PubMed] [Google Scholar]
- 77.Sadighi Akha A a, Miller R a. Signal transduction in the aging immune system. Curr Opin Immunol. 2005;17(5):486–91. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200(12):1613–22. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kovaiou RD, Grubeck-Loebenstein B. Age-associated changes within CD4+ T cells. Immunol Lett. 2006;107(1):8–14. doi: 10.1016/j.imlet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 80.Fulop T, Larbi A, Wikby A, Mocchegiani E, Hirokawa K, Pawelec G. Dysregulation of T-cell function in the elderly: scientific basis and clinical implications. Drugs Aging. 2005;22(7):589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- 81.Larbi A, Douziech N, Dupuis G, Khalil A, Pelletier H, Guerard K-P, et al. Age-associated alterations in the recruitment of signal-transduction proteins to lipid rafts in human T lymphocytes. J Leukoc Biol. 2004;75(2):373–81. doi: 10.1189/jlb.0703319. [DOI] [PubMed] [Google Scholar]
- 82.Shearer GM. Th1/Th2 changes in aging. Mech Ageing Dev. 1997;94(1-3):1–5. doi: 10.1016/s0047-6374(96)01849-0. [DOI] [PubMed] [Google Scholar]
- 83.Uciechowski P, Kahmann L, Plümäkers B, Malavolta M, Mocchegiani E, Dedoussis G, et al. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp Gerontol. 2008;43(5):493–8. doi: 10.1016/j.exger.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Li SP, Miller RA. Age-associated decline in IL-4 production by murine T lymphocytes in extended culture. Cell Immunol. 1993;151(1):187–95. doi: 10.1006/cimm.1993.1230. [DOI] [PubMed] [Google Scholar]
- 85.Huang H, Patel DD, Manton KG. The immune system in aging: roles of cytokines, T cells and NK cells. Front Biosci. 2005;10(4):192–215. doi: 10.2741/1521. [DOI] [PubMed] [Google Scholar]
- 86.Zanni F, Vescovini R, Biasini C, Fagnoni F, Zanlari L, Telera A, et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp Gerontol. 2003;38(9):981–7. doi: 10.1016/s0531-5565(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 87.Yung R, Powers D, Johnson K, Amento E, Carr D, Laing T, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97(12):2866–71. doi: 10.1172/JCI118743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183–5. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 89.Tesar BM, Du W, Shirali AC, Walker WE, Shen H, Goldstein DR. Aging augments IL-17 T-cell alloimmune responses. Am J Transplant. 2009;9(1):54–63. doi: 10.1111/j.1600-6143.2008.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mo R, Chen J, Han Y, Bueno-Cannizares C, Misek DE, Lescure PA, et al. T cell chemokine receptor expression in aging. J Immunol. 2003;170(2):895–904. doi: 10.4049/jimmunol.170.2.895. [DOI] [PubMed] [Google Scholar]
- 91.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21(10):1105–11. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 92.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 93.Taams LS, Akbar AN. Peripheral generation and function of CD4+CD25+ regulatory T cells. Curr Top Microbiol Immunol. 2005;293:115–31. doi: 10.1007/3-540-27702-1_6. [DOI] [PubMed] [Google Scholar]
- 94.Dejaco C, Duftner C, Schirmer M. Are regulatory T-cells linked with aging? Exp Gerontol. 2006;41(4):339–45. doi: 10.1016/j.exger.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 95.Tsaknaridis L, Spencer L, Culbertson N, Hicks K, LaTocha D, Chou YK, et al. Functional assay for human CD4+CD25+ Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res. 2003;74(2):296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- 96.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, et al. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140(3):540–6. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gupta S. Molecular mechanisms of apoptosis in the cells of the immune system in human aging. Immunol Rev. 2005;205:114–29. doi: 10.1111/j.0105-2896.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 98.Potestio M, Pawelec G, Di Lorenzo G, Candore G, D’Anna C, Gervasi F, et al. Age-related changes in the expression of CD95 (APO1/FAS) on blood lymphocytes. Exp Gerontol. 1999;34(5):659–73. doi: 10.1016/s0531-5565(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 99.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160(4):1627–37. [PubMed] [Google Scholar]
- 100.Gupta S, Gollapudi S. CD95-mediated apoptosis in naïve, central and effector memory subsets of CD4+ and CD8+ T cells in aged humans. Exp Gerontol. 2008;43(4):266–74. doi: 10.1016/j.exger.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 101.Yang Y, An J, Weng N. Telomerase is involved in IL-7-mediated differential survival of naive and memory CD4+ T cells. J Immunol. 2008;180(6):3775–81. doi: 10.4049/jimmunol.180.6.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cancro MP, Hao Y, Scholz JL, Riley RL, Frasca D, Dunn-Walters DK, et al. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30(7):313–8. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171(5):2326–30. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 104.Riley RL, Blomberg BB, Frasca D. B cells, E2A, and aging. Immunol Rev. 2005;205:30–47. doi: 10.1111/j.0105-2896.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 105.Labrie JE, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200(4):411–23. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frasca D, Van der Put E, Landin AM, Gong D, Riley RL, Blomberg BB. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175(10):6633–44. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- 107.Johnson KM, Owen K, Witte PL. Aging and developmental transitions in the B cell lineage. Int Immunol. 2002;14(11):1313–23. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- 108.Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol. 1999;162(6):3342–9. [PubMed] [Google Scholar]
- 109.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7(3):213–9. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 110.Chumley MJ, Dal Porto JM, Cambier JC. The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J Immunol. 2002;169(4):1735–43. doi: 10.4049/jimmunol.169.4.1735. [DOI] [PubMed] [Google Scholar]
- 111.Gibson KL, Wu Y-C, Barnett Y, Duggan O, Vaughan R, Kondeatis E, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8(1):18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Colonna-Romano G, Bulati M, Aquino A, Scialabba G, Candore G, Lio D, et al. B cells in the aged: CD27, CD5, and CD40 expression. Mech Ageing Dev. 2003;124(4):389–93. doi: 10.1016/s0047-6374(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 113.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 114.Harless SM, Lentz VM, Sah AP, Hsu BL, Clise-Dwyer K, Hilbert DM, et al. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol. 2001;11(24):1986–9. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 115.Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160(1):63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 116.Han S, Marinova E, Zheng B. Rectification of age-related impairment in Ig gene hypermutation during a memory response. Int Immunol. 2004;16(4):525–32. doi: 10.1093/intimm/dxh054. [DOI] [PubMed] [Google Scholar]
- 117.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114(1):37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aydar Y, Balogh P, Tew JG, Szakal AK. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. J Immunol. 2003;171(11):5975–87. doi: 10.4049/jimmunol.171.11.5975. [DOI] [PubMed] [Google Scholar]
- 119.Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172(4):2155–62. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- 120.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227(1):221–33. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 121.Medzhitov R, Janeway C a. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 122.Murphy SP, Porrett PM, Turka L a. Innate immunity in transplant tolerance and rejection. Immunol Rev. 2011;241(1):39–48. doi: 10.1111/j.1600-065X.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 123.Rocha PN, Plumb TJ, Crowley SD, Coffman TM. Effector mechanisms in transplant rejection. Immunol Rev. 2003;196:51–64. doi: 10.1046/j.1600-065x.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 124.Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 125.Kroemer A, Edtinger K, Li XC. The innate natural killer cells in transplant rejection and tolerance induction. Curr Opin Organ Transplant. 2008;13(4):339–43. doi: 10.1097/MOT.0b013e3283061115. [DOI] [PubMed] [Google Scholar]
- 126.Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL. Effect of aging on bone marrow-derived murine CD11c+CD4-CD8alpha-dendritic cell function. J Gerontol A Biol Sci Med Sci. 2006;61(10):1039–47. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- 127.Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, et al. Murine [corrected] myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5(6):473–86. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 128.Lung TL, Saurwein-Teissl M, Parson W, Schönitzer D, Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18(16):1606–12. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 129.Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122(2):220–8. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 130.Sprecher E, Becker Y, Kraal G, Hall E, Harrison D, Shultz LD. Effect of aging on epidermal dendritic cell populations in C57BL/6J mice. J Invest Dermatol. 1990;94(2):247–53. doi: 10.1111/1523-1747.ep12874586. [DOI] [PubMed] [Google Scholar]
- 131.Fujihashi K, McGhee JR. Mucosal immunity and tolerance in the elderly. Mech Ageing Dev. 2004;125(12):889–98. doi: 10.1016/j.mad.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 132.Varas A, Sacedón R, Hernandez-López C, Jiménez E, García-Ceca J, Arias-Díaz J, et al. Age-dependent changes in thymic macrophages and dendritic cells. Microsc Res Tech. 2003;62(6):501–7. doi: 10.1002/jemt.10411. [DOI] [PubMed] [Google Scholar]
- 133.Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28(10):1507–21. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 134.Linton P-J, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T-cell responses in aging. Immunol Rev. 2005;205:207–19. doi: 10.1111/j.0105-2896.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 135.Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol. 2008;28(1):14–20. doi: 10.1007/s10875-007-9127-6. [DOI] [PubMed] [Google Scholar]
- 136.Donnini A, Argentati K, Mancini R, Smorlesi A, Bartozzi B, Bernardini G, et al. Phenotype, antigen-presenting capacity, and migration of antigen-presenting cells in young and old age. Exp Gerontol. 2002;37(8-9):1097–112. doi: 10.1016/s0531-5565(02)00087-6. [DOI] [PubMed] [Google Scholar]
- 137.Grewe M. Chronological ageing and photoageing of dendritic cells. Clin Exp Dermatol. 2001;26(7):608–12. doi: 10.1046/j.1365-2230.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- 138.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7(1):19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 139.Herrero C, Sebastián C, Marqués L, Comalada M, Xaus J, Valledor AF, et al. Immunosenescence of macrophages: reduced MHC class II gene expression. Exp Gerontol. 2002;37(2-3):389–94. doi: 10.1016/s0531-5565(01)00205-4. [DOI] [PubMed] [Google Scholar]
- 140.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3(4):161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 141.De La Fuente M. Changes in the macrophage function with aging. Comp Biochem Physiol A Comp Physiol. 1985;81(4):935–8. doi: 10.1016/0300-9629(85)90933-8. [DOI] [PubMed] [Google Scholar]
- 142.Khare V, Sodhi A, Singh SM. Effect of aging on the tumoricidal functions of murine peritoneal macrophages. Nat Immun. 1996;15(6):285–94. [PubMed] [Google Scholar]
- 143.Davila DR, Edwards CK, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990;4(11):2906–11. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- 144.Ding a, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153(5):2146–52. [PubMed] [Google Scholar]
- 145.Kissin E, Tomasi M, McCartney-Francis N, Gibbs CL, Smith PD. Age-related decline in murine macrophage production of nitric oxide. J Infect Dis. 1997;175(4):1004–7. doi: 10.1086/513959. [DOI] [PubMed] [Google Scholar]
- 146.Chen LC, Pace JL, Russell SW, Morrison DC. Altered regulation of inducible nitric oxide synthase expression in macrophages from senescent mice. Infect Immun. 1996;64(10):4288–98. doi: 10.1128/iai.64.10.4288-4298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rollo EE, Denhardt DT. Differential effects of osteopontin on the cytotoxic activity of macrophages from young and old mice. Immunology. 1996;88(4):642–7. doi: 10.1046/j.1365-2567.1996.d01-691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu D, Hayek MG, Meydani S. Vitamin E and macrophage cyclooxygenase regulation in the aged. J Nutr. 2001;131(2):382S–8S. doi: 10.1093/jn/131.2.382S. [DOI] [PubMed] [Google Scholar]
- 149.Harizi H, Grosset C, Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J Leukoc Biol. 2003;73(6):756–63. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 150.Wood JJ, Grbic JT, Rodrick ML, Jordan A, Mannick JA. Suppression of interleukin 2 production in an animal model of thermal injury is related to prostaglandin synthesis. Arch Surg. 1987;122(2):179–84. doi: 10.1001/archsurg.1987.01400140061007. [DOI] [PubMed] [Google Scholar]
- 151.Hilkens CM, Snijders A, Snijdewint FG, Wierenga EA, Kapsenberg ML. Modulation of T-cell cytokine secretion by accessory cell-derived products. Eur Respir J Suppl. 1996;22:90s–94s. [PubMed] [Google Scholar]
- 152.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 153.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75(2):342–9. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 154.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169(9):4697–701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 155.Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest. 1998;78(1):47–58. [PubMed] [Google Scholar]
- 156.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79(12):1479–87. [PubMed] [Google Scholar]
- 157.Kmiec Z, Myśliwska J, Rachón D, Kotlarz G, Sworczak K, Myśliwski A. Natural killer activity and thyroid hormone levels in young and elderly persons. Gerontology. 2001;47(5):282–8. doi: 10.1159/000052813. [DOI] [PubMed] [Google Scholar]
- 158.Myśliwska J, Bryl E, Zorena K, Balon J, Foerster J, Myśliwski A. Overactivity of tumor necrosis factor-alpha but not interleukin 6 is associated with low natural killer cytotoxic activity in the elderly. Gerontology. 1997;43(3):158–67. doi: 10.1159/000213845. [DOI] [PubMed] [Google Scholar]
- 159.Ravaglia G, Forti P, Maioli F, Bastagli L, Facchini A, Mariani E, et al. Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged >/=90 y. Am J Clin Nutr. 2000;71(2):590–8. doi: 10.1093/ajcn/71.2.590. [DOI] [PubMed] [Google Scholar]
- 160.Hsueh CM, Chen SF, Ghanta VK, Hiramoto RN. Involvement of cytokine gene expression in the age-dependent decline of NK cell response. Cell Immunol. 1996;173(2):221–9. doi: 10.1006/cimm.1996.0271. [DOI] [PubMed] [Google Scholar]
- 161.Mariani E, Mariani AR, Meneghetti A, Tarozzi A, Cocco L, Facchini A. Age-dependent decreases of NK cell phosphoinositide turnover during spontaneous but not Fc-mediated cytolytic activity. Int Immunol. 1998;10(7):981–9. doi: 10.1093/intimm/10.7.981. [DOI] [PubMed] [Google Scholar]
- 162.Fernandes G, Gupta S. Natural killing and antibody-dependent cytotoxicity by lymphocyte subpopulations in young and aging humans. J Clin Immunol. 1981;1(3):141–8. doi: 10.1007/BF00922755. [DOI] [PubMed] [Google Scholar]
- 163.Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82(9):2767–73. [PubMed] [Google Scholar]
- 164.Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3(4):177–84. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 165.Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34(2):253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 166.Mariani E, Meneghetti A, Neri S, Ravaglia G, Forti P, Cattini L, et al. Chemokine production by natural killer cells from nonagenarians. Eur J Immunol. 2002;32(6):1524–9. doi: 10.1002/1521-4141(200206)32:6<1524::AID-IMMU1524>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 167.Born J, Uthgenannt D, Dodt C, Nünninghoff D, Ringvolt E, Wagner T, et al. Cytokine production and lymphocyte subpopulations in aged humans. An assessment during nocturnal sleep. Mech Ageing Dev. 1995;84(2):113–26. doi: 10.1016/0047-6374(95)01638-4. [DOI] [PubMed] [Google Scholar]
- 168.Chatta GS, Andrews RG, Rodger E, Schrag M, Hammond WP, Dale DC. Hematopoietic progenitors and aging: alterations in granulocytic precursors and responsiveness to recombinant human G-CSF, GM-CSF, and IL-3. J Gerontol. 1993;48(5):M207–12. doi: 10.1093/geronj/48.5.m207. [DOI] [PubMed] [Google Scholar]
- 169.Biasi D, Carletto A, Dell’Agnola C, Caramaschi P, Montesanti F, Zavateri G, et al. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation. 1996;20(6):673–81. doi: 10.1007/BF01488803. [DOI] [PubMed] [Google Scholar]
- 170.Fransson C, Mooney J, Kinane DF, Berglundh T. Differences in the inflammatory response in young and old human subjects during the course of experimental gingivitis. J Clin Periodontol. 1999;26(7):453–60. doi: 10.1034/j.1600-051x.1999.260707.x. [DOI] [PubMed] [Google Scholar]
- 171.Damtew B, Spagnuolo PJ, Goldsmith GG, Marino JA. Neutrophil adhesion in the elderly: inhibitory effects of plasma from elderly patients. Clin Immunol Immunopathol. 1990;54(2):247–55. doi: 10.1016/0090-1229(90)90086-6. [DOI] [PubMed] [Google Scholar]
- 172.Corberand J, Ngyen F, Laharrague P, Fontanilles AM, Gleyzes B, Gyrard E, et al. Polymorphonuclear functions and aging in humans. J Am Geriatr Soc. 1981;29(9):391–7. doi: 10.1111/j.1532-5415.1981.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 173.Niwa Y, Kasama T, Miyachi Y, Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 1989;44(22):1655–64. doi: 10.1016/0024-3205(89)90482-7. [DOI] [PubMed] [Google Scholar]
- 174.Seres I, Csongor J, Mohácsi A, Leövey A, Fülöp T. Age-dependent alterations of human recombinant GM-CSF effects on human granulocytes. Mech Ageing Dev. 1993;71(1-2):143–54. doi: 10.1016/0047-6374(93)90042-p. [DOI] [PubMed] [Google Scholar]
- 175.Emanuelli G, Lanzio M, Anfossi T, Romano S, Anfossi G, Calcamuggi G. Influence of age on polymorphonuclear leukocytes in vitro: phagocytic activity in healthy human subjects. Gerontology. 1986;32(6):308–16. doi: 10.1159/000212809. [DOI] [PubMed] [Google Scholar]
- 176.Mege JL, Capo C, Michel B, Gastaut JL, Bongrand P. Phagocytic cell function in aged subjects. Neurobiol Aging. 1988;9(2):217–20. doi: 10.1016/s0197-4580(88)80054-x. [DOI] [PubMed] [Google Scholar]
- 177.Fu YK, Arkins S, Li YM, Dantzer R, Kelley KW. Reduction in superoxide anion secretion and bactericidal activity of neutrophils from aged rats: reversal by the combination of gamma interferon and growth hormone. Infect Immun. 1994;62(1):1–8. doi: 10.1128/iai.62.1.1-8.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tortorella C, Polignano A, Piazzolla G, Serrone M, Jirillo E, Antonaci S. Lipopolysaccharide-, granulocyte-monocyte colony stimulating factor and pentoxifylline-mediated effects on formyl-methionyl-leucine-phenylalanine-stimulated neutrophil respiratory burst in the elderly. Microbios. 1996;85(344):189–98. [PubMed] [Google Scholar]
- 179.Fülöp T, Varga Z s, Jacob MP, Robert L. Effect of lithium on superoxide production and intracellular free calcium mobilization in elastin peptide (kappa-elastin) and FMLP stimulated human PMNS. Effect of age. Life Sci. 1997;60(22):325–32. doi: 10.1016/s0024-3205(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 180.Rao KM, Currie MS, Padmanabhan J, Cohen HJ. Age-related alterations in actin cytoskeleton and receptor expression in human leukocytes. J Gerontol. 1992;47(2):B37–44. doi: 10.1093/geronj/47.2.b37. [DOI] [PubMed] [Google Scholar]
- 181.Alvarez E, Ruiz-Gutiérrez V, Sobrino F, Santa-María C. Age-related changes in membrane lipid composition, fluidity and respiratory burst in rat peritoneal neutrophils. Clin Exp Immunol. 2001;124(1):95–102. doi: 10.1046/j.1365-2249.2001.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol. 2006;79(5):1061–72. doi: 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- 183.Terasaki PI, Gjertson DW, Cecka JM, Takemoto S, Cho YW. Significance of the donor age effect on kidney transplants. Clin Transplant. 1997;11(5 Pt 1):366–72. [PubMed] [Google Scholar]
- 184.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333(6):333–6. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 185.Cecka JM. Living donor transplants. Clin Transpl. 1995;11:363–77. [PubMed] [Google Scholar]
- 186.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232(2):194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 187.Epstein M. Aging and the kidney. J Am Soc Nephrol. 1996;7(8):1106–22. doi: 10.1681/ASN.V781106. [DOI] [PubMed] [Google Scholar]
- 188.Boom H, Mallat MJ, De Fijter JW, Zwinderman AH, Paul LC. Delayed graft function influences renal function, but not survival. Kidney Int. 2000;58(2):859–66. doi: 10.1046/j.1523-1755.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 189.Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys. 2003;420(2):287–97. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 190.Okaya T, Blanchard J, Schuster R, Kuboki S, Husted T, Caldwell CC, et al. Age-dependent responses to hepatic ischemia/reperfusion injury. Shock. 2005;24(5):421–7. doi: 10.1097/01.shk.0000181282.14050.11. [DOI] [PubMed] [Google Scholar]
- 191.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, et al. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci. 2005;60(7):830–9. doi: 10.1093/gerona/60.7.830. [DOI] [PubMed] [Google Scholar]
- 192.Kusaka J, Koga H, Hagiwara S, Hasegawa A, Kudo K, Noguchi T. Age-dependent responses to renal ischemia-reperfusion injury. J Surg Res. 2012;172(1):153–8. doi: 10.1016/j.jss.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 193.Li X, Cooley BC, Gould JS. Effect of age upon ischemia/reperfusion injury in rat muscle free flaps. J Surg Res. 1993;55(2):193–200. doi: 10.1006/jsre.1993.1129. [DOI] [PubMed] [Google Scholar]
- 194.Mowlavi A, Reynolds C, Neumeister MW, Wilhelmi BJ, Song Y-H, Naffziger R, et al. Age-related differences of neutrophil activation in a skeletal muscle ischemia-reperfusion model. Ann Plast Surg. 2003;50(4):403–11. doi: 10.1097/01.SAP.0000041663.28703.54. [DOI] [PubMed] [Google Scholar]
- 195.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 196.Halloran PF, Homik J, Goes N, Lui SL, Urmson J, Ramassar V, et al. The “injury response”: a concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc. 1997;29(1-2):79–81. doi: 10.1016/s0041-1345(96)00015-2. [DOI] [PubMed] [Google Scholar]
- 197.Reutzel-Selke A, Jurisch A, Denecke C, Pascher A, Martins PN a, Kessler H, et al. Donor age intensifies the early immune response after transplantation. Kidney Int. 2007;71(7):629–36. doi: 10.1038/sj.ki.5002098. [DOI] [PubMed] [Google Scholar]
- 198.Ordemann R, Hutchinson R, Friedman J, Burakoff SJ, Reddy P, Duffner U, et al. Enhanced allostimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J Clin Invest. 2002;109(9):1249–56. doi: 10.1172/JCI14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Castle SC, Uyemura K, Crawford W, Wong W, Makinodan T. Antigen presenting cell function is enhanced in healthy elderly. Mech Ageing Dev. 1999;107(2):137–45. doi: 10.1016/s0047-6374(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 200.Sidman CL, Luther EA, Marshall JD, Nguyen KA, Roopenian DC, Worthen SM. Increased expression of major histocompatibility complex antigens on lymphocytes from aged mice. Proc Natl Acad Sci U S A. 1987;84(21):7624–8. doi: 10.1073/pnas.84.21.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Agrawal A, Agrawal S, Cao J-N, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178(11):6912–22. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 202.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17(5):457–62. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 204.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 205.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 206.Basar H, Soran A, Shapiro R, Vivas C, Scantlebury VP, Jordan ML, et al. Renal transplantation in recipients over the age of 60: the impact of donor age. Transplantation. 1999;67(8):1191–3. doi: 10.1097/00007890-199904270-00019. [DOI] [PubMed] [Google Scholar]
- 207.Waiser J, Schreiber M, Budde K, Fritsche L, Böhler T, Hauser I, et al. Age-matching in renal transplantation. Nephrol Dial Transplant. 2000;15(5):696–700. doi: 10.1093/ndt/15.5.696. [DOI] [PubMed] [Google Scholar]
- 208.Frei U, Noeldeke J, Machold-Fabrizii V, Arbogast H, Margreiter R, Fricke L, et al. Prospective age-matching in elderly kidney transplant recipients--a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant. 2008;8(1):50–7. doi: 10.1111/j.1600-6143.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- 209.Reutzel-Selke A, Filatenkov A, Jurisch A, Denecke C, Martins PNA, Pascher A, et al. Grafts from elderly donors elicit a stronger immune response in the early period posttransplantation: a study in a rat model. Transplant Proc. 2005;37(1):382–3. doi: 10.1016/j.transproceed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 210.Tullius SG, Reutzel-Selke A, Egermann F, Nieminen-Kelhä M, Jonas S, Bechstein WO, et al. Contribution of prolonged ischemia and donor age to chronic renal allograft dysfunction. J Am Soc Nephrol. 2000;11(7):1317–24. doi: 10.1681/ASN.V1171317. [DOI] [PubMed] [Google Scholar]
- 211.Denecke C, Ge X, Jurisch A, Kleffel S, Kim IK, Padera RF, et al. Modified CD4(+) T-cell response in recipients of old cardiac allografts. Transpl Int. 2012;25(3):328–36. doi: 10.1111/j.1432-2277.2011.01417.x. [DOI] [PubMed] [Google Scholar]
- 212.Moreso F, Serón D, Gil-Vernet S, Riera L, Fulladosa X, Ramos R, et al. Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrol Dial Transplant. 1999;14(4):930–5. doi: 10.1093/ndt/14.4.930. [DOI] [PubMed] [Google Scholar]
- 213.Halloran PF, Melk A, Barth C. Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol. 1999;10(1):167–81. doi: 10.1681/ASN.V101167. [DOI] [PubMed] [Google Scholar]
- 214.Meier-Kriesche H-U, Schold JD, Gaston RS, Wadstrom J, Kaplan B. Kidneys from deceased donors: maximizing the value of a scarce resource. Am J Transplant. 2005;5(7):1725–30. doi: 10.1111/j.1600-6143.2005.00923.x. [DOI] [PubMed] [Google Scholar]
- 215.Smits JMA, Persijn GG, Van Houwelingen HC, Claas FHJ, Frei U. Evaluation of the Eurotransplant Senior Program. The results of the first year. Am J Transplant. 2002;2(7):664–70. doi: 10.1034/j.1600-6143.2002.20713.x. [DOI] [PubMed] [Google Scholar]
- 216.Meier-Kriesche HU, Friedman G, Jacobs M, Mulgaonkar S, Vaghela M, Kaplan B. Infectious complications in geriatric renal transplant patients: comparison of two immunosuppressive protocols. Transplantation. 1999;68(10):1496–502. doi: 10.1097/00007890-199911270-00012. [DOI] [PubMed] [Google Scholar]
- 217.Meier-Kriesche HU, Ojo A, Hanson J, Cibrik D, Lake K, Agodoa LY, et al. Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 2000;69(5):885–9. doi: 10.1097/00007890-200003150-00037. [DOI] [PubMed] [Google Scholar]
- 218.Danpanich E, Kasiske BL. Risk factors for cancer in renal transplant recipients. Transplantation. 1999;68(12):1859–64. doi: 10.1097/00007890-199912270-00008. [DOI] [PubMed] [Google Scholar]
- 219.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905–13. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 220.Danovitch GM, Gill J, Bunnapradist S. Immunosuppression of the elderly kidney transplant recipient. Transplantation. 2007;84(3):285–91. doi: 10.1097/01.tp.0000275423.69689.dc. [DOI] [PubMed] [Google Scholar]
- 221.Bernardo JF, McCauley J. Drug therapy in transplant recipients: special considerations in the elderly with comorbid conditions. Drugs Aging. 2004;21(5):323–48. doi: 10.2165/00002512-200421050-00004. [DOI] [PubMed] [Google Scholar]
- 222.Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O’Sullivan EJ, Johnson MR, Heroux AL, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. 1990;323(25):1723–8. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- 223.Thistlethwaite JR, Stuart JK, Mayes JT, Gaber AO, Woodle S, Buckingham MR, et al. Complications and monitoring of OKT3 therapy. Am J Kidney Dis. 1988;11(2):112–9. doi: 10.1016/s0272-6386(88)80192-6. [DOI] [PubMed] [Google Scholar]
- 224.Johnson DW, Nicol DL, Preston JM, Brown AM, Hawley CM, Campbell SB, et al. Use of mycophenolate mofetil in immunosuppressive protocols in elderly renal transplant recipients. Transplantation. 2003;76(3):619. doi: 10.1097/01.TP.0000074314.46438.25. [DOI] [PubMed] [Google Scholar]
- 225.Meier-Kriesche H-U, Morris JA, Chu AH, Steffen BJ, Gotz VP, Gordon RD, et al. Mycophenolate mofetil vs azathioprine in a large population of elderly renal transplant patients. Nephrol Dial Transplant. 2004;19(11):2864–9. doi: 10.1093/ndt/gfh445. [DOI] [PubMed] [Google Scholar]
- 226.Emparan C, Wolters H, Laukötter M, Senninger N. Long-term results of calcineurin-free protocols with basiliximab induction in “old-to-old” programs. Transplant Proc. 2004;36(9):2646–8. doi: 10.1016/j.transproceed.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 227.Oberbauer R, Segoloni G, Campistol JM, Kreis H, Mota A, Lawen J, et al. Early cyclosporine withdrawal from a sirolimus-based regimen results in better renal allograft survival and renal function at 48 months after transplantation. Transpl Int. 2005;18(1):22–8. doi: 10.1111/j.1432-2277.2004.00052.x. [DOI] [PubMed] [Google Scholar]
- 228.Badowski M, Gurk-Turner C, Cangro C, Weir M, Philosophe B, Klassen D, et al. The impact of reduced immunosuppression on graft outcomes in elderly renal transplant recipients. Clin Transplant. 2009;23(6):930–7. doi: 10.1111/j.1399-0012.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- 229.Kahan BD, Yakupoglu YK, Schoenberg L, Knight RJ, Katz SM, Lai D, et al. Low incidence of malignancy among sirolimus/cyclosporine-treated renal transplant recipients. Transplantation. 2005;80(6):749–58. doi: 10.1097/01.tp.0000173770.42403.f7. [DOI] [PubMed] [Google Scholar]
- 230.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80(7):883–9. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 231.Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev. 2009;130(10):709–12. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 232.Leng Q, Bentwich Z, Borkow G. CTLA-4 upregulation during aging. Mech Ageing Dev. 2002;123(10):1419–21. doi: 10.1016/s0047-6374(02)00077-5. [DOI] [PubMed] [Google Scholar]
- 233.Agrawal A, Sridharan A, Prakash S, Agrawal H. Dendritic cells and aging: consequences for autoimmunity. Expert Rev Clin Immunol. 2012;8(1):73–80. doi: 10.1586/eci.11.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hasler P, Zouali M. Immune receptor signaling, aging, and autoimmunity. Cell Immunol. 2005;233(2):102–8. doi: 10.1016/j.cellimm.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 235.Martins PN. Impact of donor and recipient age on allograft tolerance. Exp Clin Transplant. 2009;7(2):67–77. [PubMed] [Google Scholar]
- 236.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30(7):351–9. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Thomas DR. Age-related changes in wound healing. Drugs Aging. 2001;18(8):607–20. doi: 10.2165/00002512-200118080-00005. [DOI] [PubMed] [Google Scholar]
- 238.Gosain A, DiPietro L a. Aging and wound healing. World J Surg. 2004;28(3):321–6. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]