Abstract
Background
Epigenetic mechanisms have been implicated in psychiatric disorders, including alcohol dependence. However, the epigenetic basis and role of specific histone deacetylase (HDAC) isoforms in the genetic predisposition to anxiety and alcoholism is unknown.
Methods
We measured amygdaloid HDAC activity, levels of HDAC isoforms and histone H3 acetylation in selectively-bred alcohol-preferring (P) and -nonpreferring (NP) rats. We employed HDAC2 siRNA infusion into the central nucleus of amygdala (CeA) of P rats to determine the causal role of HDAC2 in anxiety-like and alcohol-drinking behaviors. Chromatin immunoprecipitation analysis was performed to examine the histone acetylation status of brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton associated protein (Arc) genes. Golgi-Cox staining was performed to measure dendritic spine density.
Results
We found that P rats innately display higher nuclear HDAC activity and HDAC2, but not HDAC 1, 3, 4, 5, and 6 protein levels, and lower acetylation of H3-K9, but not H3-K14, in the CeA and medial nucleus of amygdala (MeA) compared with NP rats. Acute ethanol exposure decreased amygdaloid HDAC activity and HDAC2 protein levels, increased global and gene (BDNF and Arc)-specific histone acetylation and attenuated anxiety-like behaviors in P rats, but had no effects in NP rats. HDAC2 knockdown in the CeA attenuated anxiety-like behaviors and voluntary alcohol, but not sucrose, consumption in P rats and increased histone acetylation of BDNF and Arc with a resultant increase in protein levels that correlated with increased dendritic spine density.
Conclusions
These novel data demonstrate the role of HDAC2-mediated epigenetic mechanisms in anxiety and alcoholism.
Keywords: Epigenetic, HDAC2, Amygdala, Anxiety and Alcoholism, BDNF, Dendritic spines
Alcoholism is a multifactorial psychiatric disorder driven by underlying genetic and environmental factors (1, 2). Evidence from animal and human studies indicates that negative emotionality is clinically relevant in alcohol dependence, and anxiety sensitivity may serve as a risk factor for the development of alcohol use disorders (3–9). Anxiety may promote alcohol consumption due to its ability to reduce dysphoria, leading to self-medication (4–11). The alcohol-preferring (P) and -nonpreferring (NP) rat lines have been selectively-bred for higher and lower alcohol preference, respectively (12). P rats innately display anxiety-like behaviors compared to NP rats, thus providing a suitable animal model for human alcoholism with comorbid anxiety (13–17). The central nucleus of amygdala (CeA), a part of the extended amygdala, appears to serve as a major neuroanatomical substrate underlying the interaction between anxiety and alcoholism (5, 11).
Identifying the epigenetic mechanisms in specific neurocircuitries underlying anxiety and alcoholism is crucial to understand the regulation of molecular pathways leading to alcohol addiction. Covalent modifications of histones, such as acetylation, dynamically regulate gene transcription that is involved in the regulation of neuronal functions (18–23). Histone deacetylases (HDACs) are enzymes that deacetylate histone proteins, regulating chromatin structure. They are grouped into four classes based on cellular localization and functional regulation (23–25). Recent studies suggest aberrant regulation of class I and II HDACs may play a role in psychiatric disorders, including alcohol dependence, thereby serving as therapeutic targets for these disorders (18–20,26–33). Recently we showed that in an unselected stock of Sprague Dawley (SD) rats, withdrawal from chronic ethanol exposure resulted in anxiety-like behaviors that were associated with an increase in HDAC activity and a reduction in histone H3-K9 acetylation in the CeA and MeA (28). Treatment of ethanol-withdrawn rats with trichostatin A, an HDAC inhibitor, attenuated anxiety-like behaviors, and normalized HDAC activity and deficits in histone acetylation (28). We further identified the involvement of amygdaloid HDAC-mediated changes in histone acetylation in the development of rapid tolerance to the anxiolytic effects of ethanol in SD rats (27).
Epigenetic mechanisms have been implicated in the regulation of brain-derived neurotrophic factor (BDNF) gene expression and associated synaptic plasticity (26, 33, 34). BDNF signaling is involved in the regulation of synaptic plasticity via induction of activity-regulated cytoskeleton (Arc) associated protein expression (35–41). BDNF and Arc are also implicated to play a causative role in both anxiety and alcoholism (13, 42–47). Previously, we found that in comparison to NP rats, P rats have innately lower levels of BDNF, Arc and dendritic spine density in the CeA and medial nucleus of amygdala (MeA) which are increased by acute ethanol treatment (13, 45). However, the role of HDAC isoform-specific chromatin remodeling in the amygdala underlying the genetic predisposition to anxiety and alcoholism is currently unknown. We therefore investigated the role of amygdaloid HDAC2-mediated histone modifications in the regulation of synaptic plasticity associated events and the phenotypes of anxietylike and alcohol-drinking behaviors using P and NP rats.
Materials and Methods
Animals
Adult male P and NP rats (Indiana Alcohol Research Center at Indiana University, Indianapolis, IN) were housed in a temperature- and humidity-controlled facility under a 12 h light/dark cycle, with ad libitum food and water access. Age-matched rats weighing 330–400 g were used. Animal protocols were approved by the Institutional Animal Care and Use Committee.
Acute Ethanol Treatment
Rats were housed individually 1 day before acute ethanol exposure and injected intraperitoneally (IP) with ethanol (diluted to 20% w/v in n-saline; 1 g/kg) or n-saline alone. The procedure and ethanol dose are based on our and other studies in the field (13, 15, 27, 28). One hour following injection, anxiety-like behaviors were measured followed by anesthetization with pentobarbital (50 mg/kg). The rats were then either perfused for the collection of brains or directly decapitated and amygdaloid tissue containing predominantly CeA and MeA was punched out for neurochemical measures. Blood was collected to measure ethanol levels using the Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Stereotaxic Cannulation Surgery and HDAC2 siRNA Infusion
For siRNA infusion experiments, rats were surgically implanted with CMA/11 guide cannulae targeting CeA (CMA Microdialysis, North Chelmsford, MA) using coordinates as described previously by us (14, 47, 48). Rats were housed individually after surgery and one week after recovery, bilateral infusions into the CeA with 0.5 µL of HDAC2 siRNA, negative control siRNA or vehicle was performed using a microdialysis probe. siRNAs were dissolved in iFect solution (Neuromics, Edina, MN), a cationic lipid-based transfection solution, such that the final concentration was 2 µg/µL. The sequence of the HDAC2 siRNA (Qiagen, Valencia, CA) was as follows: sense, 5’-CAAGUUUCUACGAUCAAUATT-3’; antisense 5’-UAUUGAUCGUAGAAACUUGAT-3’. In some cases, HDAC2 siRNA was tagged with a 3’ AlexaFluor-488 fluorescent probe. The control siRNA used was AllStars Negative Control siRNA (Qiagen). Vehicle was prepared by mixing RNase-free water in iFect solution instead of siRNA. Anxiety-like behaviors were tested 16 hours after infusion, followed immediately by collection of brains for epigenetic measures.
Measurement of Anxiety-like Behaviors
Rats were tested for anxiety-like behaviors using the elevated plus maze (EPM) or light/dark box (LDB) exploration test as reported earlier by us (13, 27, 28).
HDAC2 siRNA Infusion and Voluntary Alcohol and Sucrose Consumption
Alcohol-naïve P rats were bilaterally cannulated and then ethanol or sucrose consumption was measured using the two-bottle free-choice paradigm as described previously (14, 48). Cannulated P rats were housed individually and habituated to drink water equally from two bottles. Rats were then given one bottle containing ethanol or sucrose solution and another bottle of water. Rats were given 7% ethanol for 3 days or 2% sucrose for 2 days, followed by 9% ethanol or 4% sucrose. The concentration of sucrose solution is based on studies in rats used for sucrose drinking behaviors (49, 50). Bilateral HDAC2 siRNA or vehicle infusions into CeA were performed following the third day of 9% ethanol or 4% sucrose consumption and intake was monitored for several days. The alcohol or sucrose intake was calculated as g/kg/day.
HDAC Activity, Immunohistochemistry, in situ RT-PCR and Golgi-Cox Staining
HDAC activity, immunohistochemistry, in situ RT-PCR and Golgi-Cox staining were performed as described previously (27, 28, 45–48). The nuclear and cytosolic fractions of the amygdala were used to measure HDAC activity by a colorimetric HDAC activity assay (Biovision Research, Mountain View, CA). Immunohistochemistry was performed using the following primary antibodies: anti-Arc and anti-BDNF (Santa Cruz Biotechnology, Santa Cruz, CA); anti-HDAC2, anti-HDAC4 (MBL International, Woburn, MA) and anti-HDAC (1, 3, 5, and 6) (BioVision, Milpitas, CA); anti-acetyl histone H3-K9 and H3-K14 (Millipore, Billerica, MA); anti-NeuN (Millipore). Gold particle-conjugated anti-rabbit IgG (Nanoprobes, Yaphank, NY) and AlexaFluor-568-conjugated goat anti-mouse IgG (Invitrogen, Eugene, OR) were used as secondary antibodies for gold-immunolabeling (13, 28) and immunofluorescence ( 27, 48) labeling, respectively. In situ RT-PCR was performed as published previously (13, 45). The primers for HDAC2 (Integrated DNA Technologies, Coralville, IO) were: Forward, 5’-CGGTGGCTCAGTTGCTGGGG-3’; Reverse, 5’-GGCCTCTGACTTCTTGGCGTGG-3’. Golgi-Cox staining was performed according to the user manual for the FD Rapid Golgi stain TM Kit (FD Neuro Technologies, Baltimore, MD) and as published (13, 46).
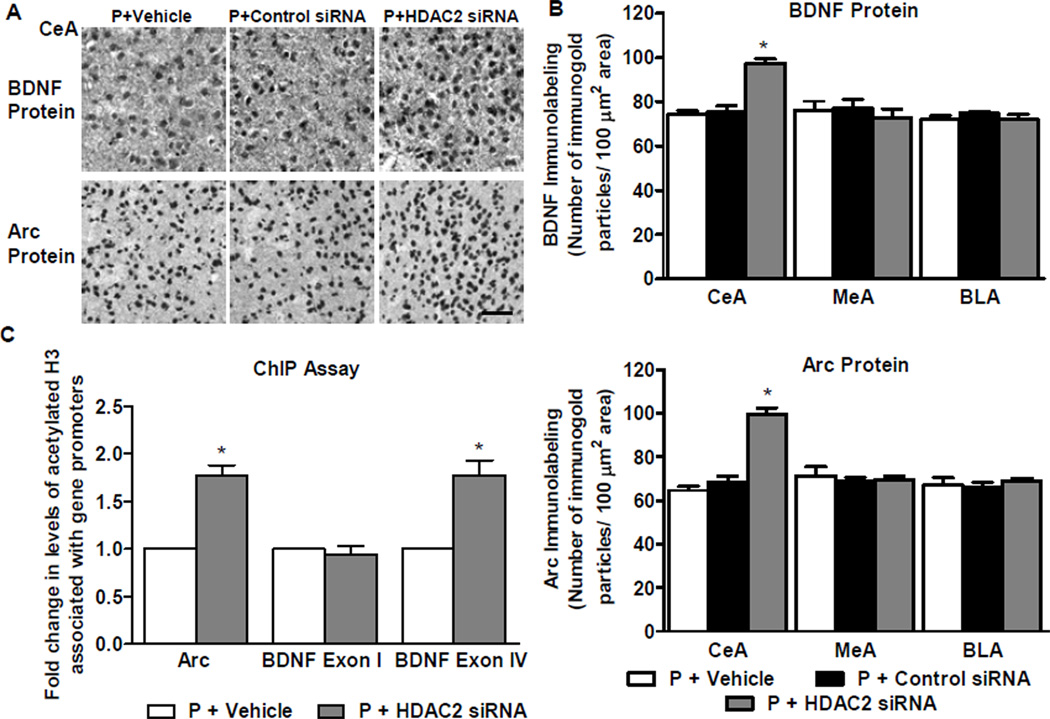
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed following manufacturer instructions for the ChIP-IT express kit (Active Motif, Carlsbad, CA). Amygdaloid tissue was fixed, homogenized, and subjected to DNA shearing and the amount of sample normalized to contain equivalent protein amounts. Chromatin was immunoprecipitated with anti-acetyl histone H3-K9/14 antibody (Millipore) or IgG (Active Motif). Associated DNA fragments were quantified using qPCR with the RT2 Sybr Green Master Mix (Super Array Biosciences, Frederick, MD) with primers for BDNF exons I and IV, and Arc designed within or adjacent to the promoter region. The primer sequences were as follows: Arc, Forward, 5’-AGTGCTCTGGCGAGTAGTCC-3’, Reverse, 5’-TCGGGACAGGCTAAGAACTC-3’; BDNF exon I, Forward, 5’-GCGCCCAAAGCCCACCTTCT-3’, Reverse, 5’-GCGTCGGCTCCGTGCTTCTT-3’; BDNF exon IV, Forward, 5’-GTTCGCTAGGACTGGAAGTGG-3’, Reverse, 5’- CCTCTGCCTCGAAATAGACAC-3’. Relative quantification of acetylated H3-associated genes in various groups was calculated by the ΔΔCt method (51). The ΔCt for each sample was calculated from their input DNA and then ΔΔCt and fold changes were calculated batch-wise from ΔCt of respective groups.
Statistical Analyses
Statistical differences between groups were evaluated by a one or two-way ANOVA test or Student’s t-test. Two-way repeated measures ANOVA was performed for evaluation of alcohol and sucrose drinking behaviors. Post hoc comparisons were performed using Tukey’s test. A p < 0.05 value was considered significant.
Results
Effects of Acute Ethanol Exposure on Anxiety Measures and HDAC Activity
P and NP rats were injected with saline or ethanol (1 g/kg; IP) and anxiety-like behaviors were measured 1hr after injections using the LDB exploration test [mean ± SEM blood ethanol levels: P = 100.8± 5.0 mg% (n=13); NP= 101.1± 5.2 mg% (n=13)]. Similar to our previous findings (13) we found that at baseline, P rats spent significantly (p<0.001) less time in the light compartment than NP rats. Acute ethanol treatment produced an anxiolytic effect in P, but not NP rats, as evidenced by a significant (p<0.001) increase in the percentage of time spent in the light compartment (Figure 1A). The total number of ambulations did not differ significantly between the groups (data not shown).
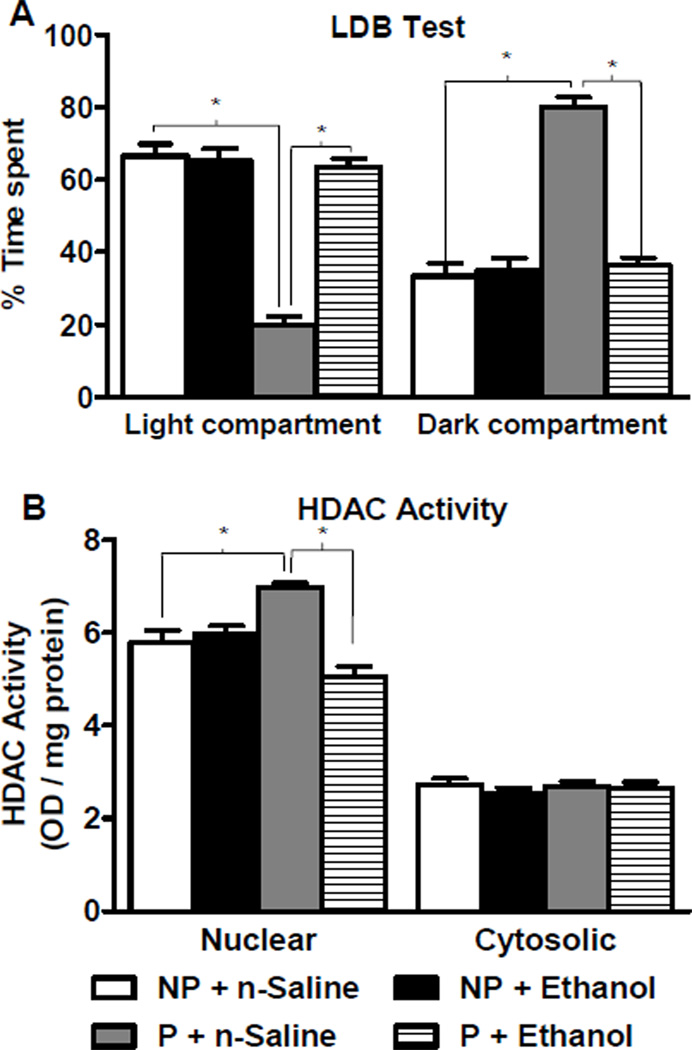
Figure 1. Effects of acute ethanol exposure on anxiety-like behaviors and HDAC activity in the amygdala of P and NP rats.
A. The light/dark box (LDB) exploration test showed that alcohol-preferring (P) rats display innate anxiety-like behaviors in comparison to alcohol-nonpreferring (NP) rats. Acute ethanol treatment (1 g/kg) produced anxiolytic effects in P rats, but not NP rats. Values are represented as the mean ± SEM of the percentage of time spent in each compartment averaged from 13 rats per group. *Significantly different from respective control groups (Two-way ANOVA; group × treatment, F1,48 = 58.6, p<0.001 followed by post hoc analysis by Tukey’s test, p<0.001).
B. The nuclear fraction, but not cytosolic fraction, of the amygdala from P rats displays higher histone deacetylase (HDAC) activity at baseline compared to NP rats. Acute ethanol treatment inhibited the nuclear HDAC activity in the amygdala of P rats, without any effect in NP rats. Values are represented as the mean ± SEM of the optical density (OD) per mg of protein from 6 rats per group. *Significantly different from respective control groups (Nuclear HDAC activity, two-way ANOVA; group × treatment, F1, 20 = 29.6, p<0.001 followed by post hoc analysis by Tukey’s test, p<0.001).
We examined effects of acute ethanol exposure on HDAC activity and found that at baseline, compared to NP rats, P rats had significantly (p<0.001) higher nuclear, but not cytosolic, amygdaloid HDAC activity. Acute ethanol significantly (p<0.001) inhibited HDAC activity only in the nuclear fraction of P rats without any effect in NP rats (Figure 1B). These data suggest that the anxiety-like behaviors of P rats may be related to innately higher nuclear HDAC activity in the amygdala, and the anxiolytic effects of acute ethanol may be related to inhibition of nuclear HDAC activity.
Effects of Acute Ethanol on Amygdaloid HDAC Protein Levels
Since P and NP rats showed differences in nuclear HDAC activity in the amygdala at baseline and during acute ethanol treatment, we explored the possibility that class I HDAC proteins, such as HDAC 1, 2, and 3 which are mainly localized in the nucleus (25), may be differentially expressed in the amygdala of P and NP rats. We also studied the differences in protein levels of class II HDACs, such as HDAC 4, 5, and 6, which are mainly cytosolic (25). Differences in amygdaloid HDAC 2 and 4 protein levels in P and NP rats following acute ethanol exposure was also investigated.
We found that at baseline, P rats had significantly (p<0.001) higher HDAC 2, but not HDAC 1, 3, 4, 5, and 6 protein levels, in the CeA and MeA compared to NP rats. The protein levels of all measured HDAC isoforms at baseline did not differ in the BLA of P and NP rats (Figure 2A, B, and Figure S1). Acute ethanol treatment resulted in a significant (p<0.001) reduction in HDAC2, but not HDAC4 protein levels, in P, but not NP rats (Figure 2A, B). These data suggest that in P rats, higher HDAC2 protein levels may be responsible for higher nuclear HDAC activity and that acute ethanol may inhibit HDAC activity via reductions in HDAC2 protein levels in the CeA and MeA.
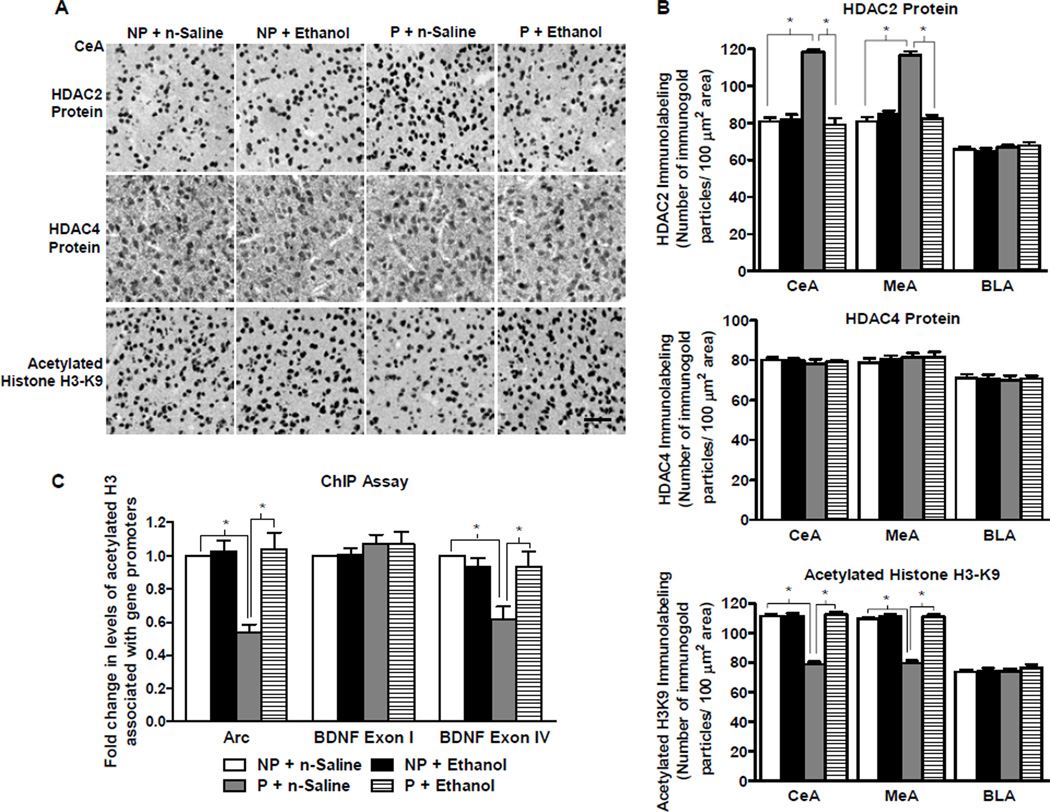
Figure 2. The effect of acute ethanol exposure on amygdaloid expression of histone deacetylases (HDAC) 2 and 4, and histone H3-K9 acetylation in P and NP rats.
A. Representative low-magnification photomicrographs (Scale bar = 50 µm) of gold-immunolabeling of HDAC2, HDAC4, and H3-K9 acetylation in the central nucleus of amygdala (CeA) of alcohol-preferring (P) and -nonpreferring (NP) rats treated with either n-saline or ethanol (1 g/kg).
B. HDAC2 protein levels, but not HDAC4 protein levels, were innately higher in the CeA (Group × treatment, F1, 20 = 64.8, p<0.001) and MeA (Group × treatment, F1, 20 = 84.2, p<0.001), but not BLA, of P rats in comparison to NP rats. Acute ethanol exposure reduced HDAC2 expression, but did not modulate HDAC4 expression, in the CeA and MeA of P rats, but not NP rats. Histone H3-K9 acetylation was also significantly different between the groups in the CeA and MeA, but not BLA. Acetylated H3-K9 levels were lower in the CeA (Group × treatment, F1, 20 = 104.7, p<0.001) and MeA (Group × treatment, F1, 20 = 101.7, p<0.001), but not BLA, of P rats in comparison to NP rats at baseline. Treatment with acute ethanol increased levels of histone H3-K9 acetylation in the CeA and MeA of P rats, but not NP rats. Values are represented as the mean ± SEM of the number of immunogold particles per 100 µm2 area from 6 rats per group. *Significantly different from their respective control groups (p<0.001; two-way ANOVA followed by post hoc analysis by Tukey’s test).
C. Chromatin Immunoprecipitation (ChIP) analysis identified significant differences between the groups for acetylated histone H3-K9/14 associated BDNF exon IV and Arc, but not BDNF exon I (BDNF exon IV: group × treatment, F1, 20 = 8.6, p<0.01; Arc: group × treatment F1, 20 = 14.8, p<0.001). The levels of acetylated histone H3-associated gene promoter of BDNF exon IV and Arc, but not BDNF exon I, were lower in tissue homogenates composed primarily of CeA and MeA in P rats than in NP rats at baseline. Treatment with acute ethanol (1 g/kg) increased amygdaloid levels of acetylated histone H3 associated BDNF exon IV and Arc, but not BDNF exon I, in P rats, but not NP rats. Values are represented as the mean ± SEM of the fold change of acetylated histone levels associated with the aforementioned genes normalized to the NP + n-saline group and derived from 6 rats per group. *Significantly different from their respective control groups (p<0.01–0.001; two-way ANOVA followed by post hoc analysis by Tukey’s test).
Effects of Acute Ethanol on Amygdaloid Histone H3 Acetylation
We also measured baseline histone acetylation status of H3-K9 and H3-K14 and found that acetylated H3-K9 but not H3-K14 protein levels were significantly (p<0.001) lower in the CeA and MeA, but not BLA, of P in comparison to NP rats (Figure 2A, B, and Figure S1). Acute ethanol exposure resulted in a significant (p<0.001) increase in acetylated H3-K9 protein levels in the CeA and MeA of P rats, but was not significantly altered in NP rats (Figure 2A, B). These data suggest that innately high levels of amygdaloid HDAC2 could be responsible for deficits in H3-K9 acetylation in the CeA and MeA of P when compared to NP rats. Also, anxiolytic effects of ethanol may be related to the normalization of deficits in H3-K9 acetylation in the CeA and MeA of P rats.
Effects of Acute Ethanol on the Levels of Acetylated Histone H3 of BDNF and Arc Genes
We performed ChIP analysis of amygdaloid tissues using an H3-K9/14 antibody and quantified acetylated histone H3 levels of BDNF exons I and IV, and Arc genes in P and NP rats treated with either n-saline or acute ethanol. We found that at baseline, P rats had significantly lower levels of acetylated histone H3 of BDNF exon IV (p<0.001) and Arc (p<0.001) as compared to NP rats. Acute ethanol treatment increased the levels of acetylated H3 of BDNF exon IV (p<0.01) and Arc (p<0.001) in P, but not NP rats (Figure 2C). No significant baseline differences or acute ethanol-induced changes were observed in the levels of acetylated histone H3 of BDNF exon I in the amygdala of P and NP rats (Figure 2C).
Effects of HDAC2 siRNA Infusion on Anxiety-like and Alcohol Drinking Behaviors
In order to determine the direct role of HDAC2 on anxiety-like and alcohol-drinking behaviors of P rats, we infused vehicle, HDAC2 siRNA, or negative control siRNA into the CeA of P rats. Using the LDB exploration test, we found that P rats treated with HDAC2 siRNA spent significantly (p<0.001) more time in the light versus the dark compartment as compared to rats infused with either vehicle or control siRNA (Figure 3A). General activity as measured by total ambulations in the LDB apparatus was not significantly different among the groups (data not shown).
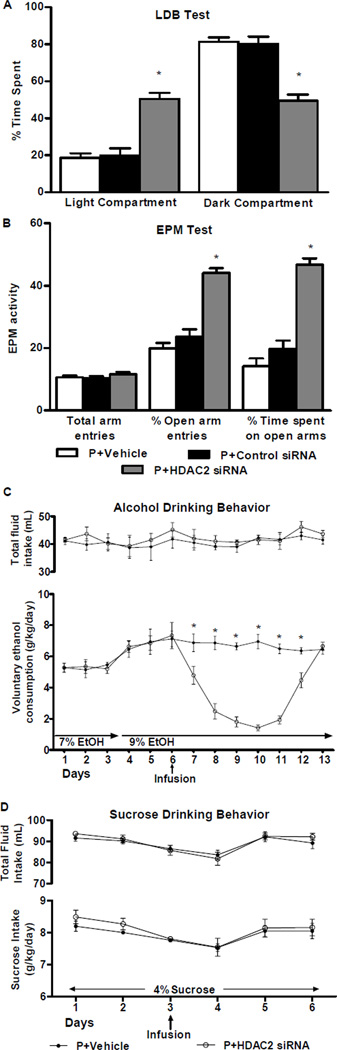
Figure 3. Effects of HDAC2 siRNA infusion into the CeA on anxiety-like behaviors and alcohol and sucrose intake in P rats.
A. The light/dark box (LDB) exploration test showed that HDAC2 siRNA infusion attenuated anxiety-like behaviors of P rats in comparison to vehicle- or control siRNA-infused rats. The percentage of time spent in the light and dark compartments was significantly different among the treatment groups (F2, 36 = 36.3, p<0.001). HDAC2 siRNA infused rats spent more time in the light compartment and less time in the dark compartment than vehicle- and control siRNA-infused rats. Values are represented as the mean ± SEM of the percentage of time spent in each compartment averaged from 5–17 P rats per group. *Significantly different from control groups (p<0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test).
B. The elevated plus maze (EPM) exploration test also showed that HDAC2 siRNA infusion into the CeA resulted in a reduction in the anxiety-like behaviors of P rats. P rat performance on the EPM test was significantly different between the treatment groups in percentage of open arm entries (F2,21 = 46.7, p< 0.001) and the percentage of time spent in the open arms (F2,21 = 52.2, p < 0.001). P rats infused with HDAC2 siRNA showed a higher percentage of open arm entries and time spent in the open arms than those infused with vehicle or control siRNA. The number of total arm entries does not differ significantly between the groups, suggesting that there are no effects of HDAC2 siRNA infusion on the general activity of P rats. Values represent the mean ± SEM of the percentage of open arm entries, percentage of time spent in the open arm, and number of total arm entries from 8 rats per group. *Significantly different from control groups (p<0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test).
C. Voluntary ethanol consumption as measured by the two-bottle free choice paradigm was reduced by infusion of HDAC2 siRNA, but not vehicle, into the CeA of P rats. Analysis by two-way repeated measures ANOVA identified a significant difference in the amount of ethanol consumed between the treatment groups overall and daily, as indicated by the group × day interaction (Group: F1,120 = 71.9, p<0.001; Group × Day: F12,120 = 13.8, p<0.001). P rats were given access to water and 7% ethanol followed by water and 9% ethanol. Following the sixth day of ethanol access, P rats received infusion of vehicle or HDAC2 siRNA and consumption of water and 9% ethanol were monitored for several days. Total fluid intake did not significantly differ between the groups. Values are represented as the mean ± SEM (6 P rats per group) of the ethanol consumption (g/kg/day) or total fluid intake (mL) plotted daily. *Significantly different between the groups (p<0.01–0.001; post hoc analysis of the group by day interaction by Tukey’s test).
D. Voluntary sucrose consumption as measured by the two-bottle free choice paradigm was unaltered by the infusion of HDAC2 siRNA and vehicle into the CeA of P rats. Analysis by two-way repeated measures ANOVA indicated no significant differences between groups. P rats were given access to water and sucrose solution as described in the methods section. Following the third day of 4% sucrose intake, P rats received infusion of vehicle or HDAC2 siRNA and consumption of water and sucrose solution were monitored for several days. Total fluid intake did not significantly differ between the groups. Values are represented as the mean ± SEM (5 P rats per group) of the sucrose intake (g/kg/day) or total fluid intake (mL) plotted daily.
In a second batch of P rats, anxiety-like behaviors were measured by the EPM test. The HDAC2 siRNA group showed a significantly (p<0.001) increased percentage of open arm entries and time spent in the open arms in comparison to controls (Figure 3B). The number of total arm entries did not differ between groups, suggesting that the treatment had no effect on the general activity level of the rats.
We utilized the two-bottle free choice paradigm of voluntary ethanol and sucrose consumption to examine the effects of HDAC2 siRNA on drinking behaviors of P rats. P rats infused with HDAC2 siRNA consumed significantly (p<0.01–0.001) less ethanol than vehicle-infused P rats, which lasted six days following a single infusion of HDAC2 siRNA (Figure 3C). However, HDAC2 siRNA infusion into the CeA had no effects on sucrose intake in P rats (Figure 3D). Total fluid intake did not significantly differ between the groups (Figure 3C, D). Taken together, these data indicate that HDAC2 siRNA infusion into the CeA attenuated anxiety-like and alcohol-drinking behaviors in P rats.
Effects of HDAC2 siRNA Infusion on HDAC2 Expression and Histone H3 Acetylation
To confirm whether the infusion of HDAC2 siRNA was effectively entering neurons, we used fluorescence-tagged HDAC2 siRNA and amygdaloid brain regions were examined using confocal microscopy. Co-localization of the fluorescence-tagged HDAC2 siRNA and neurons was analyzed via staining of a neuron-specific nuclear protein (NeuN). Qualitative analysis of the confocal images revealed that HDAC2 siRNA-linked fluorescence was restricted to the CeA and did not diffuse into surrounding areas of MeA or BLA and was also primarily co-localized with cells expressing NeuN, suggesting that the siRNA preferentially transfected the neuronal population in the CeA (Figure 4A).
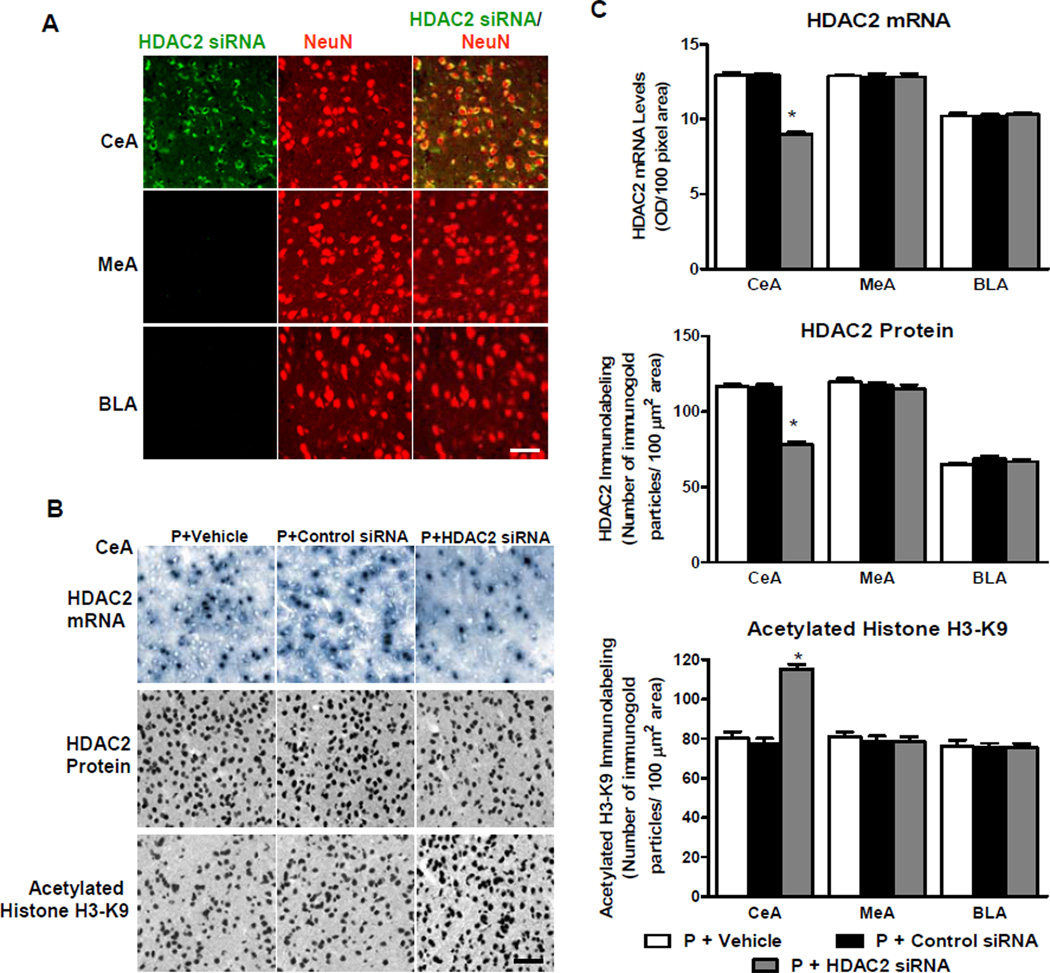
Figure 4. Effects of HDAC2 siRNA infusion into the CeA on mRNA and protein levels of HDAC2 and on histone H3-K9 acetylation in amygdaloid structures of P rats.
A. Representative confocal photomicrographs reveal that HDAC2 siRNA penetrates neurons as evidenced by co-localization of fluorescence-tagged HDAC2 siRNA and neuron-specific nuclear protein (NeuN) in CeA, but not MeA or BLA. Scale bar = 50 µm.
B. Representative low-magnification photomicrographs illustrating cells of in situ RT-PCR for HDAC2 mRNA and immunohistochemical staining (gold immunolabeling) for HDAC2 protein and acetylated histone H3-K9 in CeA. In comparison to vehicle and control siRNA infusion, HDAC2 siRNA infusion reduced HDAC2 mRNA and protein levels, and increased acetylated histone H3-K9 protein levels in the CeA. Scale bar = 50 µm.
C. HDAC2 mRNA and protein, and histone H3-K9 acetylation levels, were significantly different between the treatment groups in the CeA, but not MeA or BLA (HDAC2 mRNA: CeA, F2, 12 = 161.0, p<0.001; HDAC2 protein: CeA, F2, 17 = 127.0, p<0.001; Acetylated histone H3-K9: CeA, F2, 17 = 70.0, p<0.001). HDAC2 mRNA and protein levels were lower in the CeA, but not MeA or BLA, of P rats infused with HDAC2 siRNA than those infused with vehicle or control siRNA. These results confirm that HDAC2 siRNA infusion causes a significant reduction in HDAC2 mRNA levels in the CeA. HDAC2 siRNA infusion into CeA also increased the levels of histone H3-K9 acetylation in the CeA, but not MeA or BLA. Values are represented as the mean ± SEM derived from 5–7 P rats per group. *Significantly different from control groups (p<0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test).
To determine the functional effects of HDAC2 siRNA infusion, we measured HDAC2 mRNA and protein levels, and associated changes in histone H3-K9 acetylation in amygdaloid brain regions. In comparison to P rats infused with vehicle or negative control siRNA, HDAC2 siRNA infusion resulted in a significant (p<0.001) reduction in HDAC2 mRNA and protein levels and a significant (p<0.001) increase in levels of acetylated H3-K9 in the CeA, but not MeA or BLA (Figure 4B, C). These data indicate that HDAC2 siRNA infusion resulted in reductions in HDAC2 mRNA and protein levels thereby, increasing histone H3-K9 acetylation in the CeA of P rats.
Effects of HDAC2 siRNA Infusion on BDNF and Arc Levels and Acetylated Histone H3 Levels of BDNF and Arc Genes
We next examined whether HDAC2 could play a role in the regulation of BDNF and Arc expression. We found HDAC2 siRNA infused P rats showed significantly (p<0.001) higher levels of BDNF and Arc protein in the CeA than rats infused with vehicle or negative control siRNA (Figure 5A, B). These data suggest an association between knockdown of HDAC2 expression and increased protein levels of BDNF and Arc.
Figure 5. Effects of HDAC2 siRNA infusion into the CeA on protein levels of brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated (Arc) protein and on acetylated histone H3 levels of BDNF exons I, IV and Arc genes in the amygdala of P rats.
A. Representative low-magnification photomicrographs showing immunohistochemical staining (gold immunolabeling) for BDNF and Arc protein in CeA. Scale bar = 50 µm.
B. Analysis of BDNF and Arc protein levels identified a significant difference between the vehicle-, HDAC2 siRNA-, and control siRNA-infused P rat groups in the CeA, but not MeA or BLA (BDNF: CeA, F2,11 = 34.5, p<0.001; Arc: CeA, F2,14 = 57.8, p<0.001). HDAC2 siRNA infusion into CeA resulted in an increase of BDNF and Arc protein in the CeA, but not MeA or BLA, of P rats in comparison to those infused with vehicle or control siRNA. Values (number of the immunogold particles per 100 µm2 area) are represented as the mean ± SEM and derived from 4–6 rats per group. *Significantly different from control groups (p<0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test).
C. ChIP analysis revealed that HDAC2 siRNA infusion increased the levels of acetylated histone H3-K9&14 of BDNF exon IV and Arc, but not BDNF exon I. Values represent mean ± SEM derived from 6 P rats per group. *Significantly different from vehicle-infused P rats (p<0.001; Student’s t-test).
We utilized the ChIP assay to determine whether there is a change in the acetylation level of histone H3 associated with the transcriptional control regions of the BDNF and Arc genes. To further determine if there is an exon-specific effect, we looked at BDNF exons I and IV. The results revealed that HDAC2 siRNA infusion increased (p<0.001) acetylated histone H3 levels of BDNF exon IV and Arc, but not BDNF exon I (Figure 5C). This suggests that the increases in BDNF and Arc levels associated with HDAC2 knockdown in the CeA could be related to increased histone H3 acetylation of BDNF exon IV and Arc genes.
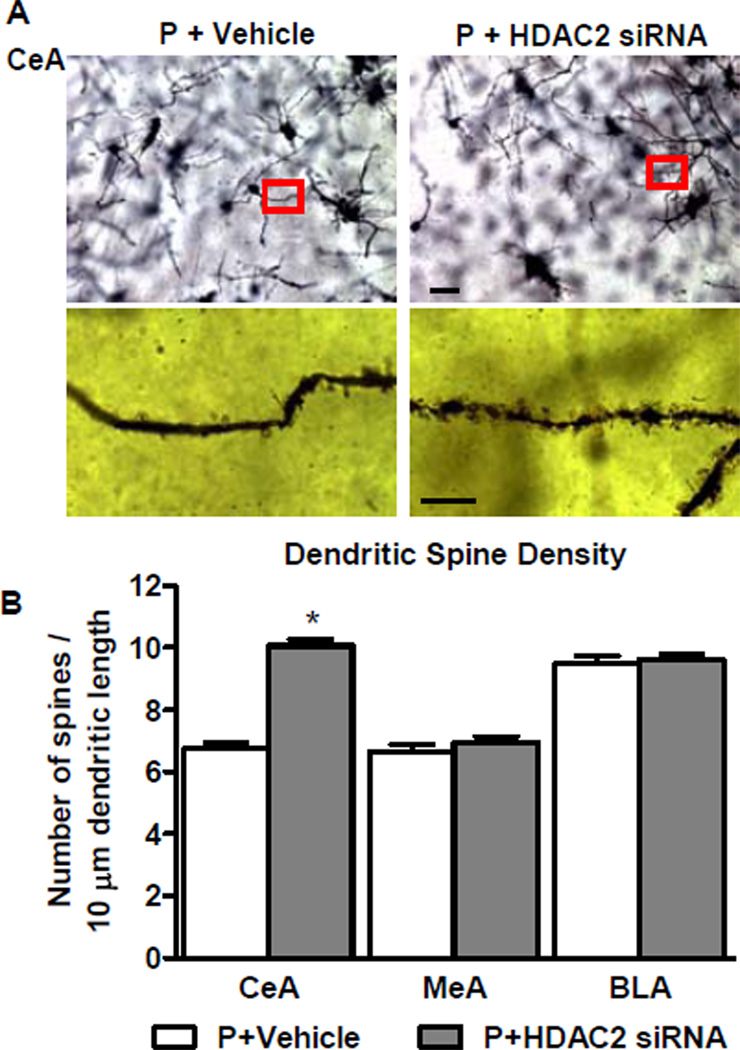
Effects of HDAC2 siRNA Infusion on Dendritic Spines in the CeA
We performed Golgi-Cox staining to quantify the dendritic spines of P rats to examine whether HDAC2 siRNA-infusion had a downstream effect on dendritic spines. Compared to vehicle-infused rats, HDAC2 siRNA infusion significantly increased (p<0.001) the dendritic spine density of P rats in the CeA, but not MeA or BLA (Figure 6A, B). These data imply that lower dendritic spine density in the CeA of P as compared to NP rats, as reported earlier by us (13), could be due to higher HDAC2 levels.
Figure 6. Effects of HDAC2 siRNA infusion into the CeA on dendritic spine density in the amygdaloid structures of P rats.
A. Representative low-magnification photomicrographs (Scale bar = 50 µm) showing Golgi-impregnated neurons in the CeA of P rats infused with HDAC2 siRNA or vehicle. The boxed areas of the low magnification photographs are shown at high magnification (Scale bar = 10 µm) in adjacent photomicrographs showing dendritic spines.
B. HDAC2 siRNA infusion into the CeA of P rats resulted in increased dendritic spine density in the CeA, but not MeA or BLA in comparison to vehicle-infused rats. Values (number of dendritic spines per 10 µm of dendritic length) are represented as the mean ± SEM derived from 6 rats per group. *Significantly different from control groups (p<0.001; Student’s t-test).
Discussion
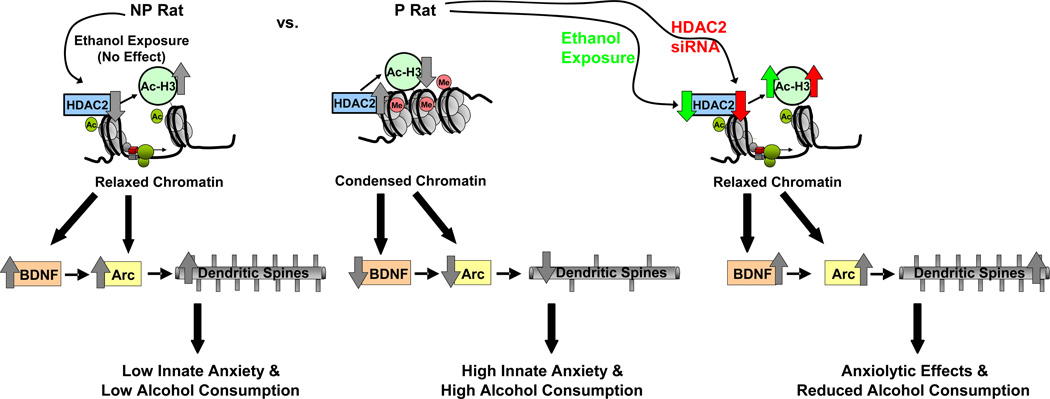
Our data demonstrate that innate abnormalities in chromatin structure and related deficits in synaptic plasticity within specific circuitry of the amygdala could play a role in alcohol drinking and anxiety-like behaviors. At the baseline level, P rats have higher nuclear HDAC activity and higher HDAC2, but not HDAC 1, 3, 4, 5, or 6, protein levels and lower acetylation of histone H3-K9, but not H3-K14 in the CeA and MeA compared to NP rats. Acute ethanol exposure caused an inhibition of HDAC activity and a reduction in HDAC2 levels resulting in increased histone H3 acetylation of BDNF and Arc genes as well as increased dendritic spines (13) in the amygdaloid brain regions of P, but not NP rats, indicating a direct correlation between histone acetylation and synaptic regulation. Additionally, HDAC2 siRNA infusion into the CeA produced anxiolytic effects and attenuated voluntary alcohol, but not sucrose, consumption in P rats. This was associated with an increase in BDNF and Arc expression and dendritic spines, resulting most likely from increased histone H3 acetylation of BDNF exon IV and Arc promoters. These novel results suggest that higher HDAC2 expression in the CeA may be involved in chromatin and synaptic remodeling which might play a fundamental role in the genetic predisposition to alcoholism and anxiety (Figure 7).
Figure 7.
Hypothetical model of baseline differences between P and NP rats, and the effect of ethanol treatment or HDAC2 siRNA infusion into CeA with regards to chromatin structure, synaptic proteins and dendritic spines, and anxiety-like and alcohol drinking behaviors. In comparison to alcohol-nonpreferring (NP) rats, alcohol-preferring (P) rats have higher levels of histone deacetylase isoform 2 (HDAC2) in the central (CeA) and medial amygdala (MeA) resulting in deficits in histone H3-K9 acetylation. The innately higher HDAC2 levels are associated with lower histone H3 acetylation of the synaptic genes, brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated (Arc) protein in the amygdala. This suggests that HDAC2-induced chromatin condensation, due to lower histone H3 acetylation levels, results in reduced BDNF and Arc expression and lower dendritic spine density (13) in the CeA and MeA which may play a role in anxiety-like and alcohol drinking behaviors of P rats as compared to NP rats. In P rats, the anxiolytic effects of acute ethanol exposure are associated with a reduction in HDAC2 levels in the CeA and MeA which result in increased histone H3 acetylation and relaxation of chromatin of BDNF and Arc genes. The resulting upregulation of protein levels of these genes may be responsible for the increased dendritic spine density in the CeA and MeA. Knockdown of HDAC2 via infusion of HDAC2 siRNA into the CeA results in similar effects on chromatin and synaptic remodeling, and decreases the anxiety-like and alcohol drinking behaviors of P rats. These results implicate a crucial role for HDAC2-induced chromatin and synaptic remodeling in the CeA in the regulation of anxiety-like and alcohol drinking behaviors. Ac, acetylation; Me, methylation; Ac-H3, histone H3 acetylation.
Recent studies have shown that regulation of specific HDAC isoforms may play a role in brain function and disease (26, 32, 40, 52–58). Increased HDAC2 expression has been linked to depression, Alzheimer’s and Huntington’s disease, and pharmacological reduction of HDAC2 appears to correct the deficits associated with these disorders (32, 58, 59). Similarly, we found that innately higher HDAC2 expression in the amygdala appears to be responsible for anxietylike and alcohol drinking behaviors in P rats, whereas knockdown of HDAC2 expression corrects these behavioral phenotypes. The innate expression of other HDAC isoforms, such as HDAC 1, 3, 4, 5, and 6 did not differ in the amygdaloid structures of P and NP rats. Taken together, these studies specifically implicate HDAC2 in the molecular mechanisms of various psychiatric disorders including alcoholism.
Interestingly HDAC2, which is predominantly expressed in neurons (60), has also been found to play an important role in the dynamic regulation of synaptic plasticity and associated gene expression and memory formation (33). For example, neuron-specific overexpression of HDAC2, but not HDAC1, resulted in decreased hippocampal synaptic plasticity (dendritic spines and synapse number) and impaired memory formation whereas, HDAC2 deficiency increased dendritic spines and synapse number in the hippocampus and enhanced memory formation in mice (33). We previously reported a deficit in amygdaloid BDNF and Arc proteins, and lower dendritic spine density in the CeA and MeA of P compared to NP rats (13, 45). The BDNF gene shows complex expression patterns dictated in part by differential exon-specific regulation (61). Epigenetic regulation has been found to play a role in the regulation of BDNF exon-specific transcription (26, 34, 62–64). BDNF exons appear to play a significant role in the regulation of synaptic plasticity and have been shown to be dynamically regulated through histone acetylation (26, 63–66). Here we observed that ethanol-induced increases in BDNF and Arc expression as well as dendritic spines in P rats (13) could be related to the increase in acetylated histone H3 levels of BDNF exon IV and Arc, but not BDNF exon I. We also reported that the anxiolytic effects of acute ethanol treatment were associated with increases in BDNF, Arc and dendritic spines in the CeA and MeA, suggesting that the modulation of amygdaloid synaptic plasticity could be related to the anxiety-like behaviors of P rats (13).
As observed, higher expression of HDAC2 and lower acetylation of histone H3-K9, but not H3-K14 may be responsible for deficits in BDNF and Arc expression in the amygdaloid structures of P as compared with NP rats. Ethanol also inhibited HDAC activity, reduced HDAC2 protein levels and increased histone H3-K9 acetylation in the amygdaloid brain regions of P but not NP rats. These results are clearly suggestive of a specific role for CeA HDAC2-induced chromatin remodeling in mediating anxiety-like and alcohol drinking behaviors in a manner that could be related to downstream changes in BDNF-Arc signaling and dendritic spines. Our data also suggest that HDAC2-induced chromatin remodeling in the CeA and MeA, but not BLA may be involved in controlling baseline anxiety or anxiolytic effects of ethanol in P rats compared to NP rats. Recently, an optogenetic study indicated that manipulations of BLA projections in the CeA, but not the BLA somata, were able to control anxiety-like behaviors (67). Also, abnormal neuropeptide Y (NPY) and GABAAα2-regulated systems in CeA have been implicated in anxiety-like and alcohol-drinking behaviors of P rats (14, 48, 68). Future studies are needed to evaluate the epigenetic regulation of these and other genes in the amygdaloid circuitry of P rats contributing to anxiety and alcoholism.
Acute ethanol sensitivity in P and NP rats has been well characterized (12, 69). For example, P rats are less sensitive to the ataxic, aversive, and sedative/hypnotic effects of ethanol and more sensitive to its stimulatory and anxiolytic effects as compared with NP rats (12–16, 69–71). The present study raises the question as to why amygdaloid HDAC2-induced histone modifications and anxiety-measures are not affected in NP rats but modulated in P and SD rats (27, 28) by ethanol (1g/kg) exposure. This finding could be suggestive of resilience or a ceiling effect in NP rats to this dose of ethanol and differences in innate anxiety-like behaviors and anti-anxiety responses of ethanol in P and NP rats may contribute to differences in alcohol intake between these lines of rats. It has been shown that P rats consume higher amounts (>5 g/kg/day) of alcohol compared to NP (<1.5 g/kg/day) rats (12, 14, 69, 72). Interestingly, infusion of HDAC2 siRNA into the CeA of P rats caused a drastic reduction in ethanol (from about 7g/kg/day to 1.4 g/kg/day) but not sucrose consumption, suggesting HDAC2-mediated regulation of motivational aspects of alcohol drinking behaviors. Furthermore, the anxiolytic effects of HDAC2 siRNA infusion into CeA and ethanol-induced HDAC2 inhibition in the CeA and MeA observed in this study support the notion that P rats consume alcohol to attenuate anxiety-like behaviors. We have shown previously that voluntary ethanol drinking and acute ethanol exposure increased cAMP responsive element-binding protein phosphorylation and expression of NPY and BDNF in the CeA and MeA of P, but not NP rats (13, 14, 48). Here, we provide additional evidence of the complex and dynamic regulation of molecular pathways by epigenetic mechanisms leading to alcoholism and anxiety.
Conclusion
The present study identifies a causal role of HDAC2-mediated histone modifications in the CeA in the regulation of anxiety-like and alcohol drinking behaviors in P rats (Figure 7) and suggests that HDAC2-specific inhibitors may serve as useful pharmacotherapeutic agents for the treatment of both alcoholism and anxiety.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grants AA-013341, AA-016690, AA-019971, and AA-010005 and by the Department of Veterans Affairs (Merit Review Grant; Research Career Scientist award) to SCP. The breeding of P and NP rats was supported by the Alcohol Resource Award (R24AA-015512) from the NIAAA to Indiana University.
Dr. Pandey reports grand rounds presentations at the School of Medicine North Carolina at Chapel Hill; Department of Pharmacology, Howard University, Washington, DC; Department of Neurobiology, University of Texas at Austin; and the Mayo clinic Rochester, MN. He also served as an expert on the research workshop on pathological gambling organized by the Ministry of Youth and Sports, Singapore. In addition, he served as a member on NIH study sections. Dr. Pandey also reports that a US patent application on a related topic (serial number 60/848237 filed on September 29th, 2006) is currently pending. All other authors reported no biomedical financial interests or potential conflicts of interest.
The data of the manuscript has been presented at both the Research Society on Alcoholism and the American College of Neuropsychopharmacology scientific meetings and is a part of the PhD dissertation of SM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 2.Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 3.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and cooccurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 4.Bolton JM, Robinson J, Sareen J. Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord. 2009;115:367–375. doi: 10.1016/j.jad.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 6.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt NB, Buckner JD, Keough ME. Anxiety sensitivity as a prospective predictor of alcohol use disorders. Behav Modif. 2007;31:202–219. doi: 10.1177/0145445506297019. [DOI] [PubMed] [Google Scholar]
- 8.Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. J Abnorm Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- 10.Robinson J, Sareen J, Cox BJ, Bolton J. Self-medication of anxiety disorders with alcohol and drugs: Results from a nationally representative sample. J Anxiety Disord. 2009;23:38–45. doi: 10.1016/j.janxdis.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Pandey SC. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci. 2003;24:456–460. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- 12.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 13.Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- 16.McKinzie DL, Sajdyk TJ, McBride WJ, Murphy JM, Lumeng L, Li TK, Shekhar A. Acoustic startle and fear-potentiated startle in alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 2000;65:691–696. doi: 10.1016/s0091-3057(99)00252-x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang BH, Stewart R, Zhang JK, Lumeng L, Li TK. Corticotropin-releasing factor gene expression is down-regulated in the central nucleus of the amygdala of alcohol-preferring rats which exhibit high anxiety: a comparison between rat lines selectively bred for high and low alcohol preference. Brain Res. 2004;1026:143–150. doi: 10.1016/j.brainres.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 20.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 22.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 23.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- 25.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 26.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 27.Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res. 2012;36:61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renthal W, Nestler EJ. Chromatin regulation in drug addiction and depression. Dialogues Clin Neurosci. 2009;11:257–268. doi: 10.31887/DCNS.2009.11.3/wrenthal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayson DR, Kundakovic M, Sharma RP. Is there a future for histone deacetylase inhibitors in the pharmacotherapy of psychiatric disorders? Mol Pharmacol. 2010;77:126–135. doi: 10.1124/mol.109.061333. [DOI] [PubMed] [Google Scholar]
- 32.Covington HE, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, 3rd, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 39.Soulé J, Messaoudi E, Bramham CR. Brain-derived neurotrophic factor and control of synaptic consolidation in the adult brain. Biochem Soc Trans. 2006;34:600–604. doi: 10.1042/BST0340600. [DOI] [PubMed] [Google Scholar]
- 40.Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang UE, Hellweg R, Kalus P, Bajbouj M, Lenzen KP, Sander T, et al. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180:95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- 44.Matsushita S, Kimura M, Miyakawa T, Yoshino A, Murayama M, Masaki T, Higuchi S. Association study of brain-derived neurotrophic factor gene polymorphism and alcoholism. Alcohol Clin Exp Res. 2004;28:1609–1612. doi: 10.1097/01.alc.0000145697.81741.d2. [DOI] [PubMed] [Google Scholar]
- 45.Prakash A, Zhang H, Pandey SC. Innate differences in the expression of brain-derived neurotrophic factor in the regions within the extended amygdala between alcohol preferring and nonpreferring rats. Alcohol Clin Exp Res. 2008;32:909–920. doi: 10.1111/j.1530-0277.2008.00650.x. [DOI] [PubMed] [Google Scholar]
- 46.Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008;28:2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Sakharkar AJ, Shi G, Ugale R, Prakash A, Pandey SC. Neuropeptide Y signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol: Clin Exp Res. 2010;34:451–461. doi: 10.1111/j.1530-0277.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour,and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 50.West CH, Weiss JM. Intake of ethanol and reinforcing fluids in rats bred for susceptibility to stress. Alcohol. 2006;38:13–27. doi: 10.1016/j.alcohol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 52.Ding H, Dolan PJ, Johnson GV. Histone deacetylase 6 interacts with the microtubule-associated protein tau. J Neurochem. 2008;106:2119–2130. doi: 10.1111/j.1471-4159.2008.05564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukada M, Hanai A, Nakayama A, Suzuki T, Miyata N, Rodriguiz RM, et al. Loss of deacetylation activity of hdac6 affects emotional behavior in mice. PLoS One. 2012;7:30924. doi: 10.1371/journal.pone.0030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, et al. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 56.Su M, Shi JJ, Yang YP, Li J, Zhang YL, Chen J, et al. HDAC6 regulates aggresome-autophagy degradation pathway of alpha-synuclein in response to MPP+-induced stress. J Neurochem. 2011;117:112–120. doi: 10.1111/j.1471-4159.2011.07180.x. [DOI] [PubMed] [Google Scholar]
- 57.Morris MJ, Karra AS, Monteggia LM. Histone deacetylases govern cellular mechanisms underlying behavioral and synaptic plasticity in the developing and adult brain. Behav Pharmacol. 2010;21:409–419. doi: 10.1097/FBP.0b013e32833c20c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gräff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mielcarek M, Benn CL, Franklin SA, Smith DL, Woodman B, Marks PA, Bates GP. SAHA decreases HDAC 2 and 4 levels in vivo and improves molecular phenotypes in the R6/2 mouse model of Huntington’s disease. PLoS One. 2011;6:27746. doi: 10.1371/journal.pone.0027746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacDonald JL, Roskams AJ. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Dev Dyn. 2008;237:2256–2267. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
- 61.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig. 2010;7:251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He DY, Neasta J, Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. J Biol Chem. 2010;285:19043–19050. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakata K, Woo NH, Martinowich K, Greene JS, Schloesser RJ, Shen L, Lu B. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci USA. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, et al. Binge alcohol drinking is associated with GABA A α2-regulated toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci USA. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McBride WJ, Li T-K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobio. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 70.Lumeng L, Waller MB, McBride WJ, Li TK. Different sensitivities to ethanol in alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 1982;16:125–130. doi: 10.1016/0091-3057(82)90023-5. [DOI] [PubMed] [Google Scholar]
- 71.Kurtz DL, Stewart RB, Zweifel M, TK Li, Froehlich JC. Genetic differences in tolerance and senitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53:585–591. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- 72.Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol. 1987;(Suppl 1):91–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.