Abstract
Background
Patients with diabetes frequently use complimentary and alternative medications including Ayurvedic medications and hence it is important to determine their efficacy and safety.
Objectives
To assess the effects of Ayurvedic treatments for diabetes mellitus.
Search methods
We searched The Cochrane Library (issue 10, 2011), MEDLINE (until 31 August 2011), EMBASE (until 31 August 2011), AMED (until 14 October 2011), the database of randomised trials from South Asia (until 14 October 2011), the database of the grey literature (OpenSigle, until 14 October 2011) and databases of ongoing trials (until 14 October 2011). In addition we performed hand searches of several journals and reference lists of potentially relevant trials.
Selection criteria
We included randomized trials of at least two months duration of Ayurvedic interventions for diabetes mellitus. Participants of both genders, all ages and any type of diabetes were included irrespective of duration of diabetes, antidiabetic treatment, comorbidity or diabetes related complications.
Data collection and analysis
Two authors independently extracted data. Risk of bias of trials was evaluated as indicated in the Cochrane Handbook for Systematic Reviews of Intervention.
Main results
Results of only a limited number of studies could be combined, in view of different types of interventions and variable quality of data. We found six trials of proprietary herbal mixtures and one of whole system Ayurvedic treatment. These studies enrolled 354 participants ( 172 on treatment, 158 on controls, 24 allocation unknown). The treatment duration ranged from 3 to 6 months. All these studies included adults with type 2 diabetes mellitus.
With regard to our primary outcomes, significant reductions in glycosylated haemoglobin A1c (HbA1c), fasting blood sugar (FBS) or both were observed with Diabecon, Inolter and Cogent DB compared to placebo or no additional treatment, while no significant hypoglycaemic response was found with Pancreas tonic and Hyponidd treatment. The study of whole system Ayurvedic treatment did not provide data on HbA1c and FBS values. One study of Pancreas tonic treatment did not detect a significant change in health-related quality of life. The main adverse effects reported were drug hypersensitivity (one study, one patient in the treatment arm); hypoglycaemic episodes (one study, one participant in the treatment arm; none had severe hypoglycaemia) and gastrointestinal side effects in one study (1 of 20 in the intervention group and 0 of 20 participants in the control group). None of the included studies reported any deaths, renal, hematological or liver toxicity.
With regard to our secondary outcomes, post prandial blood sugar (PPBS) was lower among participants treated with Diabecon, was unchanged with Hyponidd and was higher in patients treated with Cogent DB. Treatment with Pancreas tonic and Hyponidd did not affect lipid profile significantly, while patients treated with Inolter had significantly higher HDL- and lower LDL-cholesterol as well as lower triglycerides. Cogent DB treated participants also had lower total cholesterol and triglycerides.
Studies of treatment with Diabecon reported increased fasting insulin levels; one study of treatment with Diabecon reported higher stimulated insulin levels and fasting C-peptide levels in the treatment group. There was no significant difference in fasting and stimulated C-peptide and insulin levels with Hyponidd, Cogent DB and Pancreas tonic treatment. The study of Inolter did not assess these outcomes.
No study reported on or was designed to investigate diabetic complications, death from any cause and economic data.
Authors’ conclusions
Although there were significant glucose-lowering effects with the use of some herbal mixtures, due to methodological deficiencies and small sample sizes we are unable to draw any definite conclusions regarding their efficacy. Though no significant adverse events were reported, there is insufficient evidence at present to recommend the use of these interventions in routine clinical practice and further studies are needed.
PLAIN LANGUAGE SUMMARY
Ayurvedic treatments for diabetes mellitus
People with diabetes and other chronic diseases often use complementary and alternative medicines. This review examines the efficacy and safety of the use of various Ayurvedic treatments for diabetes mellitus. We found seven trials which included 354 participants (172 on treatment, 158 on control, 24 could not be classified). All these studies included adults with type 2 diabetes mellitus. Six studies tested five different types of herbal mixtures (proprietary drugs) and only one tested ‘whole system’ Ayurvedic treatment. The duration of treatment ranged from three to six months. One study each of Diabecon, Inolter and Cogent DB (proprietary herbal mixtures) found significantly lower glycosylated haemoglobin A1c (HbA1C) levels at the end of the treatment period compared to controls. Two studies of Diabecon, and one study of Cogent DB (proprietary herbal mixtures) found significantly lower fasting blood sugar levels at the end of the study period in the treatment group. No deaths were observed in these trials and side effects did not differ significantly between intervention and control groups. One study of Pancreas tonic reported no significant change in health-related quality of life. No study reported on or was designed to investigate diabetic complications, death from any cause and costs. Despite positive results in some studies, and absence of serious side effects, firm conclusions cannot be drawn due to weak methods and small number of participants in the evaluated studies. Further research is needed to assess the efficacy of these treatments. Ayurvedic physicians generally use a mixture of various herbs or proprietary preparations along with diet, exercise and mode of living. The treatments are usually individualised taking into account the balance of three ‘doshas’. It is possible that the interventions in the trials analysed have not replicated actual Ayurvedic practice but only assessed some components individually.
BACKGROUND
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long-term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under ‘Additional information’ in the information on the Metabolic and Endocrine Disorders Group in The Cochrane Library (see ‘About’, ‘Cochrane Review Groups (CRGs)’). For an explanation of methodological terms, see the main glossary in The Cochrane Library.
There are two types of diabetes mellitus: type 1 insulin dependent (IDDM) and type 2 non-insulin dependent (NIDDM). Type 2 diabetes mellitus, the most common form, is the fourth leading cause of death in developed countries with a two fold excess mortality and two to four fold increased risk of coronary heart disease and stroke (McKinlay 2000). Diabetes affects women and men of all ages and every ethnic category, and many cases of diabetes remain undiagnosed for an average of 4 to 7 years (McKinlay 2000). Diabetes profoundly affects health-related quality of life and represents a life-long burden on a patient’s social support system. Diabetes places large financial demands on the health care system (Alberti 1998).
Description of the intervention
Ayurveda which means ‘Science of life’ is derived from the Sanskrit words ‘Ayur’ meaning life and ‘Veda’ meaning knowledge. It takes an integrated view of the interactions of the physical, mental, spiritual and social aspects of the life of human beings. Ayurveda was first referred to in the Vedas (Rigveda and Atharva Veda 1500 BC). It was originally composed by Agnivesa around 1000 BC, and subsequently comprehensively documented in the Charaka Samhita around 300 BC ( Ayush 2007; Subbarayappa 2001). According to Ayurveda all objects and living bodies are composed of five basic structural elements (Panchamahabhutas), namely earth, water, fire, air and vacuum (ether). Ayurveda believes in the theory of tridoshas, namely vata (ether and air), pitha (fire) and kapha (earth and water). These three doshas are physiological entities in living beings. Ayurveda aims to keep the structural and physiological entities in a state of equilibrium, which signifies good health. Any imbalance due to internal or external factors may cause disease (Ayush 2007). Ayurvedic treatment aims to restore the equilibrium through various techniques, procedures, regimens, diet and medicines. Ayurvedic treatment consists of drugs, diet, exercise and general mode of life. Ayurveda largely uses plants as raw material for the manufacture of drugs, though materials of animal and marine origin, metals and minerals are also used.
Diabetes mellitus (Madhumeha) was known to ancient Indian physicians and an elaborate description of its clinical features and management appears in Ayurvedic texts (Upadhyay 1984). Ayurvedic practitioners treat diabetes with a multi-pronged approach, using diet modification, Panchkarma to cleanse the system, herbal preparations, yoga and breathing exercises. The herbs which are used to treat diabetes include shilajit, turmeric, neem, coccinea indica, amalaki, triphala, bitter gourd, rose apple, leaves of bilva, cinnamon, gymnema, fenugreek, bay leaf and aloe vera (McWhorter 2001; Saxena 2004). Decoctions of triphala, fenugreek and Shilajit are commonly used. Powders (Churana) used include Amalaki Churna, Haldi powder (Turmeric powder) and Naag Bhasma. The Ayurvedic preparations ‘Vasanta Kusumakar Ras’ and ‘Chandraprabhavati’ are believed to lower sugar levels. Proprietary Ayurvedic medications are also used to treat diabetes.
How the intervention might work
It is postulated that Ayurvedic medications may act through potential pancreatic as well as extrapancreatic effects. The probable mechanisms of action include: delaying gastric emptying, slowing carbohydrate absorption, inhibition of glucose transport, increasing the erythrocyte insulin receptors and peripheral glucose utilization, increasing glycogen synthesis, modulating insulin secretion, decreasing blood glucose synthesis through depression of the enzymes glucose-6-phosphatase, fructose-1, and 6-bisphosphatase, and enhanced glucose oxidation by the enzyme glucose-6-phosphatase-dehydrogenase pathway (McWhorter 2001).
Adverse effects of the intervention
There are reports of heavy metal contamination (such as lead) in herbal preparations resulting in intoxication (Keen 1994). There are also reports of spurious herbal products contaminated with oral hypoglycaemic agents (Kulambil 2009) which could lead to adverse effects, such as hypoglycaemic episodes. The safety profile of these drugs has not been fully investigated. It is also not clear, whether these preparations might interact with other drugs or diagnostic tests.
Why it is important to do this review
There is a constant need for better treatments for diabetes mellitus. Patients with diabetes and other common chronic medical conditions are more likely to use complimentary and alternative medications (CAM) than individuals in the general population (Egede 2002; Garrow 2006). Earlier systematic reviews (AHRQ 2001; Yeh 2003) have highlighted the methodological weaknesses of trials of Ayurvedic interventions for diabetes mellitus such as lack of high quality RCTs and CCTs, lack of power calculations to detect treatment effects, extremely small numbers of participants in many studies and lack of appropriate statistical methods in reporting the results. However, these reviews were published in 2001 and 2003. Hence, there is a need for an up-to-date systematic review to assess the benefits and possible adverse effects of Ayurvedic medications used in the treatment of diabetes. If Ayurvedic treatments are effective, it would give an additional option for persons with diabetes. If sufficient studies are not found it would highlight the gaps in our knowledge and possibly lead to the conduct of adequate randomized controlled trials.
OBJECTIVES
To assess the effects of Ayurvedic treatments for diabetes mellitus.
METHODS
Criteria for considering studies for this review
Types of studies
We included randomized controlled clinical trials and quasi-randomized trials with a minimum treatment period of two months.
Types of participants
We included men or women, adults or children with any type of diabetes mellitus, irrespective of duration of diabetes and associated comorbidity or diabetes related complications. Participants with recently detected diabetes (not treated with antidiabetic drugs) as well as those treated earlier with antidiabetic drugs were included. Trials in which the major goal of the intervention was the treatment of diabetic complications were excluded.
Diagnostic criteria for diabetes
To be consistent with changes in classification and diagnostic criteria of diabetes mellitus through the years, the diagnosis should have been established using the standard criteria valid at the time of the beginning of the trial (for example ADA 1997; ADA 1999; ADA 2003; ADA 2010; WHO 1980; WHO 1985; WHO 1998;WHO 2006). For studies, wherein the diagnostic criteria was not described, we used authors’ definition of diabetes mellitus. We planned to subject diagnostic criteria to a sensitivity analysis.
Types of interventions
Intervention
Ayurvedic treatment, with or without oral hypoglycaemic drugs or insulin.
The intervention of Ayurvedic treatments may include extracts from botanicals, single botanical agents, Ayurvedic proprietary medicines or a mixture of the above, prescribed by an Ayurvedic practitioner (individualised treatment).
Control
placebo;
non-pharmacological intervention (for example exercise, diet or both);
any active intervention used with the intention of lowering blood glucose levels (for example oral hypoglycaemic drugs, insulin);
no intervention.
Co-interventions were allowed as long as both arms of the randomized trial received the same co-intervention(s).
Types of outcome measures
Primary outcomes
glycaemic control (measured by glycosylated haemoglobin A1c (HbA1c) and fasting blood glucose levels);
health-related quality of life;
adverse effects (including hypoglycaemia).
Secondary outcomes
diabetic complications such as ketosis, nephropathy, neuropathy, retinopathy, vascular complications (myocardial infarction, stroke or peripheral vascular disease);
hospitalisation due to any cause;
weight or body mass index (BMI) changes;
post prandial blood sugar;
fasting and stimulated insulin levels;
fasting and stimulated C-peptide levels;
lipid profile;
death from any cause;
economic data (costs).
Covariates, effect modifiers and confounders
age;
gender.
Timing of outcome measurement
For outcomes such as glycaemic control, we considered trials of short duration (at least two months), while for outcomes such as diabetic complications, death from any cause and health-related quality of life we planned to consider trials of longer duration (more than six months).
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Library (issue 10, 2011);
MEDLINE (until 31 August 2011);
EMBASE (until 31 August 2011);
AMED (Allied and Complementary Medicine Database) (until 14 October 2011);
Database of randomized trials from South Asia, available at http://www.cochrane-sadcct.org/ (until 14 October 2011)
Database of the grey literature www.opengrey.eu (OpenSigle) (until 14 October 2011).
We also searched databases of ongoing trials: ‘Current Controlled Trials’ (www.controlled-trials.com - with links to other databases of ongoing trials) on October 14, 2011.
For detailed search strategies please see under Appendix 1. Additional key words of relevance could have been detected during any of the electronic or other searches. If this was the case, electronic search strategies would have been modified to incorporate these terms. Studies published in any language were included.
Searching other resources
We hand searched the following journals (from the earliest publication date available):
The International Journal of Diabetes in Developing Countries;
The Asian Journal of Diabetology;
Journal of Research in Ayurveda and Siddha
Theoretical and Experimental Journal of Ayurveda and Siddha (TEJAS; published between 1981 and 2008 as Ancient Science of Life);
The Journal of Research & Education in Indian Medicine (JREIM);
AYU (published quarterly by Ayurvedic University);
The International Journal for Ayurveda Research (published quarterly);
Indian Journal of Traditional knowledge;
Journal of Drug Research in Ayurveda;
Journal of Alternative and Complementary Medicine.
Experts in the field of Ayurveda including the department of AYUSH (earlier called as Department of Indian Systems of Medicine & Homeopathy), the Indian Medicinal Pharmaceutical Corporation Ltd, Central Council for Research in Ayurveda & Siddha, which function under the Ministry of Health and Family Welfare, Govt of India were contacted to find out any relevant trials.
Authors of relevant identified studies were contacted in order to obtain additional references, unpublished trials, ongoing trials or to obtain missing data not reported in the original trials. Similarly, manufacturers of the reviewed Ayurvedic medicines were contacted in order to retrieve information on other trials, published and unpublished.
We tried to identify additional studies by searching the reference lists of included trials and systematic reviews, meta-analyses and health technology assessment reports noticed.
Data collection and analysis
Selection of studies
To determine the studies to be assessed further, two authors (KS, RM) independently scanned the abstract, title or both sections of every record retrieved. Full articles were retrieved for further assessment if the information given suggested that the study:
included patients with diabetes mellitus;
compared Ayurvedic treatment with placebo or any other active intervention;
assessed one or more relevant outcome measure;
used (quasi) random allocation to the comparison groups.
If the information in the title or abstract was not clear, the full articles were retrieved for clarification. Full text of all potentially relevant articles were obtained. Interrater agreement for study selection was measured using the kappa statistic (Cohen 1960). Differences were marked and if these studies were later on included, we planned to study the influence of the primary choice by means of a sensitivity analysis. Where differences in opinion existed, they were resolved by a third author (SR). If resolving disagreement was not possible, the article was added to those ‘awaiting assessment’ and authors were contacted for clarification. An adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow-chart of study selection is attached (Liberati 2009).
Data extraction and management
For studies that fulfilled inclusion criteria, two authors (KS, RM) independently extracted data using standard data extraction templates (for details see Characteristics of included studies, Table 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5) , Disagreements were resolved by discussion.. We contacted the original authors of the study for any relevant missing information.
Table 1.
Overview of study populations
| Character- istic Study ID |
Interven- tion(s) & control(s) |
[n] screened |
[n] ran- domized |
[n] safety | [n] ITT | [n] finish- ing study |
[%] of ran- dom- ized partici- pants finishing study |
Comments |
|---|---|---|---|---|---|---|---|---|
| Agrawal 2002 | I: Inolter® C: Placebo |
I: - C: - T: 90 |
I: 30 C: 30 T: 60 |
I: 30 C: 30 T: 60 |
I: 30 C: 30 T: 60 |
I: 30 C: 30 T: 60 |
100% | |
| Dubey 1993 | I: Diabecon ® (D-400) C: Placebo |
I: 14 C: 14 T: 28 |
I: 14 C: 14 T: 28 |
I: 14 C: 14 T: 28 |
I: 14 C: 14 T: 28 |
I: 14 C: 14 T: 28 |
100% | no with- drawals re- ported |
| Elder 2006 | I: MA471 (mixture of herbs) + meditation, diet, exercise C: Usual care + diabetes ed- ucation |
I: - C: - T: 89 |
I: 30 C: 30 T: 60 |
I: 27 C: 28 T: 55 |
I: 26 C: 28 T: 54 |
I: 26 C: 28 T: 54 |
90% | 3 participants in Vedic and 2 in control groups with- drew due to time con- straints, 1 in Vedic group did not complete 6 month fol- low-up |
| Hsia 2004 | I: Pancreas tonic (herbal mixture) C: Placebo |
I: - C: - T: 63 |
I: 31 C: 16 T: 47 |
- | I: 23 C: 13 T: 36 |
I: 23 C: 13 T: 36 |
76.6% | 1 in placebo and 4 in treatment group with- drawn due to non-com- pliance, oth- erswithdrew mainly due to inconve- nience |
| Mohan 1998 | I: Diabecon ® (D-400) C: Placebo |
I: 20 C: 20 T: 40 |
I: 20 C: 20 T: 40 |
I: 19 C: 18 T: 37 |
- | I: 17 C: 15 T: 32 |
80% | 8 withdrew - 2 in placebo and 1 in treatment group due to non-com- pliance, 2 in treatment group due to gastritis and skin rashes, 3 in placebo group due to uncon- trolled dia- betes |
| Poongothai 2002 | I: Hyponidd ® C: Placebo |
I: 20 C: 20 T: 40 |
I: 20 C: 20 T: 40 |
I: - C: - T: 35 |
I: 16 C:16 T: 32 |
I: 16 C: 16 T: 32 |
80% | 8 with- drawals - 5 due to non- compliance, 2 had severe hypergly- caemia and 1 due to el- evated liver enzymes |
| Shekhar 2002 | I: Cogent db ® C: Usual care (antidi- abetic drugs) |
I: 39 C: 40 T: 79 |
I: 39 C: 40 T: 79 |
I: 30 C: 30 T: 60 |
I: 30 C:30 T: 60 |
I: 30 C: 30 T: 60 |
75.9% | 19 with- drawals - 4 due to non-com- pliance with medication, 15 due to with- drawn con- sent |
| Total |
I: 184
C: 170 T: 354 |
I: 156
C: 146 T: 302 |
“-” denotes not reported
C: control; I: intervention; ITT: intention-to-treat; T: Total
We resolved any differences in data extraction by consensus, and by referring back to the original article. When necessary, information was sought from the authors of the primary studies.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we tried to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) was given priority.
Assessment of risk of bias in included studies
Two authors (KS, RM) assessed each trial independently. We resolved any disagreements by consensus, or with consultation of a third party (SR) in case of disagreement. Interrater agreement for key bias indicators (e.g. allocation concealment, incomplete outcome data) was calculated using the kappa statistic (Cohen 1960). In cases of disagreement, the rest of the group was consulted and a judgement was made based on consensus.
We assessed risk of bias using the Cochrane Collaboration’s tool (Higgins 2008). We used the following criteria:
was the allocation sequence adequately generated?
was the allocation adequately concealed?
was knowledge of the allocated intervention adequately prevented during the study?
were incomplete outcome data adequately addressed?
are reports of the study free of suggestion of selective outcome reporting?
was the study apparently free of other problems that could put it at a high risk of bias?
We used individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). A ‘risk of bias graph’ figure and ‘risk of bias summary’ figure are attached. We planned to explore the influence of individual risk of bias criteria in a sensitivity analysis.
Measures of treatment effect
Relative risks and odds ratios were used for measuring the treatment effect for binary outcomes. Mean differences were used for treatment effects involving continuous outcomes. 95% confidence intervals (CI) were applied to measure the precision. We planned to calculated the number needed to treat (NNT) and number needed to harm (NNH), where appropriate.
Unit of analysis issues
We took into account the level at which randomization occurred, such as cross-over trials, cluster-randomized trials and multiple observations for the same outcome.
Dealing with missing data
We obtained relevant missing data from authors, if feasible and carefully performed evaluation of important numerical data such as screened, randomized patients as well as intention-to-treat (ITT), as-treated and per-protocol (PP) population. We investigated attrition rates, for example drop-outs, losses to follow up and withdrawals and critically appraised issues of missing data and imputation methods (for example, last-observation-carried-forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical or methodological or statistical heterogeneity, study results were not reported as meta-analytically pooled effect estimates.
Clinical heterogeneity (diversity) were assessed by visual scanning of participant characteristics. We identified heterogeneity by visual inspection of the forest plots, by using a standard Chi2 test and a significance level of α = 0.1, in view of the low power of such tests. We specifically examined heterogeneity with the I2 statistic (Higgins 2002), with categories ‘might not be important’ (0% to 40%), ‘moderate’ (30% to 60%), ‘substantial’ (50% to 90%) and ‘considerable’ (75% to 100%) (Higgins 2008).
When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We used funnel plots to assess for the potential existence of small study bias. There may be a number of explanations for the asymmetry of a funnel plot (Sterne 2001). Therefore, we carefully interpreted results (Lau 2006).
Data synthesis
We summarised the data statistically if they were available, sufficiently similar and of sufficient quality. We performed statistical analyses according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses if one of the primary outcome parameters demonstrated statistically significant differences between intervention groups. In any other case subgroup analyses were planned to be clearly marked as a hypothesis generating exercise.
The following subgroup analyses were planned:
glycosylated haemoglobin A1c (HbA1c) level at baseline (if this was unavailable, mean level of fasting plasma glucose at baseline would have been used);
age (less than 18 years, 18 to 40 years, 41 to 64 years, older than 65 years);
gender;
weight (BMI: women less than 25 kg/m2, men less than 27 kg/m2; BMI: women 25 to 30 kg/m2, men 27 to 30 kg/m2; BMI more than 30 kg/m2);
type of diabetes.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of risk of bias, as specified above;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (relative risk, odds ratio etc.) and different statistical models (fixed-effect model and random-effects model).
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
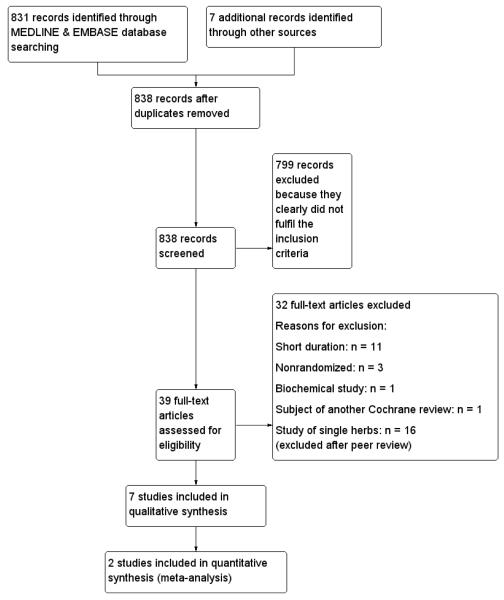
We found 831 potentially relevant studies by search of MEDLINE and EMBASE. After perusal of the titles and abstracts, 799 studies were excluded because they were studies of herbs which are not used in Ayurveda (Western and Chinese herbs), non-randomised studies, or had study objectives different from this review. Thirty-two were found potentially eligible at this stage and the full papers were obtained, among which we found 19 studies, fulfilling our initial inclusion criteria. We identified seven other studies (Crawford 2009; Dubey 1993; Faizal 2009; Maji 1995; Mohan 1998; Poongothai 2002; Yadav 2001) through additional searches, out of which four (Crawford 2009, Dubey 1993; Mohan 1998; Poongothai 2002) were found eligible for inclusion. After feed back from peer reviewers, it was decided to exclude 16 studies of single herbs (Altschuler 2007; Anderson 1999; Aro 1981; Blevins 2007; Cohen 1980; Crawford 2009; Gupta 2001; Holman 1987; ICMR Study Group 2005; Kuriyan 2008; Lalor 1990; Mang 2006; Niemi 1988; Uusitupa 1984; Uusitupa 1989;Ziai 2005) and hence only seven studies were ultimately included in the final analysis (Agrawal 2002; Dubey 1993, Elder 2006; Hsia 2004; Mohan 1998; Poongothai 2002; Shekhar 2002). An adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow-chart of study selection is attached Figure 1
Figure 1.
Adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of study selection
Included studies
We found seven eligible studies, six testing five different proprietary Ayurvedic drugs: Diabecon (Dubey 1993; Mohan 1998), Hyponidd (Poongothai 2002), Cogent DB (Shekhar 2002), Inolter (Agrawal 2002) and Pancreas Tonic (Hsia 2004) and one study of ‘whole system’ Ayurvedic treatment (Elder 2006). Together these studies enrolled 354 participants ( 172 on treatment, 158 on control, 24 allocation unknown). The duration of treatment ranged from three to six months. All these studies included adults with type 2 diabetes mellitus. The details of the studies are given in Characteristics of included studies, Appendix 2, Appendix 3, Appendix 4 and Appendix 5.
Excluded studies
Among potentially relevant studies, we excluded 32 studies, the details of which are given in the table Characteristics of excluded studies. Eleven studies were of short duration ranging from one to six weeks (Agrawal 1996; Akhtar 2010; Bandara 2009; Fuessl 1987; Khan 1980; Khan 2003; Krarup 1980; Serraclara 1998; Stokholm 1981; Vuorinen-Markkola 1992; Yadav 2001), three were non-randomised (Faizal 2009, Maji 1995, Upadhyay 2004), one was a biochemical study (Sadhukhan 1994) and another study of Mormodia charantia (John 2003) was already evaluated in another Cochrane review (Ooi 2010). We also excluded 16 other studies of single herbs (Altschuler 2007; Anderson 1999; Aro 1981; Blevins 2007; Cohen 1980; Crawford 2009; Gupta 2001; Holman 1987; ICMR Study Group 2005; Kuriyan 2008; Lalor 1990; Mang 2006; Niemi 1988; Uusitupa 1984; Uusitupa 1989; Ziai 2005) since the use of single herbs is not considered the usual practice in Ayurveda. Moreover, a Cochrane protocol on Cinnamon (Leach 2008) and a Cochrane review on Momordica charantia (Ooi 2010) have been published in The Cochrane Library. We also excluded studies investigating the use of Western herbal preparations, which are not used in Ayurveda. Two Ayurvedic physicians were consulted to ensure that the interventions included are used in Ayurveda.
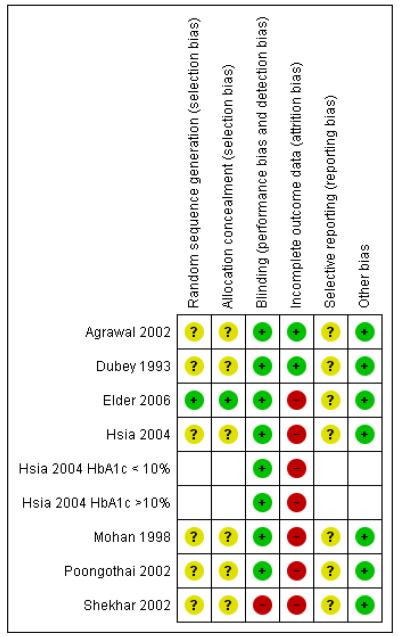
Risk of bias in included studies
The risk of bias assessments are summarised in Figure 2 and Figure 3
Figure 2.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
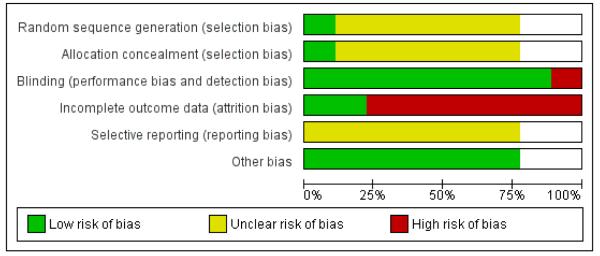
Allocation
Sequence generation and concealment of randomisation was adequate in one study only (Elder 2006), while in the other studies, randomisation methods were not described. In the study by Elder 2006, the randomisation was achieved in blocks of 4 to 8. Sixty sequential cards were printed each displaying randomisation number and assigned study arm. These were placed in consecutively numbered envelopes. The study nurse, to whom the sequence was concealed and after ascertaining the eligibility, opened the envelopes sequentially and assigned the treatment.
Blinding
Six studies were blinded. Both the patient and investigator were blinded in five studies (Agrawal 2002; Dubey 1993; Hsia 2004; Mohan 1998; Poongothai 2002). In general, the blinding was achieved by using identical looking treatment and placebo tablets or capsules. It is possible that in some studies, patients and investigators may have become unblinded, due to differences in physical characteristics of the compounds. In one study, only the outcome assessor (laboratory) was blinded (Elder 2006) and one study had an open design (Shekhar 2002).
Incomplete outcome data
The percentage of randomised participants completing the study ranged from 76% to 100% (Table 1). No drop-outs were reported in two studies (Agrawal 2002; Dubey 1993). In four studies 75% to 80% of randomized participants completed the study (Hsia 2004; Mohan 1998; Poongothai 2002; Shekhar 2002), while in the other study (Elder 2006) 90% of randomised participants completed the study. An intention-to-treat analysis was probably not performed in any of these studies and the participants who withdrew from the study were apparently excluded from analysis.
Selective reporting
Almost all studies reported data on the outcomes proposed to be studied. However, the results were not provided in a uniform manner. The study on whole system Ayurvedic approach (Elder 2006) reported adjusted treatment effects, which were difficult to interpret or combine with other studies.
Other potential sources of bias
We did not detect unambiguous other sources of bias. We should like to point out, however, that pre-trial estimation of sample size was not done in any study. Moreover, pharmaceutical funding or association was reported in four studies (Agrawal 2002; Mohan 1998; Poongothai 2002; Shekhar 2002). Two studies were funded by non-pharmaceutical sources, such as research organizations (Elder 2006; Hsia 2004). Information regarding funding was not available in one study (Dubey 1993).
Effects of interventions
Effect of proprietary Ayurvedic drugs (herbal mixtures)
No study reported on or was designed to investigate diabetic complications, hospitalisations, death from any cause and economic data.
Adverse effects
Adverse events were not uniformly reported in many studies. Serious adverse events leading to withdrawal from the study were reported in two studies (Mohan 1998; Poongothai 2002). In one study (Mohan 1998) two participants among the intervention group and none in the control group withdrew from the study due to adverse effects. In the other study (Poongothai 2002) three participants withdrew due to adverse effects, but it is not clear whether they were in the treatment or control arm. No renal, haematological or liver toxicity was reported in any of the studies. One study reported drug hypersensitivity in one patient in the intervention group (Mohan 1998). Hypoglycaemia was reported in 1 of 31 patients in the intervention group in one study (Hsia 2004), however none had severe hypoglycaemia. Hypoglycaemia was not reported in any of the controls. Gastrointestinal side effects were reported in one study (Mohan 1998). The details of the adverse events are given in Appendix 5.
Diabecon (proprietary drug)
Two studies investigated the effects of six months treatment with Diabecon (Dubey 1993; Mohan 1998). The first study analysed the results among 14 participants each in the treatment and placebo groups (Dubey 1993). The second study analysed the results among 17 participants in the treatment group and 14 in placebo group (Mohan 1998).
Primary outcomes
HbA1c at end of study
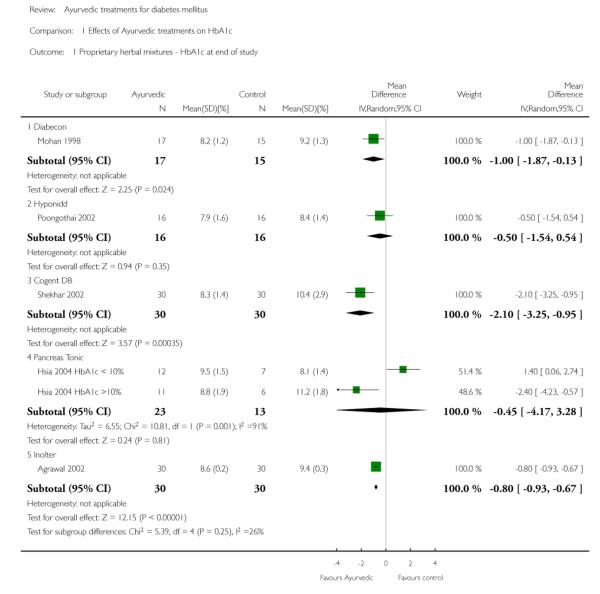
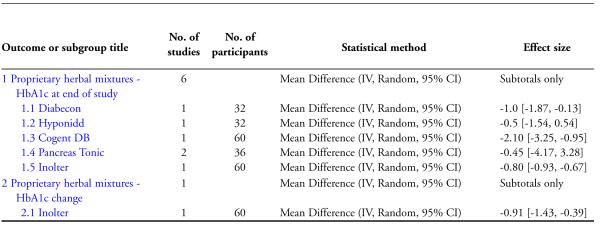
One study (Mohan 1998 ) with 32 participants (17 treatment / 15 controls) found a significant reduction in glycosylated haemoglobin A1c (HbA1c) with Diabecon (MD −1 %, 95% CI −1.9 to −0.1; Analysis 1.1).
Fasting blood sugar at end of study
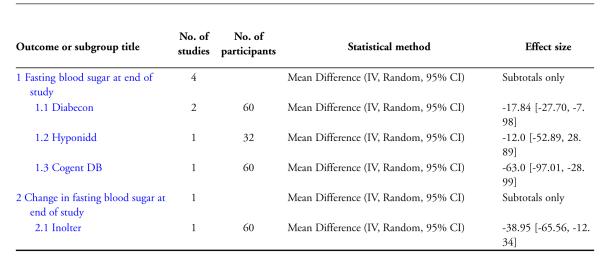
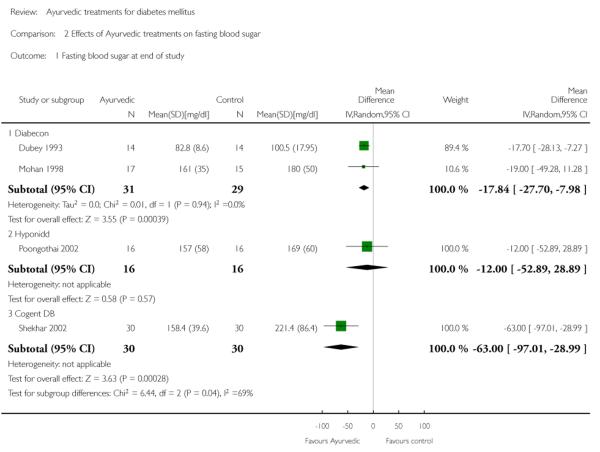
Two studies (Dubey 1993; Mohan 1998) analysing 60 patients (31 treatment / 29 controls) found significantly lower fasting blood glucose (FBS) levels in the Diabecon group at the end of the study period (MD −18 mg/dl; 95% CI −28 to −8). The variance in one study (Dubey 1993) was found to be extremely narrow; we assumed that this could be a SEM instead of a SD, consequently calculated the SD and analysed data accordingly. Despite our best efforts we were unable to contact the author for clarification. When this study was excluded, the results of the other study did not reveal any significant differences in FBS (MD −19 mg/dl, 95% CI −49 to 11; Analysis 2.1).
Post prandial blood sugar at end of study
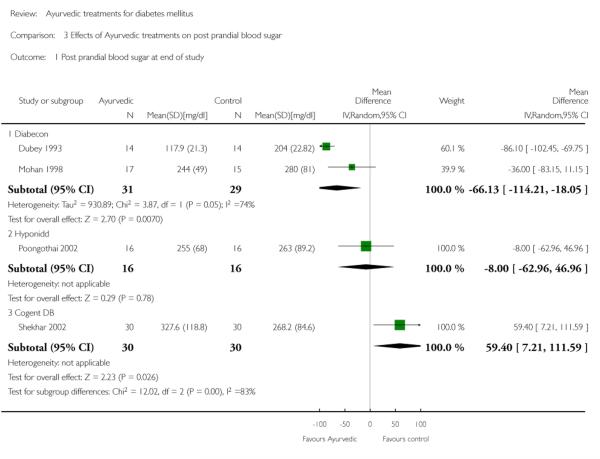
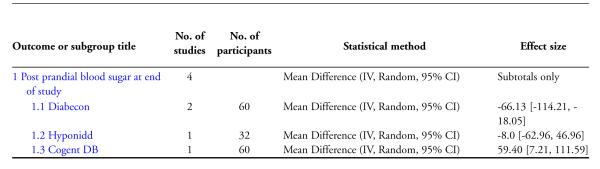
Two studies (Dubey 1993; Mohan 1998) showed significantly lower post prandial blood sugar (PPBS) in the treatment group compared to controls (MD −66 mg/dl, 95% CI −114 to −18; Analysis 3.1). When one study (Dubey 1993) was excluded (see above), the results of the other study did not reveal any significant difference in PPBS (MD −36 mg/dl, 95% CI −83 to 11).
Secondary outcomes
Serum insulin and C-peptide levels
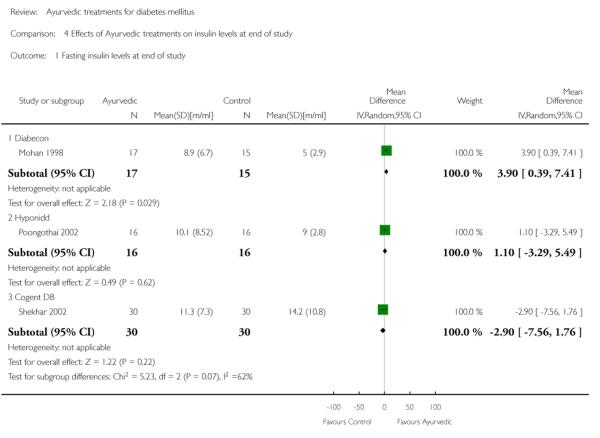
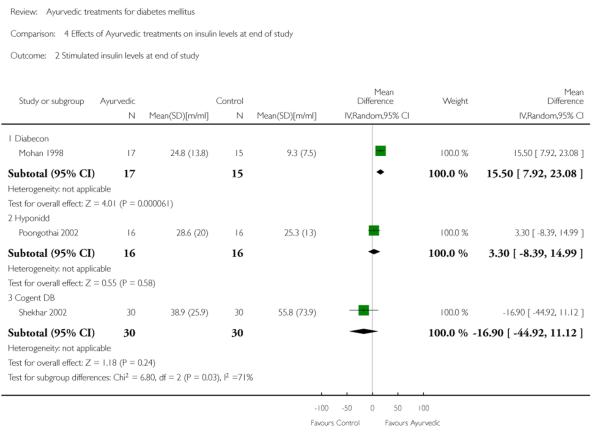
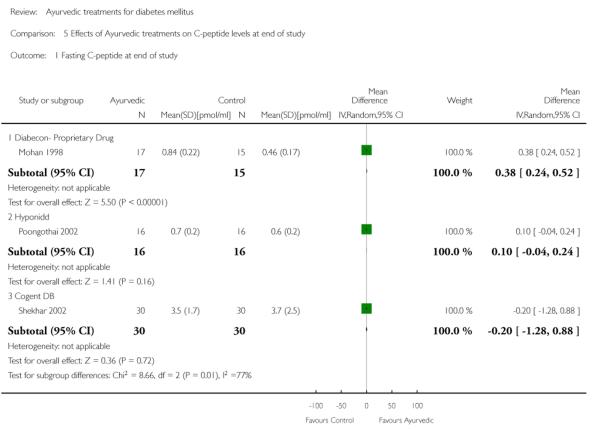
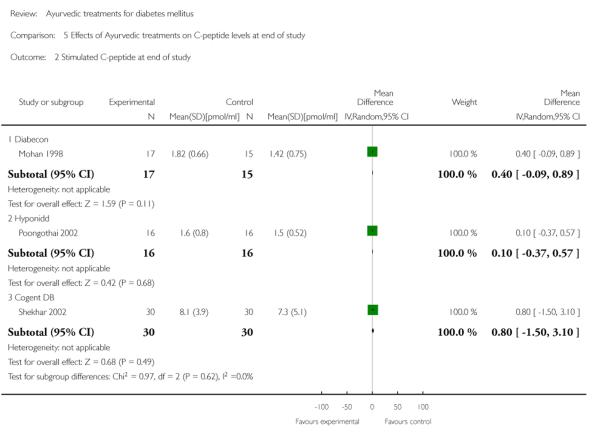
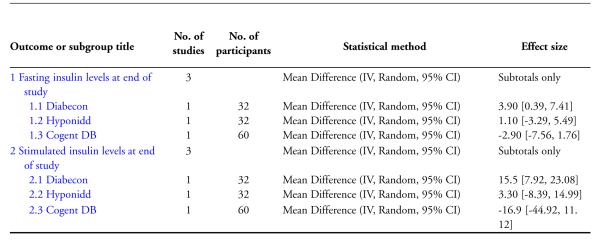
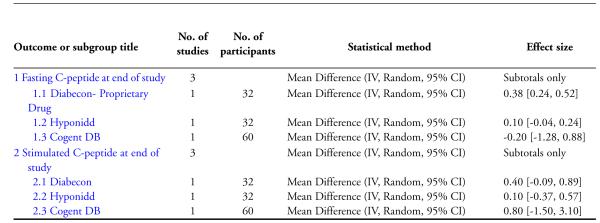
One study (Mohan 1998) showed significantly higher levels of fasting serum insulin (MD 3.90 μm/ml, 95% CI 0.39 to 7.41; Analysis 4.1). Stimulated insulin levels (MD 15.50 μm/ml, 95% CI 7.92 to 23.08; Analysis 4.2) and C-peptide levels (MD 0.38 pmol/ml, 95% CI 0.24 to 0.52; Analysis 5.1) revealed significant differences with Diabecon treatment compared to placebo. However, there was no significant difference in stimulated C-peptide levels (MD 0.40 pmol/ml; 95% CI −0.09 to 0.89) at the end of study (Analysis 5.2).
No data were available regarding changes in lipid profile in both the studies.
Hyponidd (proprietary drug)
One study (Poongothai 2002) investigated the effects of 12 weeks treatment with the proprietary Ayurvedic drug Hyponidd, analysing the results of 32 participants (16 treatment /16 controls).
Primary outcomes
The study found no significant differences in HbA1c (MD −0.5 %, 95% CI −1.5 to 0.5; Analysis 1.1), FBS (MD −12 mg/dl, 95% CI −53 to 29; Analysis 2.1), PPBS (MD −8 mg/dl, 95% CI −63 to 47; Analysis 3.1).
Secondary outcomes
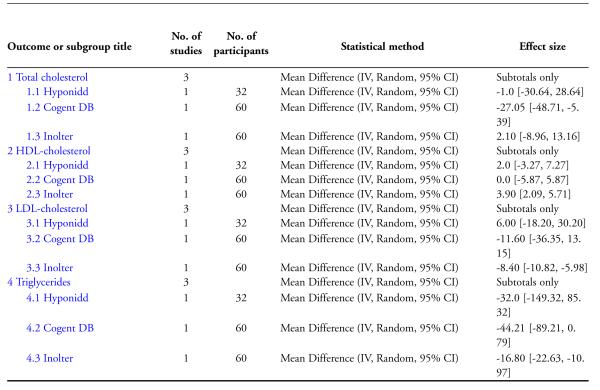
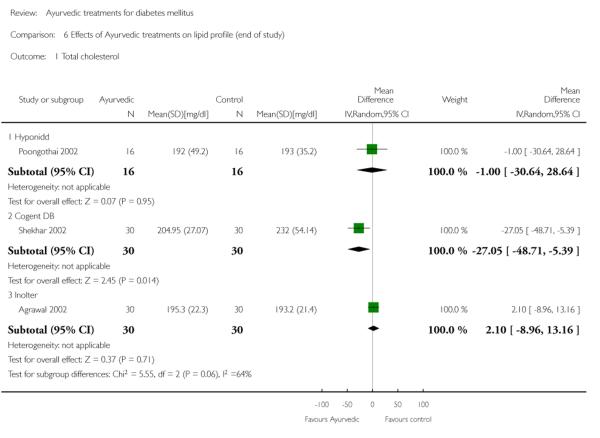
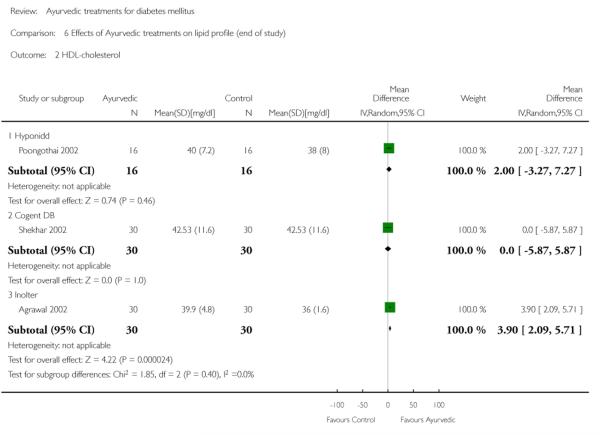
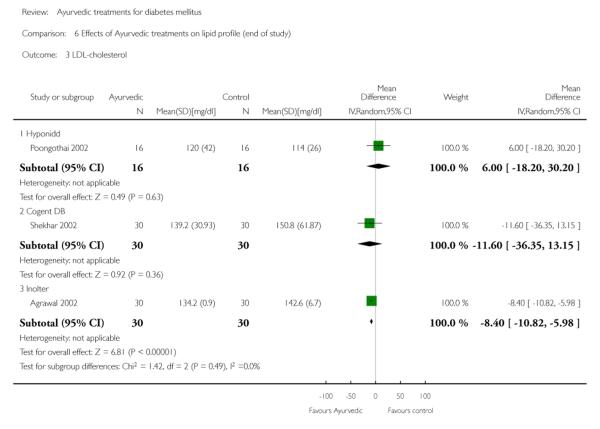
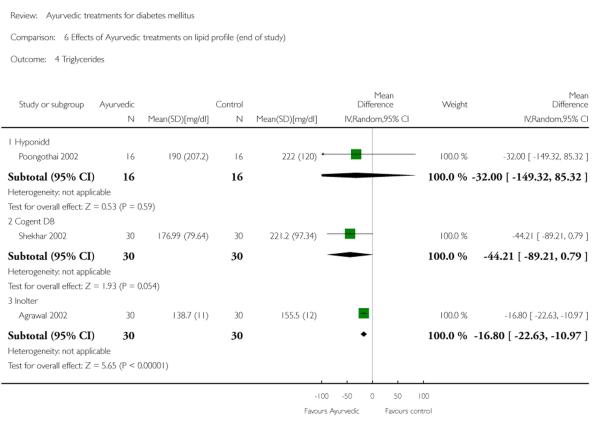
The study found no significant differences at the end of the study period between intervention groups and controls in: Fasting serum insulin (MD 1.10 μm/ml, 95% CI −3.29 to 5.49; Analysis 4.1); stimulated serum insulin (MD 3.30 μm/ml, 95% CI −8.39 to 14.99; Analysis 4.2), fasting C-peptide (MD 0.10 pmol/ml, 95% CI −0.04 to 0.24; Analysis 5.1); stimulated C-peptide (MD 0.10 pmol/ml, 95% CI −0.37 to 0.57; Analysis 5.2); total cholesterol (MD −1.00 mg/dl, 95% CI −30.64 to 28.64; Analysis 6.1); HDL-cholesterol (MD 2 mg/dl, 95% CI −3 to 7; Analysis 6.2); LDL-cholesterol (MD 6 mg/dl, 95% CI −18 to 30; Analysis 6.3) or triglyceride levels (MD −32 mg/dl, 95% CI −149 to 85; Analysis 6.4).
Inolter (proprietary drug)
One study (Agrawal 2002) analysed 60 participants (30 treatment, 30 controls) treated for three months with Inolter or placebo.
Primary outcomes
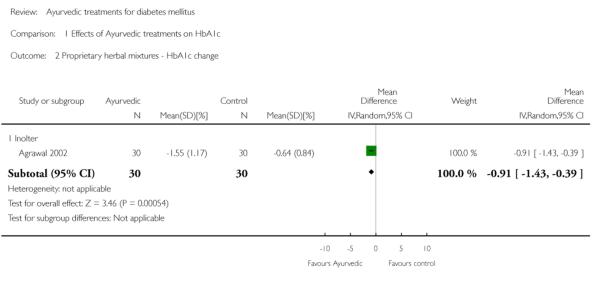
The study found significantly lower HbA1c levels with Inolter treatment compared to placebo (MD −0.8%, 95% CI −0.9 to −0.7; Analysis 1.1) and also significant reductions in HbA1c compared to baseline favouring the treatment group (MD −0.9 %, 95% CI −1 to −0.4; Analysis 1.2).
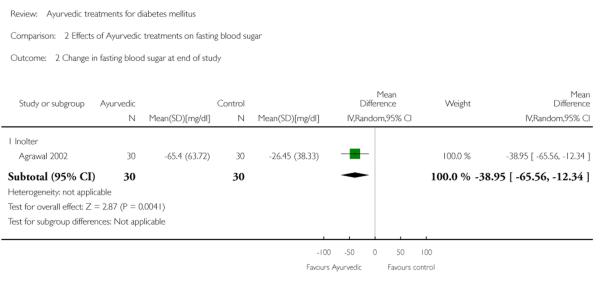
The FBS levels at the end of the three months treatment period compared to baseline were also significantly lower in the Inolter group (MD −39 mg/dl, 95% CI −66 to −12; Analysis 2.1).
Secondary outcomes
Results for the treatment group compared to controls at the end of the study period were as follows: Total cholesterol (MD 2 mg/dl, 95% CI −9 to 13; Analysis 6.1); a significant increase in HDL-cholesterol (MD 4 mg/dl, 95% CI 2 to 6; Analysis 6.2); a significant reduction in LDL-cholesterol (MD −8 mg/dl, 95% CI −11 to −6; Analysis 6.3); triglycerides (MD −17 mg/dl, 95% CI −23 to −11; Analysis 6.4).
Effects on plasma insulin and C-peptide levels were not investigated.
Cogent DB (proprietary drug)
One study (Shekhar 2002) analysed the results of 60 participants treated for three months with Cogent DB or placebo (30 in each group).
Primary outcomes
The study reported significantly lower HbA1c levels (MD −2.1 %, 95% CI −3.3 to −1; Analysis 1.1), and FBS values (MD −63 mg/dl, 95% CI −97 to −29; Analysis 2.1) at the end of three months treatment. However, this study reported significant increases in PPBS in the treatment group (MD 59 mg/dl, 95% CI 7 to 112; Analysis 3.1).
Secondary outcomes
There were no significant differences between the Cogent DB and control groups for the following outcomes: Fasting serum insulin (MD −2.90 μm/ml, 95% CI −7.56 to 1.76; Analysis 4.1); stimulated serum insulin (MD −16.90 μm/ml, 95% CI −44.92 to 11.12; Analysis 4.2); C-peptide (MD −0.20 pmol/ml, 95% CI −1.28 to 0.88; Analysis 5.1) and stimulated C-peptide levels (MD 0.80 pmol/ml, 95% CI −1.50 to 3.10; Analysis 5.2). The serum cholesterol (MD −27 mg/dl, 95% CI −49 to −5; Analysis 6.1) and triglyceride levels (MD −44 mg/dl, 95% CI −89 to 0.8; Analysis 6.4) were significantly lower compared to controls, but there was no significant difference in HDL-cholesterol (MD 0 mg/d, 95% CI −6 to 6; Analysis 6.2) or LDL-cholesterol (MD −12 mg/dl, 95% CI −36 to 13; Analysis 6.3) levels at the end of the study period.
Pancreas tonic (proprietary drug)
One study (Hsia 2004) analysed the results of 36 participants, 23 were treated with Pancreas tonic and 13 with placebo for three months.
Primary outcomes
The study found no significant differences in HbA1c values with Pancreas tonic (MD −0.5 %, 95% CI −4.2 to 3.3), but reported significantly lower HbA1c results among a subgroup whose baseline HbA1c was more than 10% (MD −2.4%, 95% CI −4.2 to −0.6), compared to those who had baseline HbA1c levels less than 10% (significant increase with a MD of 1.4%, 95% CI 0.1 to 2.7; Analysis 1.1).
This study also assessed health-related quality of life using the modified SF 36 questionnaire and found no significant changes in treatment satisfaction indices pertaining to symptomatology, general feelings, or perceived health status in either group.
Secondary outcomes
The study reported that there were no significant differences for the secondary outcomes (lipids, body mass index (BMI), body composition, blood pressure, insulin sensitivity, glucose and insulin responses to a meal challenge, safety indices) between treatment groups, but did not give the actual values
Effect of whole system Ayurvedic treatment
The single study investigating the effect of ‘whole system’ Ayurvedic treatment did not give the actual values of HbA1c for analysis, but reported treatment effects adjusted for gender and baseline value (Elder 2006). The study reported no significant differences for HbA1c, FBS, blood pressure, lipids or body weight. Secondary analysis suggested possible benefits for individuals with baseline HbA1c values above 6.5%. The study found no significant study related adverse events or toxicity.
Subgroup and sensitivity analyses
We were unable to do subgroup or sensitivity analysis due to lack of adequate number of trials.
DISCUSSION
Summary of main results
We found seven trials, six tested five different types of proprietary drugs (herbal mixtures) and one tested whole system Ayurvedic treatment. These studies enrolled 354 participants (172 on treatment, 158 on controls, 24 allocation unknown). The duration of treatment ranged from three to six months. No study reported on or was designed to investigate diabetic complications, hospitalisations, death from any cause and economic data.Analysis of two studies of Diabecon found significantly lower glycosylated haemoglobin A1c (HbA1c), fasting blood glucose (FBS), post prandial blood sugar (PPBS) levels at the end of study. One study found higher C-peptide, fasting insulin and stimulated insulin levels in the treatment group but no difference in stimulated C-peptide levels at the end of study. One study of Inolter treatment found significantly lower HbA1c and significant reductions (compared to baseline) in HbA1c and FBS levels compared to the control group at the end of study. Other outcomes were not assessed. One study of treatment with Hyponidd found no significant differences in HbA1c, FBS, PPBS, total cholesterol, HDL- and LDL-cholesterol, triglycerides, fasting and stimulated C-peptide and insulin levels in the treatment group compared to controls at the end of study. One study of Cogent DB treatment found significantly lower HbA1c, FBS and total cholesterol but significantly higher PPBS levels compared to the control group at the end of study. There were no significant differences in HDL- and LDL-cholesterol, triglycerides, fasting and stimulated C-peptide and insulin levels at end of study. One study of treatment with Pancreas tonic found no significant reductions in HbA1c in the treatment group at the end of the study. However, subgroup analysis of participants with HbA1c more than 10% showed significant reductions. The study reported no significant treatment related differences in FBS, lipids, insulin sensitivity but did not provide the actual values. One study of whole system Ayurvedic treatment did not provide sufficient data to assess the effects on HbA1c and FBS at the end of study. Only one study investigated the effects on health-related quality of life and found no significant changes (Hsia 2004). No deaths were reported in any of the studies. Serious adverse events leading to study withdrawal were reported in five patients (two in the intervention group and allocation unknown for the other three). The main side effects reported were drug hypersensitivity (one study, one patient in treatment arm); hypoglycaemic episodes (one study, one participant in treatment arm, none had severe hypoglycaemia); gastrointestinal side effects in one study (one of 20 in intervention group and none of 20 participants in control group). No renal, hematological or liver toxicity was reported.
Overall completeness and applicability of evidence
Seven trials (all parallel group studies) were selected for the review. The trials used varying interventions: Five different proprietary Ayurvedic drugs were used in six studies. Only one study investigated ‘whole system’ Ayurvedic treatment. These studies were dispersed in time with some trials being conducted more than 18 years ago. The number of participants enrolled varied from 28 to 79, with only three studies enrolling more than 50 participants. Power calculations to determine sample size were not employed in most studies. The interventions in the control groups varied - placebo in five studies, no treatment or usual care in two studies. In addition, the time point for assessment of the effect of intervention for the trials were not uniform, ranging from three months to six months.
Ayurvedic physicians generally use a mixture of various herbs or proprietary preparations along with diet, exercise and mode of living. The treatments are usually individualised taking into account the balance of three ‘doshas’. It is possible that the interventions in the trials analysed have not replicated actual Ayurvedic practice but only assessed some components individually. This limits our ability to draw reliable conclusions regarding the effectiveness of traditional Ayurvedic treatment for diabetes.
Quality of the evidence
Sequence generation and concealment of randomisation was adequate in one study only (Elder 2006), while in the other studies, randomisation methods were not described. Only five of the seven studies were double-blind, outcome assessors alone were blinded in one study and one was not blinded. The percentage of randomised participants completing the study ranged from 76% to 100%. No drop-outs were reported in two studies. The outcomes studied in these studies were not same. The results were not provided in an uniform manner.
Potential biases in the review process
This systematic review has several limitations. There may be a publication bias with negative studies not being reported. There were not enough studies for each intervention to perform a reliable meta-analysis and investigate funnel plots. The majority of the included trials also suffer from methodological deficiencies. Poor quality of randomisation and blinding may be associated with exaggerated effects of the interventions due to systematic errors (bias) occurring at different stages of the study such as selection of patients, administration of treatment and assessment of outcomes. Diagnostic criteria for diabetes mellitus were not specified in most studies. The small sample size of many of the trials may lead to diminished power to detect a treatment effect. All the trials reported end-of treatment responses, ranging from three to six months and long-term responses beyond this period are not known. Specifically long-term safety and adverse events are not addressed in these short-term studies. The different types of interventions tested make it difficult to combine data. Pharmaceutical funding or association was reported in four of the seven studies. Greater beneficial response was also found in the pharmaceutical company funded studies of proprietary Ayurvedic drugs. Treatment with proprietary Ayurvedic drugs was found to reduce HBA1c in three and FBS in two of the of the five drugs tested. Participants who withdrew from the study were excluded from the analysis in most studies. We also excluded studies wherein the treatment duration was less than eight weeks, since they would not contribute adequately to the assessment of main outcomes.
Agreements and disagreements with other studies or reviews
Our findings are similar to the conclusions of two previous systematic reviews. Yeh 2003 analysed the effects of 42 randomized and 16 non-randomized trials of 36 herbs (given single or in combination) and nine vitamin/mineral supplements, involving 4565 patients with type 2 diabetes or impaired glucose tolerance. The authors found that the studies were heterogenous and hence did not perform a formal meta-analysis. The authors found evidence for improved glucose control with very few adverse effects, warranting further study. Another comprehensive review also pointed out methodological weaknesses of the studies conducted in this area (AHRQ 2001). This review also emphasized the need for testing Ayurveda as a whole system or at least test multiple modalities at the same time.
AUTHORS’ CONCLUSIONS
Implications for practice
Though there were significant reduction in glycosylated haemoglobin A1c (HbA1c) levels at the end of treatment periods with the use of proprietary Ayurvedic herbal mixtures (Cogent DB, Diabecon and Inolter), due to methodological deficiencies and small sample sizes we are unable to draw any definite conclusions regarding the efficacy of these treatments. Though no significant adverse events were reported, there is insufficient evidence at present to recommend the use of these interventions.
Implications for research
Further well designed studies are needed to evaluate the potential benefits and tolerability or safety of botanical agents or mixture of herbs as in proprietary preparations as well as the ‘whole system Ayurvedic treatment’. The studies should enrol adequate number of participants and use valid methods of random allocation and concealment of randomisation. Adequate blinding methods should be used wherever possible. Even if the participant or treating physician cannot be blinded, outcome assessors should be blinded. The enrolled participants should fulfil the currently accepted diagnostic criteria for diabetes mellitus. The treatment period should be at least three months to assess the hypoglycaemic efficacy and a few years to determine long-term safety. The Ayurvedic treatment may be used in newly diagnosed patients or as add-on to antidiabetic drugs. Withdrawals from the study should be minimized. It is preferable to test these treatments in different ethnic groups, to assess the cultural differentials of these treatments.
CHARACTERISTICS OF STUDIES.
Agrawal 2002
| Methods | PARALLEL RANDOMIZED CONTROLLED CLINICAL TRIAL |
| Participants | INCLUSION CRITERIA: Newly diagnosed Type 2 diabetes mellitus |
| EXCLUSION CRITERIA: Not reported | |
| DIAGNOSTIC CRITERIA: Not reported | |
| CO-MORBIDITIES: Not reported | |
| CO-MEDICATIONS: Apparently none | |
| Interventions | NUMBER OF STUDY CENTRES: Single |
| COUNTRY/ LOCATION: India | |
| SETTING: Outpatients attending a Medical College Hospital | |
|
INTERVENTION(ROUTE,TOTALDOSE/DAY, FREQUENCY):Treatment group received Inolter (each capsule of Inolter contains Mormordica charantia 1OO mg, Trigonella Faenum Graecum 100 mg, Asphalt 100 mg, Gymnema Sylvestre 100 mg, Eugenia Jambolina 100 mg). Dose and frequency of administration not mentioned |
|
|
CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Controls received identical looking placebo - dose and frequency of administration not mentioned. Diet and exercise were continued for both groups |
|
| TREATMENT BEFORE STUDY: None for diabetes | |
| TITRATION PERIOD: Not reported | |
| Outcomes |
PRIMARY OUTCOME(S) (as stated in the publication): Glycaemic control (FBS, HbA1c) and changes in lipid profile |
| SECONDARY OUTCOMES (as stated in the publication): None mentioned | |
| ADDITIONAL OUTCOMES: None reported | |
| Notes | No withdrawals reported |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | coded and identical looking active medication or placebo |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | no withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | nothing detected |
Dubey 1993
| Methods | CONTROLLED CLINICAL TRIAL (CCT) |
| Not mentioned as randomized study | |
| Parallel RCT | |
| Participants |
INCLUSION CRITERIA: People over the age of 30 years with persistent postprandial hyperglycaemia, freshly diagnosed and not on any oral hypoglycaemic |
|
EXCLUSION CRITERIA: Patients with Cushing’s syndrome, hypothyroidism, severe hypertension, angina, myocardial infarction, cerebrovascular accident or renal failure in the preceding 6 months |
|
| DIAGNOSTIC CRITERIA: Not reported | |
| CO-MORBIDITIES: Not reported | |
| CO-MEDICATIONS: None | |
| 28 adults with persistent postprandial hyperglycaemia (14 in Diabecon group and 14 in placebo group) |
|
| Interventions | NUMBER OF STUDY CENTRES: 1 |
| COUNTRY/ LOCATION: India | |
| SETTING: Not mentioned- probably university hospital outpatients | |
|
INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Diabecon, 2 tablets oral twice a day for 6 months. Diabecon (D-400) - a proprietary herbal preparation, (manufactured by Himalaya drug company) containing Eugenia jambulana, Tinospora cordifolia, Pterocarpus marsupium. Ficus glomerulata,Momordica charantia and Ocimum sanctum as its main ingredients |
|
|
CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Identical looking placebo, 2 tablets oral twice a day for 6 months |
|
| TREATMENT BEFORE STUDY: None | |
| TITRATION PERIOD: None | |
| Outcomes |
PRIMARY OUTCOME(S) (as stated in the publication): Fasting blood sugar, postprandial blood sugar, 6, 12 & 24 weeks after start of treatment SECONDARY OUTCOMES (as stated in the publication): Body weight at 6, 12 & 24 weeks after start of treatment |
| ADDITIONAL OUTCOMES: Liver, kidney, haemopoietic function, ECG | |
| Notes | No withdrawals reported |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Unclear risk | “Out of 28 cases, 14 were in the Diabecon -treated group, while the other 14 received identical-looking placebo tablets” |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | identical looking placebo |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | no withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | nothing detected |
Elder 2006
| Methods | PARALLEL RANDOMIZED CONTROLLED CLINICAL TRIAL |
| Participants |
WHO PARTCIPATED: 60 adult patients from a group model health maintenance organization |
|
INCLUSION CRITERIA: People with FBS > 125,HbA1c 6-8%fromKaiser northwest diabetes registry database |
|
|
EXCLUSION CRITERIA: Psychosis, depression, serious medical conditions, partic- ipants on warfarin or steroids, pregnant or nursing women, residing outside Portland metropolitan area and unable to comply with treatment protocol |
|
| DIAGNOSTIC CRITERIA: Not reported | |
| CO-MORBIDITIES: Not reported | |
|
CO-MEDICATIONS: Anti-hyperlipidaemic and anti hypertensive medications were allowed and could be adjusted during trial |
|
| Interventions | NUMBER OF STUDY CENTRES: Single |
|
COUNTRY/LOCATION: USA - Kaiser Permanente Center forHealth Research clinic, Portland, Oregan |
|
| SETTING: Group model health maintenance organisation. | |
|
INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Whole system Ayurvedic protocol comprising: Herbal supplement “MA471” (containing Phylanthus Niruri, Arjuna Myrobalan, Enicostema Littorale, Bael Fruit, Neem Leaf, Bitter Gourd, Black Berry) + meditation instruction + diet + exercise and daily routine MA 471 was supplied byMaharishi Ayurveda Products International, Colorado Springs, Colorado, USA |
|
|
CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): 4 sessions of diabetic education given by a nurse, and usual care from primary physician |
|
| TREATMENT BEFORE STUDY: Probably none | |
| TITRATION PERIOD: Not reported | |
| Outcomes | PRIMARY OUTCOME(S) (as stated in the publication): HbA1C, FBS |
|
SECONDARY OUTCOMES (as stated in the publication): Lipids, weight, blood pres- sure and pulse |
|
|
ADDITIONAL OUTCOMES: Safety - Blood urea nitrogen,creatinine, liver enzymes, blood count |
|
| Notes | 6 participants withdrew 4 from Vedic group and 3 from control group; baseline characteristics of the two groups differed in sex distribution, number receiving BP medication and triglyceride levels |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Low risk | Block randomization in blocks of 4 to 8 |
| Allocation concealment (selection bias) | Low risk | “60 sequential cards were printed each displaying randomization number and assigned study arm. These were placed in consecutively numbered envelopes The study nurse, after ascertaining the eligibil- ity, opened the envelopes sequentially and assigned the treatment” |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | single blinded patients not blinded, Labo- ratory -assessing biochemical outcome was blinded |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Drop outs from study probably excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | nothing detected |
Hsia 2004
| Methods |
PARALLEL RANDOMIZED CONTROLLED CLINICAL TRIAL randomization RATIO: 2:1 randomization was stratified according to HbA1C levels - a lower stratum of HbA1c (8. 0% to 9.9%) or a higher stratum (10.0% to 12.0%) |
| Participants |
INCLUSION CRITERIA: Diagnosis of type 2 diabetes mellitus (according to the ADA criteria) for at least 1 year prior to study entry with a stable dose of oral antidiabetes agents, or a stable dietary and lifestyle regimen without pharmacotherapy, for at least 3 months. Adults aged between 18 and 70; HbA1c levels between 8% and 12% EXCLUSION CRITERIA: People treated with insulin; pregnant or planning to become pregnant or lactating women; recent use of any investigational drug; hospitalizations or emergency room visits for hyperglycaemia or 2 or more severe hypoglycaemic episodes; recreational drug or alcohol dependence; significant cardiac, renal, hepatic, neurological disease, autonomic involvement; active infections (e.g., human immunodeficiency virus [HIV]); recent major surgical procedure; any history of malignancy; haemoglobin level < 11.0 G/dl) DIAGNOSTIC CRITERIA: ADA criteria CO-MORBIDITIES: Not reported CO-MEDICATIONS:Concurrent oral antidiabetes drugs (sulphonylurea and/or met- formin) BMI: Controls = 34.8 ± 9.7; Treatment group 31.4 ± 5.7 |
| Interventions |
NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: USA SETTING: 3 Diabetes Clinics located in Los Angeles, California INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Pancreas tonic - an herbal mixture of 10 herbal extracts (Aegle marmelose (leaves) 30%, Pterocarpus marsupium (heartwood) 30%, Syzigium cumini (fruit) 10%, Momordica charantia (seeds) 7%, Gymnema sylvestre (leaves) 5%, Trigonella foenum graecum (seeds) 5%, Azadirachta indica (seeds) 5%, Ficus racemosa 5%, Tinospora cordifolia (stem) 2%, Cinnamomum tamala (leaves) 1%). Capsules of Pancreas Tonic were manufactured by US Botanicals (Bell Gardens, CA) 2 Capsules 3 Times per day CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Identical looking Placebo capsules which were also provided by US Botanicals. 2 Capsules 3 Times per day TREATMENT BEFORE STUDY: 1 Month Placebo run in TITRATION PERIOD: Not reported |
| Outcomes |
PRIMARY OUTCOME(S) (as stated in the publication):HbA1C SECONDARY OUTCOMES (as stated in the publication):Fasting plasma glucose,lipid and lipoprotein parameters, blood pressure, BMI, body composition by BIA (total fat and fat-free mass), Quality of life by SF 36 ADDITIONAL OUTCOMES: Safety analysis of hematologic and biochemical param- eters, ECG, Changes of insulin sensitivity (Si), glucose effectiveness (Sg), acute (first- phase) insulin response to intravenous glucose (AIRg, defined as the first 10 minutes after the glucose bolus), and the disposition index (DI, defined as the product of Si and AIRg) were obtained from the FSIVGTT using the Bergman Minimal Model. Changes in the glucose and insulin areas under the curve (AUC) above the baseline values were obtained for the meal challenge tests using the trapezoidal method, with baseline values defined as the average of the 15 and 0 minute measurements. |
| Notes | 11 withdrew from the study - 3 from placebo and 8 from intervention group |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | Pancreas Tonic and placebo were dispensed as Identical looking capsules |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | participants who withdrew probably ex- cluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | nothing detected |
Hsia 2004 HbA1c < 10%
| Methods | Created to enable analysis of the results of two strata reported in the study |
| Participants | Same as Hsia 2004 |
| Interventions | Same as Hsia 2004 |
| Outcomes | Same as Hsia 2004 |
| Notes |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | |
| Incomplete outcome data (attrition bias) All outcomes |
High risk |
Hsia 2004 HbA1c >10%
| Methods | Created to enable analysis of the results of two strata reported in the study |
| Participants | Same as Hsia 2004 |
| Interventions | Same as Hsia 2004 |
| Outcomes | Same as Hsia 2004 |
| Notes |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | |
| Incomplete outcome data (attrition bias) All outcomes |
High risk |
Mohan 1998
| Methods | PARALLEL RANDOMIZED CONTROLLED CLINICAL TRIAL |
| Participants |
WHO PARTCIPATED: 40 adults with diabetes of 10-15 years duration INCLUSION CRITERIA: NIDDM diagnosed according to WHO Study Group Classification, non obese adults aged over 30 years, failure to respond to OHA even in maximal doses of combination therapy i.e. glibenclamide 15 mg plus metformin 1000 mg/ day for over 3 months despite good diet control and absence of infections EXCLUSION CRITERIA: Patients who are obese (weight more than 20% above ideal body weight) DIAGNOSTIC CRITERIA: WHO study group classification CO-MORBIDITIES: Not reported CO-MEDICATIONS: Not reported, except OHA |
| Interventions |
NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: India SETTING: Outpatient diabetic speciality clinic INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Diabecon D- 400 -DiabeconD-400 (containsGymnema sylvestre(Meshashringi), Eugenia jambolana (Jambu), Tinospora cordifolia, Pterocarpus marsupium, Ficus glomerata, Momordica charantia (Karela), Ocimum sanctum (Vishnu priya)), 2 tablets 3 times a day CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): matching placebo tablets, 2 tablets 3 times a day TREATMENT BEFORE STUDY: Oral hypoglycaemic Agents TITRATION PERIOD: Not reported Patients in both groups received similar diet instructions from the dietician |
| Outcomes |
PRIMARY OUTCOME(S) (as stated in the publication): Not stated SECONDARY OUTCOMES (as stated in the publication): Not Stated ADDITIONAL OUTCOMES: Fasting plasma glucose, postprandial glucose, glycosylated haemoglobin HbA1c, fasting insulin assay, stimulated insulin assay, fasting C-peptide, stimulated C-peptide, body weight, urea, creatinine, hemogram |
| Notes | 8 participants withdrew 3 from intervention group and 5 from placebo group |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Unclear risk | “Patients were randomly allocated to either group A or B” |
| Allocation concealment (selection bias) | Unclear risk | not known |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “The drug and placebo looked identical and were packed in different boxes coded A and B” |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | participants who withdrew were probably excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | nothing detected |
Poongothai 2002
| Methods | PARALLEL RANDOMIZED CONTROLLED CLINICAL TRIAL |
| Participants |
WHO PARTCIPATED: 40 type 2 diabetic patients aged between 30 to 60 years INCLUSIONCRITERIA: Adults within the age range of 30 - 60 years; secondary failure to OHA (patient had HbA1c levels > 8.5% even after supplementation of maximal dose of a combination of a sulphonylurea (15 mg glibenclamide or 160 mg gliclazide or 15 mg glipizide) and metformin 1500 mg/day) EXCLUSIONCRITERIA: Patientswith ketosis, diabetes related complications, hepatic or renal disease, pancreatitis, cardiac problems, uncontrolled hypertension,malnutrition and severe immune deficiency DIAGNOSTIC CRITERIA: Not reported CO-MORBIDITIES: Not reported CO-MEDICATIONS: Not reported |
| Interventions |
NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: India SETTING: Outpatient Diabetic Speciality Clinic INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Hyponidd - a herb-mineral formulation that has 12 blended ingredients including Vijaysar (Pterocarpus marsupium), Gurmar (Gymneme Sylvestre), Jambu Beej (syzygium Cumine), Amla (Emblica Officianale), Haldi (Gurcuma Longa), Neem (Melia Azadirachta), Trivang Bhasma and Shilajit; 2 tablets three times daily for 12 weeks CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Identical looking placebo- 2 tablets three times a day for 12 weeks TREATMENT BEFORE STUDY: Not Reported TITRATION PERIOD: Not Reported |
| Outcomes |
PRIMARY OUTCOME(S) (as stated in the publication): Not stated SECONDARY OUTCOMES (as stated in the publication): Not Stated ADDITIONAL OUTCOMES: Fasting and post prandial plasma glucose, glycosylated haemoglobin, insulin measurements, C-peptide assays and lipid profile |
| Notes | 8 participants withdrew 4 from each group |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Unclear risk | “Patients were randomly allocated to either group A or B” |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes |
Low risk | “the drug and the placebo looked identical and were placed in different boxes coded A and B respectively” |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | drop-outs excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | nothing detected |
Shekhar 2002
| Methods | PARALLEL RANDOMIZED CONTROLLED CLINICAL TRIAL: Y(see notes) |
| Participants |
WHO PARTCIPATED: 79 Malaysians with type 2 diabetes, 39 participants treated with Cogent DB and 40 controls INCLUSION CRITERIA: Adults aged between 30 and 70 years; diagnosed as type 2 diabetes mellitus with fasting blood glucose (FBG) > 126 mg and random blood glucose (RBG) > 200 mg EXCLUSIONCRITERIA: Participants taking insulin; diastolic blood pressure >100mg Hg; obesity (BMI > 30), Cushing’s syndrome; debilitating illness; significant neurologic or psychiatric illness; taking medications that may affect their mental status; glaucoma; pregnant women; and participation in other clinical trials within the preceding month DIAGNOSTIC CRITERIA: Not Stated CO-MORBIDITIES: Not Stated CO-MEDICATIONS: Not stated |
| Interventions |
NUMBER OF STUDY CENTRES: 2 COUNTRY/ LOCATION: Malaysia SETTING: Two major peripheral clinics in the Klang Valley, Malaysia INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Cogent db - a proprietary Ayurvedic drug (Cybele Herbal Laboratories [PVT] Ltd., Kochi, Kerala State, India), each tablet of which contains the following: Azadirachta indica (3.0 gm) , Phyllanthus embilica (0.7 g), Circuma longa (0.8 g),Trigonella foenum graecum linn (0.1 g), Syzygium cumini (2.0 g), Tribulus terrestris (1.0 g), Terminalia bellirica (0.7 g) , Terminalla chebula (0.7 g), and Routula aquatica (1.0 g). 2 tablets three times daily after each meal was given in addition to the regular allopathic drugs CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Controls received antidiabetic drugs alone. The allopathic drug regimen remained stable during trial. All participants were required to adhere to a prescribed diet and daily physical exercise TREATMENT BEFORE STUDY: Oral hypoglycemic drugs TITRATION PERIOD: Not stated |
| Outcomes |
PRIMARY OUTCOME(S) (as stated in the publication): Not Stated SECONDARY OUTCOMES (as stated in the publication): Not Stated ADDITIONAL OUTCOMES: Clinical improvement, blood glucose control, glycosylated haemoglobin (Hb A1c), seruminsulin, and C-peptide levels. Safety and tolerability of Cogent db was measured by recording all adverse events, laboratory investigations, including hematologic tests, liver enzymes, urea, and creatinine |
| Notes | 19 participants withdrew from study included 15 non Indian origin participants, who dropped out due to lack of belief in Ayurveda Under methods it is stated that “Each subject was assigned to the treatment or control group by a standard randomisation process”, though in the abstract it is mentioned as a “non-randomized study” |
| Risk of bias | ||
|---|---|---|
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) |
Unclear risk | “Each subjectwas assigned to the treatment or control group by a standard randomisation process” |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes |
High risk | controls received usual care |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | drop-outs excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | no protocol available |
| Other bias | Low risk | nothing detected |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Agrawal 1996 | Duration of study 4 weeks |
| Akhtar 2010 | Duration of study 3 weeks |
| Altschuler 2007 | Study of single herb or botanical agent |
| Anderson 1999 | Study of single herb or botanical agent |
| Aro 1981 | Study of single herb or botanical agent |
| Bandara 2009 | Study duration was 4 weeks |
| Blevins 2007 | Study of single herb or botanical agent |
| Cohen 1980 | Study of single herb or botanical agent |
| Crawford 2009 | Study of single herb or botanical agent |
| Faizal 2009 | Non-randomised study |
| Fuessl 1987 | Cross-over trial, participants received active treatment for 4 weeks |
| Gupta 2001 | Study of single herb or botanical agent |
| Holman 1987 | Study of single herb or botanical agent |
| ICMR Study Group 2005 | Study of single herb or botanical agent |
| John 2003 | Cochrane review already published on Mormodica charantia |
| Khan 1980 | Duration of treatment 6 weeks |
| Khan 2003 | Duration of treatment 40 days |
| Krarup 1980 | Two week cross-over study |
| Kuriyan 2008 | Study of single herb or botanical agent |
| Lalor 1990 | Study of single herb or botanical agent |
| Maji 1995 | Not randomised |
| Mang 2006 | Study of single herb or botanical agent |
| Niemi 1988 | Study of single herb or botanical agent |
| Sadhukhan 1994 | Two day biochemical study |
| Serraclara 1998 | One month treatment period |
| Stokholm 1981 | One week treatment period |
| Upadhyay 2004 | Open non-randomised study |
| Uusitupa 1984 | Study of single herb or botanical agent |
| Uusitupa 1989 | Study of single herb or botanical agent |
| Vuorinen-Markkola 1992 | Six weeks treatment period |
| Yadav 2001 Six | weeks treatment period |
| Ziai 2005 | Study of single herb or botanical agent |
Comparison 1.
Effects of Ayurvedic treatments on HbA1c
Comparison 2.
Effects of Ayurvedic treatments on fasting blood sugar
Comparison 3.
Effects of Ayurvedic treatments on post prandial blood sugar
Comparison 4.
Effects of Ayurvedic treatments on insulin levels at end of study
Comparison 5.
Effects of Ayurvedic treatments on C-peptide levels at end of study
Comparison 6.
Effects of Ayurvedic treatments on lipid profile (end of study)
Analysis 1.1.
Comparison 1 Effects of Ayurvedic treatments on HbA1c, Outcome 1 Proprietary herbal mixtures - HbA1c at end of study.
Analysis 1.2.
Comparison 1 Effects of Ayurvedic treatments on HbA1c, Outcome 2 Proprietary herbal mixtures - HbA1c change.
Analysis 2.1.
Comparison 2 Effects of Ayurvedic treatments on fasting blood sugar, Outcome 1 Fasting blood sugar at end of study.
Analysis 2.2.
Comparison 2 Effects of Ayurvedic treatments on fasting blood sugar, Outcome 2 Change in fasting blood sugar at end of study.
Analysis 3.1.
Comparison 3 Effects of Ayurvedic treatments on post prandial blood sugar, Outcome 1 Post prandial blood sugar at end of study.
Analysis 4.1.
Comparison 4 Effects of Ayurvedic treatments on insulin levels at end of study, Outcome 1 Fasting insulin levels at end of study.
Analysis 4.2.
Comparison 4 Effects of Ayurvedic treatments on insulin levels at end of study, Outcome 2 Stimulated insulin levels at end of study.
Analysis 5.1.
Comparison 5 Effects of Ayurvedic treatments on C-peptide levels at end of study, Outcome 1 Fasting C-peptide at end of study.
Analysis 5.2.
Comparison 5 Effects of Ayurvedic treatments on C-peptide levels at end of study, Outcome 2 Stimulated C-peptide at end of study.
Analysis 6.1.
Comparison 6 Effects of Ayurvedic treatments on lipid profile (end of study), Outcome 1 Total cholesterol.
Analysis 6.2.
Comparison 6 Effects of Ayurvedic treatments on lipid profile (end of study), Outcome 2 HDL-cholesterol.
Analysis 6.3.
Comparison 6 Effects of Ayurvedic treatments on lipid profile (end of study), Outcome 3 LDLcholesterol.
Analysis 6.4.
Comparison 6 Effects of Ayurvedic treatments on lipid profile (end of study), Outcome 4 Triglycerides.
ACKNOWLEDGEMENTS
Dr. Bhima Bhat, chief Ayurvedic physician, Holy Family Hospital, New Delhi and Dr. V Nagaraju (MD Ayurveda) chief Ayurvedic physician, Ayurvedic dispensary, Neyveli, Tamilnadu for their assistance in study selection.
This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM). The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
APPENDICES
Appendix 1.
Search strategies
| Search terms |
|---|
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) or asterisk (*) stand for any character(s); the question mark (?) substitutes one or no characters; ab = abstract; adj = adjacent; ot = original title; pt = publication type; rn = Registry number or Enzyme Commission number; sh = MeSH; ti = title; tw = text word |
| The Cochrane Library: |
| #1 MeSH descriptor Diabetes Mellitus explode all trees |
| #2 (diabet*) |
| #3 (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D) |
| #4 (non insulin* depend* or non insulin depend* or non insulin?depend* or non insulin?depend) |
| #5 (insulin* depend* or insulin?depend*) |
| #6 (#1 OR #2 OR #3 OR #4 OR #5) |
| #7 MeSH descriptor Diabetes Insipidus explode all trees |
| #8 (diabet* insipidus) |
| #9 (#7 OR #8) |
| #10 (#6 AND NOT #9) |
| #11 MeSH descriptor Medicine, Ayurvedic explode all trees |
| #12 MeSH descriptor Medicine, Traditional explode all trees |
| #13 (medicine near6 siddha) |
| #14 (medicine near6 Hindu) |
| #15 (medicine near6 Indian) |
| #16 MeSH descriptor Plants, Medicinal explode all trees with qualifier: TU |
| #17 MeSH descriptor Plant Extracts explode all trees with qualifier: TU |
| #18 MeSH descriptor Plants explode all trees with qualifier: TU |
| #19 MeSH descriptor Phytotherapy explode all trees |
| #20 MeSH descriptor Momordica charantia explode all trees |
| #21 MeSH descriptor Trigonella explode all trees |
| #22 (coccinea indica or pancreas tonic* or momordica charantia or Trigonella foenum graecum) |
| #23 (ayurvedic near6 diet*) |
| #24 (ayurvedic near6 herb*) |
| #25 (ayurvedic near6 extract*) |
| #26 (ayurvedic near6 plant*) |
| #27 (ayurvedic near6 preparation*) |
| #28 (ayurvedic near6 supplement*) |
| #29 (ayurvedic near6 therap*) |
| #30 (ayurvedic near6 intervention*) |
| #31 (ayurvedic near6 medicin*) |
| #32 (ayurvedic near6 drug*) |
| #33 (plant* near6 extract*) |
| #34 (plant* near6 therap*) |
| #35 (plant* near6 intervention*) |
| #36 (herb* near6 extract*) |
| #37 (herb* near6 therap*) |
| #38 (herb* near6 intervention*) |
| #39 (ethnobotan* or ethno botan* or ethnopharmacolog* or ethno pharmacolog*) |
| #40 (phytotherap* or phyto therap*) |
| #41 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR # 25 OR #26 OR #27 OR #28 OR #29 OR #30) |
| #42 (#31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40) |
| #43 (#41 OR #42) |
| #44 (#10 AND #43) |
| #45 MeSH descriptor Medicine, Chinese Traditional explode all trees |
| #46 MeSH descriptor Drugs, Chinese Herbal explode all trees |
| #47 (Chinese medicin*) |
| #48 (#45 OR #46 OR #47) |
| #49 (#44 AND NOT #48) |
| MEDLINE: |
| 1. exp Medicine, Ayurvedic/ |
| 2. *Medicine, Traditional/ |
| 3. exp Complementary medicine/tu [Therapeutic use] |
| 4. (medicine adj6 (siddha or Hindu or Indian or traditional)).tw,ot. |
| 5. ((complementary or alternative) adj3 therap*).tw,ot. |
| 6. exp Plants, Medicinal/tu [Therapeutic use] |
| 7. exp Plant Extracts/tu [Therapeutic use] |
| 8. exp Plants/tu [Therapeutic use] |
| 9. exp Ethnobotany/ |
| 10. exp Ethnopharmacology/ |
| 11. *Phytotherapy/ |
| 12. exp Momordica charantia/ |
| 13. exp Trigonella/ |
| 14. (coccinea indica or pancreas tonic* or momordica charantia or Trigonella foenum greacum).tw,ot. |
| 15. (ayurvedic adj6 (diet* or herb* or extract* or plant* or preparation* or supplement*)).tw,ot. |
| 16. (ayurvedic adj6 (therap* or intervention* or medicin* or drug*)).tw,ot. |
| 17. ((plant* or herb*) adj6 (extract* or therap* or intervention*)).tw,ot. |
| 18. (ethnobotan* or ethno botan* or ethnopharmacolog* or ethno pharmacolog*).tw,ot. |
| 19. (phytotherap* or phyto therap*).tw,ot. |
| 20. or/1-19 |
| 21. exp Drugs, Chinese Herbal/ or exp Medicine, Chinese Traditional/ |
| 22. (Chinese adj6 herbal medicin*).tw,ot. |
| 23. 21 or 22 |
| 24. 20 not 23 |
| 25. exp Diabetes Mellitus/ |
| 26. diabet$.tw,ot. |
| 27. (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D).tw,ot. |
| 28. (non insulin$ depend$ or non insulin$ depend$ or non insulin?depend$ or non insulin?depend$).tw,ot. |
| 29. (insulin$ depend$ or insulin?depend$).tw,ot. |
| 30. or/25-29 |
| 31. exp Diabetes Insipidus/ |
| 32. diabet$ insipidus.tw,ot. |
| 33. 31 or 32 |
| 34. 30 not 33 |
| 35. randomised controlled trial.pt. |
| 36. controlled clinical trial.pt. |
| 37. randomi?ed.ab. |
| 38. placebo.ab. |
| 39. clinical trials as topic.sh. |
| 40. randomly.ab. |
| 41. trial.ti. |
| 42. or/35-41 |
| 43. Meta-analysis.pt. |
| 44. exp Technology Assessment, Biomedical/ |
| 45. exp Meta-analysis/ |
| 46. exp Meta-analysis as topic/ |
| 47. hta.tw,ot. |
| 48. (health technology adj6 assessment$).tw,ot. |
| 49. (meta analy$ or metaanaly$ or meta?analy$).tw,ot. |
| 50. ((review$ or search$) adj10 (literature$ or medical database$ or MEDLINE or PubMed or EMBASE or Cochrane or CINAHL or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. |
| 51. or/43-50 |
| 52. (comment or editorial or historical-article).pt. |
| 53. 51 not 52 |
| 54. 42 or 53 |
| 55. 24 and 34 and 54 |
| 56. (animals not (animals and humans)).sh. |
| 57. 55 not 56 |
| EMBASE: |
| 1. exp Ayurveda/ |
| 2. *traditional medicine/ |
| 3. *alternative medicine/ |
| 4. (medicin* adj6 (siddha or Hindu or Indian)).tw,ot. |
| 5. *medicinal plant/ |
| 6. *plant extract/ |
| 7. exp ethnopharmacology/ or exp ethnobotany/ |
| 8. exp phytotherapy/ |
| 9. exp Momordica charantia/ or exp Momordica charantia extract/ |
| 10. exp fenugreek/ |
| 11. ayurvedic drug/ or exp coccinea indica/ or exp Trigonella foenum graecum extract/ or exp Trigonella foenum graecum extract/ |
| 12. (coccinea indica or pancreas tonic* or momordica charantia or Trigonella foenum graecum).tw,ot. |
| 13. (ayurvedic adj6 (diet* or herb* or extract* or plant* or preparation* or supplement*)).tw,ot. |
| 14. (ayurvedic adj6 (therap* or intervention* or medicine* or drug*)).tw,ot. |
| 15. ((plant* or herb*) adj6 (extract* or therap* or intervention*)).tw,ot. |
| 16. (ethnobotan* or ethno botan* or ethnopharmacolog* or ethno pharmacolog*).tw,ot. |
| 17. (phytotherap* or phyto therap*).tw,ot. |
| 18. or/1-17 |
| 19. exp Chinese herb/ or exp Chinese drug/ or exp Chinese medicine/ |
| 20. (Chinese adj2 (herbal and medicin*)).tw,ot. |
| 21. 19 or 20 |
| 22. 18 not 21 |
| 23. exp Diabetes Mellitus/ |
| 24. diabet$.tw,ot. |
| 25. (non insulin* depend* or non insulin* depend* or non insulin?depend* or non insulin?depend*).tw,ot. |
| 26. (insulin* depend* or insulin?depend*).tw,ot. |
| 27. (IDDM or NIDDM or MODY or T1DM or T2DM or T1d or T2D).tw,ot. |
| 28. or/23-27 |
| 29. exp Diabetes Insipidus/ |
| 30. diabet* insipidus.tw,ot. |
| 31. 29 or 30 |
| 32. 28 not 31 |
| 33. exp Randomized Controlled Trial/ |
| 34. exp Controlled Clinical Trial/ |
| 35. exp Clinical Trial/ |
| 36. exp Comparative Study/ |
| 37. exp Drug comparison/ |
| 38. exp Randomization/ |
| 39. exp Crossover procedure/ |
| 40. exp Double blind procedure/ |
| 41. exp Single blind procedure/ |
| 42. exp Placebo/ |
| 43. exp Prospective Study/ |
| 44. ((clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. |
| 45. (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. |
| 46. ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. |
| 47. (cross over or crossover).ab,ti. |
| 48. or/33-47 |
| 49. exp meta analysis/ |
| 50. (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. |
| 51. ((review$ or search$) adj10 (literature$ or medical database$ or MEDLINE or PubMed or EMBASE or Cochrane or CINAHL or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. |
| 52. exp Literature/ |
| 53. exp Biomedical Technology Assessment/ |
| 54. hta.tw,ot. |
| 55. (health technology adj6 assessment$).tw,ot. |
| 56. or/49-55 |
| 57. (comment or editorial or historical-article).pt. |
| 58. 56 not 57 |
| 59. 48 or 58 |
| 60. 22 and 32 and 59 |
| 61. limit 60 to human |
| AMED: |
| 1. exp clinical trials/ |
| 2. exp randomised controlled trials/ |
| 3. exp double-blind method/ or |
| 4. exp random allocation/ |
| 5. randomised controlled trial.pt. |
| 6. clinical trial.pt. |
| 7. controlled clinical trial.pt. |
| 8. clinic$ adj4 trial$).mp. or |
| 9. (random$ adj5 (assign$ or allocat$ or assort$)).mp. |
| 10. (randomi$ adj5 control$ adj5 trial$).mp. |
| 11. (crossover or cross-over).mp. |
| 12.((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).mp.) |
| 13. OR/1-12 |
| 14. exp Diabetes Mellitus/ |
| 15 13 AND 14 |
| 16. (ayrved$.mp. or ayurved$.mp. or ayruvedic.mp. or exp medicine ayurvedic/) |
| 17. 15 AND 16 |
| Database of randomized trials from South Asia and OpenSigle: |
| Ayurved* |
Appendix 2.
Description of interventions
| Study ID Characteris- tic |
Agrawal 2002 | Dubey 1993 | Elder 2006 | Hsia 2004 | Mohan 1998 | Poongothai 2002 | Shekhar 2002 |
|---|---|---|---|---|---|---|---|
| Intervention (s) |
Inolter®, oral | Diabecon® (D-400), oral, BD, 4 tablets/ day |
MA471 (mix- ture of herbs) + meditation, diet, exercise, MA471 oral |
Pancreas tonic (herbal mixture), oral, TDS, 6 cap- sules/ day |
Diabecon® (D-400) , oral, TDS, 6 tablets/day |
Hyponidd ®, oral, TDS, 6 tablets/day |
Cogent db®, oral, TDS, 6 tablets/day |
| Control(s) | Placebo, oral | Placebo, oral, BD, 4 tablets/ day |
No additional med- ication (usual care + diabetes education) |
Placebo, oral, TDS, 6 cap- sules/ day |
Placebo, oral, TDS, 6 tablets/day |
Placebo, oral, TDS, 6 tablets/day |
No additional intervention |
BD: two times daily; TDS: three times daily
Appendix 3.
Baseline characteristics
| Study ID Characteris- tic |
Agrawal 2002 | Dubey 1993 | Elder 2006 | Hsia 2004 | Mohan 1998 | Poongothai 2002 | Shekhar 2002 |
|---|---|---|---|---|---|---|---|
| Interven- tion(s) & con- trol(s) |
I: Inolter® C: Placebo |
I: Diabecon® (D-400) C: Placebo |
I: MA471 (mix- ture of herbs) , meditation, diet, exercise C: None (usual care only) |
I: Pancreas tonic C: Placebo |
I: Diabecon® (D-400) C: Placebo |
I: Hyponidd® C: Placebo |
I: Cogent DB ® C: No additional treatment |
| Participating population |
Newly diag- nosed patients with type 2 di- abetes |
Adults with persis- tent postpran- dial hypergly- caemia |
Newly diag- nosed people with presymp- tomatic type 2 diabetes |
Adults with type 2 di- abetes |
Adults with type 2 diabetes mellitus |
Adults with type 2 diabetes mellitus |
Adults with type 2 diabetes mellitus |
| Country / Lo- cation |
India | India | USA | USA | India | India | Malaysia |
| Sex [female%] | I: 27 C: 20 |
- | I: 50 C: 67 |
I: 74 C: 69 |
- | I: 38 C: 31 |
I: 41 C: 27 |
| Age [mean years (SD)] |
I: 45 (11.2) C: 47.2 (2.1) |
More than 30 | I: 53.7 (8.4) C: 53.3 (12) |
I: 47.6 (11.5) C: 47.4 (7.0) |
More than 30 | I: 53 (9) C: 54 (10) |
I: 44 (9.9) C: 35 (9.8) |
| Duration of disease [mean years (SD)] |
Newly diagnosed |
- | Newly diagnosed |
I: 3.9 (3.2) C: 4.3 (3.1) |
10-15 years | - | I: 8.9 (2.3) C: 9.1 (2.6) |
| Ethnic groups [%] |
Asian Indians | Asian Indians | Ameri- cans (ethnicity not reported) |
Ameri- cans(ethnicity not reported) |
Asian Indians | Asian Indians | I: Indians 69. 2%; Malay/ Chinese 30. 8% C: Indians 55%, Malay/ Chinese 45% |
| Duration of intervention |
3 months | 6 months | 6 months | 3 months | 6 months | 12 weeks | 3 months |
“-” denotes not reported
C: control; F: female; I: intervention; M: male
Appendix 4.
Matrix of study endpoints
| Study ID Characteris- tic |
Agrawal 2002 | Dubey 1993 | Elder 2006 | Hsia 2004 | Mohan 1998 | Poongothai 2002 | Shekhar 2002 |
|---|---|---|---|---|---|---|---|
| Interven- tion(s) & con- trol(s) |
I: Inolter® C: Placebo |
I: Diabecon® (D-400) C: Placebo |
I: MA471 (mix- ture of herbs) , meditation, diet, exercise CNone (usual care only): |
I: Pancreas Tonic C: Placebo |
I:Diabecon® (D-400) C: Placebo |
I: Hyponidd® C: Placebo |
I: Cogent DB ® C: No additional treatment |
| Primary1 end- point(s) |
- | - | HbA1c, FBS | HbA1c | - | - | - |
| Secondary2 endpoint(s) |
- | - | Lipids, body weight, BP, pulse |
BP, FBS, lipids and lipoprotein, BMI, body composition, complete blood count, ECG, blood biochemistry, insulin- modified fre- quently- sampled in- travenous glu- cose tolerance test, quality of life assessment by modified SF36 |
- | - | - |
| Other3 endpoint(s) | FBS, HbA1c, Lipid profile |
FBS, PPBS, Body weight, liver, kidney, haemopoietic function or ECG |
- | - | FBS, PPBS, HbA1c, insulin and C-peptide (fasting and stimu- lated), body weight, urea, creatinine, haemogram |
Weight, BP, pulse, ECG, FBS, PPBS, HbA1c, insulin and C-peptide as- says, lipid profile, SGOT, SGPT urea, creatinine |
Symp- toms, urinary glucose and pro- tein, complete blood counts, FBS, PPBS, urea, creatinine, al- bumin, alka- line phosphatase, LFT, HbA1c and total gly- cated haemo- globin, fasting and stimulated in- sulin assay and C-peptide assay, lipid profile |
as stated in the publication;
as stated in the publication;
not stated as primary or secondary endpoint(s) in the publication
“-” denotes not reported
BP: blood pressure; BMI: body mass index; C: control; ECG: electrocardiogram; FBS: fasting blood sugar; HbA1c: glycosylated haemoglobin A1c; HDL: high density lipoprotein cholesterol; I: intervention; PPBS: postprandial blood sugar
Appendix 5.
Adverse events
| Study ID Characteris- tic |
Agrawal 2002 | Dubey 1993 | Elder 2006 | Hsia 2004 | Mohan 1998 | Poongothai 2002 | Shekhar 2002 |
|---|---|---|---|---|---|---|---|
| Interven- tion(s) & con- trol(s) |
I: Inolter® C: Placebo |
I:Diabecon® (D-400) C: Placebo |
I: MA471 (mix- ture of herbs) , meditation, diet, exercise C: None (usual care only) |
I: Pancreas tonic C: Placebo |
I:Diabecon® (D-400) C: Placebo |
I: Hyponidd® C: Placebo |
I: Cogent DB ® C: No additional treatment |
| Diabetes related deaths [n] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
I: 0/27 C: 0/28 T: 0/55 |
I: 0/31 C: 0/16 T: 0/47 |
I: 0/20 C: 0/20 T: 0/40 |
I: 0/20 C: 0/20 T: 0/40 |
I: 0/30 C: 0/30 T: 0/60 |
| Death from any cause [n] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
I: 0/27 C: 0/28 T: 0/55 |
I: 0/31 C: 0/16 T: 0/47 |
I: 0/20 C: 0/20 T: 0/40 |
I: 0/20 C: 0/20 T: 0/40 |
I: 0/30 C: 0/30 T: 0/60 |
| Adverse events [n/N] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
- | I: 6/31 C: 5/16 T: 11/47 |
I: 2/20 C: 3/20 T: 5/40 |
I: - C: - T: 3/40 |
I: 0/30 C: 0/30 T: 0/60 |
| Serious ad- verse events [n /N] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
I: 0/27 C: 0/28 T: 0/55 |
I: 0/31 C: 0/16 T: 0/47 |
I: 2/20 C: 3/20 T: 5/40 |
I: - C: - T: 3/40 |
I: 0/30 C: 0/30 T: 0/60 |
| Drop-outs due to adverse events [n /N] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
I: 0/27 C: 0/28 T: 0/55 |
I: 0/31 C: - T: - |
I: 2/20 C: 3/20 T: 5/40 |
I: - C: - T: 3/40 |
I: 0/30 C: 0/30 T: 0/60 |
| Hospitalisa- tion due to ad- verse event [n / N] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
- | I: 0/31 C: 0/16 T: 0/47 |
- | - | I: 0/30 C: 0/30 T: 0/60 |
| Out-patient treatment [n / N] |
I: 0/30 C: 0/30 T: 0/60 |
- | - | I: 0/31 C: 0/16 T: 0/47 |
- | - | - |
| Hypo- glycemic episodes [n / N] |
I: 0/30 C: 0/30 T: 0/60 |
- | - | I:1/31 C:0/16 T:1/47 |
- | - | - |
| Severe hypo- glycaemic episodes [n / N] |
I: 0/30 C: 0/30 T: 0/60 |
- | - | I:0/31 C:0/16 T:0/47 |
- | - | - |
| Definition of severe hy- poglycaemia |
- | - | - | - | - | - | - |
| Nocturnal hy- poglycaemic episodes [n / N] |
I: 0/30 C: 0/30 T: 0/60 |
- | - | - | - | - | - |
| Hematolog- ical toxicity [n /N] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
I: 0/27 C: 0/28 T: 0/55 |
I: 0/31 C: 0/16 T: 0/47 |
I: 0/17 C: 0/15 T: 0/32 |
- | I: 0/30 C: 0/30 T: 0/60 |
| Liver toxicity [n/N] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
I: 0/27 C: 0/28 T: 0/55 |
I: 0/31 C:0/16 T: 0/47 |
- | I: 1/20 C: 0/20 T: 1/40 |
I: 0/30 C: 0/30 T: 0/60 |
| Hypersen- sitivity or drug allergy [n / N] |
I: 0/30 C: 0/30 T: 0/60 |
I: 0/14 C: 0/14 T: 0/28 |
- | I: 0/31 C: 0/16 T: 0/47 |
I: 1/20 C: 0/20 T: 1/40 |
- | - |
| Gastrointesti- nal side effects [n/N] |
I: 0/30 C: 0/30 T: 0/60 |
- | - | - | I: 1/20 C: 0/20 T: 1/40 |
- | - |
| Renal toxicity [n/N] |
- | - | - | I: 0/31 C: 0/16 T: 0/47 |
I: 0/20 C: 0/20 T: 0/40 |
I: 0/20 C: 0/20 T: 0/40 |
I: 0/30 C: 0/30 T: 0/60 |
| Other side ef- fects [n / N] |
I: 0/30 C: 0/30 T: 0/60 |
- | - | - | - | - | - |
Footnotes “-” denotes not reported
C: control; I: intervention; T: total
Footnotes
Indicates the major publication for the study
REFERENCES
References to studies included in this review
- Agrawal RP, Sharma A, Dua AS, Chandershekhar, Kochar DK, Kothari RP. A randomized placebo controlled trial of Inolter (herbal product) in the treatment of type 2 diabetes. Journal of the Association of Physicians of India. 2002;50:391–3. [PubMed] [Google Scholar]
- Dubey GP, Agrawal A, Singh A. Evaluation of Diabecon (D-400) An Indigenous Herbal Preparation, in Diabetes Mellitus. Indian Journal of Internal Medicine. 1993;6:183–6. [Google Scholar]
- Elder C, Aickin M, Bauer V, Cairns J, Vuckovic N. Randomized trial of a whole-system ayurvedic protocol for type 2 diabetes. Alternative Therapies in Health and Medicine. 2006;12(5):24–30. [PubMed] [Google Scholar]
- Hsia SH, Bazargan M, Davidson MB. Effect of Pancreas Tonic (an ayurvedic herbal supplement) in type 2 diabetes mellitus. Metabolism. 2004;53:1166–73. doi: 10.1016/j.metabol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hsia SH, Bazargan M, Davidson MB. Effect of Pancreas Tonic (an ayurvedic herbal supplement) in type 2 diabetes mellitus. Metabolism. 2004;53:1166–73. doi: 10.1016/j.metabol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hsia SH, Bazargan M, Davidson MB. Effect of Pancreas Tonic (an ayurvedic herbal supplement) in type 2 diabetes mellitus. Metabolism. 2004;53:1166–73. doi: 10.1016/j.metabol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mohan V. Evaluation of Diabecon (D-400) as an Antidiabetic Agent - A Double-Blind Placebo-Controlled Trial in NIDDM Patients with Secondary Failure to Oral Drugs. Indian Journal of Clinical Practice. 1998;8(9):18. [Google Scholar]
- Poongothai S, Karkuzhali K, Sharadha J, Deepa R, Mohan V. Evaluation of Safety and Efficacy of Hyponidd, an Ayurvedic Compound: A double blind, placebo controlled study in Type 2 Diabetic patients with secondary failure to oral drugs. International Journal of Diabetes in Developing Countries. 2002;22:19–24. [Google Scholar]
- Shekhar KC, Achike FI, Kaur G, Kumar P, Hashim R. A preliminary evaluation of the efficacy and safety of Cogent db (an ayurvedic drug) in the glycemic control of patients with type 2-diabetes. Journal of Alternative and Complementary Medicine. 2002;8:445–57. doi: 10.1089/107555302760253649. [see comment] References to studies excluded from this review
- Agrawal P, Rai V, Singh RB. Randomized placebo-controlled, single blind trial of holy basil leaves in patients with non insulin-dependent diabetes mellitus. International Journal of Clinical Pharmacology and Therapeutics. 1996;34:406–9. [PubMed] [Google Scholar]
- Akhtar MS, Naeem F, Muhammad F, Bhatty N. Effect of Butea monosperma (Palas papra) fruit on blood glucose and lipid profiles of normal and diabetic human volunteers. Pharmacology online. 2010;1:614–623. 623. [Google Scholar]
- Altschuler JA, Casella SJ, MacKenzie TA, Curtis KM. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care. 2007;30:813–6. doi: 10.2337/dc06-1871. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Allgood LD, Turner J, Oeltgen PR, Daggy BP. Effects of psyllium on glucose and serum lipid responses in men with type 2 diabetes and hypercholesterolemia. American Journal of Clinical Nutrition. 1999;70:466–73. doi: 10.1093/ajcn/70.4.466. [DOI] [PubMed] [Google Scholar]
- Aro A, Uusitupa M, Voutilainen E, Hersio K, Korhonen T, Siitonen O. Improved diabetic control and hypocholesterolaemic effect induced by long-term dietary supplementation with guar gum in type 2 (insulin-independent) diabetes. Diabetologia. 1981;21:29–33. doi: 10.1007/BF03216219. [DOI] [PubMed] [Google Scholar]
- Bandara T, Rokeya B, Khan S, Ali L, Ekanayake S, Jansz ER, et al. Effects of Gymnema lactiferum leaves on glycemic and lipidemic status in type 2 diabetes participants. Bangladesh Journal of Pharmacology. 2009;4(2):92–5. [Google Scholar]
- Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care. 2007;30:2236–7. doi: 10.2337/dc07-0098. 2007, 30: 2236-2237. [DOI] [PubMed] [Google Scholar]
- Cohen M, Leong VW, Salmon E, Martin FI. Role of guar and dietary fibre in the management of diabetes mellitus. The Medical journal of Australia. 1980;1:59–61. doi: 10.5694/j.1326-5377.1980.tb134625.x. [DOI] [PubMed] [Google Scholar]
- Crawford P. Effectiveness of Cinnamon for LoweringHemoglobin A1C in Patients with Type 2 Diabetes:A Randomized, Controlled Trial. Journal of the American Board of Family Medicine. 2009;22:507–512. doi: 10.3122/jabfm.2009.05.080093. [DOI] [PubMed] [Google Scholar]
- Faizal P, Suresh S, Satheesh KR, Augusti KT. A study on the hypoglycemic and hypolipidemic effects of an ayurvedic drug Rajanyamalakadi in diabetic patients. Indian Journal of Clinical Biochemistry. 2009;24(1):82–7. doi: 10.1007/s12291-009-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuessl HS, Williams G, Adrian TE, Bloom SR. Guar sprinkled on food: effect on glycaemic control, plasma lipids and gut hormones in non-insulin dependent diabetic patients. Diabetic Medicine. 1987;4:463–8. doi: 10.1111/j.1464-5491.1987.tb00910.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Gupta R, Lal B. Effect of Trigonella foenumgraecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. Journal of the Association of Physicians of India. 2001;49:1057–61. [PubMed] [Google Scholar]
- Holman RR, Steemson J, Darling P, Turner RC. No glycemic benefit from guar administration in NIDDM. Diabetes Care. 1987;10:68–71. doi: 10.2337/diacare.10.1.68. [DOI] [PubMed] [Google Scholar]
- Hariharan RS, Venkataraman S, Sunitha P, Rajalakshmi S, Samal KC, Routray BM, et al. Efficacy of vijayasar (Pterocarpus marsupium) in the treatment of newly diagnosed patients with type 2 diabetes mellitus: a flexible dose double-blind multicenter randomized controlled trial. Diabetologia Croatica. 2005;34:13–20. [Google Scholar]
- John AJ, Cherian R, Subhash HS, Cherian AM. Evaluation of the efficacy of bitter gourd (momordica charantia) as an oral hypoglycemic agent--a randomized controlled clinical trial. AAPPO Journal. 2003;47:363–5. [PubMed] [Google Scholar]
- Khan AK, AKhtar S, Mahtab H. Treatment of diabetes mellitus with Coccinia indica. British Medical Journal. 1980;280:1044. doi: 10.1136/bmj.280.6220.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Safdar M, li Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–18. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- Krarup T, Sestoft L. Lack of effect of guar gum (Slocose) on blood glucose in insulin-treated diabetes mellitus. Ugeskrift for laeger. 1980;142:2979–81. [PubMed] [Google Scholar]
- Kuriyan R, Rajendran R, Bantwal G, Kurpad AV. Effect of supplementation of Coccinia cordifolia extract on newly detected diabetic patients. Diabetes Care. 2008;31:216–20. doi: 10.2337/dc07-1591. [DOI] [PubMed] [Google Scholar]
- Lalor BC, Bhatnagar D, Winocour PH, Ishola M, Arrol S, Brading M, et al. Placebo-controlled trial of the effects of guar gum and metformin on fasting blood glucose and serum lipids in obese, type 2 diabetic patients. Diabetic Medicine. 1990;7:242–5. doi: 10.1111/j.1464-5491.1990.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Maji D, Singh AK. Clinical trial of D-400, a herbo mineral preparation in diabetes mellitus. Journal of the Diabetic Association of India. 1995;35(1):1–4. [Google Scholar]
- Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, et al. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. European Journal of Clinical Investigation. 2006;36:340–4. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- Niemi MK, Keinänen-Kiukaanniemi SM, Salmela PI. Long-term effects of guar gum and microcrystalline cellulose on glycaemic control and serum lipids in type 2 diabetes. European journal of clinical pharmacology. 1988;34:427–9. doi: 10.1007/BF00542449. [DOI] [PubMed] [Google Scholar]
- Sadhukhan B, Roychowdhury U, Banerjee P, Sen S. Clinical evaluation of a herbal antidiabetic product. Journal of the Indian Medical Association. 1994;92(4):115–7. [PubMed] [Google Scholar]
- Serraclara A, Hawkins F, Pérez C, Domínguez E, Campillo JE, Torres MD. Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes research and clinical practice. 1998;39:19–22. doi: 10.1016/s0168-8227(97)00112-5. [DOI] [PubMed] [Google Scholar]
- Stokholm KH, Lauritsen KB, Larsen S. Reduced glycosuria during guar gum supplementation in non-insulin-dependent diabetics. A double-blind,randomised cross-over study. Danish medical bulletin. 1981;28:41–2. [PubMed] [Google Scholar]
- Upadhyay UM, Goyal RK. Efficacy of Enicostemma littorale in Type 2 Diabetic Patients. Phytotherapy Research. 2004;18:233–5. doi: 10.1002/ptr.1434. [DOI] [PubMed] [Google Scholar]
- Uusitupa M, Tuomilehto J, Karttunen P, Wolf E. Long term effects of guar gum on metabolic control, serum cholesterol and blood pressure levels in type 2 (non-insulin-dependent) diabetic patients with high blood pressure. Annals of clinical research. 1984;16(Suppl 43):126–31. [PubMed] [Google Scholar]
- Uusitupa M, Siitonen O, Savolainen K, Silvasti M, Penttilä I, Parviainen M. Metabolic and nutritional effects of long-term use of guar gum in the treatment of non insulin-dependent diabetes of poor metabolic control. The American journal of clinical nutrition. 1989;49:345–51. doi: 10.1093/ajcn/49.2.345. [DOI] [PubMed] [Google Scholar]
- Vuorinen-Markkola H, Sinisalo M, Koivisto VA. Guar gum in insulin dependent diabetes: effects on glycemic control and serum lipoproteins. The American journal of clinical nutrition. 1992;56:1056–60. doi: 10.1093/ajcn/56.6.1056. [DOI] [PubMed] [Google Scholar]
- Yadav RK, Mishra R, Chhipa RP, Audichya KC. Clinical Trial of an indigenous compound drug Nishaamalki in the management of Madhumeha vis-a-vis Diabetes Mellitus. Ancient Science of Life. 2001;21(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Ziai SA, Larijani B, Akhoondzadeh S, Fakhrzadeh H, Dastpak A, Bandarian F, et al. Psyllium decreased serum glucose and glycosylated hemoglobin significantly in diabetic outpatients. Journal of Ethnopharmacology. 2005;102:202–7. doi: 10.1016/j.jep.2005.06.042. [DOI] [PubMed] [Google Scholar]
Additional references
- American Diabetes Association Report on the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1999;22(Suppl 1):S5–19. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Healthcare Research and Quality Ayurvedic Interventions for Diabetes Mellitus: A Systematic Review. 2001 http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1.biblist.95806.
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Department of Ayurveda, Siddha, Unani and Homeopathy (AYUSH) Ayush in India. Department of Ayurveda, Siddha, Unani and Homeopathy (AYUSH), Ministry of Health & Family Welfare, Govt of India; 2007. Ministry of Health & Family Welfare, Govt of India. [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- Egede LE, Ye X, Zheng D, Silverstein MD. The Prevalence and Pattern of Complementary and Alternative Medicine Use in Individuals With Diabetes. Diabetes Care. 2002;25:324–9. doi: 10.2337/diacare.25.2.324. [DOI] [PubMed] [Google Scholar]
- Garrow D, Egede LE. Association Between Complementary and Alternative Medicine Use, Preventive Care Practices, and Use of Conventional Medical Services Among Adults With Diabetes. Diabetes Care. 2006;29:15–9. doi: 10.2337/diacare.29.01.06.dc05-1448. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. [updated February 2008];Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. (Version 5.0.0). 2008 Available from www.cochrane-handbook.org.
- Keen RW, Deacon AC, Delves HT, Moreton JA, Frost PG. Indian herbal remedies for diabetes as a cause of lead poisoning. Postgraduate Medical Journal. 1994;70:113–4. doi: 10.1136/pgmj.70.820.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulambil Padinjakara RN, Ashawesh K, Butt S, Nair R, Patel V. Herbal remedy for diabetes: two case reports. Experimental and Clinical Endocrinology and Diabetes. 2009;117:3–5. doi: 10.1055/s-0028-1085426. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. British Medical Journal. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database of Systematic Reviews. 2008;(Issue 2) doi: 10.1002/14651858.CD007170.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic and Meta-Analyses of Studies That Evaluate Interventions: Explanation and Elaboration. PLoS Med. 1999;6(7):1–28. doi: 10.1371/journal.pmed.1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay J, Marceau L. US public health and the 21st century: diabetes mellitus. Lancet. 2000;356(9231):757–61. doi: 10.1016/S0140-6736(00)02641-6. [DOI] [PubMed] [Google Scholar]
- McWhorter LS. Biological Complementary Therapies: A Focus on Botanical Products in Diabetes. Diabetes Spectrum. 2001;14:199–208. [Google Scholar]
- Ooi CP, Yassin Z, Hamid TA. Momordica charantia for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2010;(Issue 2) doi: 10.1002/14651858.CD007845.pub2. [DOI: 10.1002/ 14651858.CD007845.pub2] [DOI] [PubMed] [Google Scholar]
- Saxena A, Vikram NK. Role of selected Indian plants in management of type 2 diabetes: a review. Journal of Alternative and Complementary Medicine. 2004;10:369–78. doi: 10.1089/107555304323062365. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care; Meta-analysis in Context. BMJ Publishing Group; London: 2001. pp. 189–208. [Google Scholar]
- Subbarayappa BV. The roots of ancient medicine : an historical outline. Journal of Biosciences. 2001;26:135–44. doi: 10.1007/BF02703637. [MEDLINE: 11426049] [DOI] [PubMed] [Google Scholar]
- Upadhyay VP, Kamla P. Ayurvedic approach to diabetes mellitus and its management by indigenous resources. In: Bajaj JS, editor. Diabetes Mellitus in developing countries. Interprint; New Delhi: 1984. pp. 375–7. [Google Scholar]
- WHO Expert Committee on Diabetes Mellitus . Second report. WHO; Geneva: 1980. (Technical Report Series 646). [PubMed] [Google Scholar]
- WHO Expert Committee on Diabetes Mellitus (Technical Report Series 727).World Health Organization. 1985 [PubMed] [Google Scholar]
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- WHO Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation. 2006 [: ISBN 92 4 159493 4 (NLM classification: WK 810)ISBN 978 92 4 159493 6] [Google Scholar]
- Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes. Diabetes Care. 2003;26:1277–94. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]