Abstract
OBJECTIVES:
At present, there are conflicting data on the ability of echocardiographic parameters to predict the exercise-induced elevation of left ventricular (LV) filling pressure. The purpose of the present study was to validate the ratio of early diastolic transmitral (E) to mitral annular velocity (e′) obtained at peak exercise in its capacity to determine the exercise-induced elevation of pulmonary capillary wedge pressure (PCWP) and to reveal new noninvasive parameters with such capacity.
METHODS:
Sixty-one patients who had undergone heart transplantation with normal LV ejection fraction underwent simultaneous exercise echocardiography and right heart catheterization.
RESULTS:
In 50 patients with a normal PCWP at rest, exercise E/e′ ≥8.5 predicted exercise PCWP ≥25 mmHg with a sensitivity of 64.3% and a specificity of 84.2% (area under the curve [AUC]=0.74). A comparable or slightly better prediction was achieved by exercise E/peak systolic mitral annular velocity (s′) ≥11.0 (sensitivity 79.3%; specificity 57.9%; AUC=0.75) and exercise E/LV systolic longitudinal strain rate ≤−105 cm (sensitivity 78.9%; specificity 78.6%; AUC=0.87). Combined, exercise E/s′ and exercise E/e′ resulted in a trend toward a slightly more precise prediction (sensitivity 53.6%; specificity 89.5%; AUC=0.78) than did either variable alone.
CONCLUSIONS:
Exercise E/e′, used as a sole parameter, is not sufficiently precise to predict the exercise-induced elevation of PCWP. Exercise E/s′, E/LV systolic longitudinal strain rate or combinations of these parameters may represent further promising possibilities for predicting exercise PCWP elevation.
Keywords: Diastolic function, Exercise echocardiography, Pulmonary capillary wedge pressure
Heart failure with normal left ventricular ejection fraction (HFNEF) is frequently observed and is associated with a poor prognosis (1). One of the main abnormalities in HFNEF is an increase in the left ventricular filling pressure (LVFP), which is one of the criteria for the diagnosis of HFNEF (2). The current guidelines of the European Society of Cardiology (2), which define HFNEF based on resting hemodynamic values, do not allow the diagnosis of this syndrome in patients in whom symptoms and pathological measures are confined only to exercise. Recently, a significant proportion of patients with unexplained exertional dyspnea or fatigue and a normal left ventricular ejection fraction (LVEF) were found to manifest elevated LVFP only during exercise (3,4). Exercise echocardiography was initially demonstrated to reveal this isolated, exclusively exercise-induced LVFP elevation by measuring the ratio of peak early diastolic transmitral flow velocity (E) to peak early diastolic mitral annular velocity (e′) (3,5). However, some recently published trials did not support the possibility of noninvasively estimating the exercise-induced elevation of LVFP using the E/e′ ratio or other echocardiographic measures (6,7). Thus, it is unclear whether the E/e′ ratio or other conventional or Doppler echocardiographic variables can be used to noninvasively determine the LVFP during exercise. Under resting conditions, several reports have described the value of new echocardiographic parameters or indexes derived from strain or strain rate analysis in predicting LVFP elevation (8–12). In contrast to velocity parameters, strain and strain rate derived from two-dimensional speckle-tracking echocardiography (2D-STE) are not angle dependent, are less influenced by tethering and better reflect myocardial function (8). Other researchers demonstrated the utility of combining echocardiographic parameters (13) and the use of natriuretic peptides (14,15) in predicting LVFP elevation. However, such analyses performed under exercise and validated by invasively measured LVFP or its surrogates in patients at risk of HFNEF are lacking.
Thus, the aims of our study are: to determine whether the E/e′ ratio obtained at peak exercise can determine the exercise-induced elevation of pulmonary capillary wedge pressure (PCWP) in a cohort of patients after orthotopic heart transplantation with a normal LVEF; to identify and validate new echocardiographic parameters and indexes for their ability to predict the exercise-induced increase in PCWP; and to assess whether combinations of these parameters can improve the prediction of exercise-induced elevation of PCWP.
METHODS
Patient population
A total of 61 patients who had undergone orthotopic heart transplantation and were referred to St Anne’s Hospital (Brno, Czech Republic) for post-transplant cardiac examination were enrolled in the present study. All of these patients were systematically followed-up at this hospital in the long-term program of care for patients after heart transplantation. Regular follow-up visits included physical examination, 12-lead electrocardiography, blood testing, conventional trans-thoracic echocardiography and, mainly, myocardial biopsy. Coronary angiography was performed 12 months after transplantation and was repeated later if clinically indicated. The inclusion criteria of the present study were: a time interval ≥6 months after heart transplantation; a sinus rhythm on the electrocardiogram; no history of myocardial infarction or angina pectoris after heart transplantation; and a conventional transthoracic echocardiogram performed on admission of acceptable quality demonstrating a normal LVEF (≥50%), no significant pericardial effusion and no significant mitral regurgitation. All patients who fulfilled the inclusion criteria entered and completed the study protocol. Eighteen age- and sex-matched healthy volunteers without any medical history and not taking any medication served as controls. The present study complied with the Declaration of Helsinki and was approved by the ethics committee at St Anne’s Hospital. All patients provided informed consent to the investigators.
Study protocol
On the day of hospital admission, conventional echocardiography was performed to determine whether the patients fulfilled echocardiographic inclusion criteria. On the second day, after a 12 h overnight fast, routine blood tests were performed, including a complete blood count and measurement of liver enzyme, urea and creatinine levels. One hour to 2 h later (from 7:30 to 8:30), supine resting, exercise and recovery echocardiography, and right heart catheterization with PCWP measurement were performed simultaneously. Resting pre-exercise measurements were initially performed in patients with both the trunk and legs in a horizontal position and then with the legs slightly elevated due to preparation for cycling. At the end of pre-exercise horizontal resting measurements, 5 min after the pre-exercise leg elevation, and 5 min after the end of exercise, blood samples for the analysis of the N-terminal segment of proatrial natriuretic peptide (NT-proANP) and brain natriuretic peptide (BNP) were drawn. In patients referred for myocardial biopsy, several myocardial specimens were collected after completion of the present study. On the day of catheterization, the morning medication was omitted. The control group of patients underwent the same exercise echocardiography and blood analysis, but without the concomitant catheterization.
Right heart catheterization
Initially, a 7 Fr Swan-Ganz thermodilution catheter (model 131HF7, Baxter Healthcare Corporation, USA) was inserted via the right jugular vein into the pulmonary artery. With the balloon on the catheter tip inflated, the catheter was advanced into the pulmonary capillary wedge position. The correct balloon position was verified by the presence of characteristic wedge pressure waveforms. PCWP was measured with a zero level at the midaxillary line. After obtaining the balloon wedge position, the resting pre-exercise catheterization echocardiography in patients at horizontal position was started. At the time of transmitral Doppler flow recording, the PCWP, heart rate and systemic blood pressure measurements were obtained using a multiparametric module Ultraview SL 91496 (Spacelabs Healthcare, USA). PCWP was averaged over pressure waveform data obtained during a 12 s interval and expressed as a mean. Measurements of PCWP, heart rate and blood pressure were repeated at rest 5 min after the patient’s leg elevation and during exercise at the end of each workload, at the time of termination of the exercise (peak exercise), and at the end of each minute of the postexercise recovery period until PCWP normalization. Resting PCWP ≥15 mmHg and peak exercise PCWP ≥25 mmHg were defined as abnormal and suggestive of HFNEF in accordance with Borlaug et al (4). These cutoff values result from previous studies in control patients free of cardiovascular diseases, who underwent invasive PCWP measurements at rest or during supine exercise (7,16,17).
Resting echocardiography
All echocardiographic examinations were performed using Vivid E9 (GE Healthcare, USA) with an M5S transducer. Grey-scale two-dimensional images (frame rate from 64 frames/s to 77 frames/s, mean 73 frames/s) were recorded from the parasternal short-axis views at the base, at the level of papillary muscles and at the apex, as well as from the apical two-, three- and four-chamber views. Three to five consecutive cardiac cycles in each view were digitally stored. Aortic and transmitral flows were recorded using pulsed-wave Doppler echocardiography. Pulsed-wave Doppler tissue imaging (DTI) of mitral annular motion was performed in the apical four-chamber view. A sample volume of 6.0 mm was placed on septal and lateral mitral annular corners. A narrow angle sector (30° to 40°) was used to obtain DTIs of individual walls at high frame rates of >150 frames/s. All Doppler recordings were performed during shallow respiration or end-expiratory apnea. Resting recordings were initially obtained in patients in a horizontal position and after leg elevation.
Exercise and recovery echocardiography
After obtaining echocardiographic views at rest, graded supine bicycle ergometry limited by the onset of symptoms was performed starting at 25 W for 2 min. The load was then increased in increments of 25 W at 2 min intervals until the occurrence of the first symptom (dyspnea or fatigue). All exercise tests were performed on an ergometer (type er900L, Ergoline GmbH, Germany). The patients were lying and cycling with their trunk in a horizontal position with their legs slightly elevated. During the exercise, LV filling was monitored in the apical four-chamber view. The following peak exercise and recovery echocardiographic recordings were obtained within 60 s to 90 s: gray-scale two-dimensional apical four-chamber view, pulsed Doppler recording of transmitral flow, and DTI of the septal and lateral mitral annular motion. The acquisition of peak exercise images started at the time of termination of cycling. The succession of the acquisition of resting, exercise and recovery echocardiographic views, as indicated above, was kept constant.
Echocardiographic parameters analyzed
Echocardiographic measurements were performed according to recommendations of the American Society of Echocardiography (18). The data were analyzed offline using EchoPAC PC versions 108.1.5 to 110.1.1 (GE Vingmed Ultrasound A/S, Norway). LV mass was estimated using the Devereux formula (19). LV and left atrial (LA) volumes were calculated by means of the biplane method of disks (18) using apical four- and two-chamber views. From the conventional pulsed-wave Doppler recordings, the peak early diastolic transmitral flow velocity (E), the peak late diastolic transmitral flow velocity (A), and the deceleration time of E wave (DT) were measured. In cases of merging E and A velocities due to exercise-induced tachycardia, echocardiographic monitoring of LV filling during exercise typically enabled the determination of whether the single peak represents E or A velocity. From DTI, peak systolic and early diastolic mitral annular velocities at the septal corner and at the lateral corner were measured and the values were averaged (s′ and e′). Conventional pulsed-wave Doppler and two-dimensional echocardiographic parameters as well as DTI parameters were obtained as a mean of three to six consecutive heart cycles. From the apical four-chamber views, global longitudinal systolic (SR-S) and early diastolic (SR-E) strain rates were determined as described previously (20). Briefly, LV endocardium was traced at end-systole and, if necessary, a software-generated region of interest was adjusted to an ideal width of the myocardial wall. The global strain rate curves were automatically created by the EchoPAC software and the values from two consecutive heart cycles were averaged (the first and last cycles were not analyzed). All Doppler and 2D-STE parameters were measured at rest with patients in a horizontal position (rest) and with slight leg elevation, at peak exercise (exe), and at the recovery period. The exercise-induced parameter changes were calculated as the peak exercise values minus the resting pre-exercise values obtained in the horizontal position. All analyses were performed offline by one experienced observer (JM), who was blinded to PCWP values. To determine the variability of Doppler and strain results, the data of 10 randomly selected patients with complete sets of initial measures were reassessed at least six months later without any knowledge of the first data set.
Measurement of BNP and NT-proANP
Six venous blood samples were collected from the right antecubital vein into tubes containing EDTA. Four pre-exercise blood samples (two obtained from patients in the horizontal position, two obtained from patients with leg elevation) were used for the analysis of NT-proANP and BNP, and were stored at 0°C to 4°C until the collection of the remaining two postexercise samples (10 min to 20 min). Immediately thereafter, all blood samples were centrifuged for 10 min at a speed of 2500 rpm and frozen. Plasma BNP analysis was performed using a commercially available assay (Architect BNP immunoassay, Abbott Laboratories, USA). Plasma NT-proANP concentrations were determined using the mean of duplicate values by a commercially available proANP (1–98) ELISA kit (Biomedica Medizinprodukte GmbH & Co KG, Austria).
Statistical analysis
Standard measures of summary statistics were used to describe the primary data: relative and absolute frequencies, and means ± SEM. A one-way ANOVA model was applied to compare the individual groups for continuous variables and a standard t test was used for a detailed mutual comparison of the groups. A maximum-likelihood χ2 test was applied to compare experimental variations in the categorical variables. Continuous parameters with asymmetric log-normal distribution were logarithmically transformed before parametric statistical analysis. The Pearson parametric coefficient of correlation was used to assess the relationship among continuous parameters, and its statistical significance was assessed using standard t statistics.
The diagnostic power of potential predictors was assessed on the basis of ROC curves. The computation was based on binormal assumption, and the significance of ROC was based on the calculated area under the curve (AUC) with the corresponding 95% CI. The AUC values were tested using the algorithm published by Hanley and McNeil (21). The sensitivities, specificities, negative and positive predictive values (PPVs) were calculated directly from 2×2 frequency tables and supplied with 95% CIs. The optimal combination of predictors for the exercise-induced elevation of PCWP ≥25 mmHg was identified using classification trees (22). The classification tree is a nonparametric data-mining technique that produces a sequence of dichotomous splits of the dataset of the best predictors (the tree). The final branches of the tree identified the predicted categories of patients, and the set of predictors in the tree nodes was considered to be the optimal combination of predictors.
RESULTS
Of the 67 initially screened patients after orthotopic heart transplantation, 61 subjects fulfilled the inclusion criteria and were included in the present study. All patients were transplanted using the bicaval technique (23) and were receiving immunosuppressive therapy. Myocardial biopsy was performed in 42 (69%) patients; according to the modified International Society for Heart and Lung Transplant histological classification of cellular rejection (24), 18 patients had grade 0, 17 patients grade 1A, five patients grade 1B and two patients had grade 2 rejection. Thirty-seven (61%) patients underwent coronary angiography. None exhibited any findings of significant coronary stenosis (luminal diameter narrowing ≥50%). According to the resting (patients in horizontal position) and peak exercise PCWP values, the patients were divided into three groups: group A, patients without HFNEF (PCWP at rest <15 mmHg, PCWP at peak exercise <25 mmHg, n=20); group B, patients with exercise-induced HFNEF (PCWP at rest <15 mmHg, PCWP at peak exercise ≥25 mmHg, n=30); and group C, patients with HFNEF at rest (PCWP at rest ≥15 mmHg, n=11). All group B and C patients reported a history of exertional dyspnea or had a low exercise tolerance (exercise duration ≤390 s) due to shortness of breath or fatigue, and had nondilated LV (end-diastolic volume index <97 mL/m2) with normal LVEF >50%, confirming the diagnosis of HFNEF (2).
Hemodynamic, echocardiographic, natriuretic peptide and catheterization measurements at rest and at peak exercise
Table 1 summarizes the baseline clinical and two-dimensional echocardiographic characteristics in groups A, B and C, and in controls. Groups A and B differed only in terms of heart rate at rest, which was lower in group B patients, and in terms of the frequency of angiotensin-converting enzyme inhibitor usage. Tables 2 and 3 show natriuretic peptide, Doppler and speckle tracking echocardiographic and PCWP results at rest (in patients lying in horizontal position) and at peak exercise, as well as parameter changes (Δ, peak exercise value minus value at rest). Groups A, B and C differed from controls in the majority of parameters. The most significant differences (P<0.01) between groups A and B were in Δpro-ANP, ΔE, DT at peak exercise (exe), PCWP at both rest and exercise, ΔPCWP, Δ(E/e′), E-exe/s′-exe, Δ(E/s′), E-exe/SR-S-exe, and Δ(E/SR-S). Mean absolute differences of intraobserver repeated measurements (JM) for resting E, e′-septal, e′-lateral, s′-septal, s′-lateral, SR-S and SR-E were 1.6 cm/s (2.2% of the mean of two absolute measurements), 0.16 cm/s (1.8%), 0.39 cm/s (3.1%), 0.12 cm/s (1.7%), 0.23 cm/s (2.4%), −0.12 1/s (13.9%) and 0.1 1/s (9.4%), respectively. Mean absolute differences of intraobserver repeated measurements (JM) for E-exe, e′-septal-exe, e′-lateral-exe, s′-septal-exe, s′-lateral-exe, SR-S-exe, and SR-E-exe were 1.1 cm/s (0.9%), 0.19 cm/s (1.4%), 0.56 cm/s (3.8%), 0.30 cm/s (3.1%), 0.34 cm/s (3.1%), −0.05 1/s (4.1%) and 0.20 1/s (11.7%), respectively.
TABLE 1.
Baseline clinical and two-dimensional echocardiographic characteristics in transplanted patients without heart failure with normal ejection fraction (HFNEF), with exercise-induced HFNEF, with HFNEF at rest and in healthy controls
| Absence of HFNEF (n=20) | Exercise-induced HFNEF (n=30) | HFNEF at rest (n=11) | Controls (n=18) | P | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, years | 53±2.3† | 57±1.8† | 57±3.1† | 44±1.4 | <0.001 |
| Age of the heart, years | 40±3.3 | 39±2.0 | 42±3.5 | 44±1.4 | 0.503 |
| Time since OHT, months | 51±11.3 | 57±10.6 | 70±15.2 | – | 0.671 |
| Men, n (%) | 19 (95.0) | 28 (93.3) | 10 (90.9) | 17 (94.4) | 0.975 |
| Hypertension, n (%) | 18 (90.0) | 27 (90.0) | 8 (72.7) | – | 0.367 |
| Hyperlipoproteinemia, n (%) | 18 (90.0) | 29 (96.7) | 9 (81.8) | – | 0.303 |
| Diabetes mellitus, n (%) | 11 (55.0) | 18 (60.0) | 5 (45.5) | – | 0.707 |
| Body mass index, kg/m2 | 27±0.8* | 28±0.5† | 28±1.1* | 24.7±0.6 | 0.001 |
| Heart rate-rest, beats/min | 80±2.8† | 74±1.7‡ | 77±3.0 | 69±2.5 | 0.020 |
| Heart rate-exe, beats/min | 110±2.9† | 103±2.5† | 109±3.3† | 123±2.1 | <0.001 |
| Systolic BP-rest, mmHg | 135±2.8† | 142±3.2† | 142±4.7† | 122±2.7 | <0.001 |
| Systolic BP-exe, mmHg | 155±3.6 | 155±3.5 | 164±5.2 | 163±3.1 | 0.249 |
| Rejection, grade 1A, 1B and 2, n (%) | 7 (46.7) | 13 (61.9) | 4 (66.7) | – | 0.580 |
| Unknown, n | 5 | 9 | 5 | ||
| Exercise duration, s | 323±12.8† | 333±11.9† | 325±21.5† | 505±15.8 | <0.001 |
| Medical therapy | |||||
| ACEi or AT II, % | 8 (40.0) | 23 (76.7)‡ | 9 (81.2)‡ | – | 0.013 |
| Beta blockers, % | 13 (65.0) | 24 (80.0) | 9 (81.8) | – | 0.416 |
| Diuretics, % | 8 (40.0) | 15 (50.0) | 4 (36.4) | – | 0.662 |
| Statins, % | 20 (100) | 27 (90.0) | 10 (90.9) | – | 0.350 |
| Acetylsalicylic acid, % | 16 (80.0) | 24 (80.0) | 11 (100) | – | 0.268 |
| Two-dimensional echocardiography | |||||
| EDVI, mL/m2 | 41±1.7 | 42±1.7 | 43±4.1 | 44±2.1 | 0.739 |
| ESVI, mL/m2 | 15±0.9 | 15±0.7 | 15±1.6 | 14±0.9 | 0.925 |
| LVEF, % | 64±1.3* | 65±1.0* | 67±1.1 | 69±0.9 | 0.041 |
| LAVI, mL/m2 | 41±1.9† | 43±2.2† | 47±3.4† | 22±1.4 | <0.001 |
| LVMI, g/m2 | 95±5.8† | 100±3.2† | 93±7.6† | 71±3.2 | <0.001 |
Data presented as mean ± SEM unless otherwise indicated. P values were calculated using statistical tests comparing all four groups;
P<0.05 versus controls;
P<0.01 versus controls;
P<0.05 versus group with the absence of heart failure with normal ejection fraction (HFNEF). Absence of HFNEF was defined as pulmonary capillary wedge pressure (PCWP) at rest <15 mmHg and PCWP at peak exercise <25 mmHg; exercise-induced HFNEF as PCWP at rest <15 mmHg, PCWP at peak exercise ≥25 mmHg; and HFNEF at rest as PCWP at rest ≥15 mmHg. ACEi Angiotensin-converting enzyme inhibitor; AT II Angiotensin II receptor blocker; BP Blood pressure; EDVI End-diastolic volume index; ESVI End-systolic volume index; exe Value obtained at peak exercise; LAVI Left atrial volume index; LVEF Left ventricular ejection fraction; LVMI Left ventricular mass index; OHT Orthotopic heart transplantation; rest Value obtained at rest
TABLE 2.
Doppler echocardiographic results and natriuretic peptide levels in transplanted patients without heart failure with normal ejection fraction (HFNEF), with exercise-induced HFNEF, with HFNEF at rest and in healthy controls
| Absence of HFNEF (n=20) | Exercise-induced HFNEF (n=30) | HFNEF at rest (n=11) | Controls (n=18) | P | |
|---|---|---|---|---|---|
| Natriuretic peptides | |||||
| Pro-ANP-rest, nmol/L | 5.2±0.7† | 5.9±0.5† | 7.3±1.1† | 1.5±0.2 | <0.001 |
| Pro-ANP-postexe, nmol/L | 6.3±0.7† | 8.2±0.5†‡ | 8.5±1.3† | 2.2±0.3 | <0.001 |
| ΔPro-ANP, nmol/L | 1.1±0.3 | 2.3±0.3†§ | 1.2±0.4 | 0.7±0.1 | <0.001 |
| BNP-rest, pmol/L | 46.7±13.8* | 57.8±7.6† | 78.4±29.6*‡ | 12.4±2.0 | 0.004 |
| BNP-postexe, pmol/L | 57.9±14.8† | 75.2±8.8†‡ | 99.8±39.7†‡ | 15.1±3.0 | 0.001 |
| ΔBNP, pmol/L | 11.2±3.4* | 17.4±3.7† | 21.4±10.5†‡ | 2.7±1.6 | 0.010 |
| Doppler parameters | |||||
| E-rest, cm/s | 73±3.9 | 71±3.1 | 88±4.7*‡ | 73±2.6 | 0.016 |
| E-exe, cm/s | 110±4.3 | 123±3.1‡ | 132±5.1*§ | 119±3.8 | 0.009 |
| ΔE, cm/s | 37±3.4 | 52±2.2*§ | 44±3.0‡ | 46±3.1 | 0.003 |
| DT-rest, ms | 163±7.2 | 151±4.4 | 137±6.1*§ | 154±4.2 | 0.016 |
| DT-exe, ms | 123±4.8 | 107±2.6†§ | 107±3.7*‡ | 124±5.5 | 0.003 |
| ΔDT, ms | −40±6.4* | −44±4.2* | −30±5.6‡ | −29±4.4 | 0.042 |
| e′-rest, cm/s | 11.2±0.4† | 10.7±0.4† | 10.7±0.6* | 15.0±0.6 | <0.001 |
| e′-exe, cm/s | 14.6±0.7† | 13.7±0.5† | 12.8±0.5* | 18.8±0.6 | <0.001 |
| Δe′, cm/s | 3.4±0.5 | 3.0±0.2* | 1.9±0.3*‡ | 3.8±0.4 | 0.037 |
| s′-rest, cm/s | 8.7±0.5† | 7.8±0.2† | 7.2±0.3*‡ | 11.2±0.5 | <0.001 |
| s′-exe, cm/s | 10.7±0.6† | 9.7±0.3† | 9.2±0.4* | 15.4±0.7 | <0.001 |
| Δs′, cm/s | 2.0±0.3† | 1.9±0.3† | 2.0±0.5* | 4.2±0.6 | <0.001 |
| Two-dimensional speckle-tracking echocardiography | |||||
| SR-E-rest, 1/s | 1.1±0.1 | 1.1±0.1 | 1.0±0.2 | 1.3±0.1 | 0.113 |
| SR-E-exe, 1/s | 1.7±0.2 | 1.5±0.1* | 1.3±0.1* | 1.9±0.1 | 0.016 |
| ΔSR-E, 1/s | 0.6±0.1 | 0.4±0.1 | 0.3±0.3 | 0.6±0.1 | 0.431 |
| SR-S-rest, 1/s | −0.9±0.1 | −0.8±0.1* | −0.8±0.1 | −1.1±0.1 | 0.001 |
| SR-S-exe, 1/s | −1.3±0.1† | −1.0±0.1†‡ | −1.0±0.1 | −1.7±0.1 | <0.001 |
| ΔSR-S, 1/s | −0.3±0.1* | −0.2±0.1† | −0.2±0.1 | −0.6±0.1 | <0.001 |
Data presented as mean ± SEM unless otherwise indicated. P values were calculated using statistical tests comparing all four groups;
P<0.05 versus controls;
P<0.01 versus controls;
P<0.05 versus group with the absence of HFNEF;
P<0.01 versus group with the absence of HFNEF. Absence of HFNEF was defined as pulmonary capillary wedge pressure (PCWP) at rest <15 mmHg and PCWP at peak exercise <25 mmHg; exercise-induced HFNEF as PCWP at rest <15 mmHg, PCWP at peak exercise ≥25 mmHg; and HFNEF at rest as PCWP at rest ≥15 mmHg. Δ Exercise value minus corresponding value at rest (horizontal patient position); BNP Brain natriuretic peptide; DT Deceleration time of E wave; E Peak early diastolic transmitral velocity; e′ Peak early diastolic mitral annular velocity; exe Value obtained at peak exercise; postexe Measured 5 min after the end of exercise; Pro-ANP Pro-atrial natriuretic peptide; rest Value obtained at rest; s′ Peak systolic mitral annular velocity; SR-E Strain rate at early diastole; SR-S Systolic strain rate
TABLE 3.
Combined echocardiographic indexes and PCWP results in transplanted patients without heart failure with normal ejection fraction (HFNEF), with exercise-induced HFNEF, with HFNEF at rest and in healthy controls
| Absence of HFNEF (n=20) | Exercise-induced HFNEF (n=30) | HFNEF at rest (n=11) | Controls (n=18) | P | |
|---|---|---|---|---|---|
| Right heart catheterization | |||||
| PCWP-rest, mmHg | 9±0.7 | 11±0.4§ | 19±1.1§ | – | <0.001 |
| PCWP-exe, mmHg | 19±1.0 | 30±0.6§ | 33±1.6§ | – | <0.001 |
| ΔPCWP, mmHg | 10±1.1 | 19±0.6§ | 13±2.3 | – | <0.001 |
| Doppler indexes | |||||
| E-rest/e′-rest | 6.6±0.4† | 6.8±0.3† | 8.9±0.8‡† | 4.9±0.1 | <0.001 |
| E-exe/e′-exe | 7.8±0.5* | 9.3±0.4†‡ | 11.0±0.8‡† | 6.4±0.2 | <0.001 |
| Δ(E/e′) | 1.2±0.3 | 2.5±0.3†§ | 2.1±0.4 | 1.5±0.2 | 0.003 |
| E-rest/s′-rest | 8.9±0.7† | 9.3±0.5† | 12.3±1.0§† | 6.7±0.3 | <0.001 |
| E-exe/s′-exe | 10.6±0.6† | 13.1±0.5†§ | 14.4±0.6§† | 8.0±0.4 | <0.001 |
| Δ(E/s′) | 1.7±0.50 | 3.8±0.5†§ | 2.1±1.1 | 1.3±0.3 | 0.003 |
| Two-dimensional speckle-tracking echocardiography | |||||
| E-rest/SR-E-rest, cm | 72.6±7.0* | 78.2±7.3* | 100.2±18.2† | 56.8±3.0 | 0.010 |
| E-exe/SR-E-exe, cm | 73.6±7.3 | 88.5±6.1† | 110.6±15.7‡† | 62.6±2.5 | <0.001 |
| Δ(E/SR-E), cm | 1.0±4.3 | 10.3±7.5 | 10.4±23.2 | 5.7±3.0 | 0.821 |
| E-exe/SR-E-rest, cm | 110.1±9.0 | 140.0±16.8* | 156.7±35.0* | 92.8±5.6 | 0.027 |
| E-rest/SR-S-rest, cm | −83.1±6.4* | −87.7±5.3† | −109.3±10.8‡† | −68.9±2.9 | 0.001 |
| E-exe/SR-S-exe, cm | −92.3±5.5† | −123.9±6.1†§ | −130.0±15.1§† | −70.6±3.1 | <0.001 |
| Δ(E/SR-S), cm | −9.2±6.1 | −36.2±6.3†§ | −20.7±16.9 | −1.7±3.6 | 0.002 |
Data presented as mean ± SEM unless otherwise indicated. P values were calculated using statistical tests comparing all four groups.
P<0.05 versus controls;
P<0.01 versus controls;
P<0.05 versus group with the absence of heart failure with normal ejection fraction (HFNEF);
P<0.01 versus group with the absence of HFNEF. Absence of HFNEF was defined as pulmonary capillary wedge pressure (PCWP) at rest <15 mmHg and PCWP at peak exercise <25 mmHg; exercise-induced HFNEF as PCWP at rest <15 mmHg, PCWP at peak exercise ≥ 25 mmHg; and HFNEF at rest as PCWP at rest ≥ 15 mmHg. Δ Exercise value minus corresponding value at rest (horizontal patient position); E Peak early diastolic transmitral velocity; e′ Peak early diastolic mitral annular velocity; exe Value obtained at peak exercise; rest Value obtained at rest; s′ Peak systolic mitral annular velocity; SR-E Strain rate at early diastole; SR-S Systolic strain rate
Complete or nearly complete fusion of early and late diastolic velocities or a poor quality of exercise images prohibited the assessment of the exercise E, DT, s′ and e′ in two, 10, three and four transplanted subjects, respectively. Good-quality exercise two-dimensional recordings for STE analysis were not obtained in 21 (for SR-S) or 22 (for SR-E) transplanted patients and in one control patient. It was not possible to evaluate SR-S-exe in six (30%) of the patients without HFNEF, in 11 (37%) of the patients with exercise-induced HFNEF, in four (36%) of the patients with HFNEF at rest and in one (6%) of the control patients. Natriuretic peptides were not analyzed in 13 patients with significant renal insufficiency (plasma creatinine levels >135 μmol/L).
Correlations of echocardiographic parameters and natriuretic peptide levels with PCWP
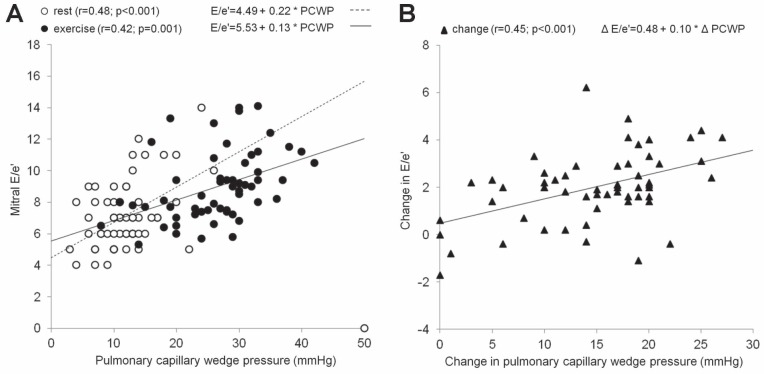
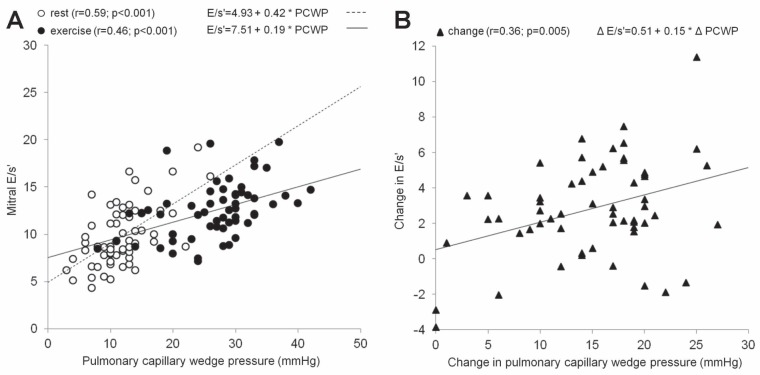
Table 4 summarizes correlations of natriuretic peptide levels and echocardiographic parameters with PCWP in patients with both normal PCWP at rest (groups A and B, n=50) and in all exercised patients (groups A, B and C, n=61). Concerning the exercise values in group A and B patients, the correlation coefficients were equal to or exceeded 0.4 for DT, E/s′, SR-S and E/SR-S. The correlation coefficient (r) between the exercise E/e′ ratio and exercise PCWP was 0.36 (P<0.05; Figure 1). However, a strong correlation between any noninvasive parameter and PCWP is not sufficient for a clinical application of this parameter in predicting the exercise PCWP. One must also find the close relationship between the exercise-induced changes of this parameter and the exercise-induced changes in PCWP (6). Thus, Table 5 demonstrates the correlations of changes of natriuretic peptides and of echocardiographic variables from the resting horizontal position to the peak of exercise with the corresponding PCWP changes. The changes in the following parameters reached r>0.40: ΔE, Δ(E/e′), Δ(E/s′) and Δ(E/SR-S). The correlations of Δ(E/e′) with ΔPCWP and Δ(E/s′) with ΔPCWP are presented in Figures 1 and 2.
TABLE 4.
Correlations of echocardiographic parameters and natriuretic peptides with pulmonary capillary wedge pressure (PCWP) in patients after orthotopic heart transplantation having normal PCWP at rest (n=50) and in all exercised patients (n=61; values in parentheses)
|
Correlation coefficients
|
||
|---|---|---|
| Values at rest in horizontal position | Peak exercise values‡ | |
| Natriuretic peptides | ||
| Pro-ANP, nmol/L | 0.31 (0.33*) | 0.30 (0.27)‡ |
| BNP, pmol/L | 0.23 (0.18) | 0.10 (0.15)‡ |
| Pulsed-wave Doppler | ||
| E, cm/s | 0.22 (0.44†) | 0.35* (0.45†) |
| DT, ms | −0.22 (−0.38†) | −0.53† (−0.50†) |
| Doppler tissue imaging and derived indexes | ||
| e′, cm/s | −0.17 (−0.18) | −0.17 (−0.19) |
| s′, cm/s | −0.41† (−0.44†) | −0.22 (−0.22) |
| E/e′ | 0.29* (0.48†) | 0.36* (0.42†) |
| E/s′ | 0.39† (0.59†) | 0.40† (0.46†) |
| Two-dimensional speckle-tracking echocardiography and derived indexes | ||
| SR-E, 1/s | 0.15 (0.03) | −0.15 (−0.26) |
| SR-S, 1/s | 0.08 (0.13) | 0.51† (0.48†) |
| E/SR-E, cm | 0.01 (0.19) | 0.25 (0.44†) |
| E/SR-S, cm | −0.39* (−0.44†) | −0.59† (−0.63†) |
P<0.05;
P<0.01;
Postexercise values. BNP Brain natriuretic peptide; DT Deceleration time of E wave; E Peak early diastolic transmitral velocity; e′ Peak early diastolic mitral annular velocity; Pro-ANP Pro-atrial natriuretic peptide; s′ Peak systolic mitral annular velocity; SR-E Strain rate at early diastole; SR-S Systolic strain rate
Figure 1).
A Correlation, both at rest (empty circles) and at peak exercise (full circles), between peak early diastolic transmitral velocity (E)/ Peak early diastolic mitral annular velocity (e′) and pulmonary capillary wedge pressure (PCWP). B Correlation between changes (from baseline to peak exercise) in E/e′ and PCWP (full triangles)
TABLE 5.
Correlations between changes in echocardiographic variables and natriuretic peptide levels from the resting horizontal position to peak exercise with the corresponding changes in pulmonary capillary wedge pressure (ΔPCWP) in patients having normal PCWP at rest (n=50) and in all exercised patients (n=61; values in parentheses)
| Correlation coefficients (r) | |
|---|---|
| Natriuretic peptides* | |
| ΔPro-ANP, nmol/L | 0.29 (0.23) |
| ΔBNP, pmol/L | 0.17 (0.16) |
| Pulsed-wave Doppler | |
| ΔE, cm/s | 0.57† (0.53†) |
| ΔDT, ms | −0.22 (−0.27) |
| Doppler tissue imaging and derived indexes | |
| Δe′, cm/s | −0.08 (−0.05) |
| Δs′, cm/s | −0.04 (−0.02) |
| Δ(E/e′) | 0.50† (0.45†) |
| Δ(E/s′) | 0.43† (0.36†) |
| Two-dimensional speckle-tracking echocardiography and derived indexes | |
| ΔSR-E, 1/s | 0.08 (−0.07) |
| ΔSR-S, 1/s | 0.34 (0.31) |
| Δ(E/SR-E), cm | 0.00 (0.17) |
| Δ(E/SR-S), cm | −0.60† (−0.56†) |
Δpro-ANP and BNP, postexercise values minus corresponding values at rest (horizontal patient position);
P<0.01. Δ Exercise value minus corresponding value at rest (horizontal patient position); BNP Brain natriuretic peptide; DT Deceleration time of E wave; E Peak early diastolic transmitral velocity; e′ Peak early diastolic mitral annular velocity; Pro-ANP Pro-atrial natriuretic peptide; s′ Peak systolic mitral annular velocity; SR-E Strain rate at early diastole; SR-S Systolic strain rate
Figure 2).
A Correlation, both at rest (empty circles) and at peak exercise (full circles), between peak early diastolic transmitral velocity (E)/ Peak systolic mitral annular velocity (s′) and pulmonary capillary wedge pressure (PCWP). B Correlation between changes (from baseline to peak exercise) in E/s′ and PCWP (full triangles)
Accuracy of echocardiographic measures, natriuretic peptides and their combinations in predicting exercise-induced elevation of PCWP
In the clinical setting, patients in whom PCWP elevation was already present at rest (group C in our study) do not require exercise to diagnose HFNEF. By contrast, exercise is of the utmost importance in patients suspected of having HFNEF with a normal or borderline PCWP at rest. Thus, the ability of noninvasive parameters to predict an exercise-induced elevation of PCWP ≥25 mmHg was analyzed in group A and B patients with resting PCWP <15 mmHg. Table 6 summarizes the sensitivities, specificities, AUCs and optimal cutoff values of individual parameters in predicting an exercise-induced elevation of PCWP ≥25 mmHg. The most successful parameters were E-exe/SR-S-exe ≤−105 cm (AUC, 0.87), SR-S-exe ≥−1.2 1/s (AUC, 0.78), E-exe/s′-exe ≥11.0 (AUC=0.75), and E-exe/e′-exe ≥8.5 or DT-exe ≤115 ms (both AUC=0.74). Except for DT-exe, all these parameters and their cutoff values were also able to separate group B patients from controls. E-exe/SR-S-exe ≤−105 cm, SR-S-exe ≥− 1.2 1/s, E-exe/s′-exe ≥11.0 and E-exe/e′-exe ≥8.5, suggestive of exercise PCWP elevation, were observed in zero, one, one and one control subjects, respectively. DT-exe ≤115 ms was observed in eight (44%) controls. Figure 3 presents an example of exercise-induced changes in E/e′ and E/s′ in a patient with a marked increase in PCWP during exercise. It also demonstrates the diagnostic potential of the new parameter – the E/s′ ratio.
TABLE 6.
Accuracy of echocardiographic parameters and natriuretic peptides in predicting exercise-induced elevation of pulmonary capillary wedge pressure ≥25 mmHg in patients with normal pulmonary capillary wedge pressure at rest
| N=50 | AUC (95% CI) | P | Cutoff | Sensitivity, % | Specificity, % |
|---|---|---|---|---|---|
| Echocardiographic parameters and indexes at peak exercise | |||||
| E-exe, cm/s | 0.72 (0.56–0.87) | 0.011 | ≥117 | 69.0 | 75.0 |
| DT-exe, ms | 0.74 (0.57–0.92) | 0.011 | ≤115 | 77.8 | 57.1 |
| e′-exe, cm/s | 0.54 (0.37–0.71) | 0.626 | ≤14.1 | 46.4 | 52.6 |
| E-exe/e′-exe | 0.74 (0.59–0.89) | 0.006 | ≥8.5 | 64.3 | 84.2 |
| s′-exe, cm/s | 0.62 (0.45–0.80) | 0.158 | ≤10.5 | 65.5 | 52.6 |
| E-exe/s′-exe | 0.75 (0.61–0.90) | 0.004 | ≥11.0 | 79.3 | 57.9 |
| SR-E-exe, 1/s | 0.60 (0.39–0.81) | 0.333 | ≤1.7 | 61.1 | 50.0 |
| E-exe/SR-E-exe, cm | 0.69 (0.49–0.88) | 0.074 | ≥75.0 | 61.1 | 50.0 |
| SR-S-exe, 1/s | 0.78 (0.62–0.94) | 0.007 | ≥−1.2 | 89.5 | 50.0 |
| E-exe/SR-S-exe, cm | 0.87 (0.73–1.00) | <0.001 | ≤−105.0 | 78.9 | 78.6 |
| Natriuretic peptide levels after exercise | |||||
| BNP-postexe, pmol/L | 0.68 (0.50–0.86) | 0.049 | ≥65.6 | 55.0 | 82.4 |
| Pro-ANP-postexe, nmol/L | 0.72 (0.56–0.89) | 0.019 | ≥7.3 | 68.2 | 70.5 |
Cutoff points and corresponding sensitivity and specificity are based on ROC analysis. AUC Area under the curve; BNP Brain natriuretic peptide; DT Deceleration time of E wave; E Peak early diastolic transmitral velocity; e′ Peak early diastolic mitral annular velocity; exe Value obtained at peak exercise; postexe Measured 5 min after the end of exercise; Pro-ANP Pro-atrial natriuretic peptide; s′ Peak systolic mitral annular velocity; SR-E Strain rate at early diastole; SR-S Systolic strain rate
Figure 3).

Example of the exercise-induced Doppler parameter changes in a patient with a marked increase in pulmonary capillary wedge pressure from 12 mmHg at rest to 37 mmHg at peak exercise. Left column, data at rest ( A, B, C) ; right column, data immediately after peak exercise (D, E, F) . Panels A and D represent transmitral Doppler filling flow (peak early diastolic transmitral velocity [E] = 61 cm/s, E at peak exercise = 142 cm/s); the remaining images represent septal ( B, E) and lateral (C, F) tissue Doppler velocities of the mitral annular motion. Average peak early diastolic mitral annular velocity (e′) rose from 12.1 cm/s to 15.1 cm/s and average peak systolic mitral annular velocity (s′) changed minimally from 7.3 cm/s to 7.2 cm/s. E/e′ exhibited exercise-induced elevation from 5.0 to 9.4, while E/s′ rose more convincingly from 8.4 to 19.7, both indicating exercise PCWP ≥25 mmHg
Of numerous noninvasive parameter combinations tested to predict the exercise-induced PCWP elevation, the most successful and potentially clinically applicable was the combination of E-exe/s′-exe ≥11.0 + E-exe/e′-exe ≥8.5. It slightly exceeded the accuracy of the individual parameters to predict an exercise PCWP ≥25 mmHg (sensitivity 53.6%, specificity 89.5%, AUC=0.78). In addition to a very good specificity, this combination also had a promising PPV of 88.2%. PPVs of E-exe/s′-exe ≥11.0 or E-exe/e′-exe ≥8.5 were 73.3% and 85.7%, respectively.
DISCUSSION
Our study leads to several important conclusions and sets some priorities. To date, it is the largest study evaluating the efficacy of echocardiographic parameters in predicting exercise-induced elevation of PCWP. It was the first to test several new echocardiographic parameters and parameter combinations for the prediction of isolated exercise-induced HFNEF. Using ROC analysis, we demonstrated only a suboptimal sensitivity of 64.3% and a specificity of 84.2% (AUC=0.74) of exercise E/e′ ≥8.5 in predicting an exercise PCWP ≥25 mmHg. We also described promising new parameters (the exercise E/s′ ratio ≥11.0 and exercise E/SR-S ≤− 105.0).
The accuracy of the currently used Doppler-derived indexes in the prediction of exercise-induced elevation of LVFP
Previous studies showed that, under resting conditions, LVFP or its elevation can be estimated noninvasively using Doppler echocardiography using the E/e′ ratio (25–27), even if the prediction of LVFP was not always optimal (28). The E/e′ ratio is a significant marker of a poor prognosis (29,30). However, very little data are available on the non-invasive assessment of exercise-induced elevation of LVFP using the E/e′ ratio and the results of published studies are contradictory. In the largest study published to date, Burgess et al (3) described a good correlation between E/e′ and LV diastolic pressure measured during exercise (r=0.59). The exercise E/e′ >13 indicated an elevation of LV diastolic pressure during exercise with a sensitivity of 73% and a specificity of 96%. However, the authors studied an unselected cohort of 37 patients who also had an LVEF <45% and a mostly significant coronary artery disease, which may have affected the power of exercise E/e′ and PCWP correlation and the E/e′ cutoff value. The ability of E/e′ to predict LVFP elevation during exercise was also described in a small study of 12 patients with exertional dyspnea and normal LVEF published by Talreja et al (5). By contrast, in 28 patients with aortic stenosis, Dalsgaard et al (6) demonstrated that exercise-induced changes in E/e′ were not related to changes in PCWP. In a study that included symptomatic patients with impaired exercise capacity and an LVEF ≥50% (ie, those with suspected HFNEF) without evidence of myocardial ischemia during the exercise, Maeder et al (7) also did not find any correlation between peak exercise E/e′ and peak exercise PCWP. The small number of patients is, however, an important drawback of these studies. In our study, we found a significant, but only weak, correlation (r=0.36) between exercise E/e′ and exercise PCWP and between exercise-induced changes in E/e′ and changes in PCWP (r=0.50). A sensitivity of 64.3% and a specificity of 84.2% of exercise E/e′ to predict the exercise PCWP elevation ≥25 mmHg was, from a clinical point of view, suboptimal. There may be several factors contributing to the above-mentioned discrepancies among studies, including various structural abnormalities in different studies with specific patient cohorts, the presence or absence of myocardial ischemia during exercise, inclusion of some patients with an LVEF <50% in some of the studies and the possibility of a partial dependence of e′ velocities on preload in the setting of high exercise-induced LV filling pressures. Concerning the individual points, myocardial ischemia was found to blunt or reverse the physiological increase of e′ during exercise (31,32), while E increased with ischemia-induced severe PCWP elevation (33), findings that may account for a higher exercise E/e′ ratio in patients with ischemia than in control patients (31). One must also take into consideration that the preload independence of e′ in patients with HFNEF is unclear (34,35), and a high LVFP may partially normalize exercise e′ velocities. Compared with the published reports testing the ability of echocardiographic exercise parameters to predict the exercise LVFP discussed above (3,5–7), our study has several important advantages, but also some limitations. It comprised, thus far, the largest cohort of patients studied. All patients had normal LVEF, nondilated LV and a high prevalence of hypertension, diabetes and hyperlipoproteinemia. Thus, they represented a group of patients at high risk of exercise-induced HFNEF. However, some of our results (mainly parameter cutoff values for predicting exercise-induced PCWP elevation) cannot be extrapolated automatically to other study cohorts, because post-heart transplant patients represent a specific population, frequently affected by myocardial rejection and coronary vasculopathy. The presence of both rejection and vasculopathy could influence the functional parameters tested in the present study. However, any substantial effect of rejection on our results appears unlikely because the majority of rejections were subclinical (grade 1A) and the rejection episodes were proportionately distributed among the patient groups. Similarly, any significant effect of ischemia appears unlikely because patients with manifest post-transplant coronary artery disease were not included. However, we cannot exclude exercise-induced silent myocardial ischemia in some patients because coronary angiographies were not consistently performed and wall motion abnormalities during exercise were not systematically followed at the time of our study. Because of the potential effect of the transplantation procedure on LA function, echocardiographic Doppler parameters characterizing late diastole were not used to predict PCWP.
New echocardiographic parameters in the prediction of exercise-induced elevation in LVFP
In our study, we described two new parameters, exercise E/SR-S and exercise E/s′, which were found to be promising for the diagnosis of isolated exercise-induced HFNEF. SR-S was obtained using 2D-STE by averaging the peak SR-S values of all six segments depicted in the apical four-chamber view. Compared with DTI, 2D-STE has the advantage of being an angle-independent method, not influenced by tethering. Thus, 2D-STE has the potential to provide parameters that more precisely and objectively reflect myocardial function. Indeed, several reports demonstrated the superiority of 2D-STE-derived parameters over DTI-derived parameters in predicting the elevation of LVFP (9–11). Our study found a relatively good sensitivity and specificity of exercise E/SR-S for identifying the exercise PCWP ≥25 mmHg. A limitation of this parameter is the fact that we were able to obtain good-quality exercise echocardiographic images for 2D-STE analysis in only 72% of the subjects including controls. However, we identified another index using the E and longitudinal systolic function variables, the exercise E/s′ ratio, which deserves further attention. The rationale for the application of E/s′ or E/SR-S in predicting LVFP results from the fact that s′ and SR-S, as parameters reflecting mainly LV longitudinal contractility (36), are likely to reflect LV elastic recoil, which constitutes the main component of LV diastolic suction. Increased LV suction due to the negative early diastolic LV pressure enables an adequate LV filling even in the short diastolic filling time during tachycardia induced pharmacologically or during exercise (37). The coupling of LV contractility and elastic recoil contributing to a fast and complete relaxation has been repeatedly proven (37,38). Thus, s′ and SR-S may serve as indirect markers of LV relaxation, which, in contrast to e′, may have the advantage of not being influenced or partially ‘pseudonormalized’ by the effect of a high LA pressure (8). The close relationship between s′ or SR-S disturbances and LVFP elevation has been repeatedly proven (8,12,39).
Combination of echocardiographic measures in the noninvasive determination of an exercise-induced increase in LVFP
Several previous reports suggest that neither E/e′ ratio nor any other echocardiographic parameter used in isolation are able to predict LVFP elevation with sufficient accuracy (28,40). In this regard, a parameter combination adds additional and more complex information that appears to provide a significant diagnostic benefit (13,41). In a population of 270 patients, Dini et al (41) demonstrated that the sequential testing by the classification and regression tree analysis of multiple echocardiographic and Doppler parameters has superior sensitivity and specificity compared with the standard approach in identifying an increased LVFP. However, the creation of the optimal model for predicting PCWP elevation required >170 patients (derivation cohort of patients). The approach using noninvasive parameter combinations was recently used to form HFNEF diagnostic guidelines (2). However, these guidelines are derived from parameters obtained only under resting conditions, and the optimal combinations of parameters and their cutoff values to diagnose exercise-induced elevation of LVFP and exercise-induced HFNEF are not known. Our study is the first to attempt to solve this problem. Neither any individual echocardiographic parameter nor natriuretic peptide levels were able to diagnose exercise-induced PCWP elevation with an accuracy sufficient for clinical decision-making. The combination of parameters appeared to provide some diagnostic benefit, but a larger study population is necessary to draw definitive conclusions on the optimal combination therapy.
Study limitations
Post-heart transplant patients are not typical HFNEF patients. The cause of elevated LVFP in post-heart transplant patients may differ from that in typical HFNEF patients. In our cohort, heart transplant patients were approximately 40 years of age on average, while the typical age of HFNEF patients is 70 to 75 years. In addition to age, the myocardial function of post-heart transplant patients can be influenced by post-transplant vasculopathy, myocardial rejection, and left atrial post-transplant structural and functional changes. Thus, our results (mainly echocardiographic cutoff values for predicting PCWP elevation) cannot be automatically extrapolated to other patient cohorts. Only 72% of the subjects had apical four-chamber images of adequate quality on exercise for strain rate analysis. Thus, strain rate-derived exercise parameters may be used to predict exercise PCWP elevation only in patients with a very good quality of 2-D echocardiographic images. A larger sample of patients is necessary for a more conclusive analysis of new parameters such as exercise E/s′ and exercise E/SR-S as well as for the selection of optimal parameter combinations.
CONCLUSIONS
In a cohort of patients after orthotopic heart transplantation with a normal LVEF, exercise E/e′, used as a sole parameter, is not sufficiently precise to predict the exercise-induced elevation of PCWP. Exercise E/s′ and E/SR-S provide comparable or slightly better prediction. However, E/SR-S ratio appears to be applicable only in patients with good-quality echocardiographic imaging. Parameter combinations (E/e′ and E/s′) may offer further improvements over parameters used in isolation. Our results and new echocardiographic indexes taking into account LV longitudinal systolic function (s′, SR-S) appear promising and deserve attention. However, their clinical utility should be confirmed in larger cohorts of patients at risk of HFNEF.
Footnotes
SOURCES OF FUNDING AND DISCLOSURES: This study was supported in part by the European Regional Development Fund – Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123). There are no other conflicts of interest.
REFERENCES
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 3.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: Hemodynamic validation and clinical significance of estimation of left ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talreja DR, Nishimura RA, Oh JK. Estimation of left ventricular filling pressure with exercise by Doppler echocardiography in patients with normal systolic function: A simultaneous echocardiographic-cardiac catheterization study. J Am Soc Echocardiogr. 2007;20:477–9. doi: 10.1016/j.echo.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Dalsgaard M, Kjaergaard J, Pecini R, et al. Left ventricular filling pressure estimation at rest and during exercise in patients with severe aortic valve stenosis: Comparison of echocardiographic and invasive measurements. J Am Soc Echocardiogr. 2009;22:343–9. doi: 10.1016/j.echo.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–63. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Assessment of left ventricular systolic function using echocardiography in patients with preserved ejection fraction and elevated diastolic pressures. Am J Cardiol. 2008;101:1766–71. doi: 10.1016/j.amjcard.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 9.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol. 2008;101:1504–9. doi: 10.1016/j.amjcard.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115:1376–83. doi: 10.1161/CIRCULATIONAHA.106.662882. [DOI] [PubMed] [Google Scholar]
- 11.Meluzin J, Spinarova L, Hude P, et al. Estimation of left ventricular filling pressures by speckle tracking echocardiography in patients with idiopathic dilated cardiomyopathy. Eur J Echocardiogr. 2011;12:11–8. doi: 10.1093/ejechocard/jeq088. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen JS, Lakkis NM, Bobek J, Goswami R, Dokainish H. Systolic and diastolic myocardial mechanics in patients with cardiac disease and preserved ejection fraction: Impact of left ventricular filling pressure. J Am Soc Echocardiogr. 2010;23:1273–80. doi: 10.1016/j.echo.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Dokainish H, Nguyen JS, Sengupta R, et al. Do additional echocardiographic variables increase the accuracy of E/e′ for predicting left ventricular filling pressure in normal ejection fraction? An echocardiographic and invasive hemodynamic study. J Am Soc Echocardiogr. 2010;23:156–61. doi: 10.1016/j.echo.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Fukuta H, Little WC. Elevated left ventricular filling pressure after maximal exercise predicts increased plasma B-type natriuretic peptide levels in patients with impaired relaxation pattern of diastolic filling. J Am Soc Echocardiogr. 2007;20:832–7. doi: 10.1016/j.echo.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Dokainish H, Zoghbi WA, Lakkis NM, et al. Optimal noninvasive assessment of left ventricular filling pressures. Circulation. 2004;109:2432–9. doi: 10.1161/01.CIR.0000127882.58426.7A. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida A, Kadota K, Kambara H, et al. Left ventricular responses to supine bicycle exercise assessed by radionuclide angiography and a Swan-Ganz catheter. Jap Circ J. 1985;49:661–71. doi: 10.1253/jcj.49.661. [DOI] [PubMed] [Google Scholar]
- 17.Firstenberg MS, Levine BD, Garcia MJ, et al. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol. 2000;36:1664–9. doi: 10.1016/s0735-1097(00)00909-8. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 20.Meluzin J, Spinarova L, Hude P, et al. Left ventricular mechanics in idiopathic dilated cardiomyopathy: Systolic-diastolic coupling and torsion. J Am Soc Echocardiogr. 2009;22:486–93. doi: 10.1016/j.echo.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Hanley JA, McNeil BJ. The meaning and use of the area under a Receiver Operating Characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 22.Breiman L, Friedman JH, Olshen RE, Stone CJ. Classification and Regression Trees. Monterey: Wadsworth & Brooks/Cole Advanced Books & Software; 1984. [Google Scholar]
- 23.Sarsam MAI, Campbell CS, Yonan NA, Deiraniya AK, Rahman AN. An alternative surgical technique in orthotopic cardiac transplantation. J Card Surg. 1993;8:344–9. doi: 10.1111/j.1540-8191.1993.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez ER. The pathology of heart transplant biopsy specimens: Revisiting the 1990 ISHLT working formulation. J Heart Lung Transplant. 2003;22:3–15. doi: 10.1016/s1053-2498(02)00575-2. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue Doppler imaging. Circulation. 1998;98:1644–50. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 26.Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction. Circulation. 2007;116:637–47. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 27.Menon SC, Gray R, Tani LY. Evaluation of left ventricular filling pressures and ventricular function by Doppler echocardiography in patients with functional single ventricle: Correlation with simultaneous cardiac catheterization. J Am Soc Echocardiogr. 2011;24:1220–5. doi: 10.1016/j.echo.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 29.Dini FL, Rosa GM, Fontanive P, et al. Combining blood flow and tissue Doppler imaging with N-terminal pro-type B natriuretic peptide for risk stratification of clinically stable patients with systolic heart failure. Eur J Echocardiogr. 2010;11:333–40. doi: 10.1093/ejechocard/jep207. [DOI] [PubMed] [Google Scholar]
- 30.Holland DJ, Prasad SB, Marwick TH. Prognostic implications of left ventricular filling pressure with exercise. Circ Cardiovasc Imaging. 2010;3:149–56. doi: 10.1161/CIRCIMAGING.109.908152. [DOI] [PubMed] [Google Scholar]
- 31.Rustad LA, Amundsen BH, Slordahl SA, Stoylen A. Upright bicycle exercise echocardiography in patients with myocardial infarction shows lack of diastolic, but not systolic, reserve: A tissue Doppler study. Eur J Echocardiogr. 2009;10:503–8. doi: 10.1093/ejechocard/jen312. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima Y, Kane GC, McCully RB, Ommen SR, Pellikka PA. Left ventricular diastolic filling pressures during dobutamine stress echocardiography: Relationship to symptoms and ischemia. J Am Soc Echocardiogr. 2009;22:947–53. doi: 10.1016/j.echo.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 33.Meluzin J, Toman J, Soucek M, et al. Variability of changes in Doppler transmitral filling pattern during stress echocardiography in patients with stable angina pectoris. Int J Cardiol. 1994;45:209–17. doi: 10.1016/0167-5273(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 34.Bhella PS, Pacini EL, Prasad A, et al. Echocardiographic indices do not reliably track changes in left-sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2011;4:482–9. doi: 10.1161/CIRCIMAGING.110.960575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumesnil JG, Paulin Ch, Pibarot P, Coulombe D, Arsenault M. Mitral annulus velocities by Doppler tissue imaging: Practical implications with regard to preload alterations, sample position, and normal values. J Am Soc Echocardiogr. 2002;15:1226–31. doi: 10.1067/mje.2002.123396. [DOI] [PubMed] [Google Scholar]
- 36.Uemura K, Kawada T, Sunagawa K, Sugimachi M. Peak systolic mitral annulus velocity reflects the status of ventricular-arterial coupling. Theoretical and experimental analyses. J Am Soc Echocardiogr. 2011;24:582–91. doi: 10.1016/j.echo.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Udelson JE, Bacharach SL, Cannon RO, III, Bonow RO. Minimum left ventricular pressure during beta-adrenergic stimulation in human subjects. Circulation. 1990;82:1174–82. doi: 10.1161/01.cir.82.4.1174. [DOI] [PubMed] [Google Scholar]
- 38.Hori M, Yellin EL, Sonnenblick EH. Left ventricular diastolic suction as a mechanism of ventricular filling. Jap Circ J. 1982;46:124–9. doi: 10.1253/jcj.46.124. [DOI] [PubMed] [Google Scholar]
- 39.Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG. Pure diastolic dysfunction is associated with long-axis systolic dysfunction. Implications for the diagnosis and classification of heart failure. Eur J Heart Failure. 2005;7:820–8. doi: 10.1016/j.ejheart.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy. Circulation. 2007;116:2702–8. doi: 10.1161/CIRCULATIONAHA.107.698985. [DOI] [PubMed] [Google Scholar]
- 41.Dini FL, Ballo P, Badano L, et al. Validation of an echo-Doppler decision model to predict left ventricular filling pressure in patients with heart failure independently of ejection fraction. Eur J Echocardiogr. 2010;11:703–10. doi: 10.1093/ejechocard/jeq047. [DOI] [PubMed] [Google Scholar]