Abstract
BACKGROUND:
The clinical outcome of patients with myocardial infarction (MI) complicated by cardiogenic shock (CS) who require mechanical ventilation (MV) is poor.
OBJECTIVE:
To analyze the impact of abciximab pretreatment in this high-risk population of MI patients.
METHODS:
The present study was a retrospective subanalysis of the multicentre randomized Routine Upfront Abciximab Versus Standard Peri-Procedural Therapy in Patients Undergoing Percutaneous Coronary Intervention for Cardiogenic Shock (PRAGUE-7) study, which included 80 MI patients in CS undergoing primary percutaneous coronary intervention (PCI). Patients were randomly assigned into group A (routine pretreatment with an abciximab bolus followed by a 1 h abciximab infusion) and group B (standard therapy). The subanalysis included 37 patients requiring MV. Seventeen patients were in group A and 20 were in group B. The primary end point (death/stroke/reinfarction/new severe renal failure) at 30 days, procedural success (thrombosis in myocardial infarction [TIMI] flow) and frequency of bleeding were assessed. The χ2 and Student’s t tests were used for statistical analysis; P<0.05 was considered to be statistically significant.
RESULTS:
The primary end point occurred in nine (53%) patients in group A and 12 (60%) patients in group B (P=0.66). TIMI flow after primary PCI was higher in group A (2.75 versus 2.31; P<0.05). Major bleeding occurred in 12% of patients in group A versus 10% of patients in group B (P=0.86). Minor or minimal bleeding was more common in group A (29%) compared with group B (5%; P<0.05).
CONCLUSION:
The results of the present study suggest that routine pretreatment with abciximab before primary PCI in mechanically ventilated patients with MI complicated by cardiogenic shock was associated with better angiographic results but also with a higher incidence of bleeding.
Keywords: Abciximab, Acute coronary syndrome, Cardiogenic shock, Intensive care, Mechanical ventilation, Percutaneous coronary intervention
Cardiogenic shock occurs in 5% to 9% of patients with acute myocardial infarction and remains associated with high mortality rates despite advances in diagnostic and therapeutic strategies (including the use of modern antithrombotics, reperfusion strategies and mechanical assist devices) (1–4). Early and successful revascularization (ie, primary percutaneous coronary intervention [PCI] in most cases) is the cornerstone of treatment, with subsequent intensive cardiac care often involving the use of invasive mechanical ventilation (MV) (5–7).
In the treatment of ST-segment elevation myocardial infarction (STEMI) patients, primary PCI has been shown to be superior to thrombolysis. However, these studies failed to demonstrate the potential superiority of fibrinolysis-facilitated PCI relative to primary PCI alone (8,9). In addition, facilitated PCI is associated with higher occurrences of bleeding complications (8,9). Recently published results from the Routine Upfront Abciximab Versus Standard Peri-Procedural Therapy in Patients Undergoing Percutaneous Coronary Intervention for Cardiogenic Shock (PRAGUE-7) study showed that in patients with myocardial infarction complicated by cardiogenic shock (CS), pretreatment with abciximab before primary PCI did not improve patient outcomes (10).
The aim of our analysis was to assess the impact of abciximab pre-treatment before primary PCI on clinical outcomes of patients with myocardial infarction and CS requiring MV, as well as on the incidence of bleeding complications.
METHODS
Study design
The original PRAGUE-7 study included 80 patients with myocardial infarction complicated by CS who were undergoing primary PCI. The study design and results have been previously described in detail (10).
Briefly, two treatment strategies were compared: pre-PCI administration of an abciximab bolus followed by a 12 h abciximab infusion plus standard therapy (group A) versus standard periprocedural therapy with selective bail-out abciximab administration at the discretion of the interventional cardiologist (group B). Patients were enrolled at four tertiary centres in the Czech Republic. Clinical and hemodynamic inclusion criteria were as follows (at least one had to be fulfilled): shock index >1, ie, sustained hypotension (systolic blood pressure (BP) <90 mmHg) and heart rate (HR) >90 beats/min (11); organ hypoperfusion (including cold, wet, diaphoretic skin) and heart rate >90 beats/min; the need for catecholamine support to maintain systolic BP >90 mmHg; or Killip class II to III and systolic BP <120 mmHg.
Patients received standard anticoagulant and antiplatelet treatment either during transport or at the catheterization laboratory (heparin, acetylsalicylic acid, and 300 mg or 600 mg of clopidogrel if possible). The recommended dose of heparin was 70 IU/kg body weight when used in conjunction with abciximab and 100 IU/kg in patients without abciximab. Patients assigned to group A received abciximab (ReoPro, Centocor, Netherlands) as an intravenous bolus of 0.25 mg/kg body weight immediately after randomization (either in the coronary care unit, emergency department or on arrival to the catheterization laboratory), followed by an infusion of 0.125 μg/kg/min abciximab (maximum 10 μg/min) for 12 h (Figure 1). PCI of the infarct-related artery was performed immediately after coronary angiography (CAG), if technically feasible. In group B, an intravenous abciximab bolus (0.25 mg/kg) followed by a 12 h abciximab infusion (0.125 ug/kg/min) was administered after CAG and only to selected patients at the discretion of the interventional cardiologist. The use of thrombolytics or anticoagulation therapy other than heparin was not permitted. Coronary angiograms were reviewed centrally by two independent investigators blinded to the clinical data and randomization.
Figure 1).
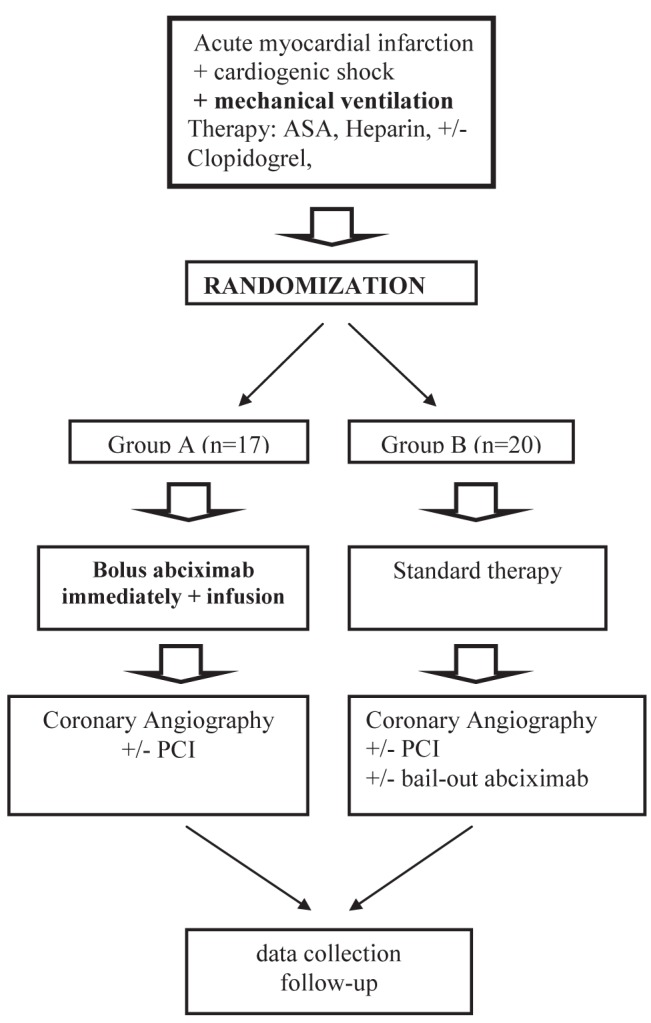
+ With; − Without; ASA Acetylsalicylic acid; PCI Primary coronary intervention
Inclusion criteria for subanalysis
Mechanically ventilated patients from the PRAGUE-7 study were analyzed. These were patients with acute myocardial infarction (STEMI, ST segment depression or bundle branch block) complicated by CS. The indication for introduction of MV was left to the discretion of the attending physician and MV was instituted either before hospital admission or in the catheterization laboratory before or during coronary angiography.
Study end points
The primary end point for the original study was the 30-day composite of all-cause mortality, reinfarction, stroke and severe acute renal failure (for definitions refer to original article) (10). In the present subanalysis, the following secondary end points were analyzed: bleeding occurrence, thrombolysis in myocardial infarction (TIMI) flow after PCI and length of hospital stay.
Bleeding was classified according to TIMI criteria as major (intracranial or overt bleeding with a decrease in hemoglobin >50 g/L or a decrease in hematocrit >15%), minor (bleeding with a decrease in hemoglobin >30 g/L, but with a <15% decrease in hematocrit) or minimal (bleeding not meeting the criteria for major or minor bleeding) (12).
Statistical analysis
Data are presented in absolute and relative values. The χ2 and Student’s t tests were used for statistical analysis; P<0.05 was considered to be statistically significant.
RESULTS
The present subanalysis included 37 mechanically ventilated patients of 80 patients from the PRAGUE-7 trial. Seventeen patients were in group A and 20 patients were in group B. The use of clopidogrel before PCI in both groups as well as other patient demographic and clinical data are presented in Table 1. In group B, bail-out abciximab after the PCI procedure was given at the discretion of the interventional cardiologist in eight of 20 cases.
TABLE 1.
Baseline demographic and clinical characteristics
| Group A (n=17) | Group B (n=20) | P | |
|---|---|---|---|
| Age, mean ± SD | 63±15 | 67±9 | 0.33 |
| Male sex | 14 (82) | 14 (70) | 0.38 |
| Diabetes | 4 (24) | 7 (35) | 0.45 |
| Previous myocardial infarction | 2 (12) | 6 (30) | 0.17 |
| Previous bleeding | 0 (0) | 2 (10) | 0.16 |
| Hypertension | 6 (35) | 16 (80) | <0.05 |
| Smoking | 7 (41) | 7 (35) | 0.71 |
| Clopidogrel | 5 (29) | 8 (40) | 0.51 |
| Preadmission CPR | 7 (41) | 13 (65) | 0.15 |
| Electrocardiograpic parameters | |||
| ST elevation | 14 (82) | 13 (65) | 0.24 |
| ST depression | 0 (0) | 5 (25) | <0.05 |
| Bundle branch block | 3 (18) | 2 (10) | 0.52 |
| IABP | 5 (29) | 5 (25) | 0.77 |
| Symptoms, min, median (1st quartile–3rd quartile) | 120 (93.6–180) | 170.4 (120–577.2) | <0.05 |
| Door to balloon, min, median (1st quartile–3rd quartile) | 53 (33.75–90.25) | 60 (30–126) | 0.51 |
Data presented as n (%) unless otherwise indicated. Bold values indicate statistical significance. CPR Cardiopulmonary resuscitation; Group A Routine preprocedural abciximab group; Group B Standard therapy group; IABP Intra-aortic balloon counterpulsation
Only a trend was observed toward a lower primary composite end point of all-cause mortality, reinfarction, stroke and severe acute renal failure at 30 days in group A (Table 2). In group A, no strokes occurred, whereas in group B three strokes occurred. The other variables included in the composite primary end point as well as hospital mortality did not differ between the groups. Length of hospital stay was comparable between the groups. TIMI grade flow after primary PCI was significantly higher in group A. The occurrence of major bleeding was similar in both groups. A significantly higher incidence of minor and minimal bleeding as well as a trend toward more transfusions were observed in group A.
TABLE 2.
Patient outcomes
| Group A (n=17) | Group B (n=20) | P | |
|---|---|---|---|
| Primary end point | 9 (53) | 12 (60) | 0.66 |
| Death at 30 days | 8 (47) | 6 (30) | 0.85 |
| Hospital mortality | 8 (47) | 10 (50) | 0.85 |
| Stroke | 0 (0) | 3 (15) | 0.09 |
| Reinfarction | 0 (0) | 0 (0) | |
| Severe acute renal failure | 1 (6) | 3 (15) | 0.62 |
| Hospital stay, days, mean | 14 | 14 | |
| Discharge at 30 days | 3 (18) | 9 (45) | 0.19 |
| Bleeding | |||
| TIMI major bleeding | 2 (12) | 2 (10) | 0.86 |
| TIMI minor or minimal bleeding | 5 (29) | 1 (5) | <0.05 |
| Transfusion | 6 (35) | 2 (10) | 0.07 |
| TIMI flow pre-percutaneous coronary intervention, n | |||
| 0 | 9 | 13 | 0.28 |
| 1 | 0 | 2 | |
| 2 | 5 | 2 | |
| 3 | 3 | 3 | |
| TIMI flow post-percutaneous coronary intervention, n | |||
| 0 | 0 | 3 | <0.05 |
| 1 | 2 | 0 | |
| 2 | 0 | 4 | |
| 3 | 14 | 12 | |
Data presented as n (%) unless otherwise indicated. Bold values indicate statistical significance. Group A Routine preprocedural abciximab group; Group B Standard therapy group; TIMI Thrombolysis in myocardial infarction bleeding criteria
DISCUSSION
The major finding of our study (a subanalysis of the PRAGUE-7 study) was that mechanically ventilated patients with acute myocardial infarction complicated by CS who were pretreated with abciximab before PCI had better angiographic results but experienced a higher incidence of TIMI minor or minimal bleeding compared with patients without abciximab pretreatment. The clinical outcomes in both groups were comparable.
Mortality among patients with acute myocardial infarction complicated by CS remains high despite the fact that the use of revascularization, modern pharmacotherapy and mechanical devices is very high (40% to 60%) (1,2,13,14) The search for new therapeutic strategies urgently continues (15).
The cornerstone of therapy is early coronary reperfusion (primary PCI in most cases). As soon as a diagnosis of STEMI is established, patients should immediately be given anticoagulant and antiplatelet therapy (16). The concept of facilitated PCI using fibrinolytic treatment before coronary angiography has been shown to have no value in several randomized trials (8–9). In addition, more bleeding complications occurred in patients who underwent thrombolytic therapy.
Another possible way to facilitate PCI is pretreatment with glycoprotein (GP) IIb/IIIa inhibitors as an adjunct to standard anticoagulant and antiplatelet therapy (17,18). However, the Bavarian Reperfusion Alternatives Evaluation (BRAVE)-3 study revealed no benefit of abciximab administration in STEMI patients pretreated using dual anti-platelet therapy (ie, acetylsalicylic acid + 600 mg clopidogrel) and, therefore, according to European Society of Cardiology 2012 guidelines for STEMI patients, GP IIb/IIIa inhibitors are only indicated for use as a bail-out therapy (16,17). Similarly, in a cohort of CS patients, upfront administration of abciximab was also shown to lack any benefits (10). In this study, more than one-half of the patients did not require MV, and this was the main reason why we performed the present subanalysis. Intravenous GP IIb/IIIa may be of clinical benefit in patients requiring MV because patients with acute myocardial infarction and CS requiring MV often do not receive thienopyridine before PCI and/or may exhibit attenuated gastrointestinal absorption of orally administered drugs. Absorption may also be decreased by the effects of vasopressors and/or opiates, or in patients with liver dysfunction, conditions that are frequently present in patients requiring MV (19). The decreased availability of clopidogrel in hemodynamically unstable patients can be confirmed by a decrease in the vasodilator-stimulated phosphoprotein index (20). In the PRAGUE-7 study, we did not measure the vasodilator-stimulated phosphoprotein index; however, 65% of mechanically ventilated patients did not receive clopidogrel before PCI. Intracoronary abciximab administration was not shown to be superior to the intravenous route of administration in the Abciximab Intracoronary versus intravenous Drug Application in STEMI (AIDA-STEMI) trial (21). In our study, we found better angiographic results (TIMI flow) in patients who were given intravenous abciximab pre-PCI, although the clinical outcome between the groups did not differ. Interestingly, there was a trend toward better TIMI flow in patients pre-treated with abciximab before PCI. Despite the abovementioned potential benefits of abciximab, its administration in patients requiring MV can increase bleeding risks. STEMI patients with bleeding complications have worse clinical outcomes compared with patients without bleeding complications (22). In the Intra-Aortic Baloon Pump (IABP) Shock II trial, Thiele et al (14) observed a 20% occurrence of moderate and severe bleeding according to the Global Use of STrategies to open Occulued coronary arteries (GUSTO) classification in STEMI patients complicated by CS. In their study, 85% of patients were on MV and approximately 40% had received GP IIb/IIIa inhibitors. Patients requiring MV in the PRAGUE-7 study were found to have similar rates of TIMI major bleeding, but more TIMI minor and minimal bleeding and a greater need for blood transfusions in group A (pretreatment with abciximab plus 12 h infusion). Moreover, the bleeding risk may have been underestimated in group B (heparin plus acetylsalicylic acid plus clopidogrel), because fewer patients were given clopidogrel and, in the abciximab-pretreatment group (group A), fewer patients were resuscitated before PCI. Compared with the entire PRAGUE-7 study patient cohort, the most striking difference was the near-doubled incidence of minor or minimal bleeding in mechanically ventilated patients pretreated with abciximab (10).
Our study has certain limitations. First, the sample size of mechanically ventilated patients in the PRAGUE-7 study was relatively small. Second, in group B, eight patients were also given abciximab as bail-out therapy after PCI (at the discretion of interventional cardiologist). Thus, our results are should be viewed as hypothesis-generating and should be reassessed in larger studies
CONCLUSION
Our results suggest that routine pretreatment with abciximab before primary PCI in mechanically ventilated patients with acute myocardial infarction complicated by cardiogenic shock may be associated with better angiographic results but also with a higher incidence of bleeding.
Footnotes
FUNDING: The study was supported by the Charles University Research Fund (project number P36) and the project Ministry of Health, Czech Republic for conceptual development of research organization 00669806 – Faculty Hospital in Pilsen.
REFERENCES
- 1.Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294:448–54. doi: 10.1001/jama.294.4.448. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RJ, Spencer FA, Gore JM, et al. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: A population-based perspective. Circulation. 2009;119:1211–9. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 4.Czarnecki A, Welsh RC, Yan RT, et al. Reperfusion strategies and outcomes of ST-segment elevation myocardial infarction patients in Canada: Observations from the Global Registry of Acute Coronary Events (GRACE) and the Canadian Registry of Acute Coronary Events (CANRACE) Can J Cardiol. 2012;28:40–7. doi: 10.1016/j.cjca.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295:2511–5. doi: 10.1001/jama.295.21.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stegman BM, Newby LK, Hochman JS, et al. Post-myocardial infarction cardiogenic shock is a systemic illness in need of systemic treatment: Is therapeutic hypothermia one possibility? J Am Coll Cardiol. 2012;59:644–7. doi: 10.1016/j.jacc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Buerke M, Lemm H, Dietz S, et al. Pathophysiology, diagnosis, and treatment of infarction-related cardiogenic shock. Herz. 2011;36:73–83. doi: 10.1007/s00059-011-3434-7. [DOI] [PubMed] [Google Scholar]
- 8.ASSENT-4 PCI investigators Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): Randomised trial. Lancet. 2006;367:569–78. doi: 10.1016/S0140-6736(06)68147-6. [DOI] [PubMed] [Google Scholar]
- 9.Ellis SG, Tendera M, de Belder MA, et al. FINESSE Investigators Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205–17. doi: 10.1056/NEJMoa0706816. [DOI] [PubMed] [Google Scholar]
- 10.Tousek P, Rokyta R, Tesarova J, et al. Routine upfront abciximab versus standard periprocedural therapy in patiens undergoing primary percutaneous coronary intervention for cardiogenic shock: The PRAGUE-7 Study. An open randomized multicentre study. Acute Cardiac Care. 2011;13:116–22. doi: 10.3109/17482941.2011.567282. [DOI] [PubMed] [Google Scholar]
- 11.Bilkova D, Motovska Z, Widimsky P, et al. Shock index: A simple clinical parameter for quick mortality risk assessment in acute myocardial infarction. Can J Cardiol. 2011;27:739–42. doi: 10.1016/j.cjca.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis In Myocardial Infarction (TIMI) trial, phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142–54. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 13.Hochman JS, Buller ChE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction – etiologies, management and outcome: A report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36:1063–1070. doi: 10.1016/s0735-1097(00)00879-2. [DOI] [PubMed] [Google Scholar]
- 14.Thiele H, Zeymer U, Neumann FJ, et al. the IABP-SHOCK II Trial Investigators Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–96. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor ChM, Rogers JG. Evidence for overturning the guidelines in cardiogenic shock. N Engl J Med. 2012;267:1349–50. doi: 10.1056/NEJMe1209601. [DOI] [PubMed] [Google Scholar]
- 16.Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 17.Mehilli J, Kastrati A, Schulz S, et al. Abciximab in patients with acute ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention after clopidogrel loading. Circulation. 2009;119:1933–40. doi: 10.1161/CIRCULATIONAHA.108.818617. [DOI] [PubMed] [Google Scholar]
- 18.Kastrati A, Mehilli J, Neumann FJ, et al. Intracoronary Stenting and Antithrombotic Regimen-Rapid Early Action for Coronary Treatment 2 Trial Investigators Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: The ISAR-REACT 2 randomized trial. JAMA. 2006;295:1531–8. doi: 10.1001/jama.295.13.joc60034. [DOI] [PubMed] [Google Scholar]
- 19.Soucková L, Opatrilová R, Suk P, et al. Impaired bioavailability and antiplatelet effect of high-dose clopidogrel in patients after cardiopulmonary resuscitation (CPR) Eur J Clin Pharmacol. 2013;69:309–17. doi: 10.1007/s00228-012-1360-0. [DOI] [PubMed] [Google Scholar]
- 20.Osmancik P, Jirmar R, Hulikova K, et al. A comparison of the VASP index between patients with hemodynamically complicated and uncomplicated acute myocardial infarction. Catheter Cardiovasc Interv. 2010;75:158–66. doi: 10.1002/ccd.22248. [DOI] [PubMed] [Google Scholar]
- 21.Thiele H, Wöhrle J, Hambrecht R, et al. Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction: A randomised trial. Lancet. 2012;379:923–31. doi: 10.1016/S0140-6736(11)61872-2. [DOI] [PubMed] [Google Scholar]
- 22.Suh JW, Mehran R, Claessen BE, et al. Impact of in-hospital major bleeding on late clinical outcomes after primary percutaneous coronary intervention in acute myocardial infarction: The HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. Am Coll Cardiol. 2011;58:1750–6. doi: 10.1016/j.jacc.2011.07.021. [DOI] [PubMed] [Google Scholar]


