Abstract
The role of oxidative stress in cardiovascular disease processes, such as atherogenesis, ischemic-reperfusion injury and cardiac remodelling, has been increasingly recognized in the past few decades. Currently, an increasing number of studies suggest that levels of oxidative stress markers in body fluids correlate with atherosclerotic disease activity. This finding may lead to novel clinical approaches in patients with coronary artery disease. Assessment of oxidative stress markers could modify risk stratification and treatment of patients with suspected coronary artery disease or myocardial infarction.
Keywords: Myocardial infarction, Myocardial remodelling, Oxidative stress, Reactive oxygen species, Reperfusion injury
Free radicals appear to be important modulators of atherovascular disease in all stages of development. They physiologically serve as signal transducers in cell communication and homeostasis including vascular gene expression and vascular cell interactions (1). When the finely regulated signalling pathways of these molecules become uninhibited, it may lead to the initiation and progression of atherosclerotic disease. New facts about the function of free radicals are emerging continuously.
OXYGEN FREE RADICALS
Reactive oxygen species
Some of the most important free radicals in biological systems are derivatives of oxygen (reactive oxygen species [ROS]) such as superoxide anion radical, hydroxyl radical, hydrogen peroxide, and triplet or singlet O2(2). ROS molecules are characterized by one or more unpaired electrons, are unstable and highly reactive, and rapidly interact with surrounding molecules, altering their structure and functions.
ROS are produced in low amounts during cellular metabolism under normal conditions: by proton leakage during oxidative phosphorylation in mitochondria; by various enzymes such as NADH/NADPH oxidase in endothelial cells, vascular smooth muscle cells and neutrophils (3), or xanthine oxidase in endothelium; cytochrome P450; lipoxygenase/cyclooxygenase pathways; and the auto-oxidation of various substances, particularly catecholamines (4). ROS participate in various physiological functions. They regulate gene expression and post-translational modifications of proteins, serving as signalling molecules in homeostasis, mitosis, apoptosis and cell differentiation (5). Potentially toxic concentrations of ROS are enclosed in the phagosomes of phagocytes, where ROS are used as defense against exogenous microorganisms (6).
Normally, these processes are largely nonpathogenic to the host organism because low levels of ROS are maintained by enzymatic (glutathione peroxidase, catalase, superoxide dismutase) or nonenzymatic (glutathione, thioredoxin) functions of endogenous antioxidants. Some exogenous compounds (vitamins A, C and E) also act as antioxidants. Cellular structures damaged by ROS are being continuously repaired or replaced.
Oxidative stress
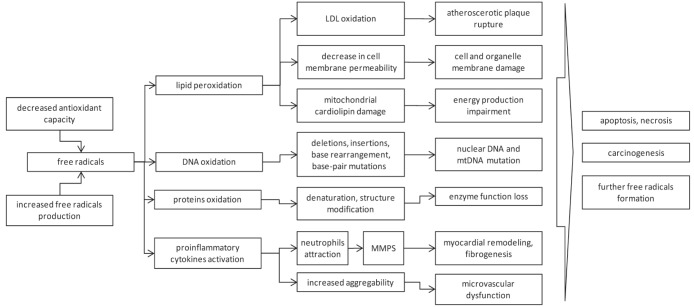
The fine balance between ROS and antioxidants is disturbed when excessive amounts of free radicals are produced or antioxidant capacity is decreased. This disturbance is known as oxidative stress and it plays an important role in cardiac pathophysiology (Figure 1).
Figure 1).
Scheme of free radicals effect on cells. LDL Low-density lipoprotein; MMPS Matrix metalloproteinases; mt Mitochondrial
Under conditions of oxidative stress, ROS attack biomolecules that are in close proximity. Mitochondrial and nuclear DNA damage, protein cross-linking and lipid peroxidation occurs, resulting in mutations, protein denaturation and loss of enzyme and membrane pump function. Damage to sarcolemmal and intracellular membranes impairs ATP-dependent Na+ and Ca2+ reuptake mechanisms. ROS decrease the activity of the sarcoplasmic reticulum membrane Ca2+ pump, which plays a crucial role in cardiac Ca2+ handling. Tumour necrosis factor-alpha (TNF-α) and interleukin (IL)-6 production triggered by ROS contribute to intracellular Ca2+ dysregulation and increase in its concentration. Cytosolic Ca2+ overload leads to myofibrillar hypercontracture, cytoskeletal damage and cell disruption via activation of Ca2+-dependent proteases and phospholipases. Mitochondrial Ca2+ overload causes inefficient ATP synthesis and utilization, leading to necrosis (7).
Apart from the direct toxic effects on surrounding molecules, excessive levels of ROS trigger a number of regulatory chain reactions. ROS are involved in apoptotic pathway control (eg, TNF-α receptor/caspase pathway) and activation of the cascade of transcriptional factors, proinflammatory cytokines (TNF-α, IL-1β, IL-6) and adhesion molecule expression (ie, intercellular adhesion molecules, vascular cell adhesion molecules, macrophage colony stimulating factor, monocyte chemoattractant protein, etc). As a result, massive recruitment of inflammatory cells, especially neutrophils, occurs (8). They penetrate the endothelium of the vessels in the affected area and highly express NADPH-oxidase, an additional important source of free radicals, which gives rise to a vicious cycle (9–11). In addition to neutrophils, smooth muscle cells, fibroblasts and T lymphocytes generate ROS. Leukocyte attraction and activation can also cause white thrombi formation in microvessels, increased platelet aggregability, microvascular cell edema and dysfunction, resulting in aggravation of ischemia and tissue stunning (12).
Laboratory assessment
To measure ROS levels in tissue or blood, various techniques can be applied (Table 1). Because of the high reactivity and very short half-life of ROS, the possibilities are limited, especially in clinical conditions. Several methods for the detection of ROS (eg, lucigenin enhanced chemiluminescence [13,14], fluorescence microtopography [15,16] or electron paramagnetic resonance spectroscopy [17]) have been described. Regarding the instability of ROS, detection using these techniques is currently only applicable under experimental conditions rather than clinical situations. The most frequently used methods detect more stable downstream products of free radicals in body fluids, such as oxidized DNA, advanced oxidation protein products (AOPPs), lipids, or assess altered defense mechanisms (superoxide dismutase levels, oxidized/reduced glutathione ratio) (18–22).
TABLE 1.
Examples of oxidative stress assessment
| Target molecule(s) | Measured oxidation product | Method | |
|---|---|---|---|
| Direct measurement of free radicals | Lucigenin enhanced chemiluminescence, fluorescence microtopography, electron paramagnetic resonance spectroscopy | ||
| Indirect measurement | |||
| Downstream oxidation products | Lipids | Malonyldialdehyde, isoprostanes | ELISA, high-performance liquid chromatography |
| Proteins, lipoproteins | AOPP, PB-DOPA, ox-LDL | ELISA | |
| DNA products | 5-OHdC, 8-OHdG, DNA strand-break frequency | ELISA, high-performance liquid chromatography variations, comet assay | |
| Decreased antioxidant capacity | Nonenzymatic | Cysteine/cystine ratio | RPLC, RPLC-MS |
| GSH/GSSG ratio, thioredoxin | HPLC, ELISA | ||
| Enzymatic | Superoxide dismutase, catalase | ELISA, RIA, spectrophotometry |
5-OHdC 5-hydroxy-2’-deoxycytidine; 8-OHdG 8-hydroxy-2′-deoxyguanosine; AOPP Advanced oxidative protein products; GSH Glutathione; GSSG Oxidized GSH; MS Mass spectrometry; ox-LDL Oxidized low-density lipoprotein; PB-DOPA Protein-bound 3,4-dihydroxyphenylalanine; RIA Radioimmunoassay; RPLC Reversed-phase liquid chromatography;
OXIDATIVE STRESS AND CORONARY ARTERY DISEASE
Oxidative stress as a marker of coronary artery disease activity
The pathological processes underlying atherovascular disease remain incompletely understood; however, there is growing evidence that oxidative stress and inflammation are positively associated with the instability of atherosclerotic plaque and the incidence of acute coronary syndrome (ACS). ROS-induced initiation of inflammatory cascades and low-density lipoprotein (LDL) oxidation leads to the formation of macrophage-derived foam cells, differentiation and proliferation of vascular smooth muscle cells, activation of vascular matrix metalloprotein-ases and impairment of the extracellular matrix (ECM) of the affected site. This may culminate in atherosclerotic plaque rupture (23,24). Ehara et al (25) demonstrated increased plasma levels of oxidized LDL in cases of ACS.
Kaneda et al (26) showed that plasma levels of AOPP were significantly higher in patients with coronary artery disease than in those without. AOPP levels correlated with severity score of CAD according to the the Gensini scoring system. In contrast, Azumi et al (9) observed that even when there was no significant difference in angiographic stenosis, the generation of ROS was significantly higher in unstable angina pectoris patients compared with stable angina patients.
Another marker of oxidative stress, thioredoxin, correlates with atherosclerotic activity. Thioredoxin is a stress-inducible protein that contains a redox-active dithiol/disulfide in the active site and provides cytoprotection against oxidative stress. Plasma thioredoxin levels are significantly increased in patients with unstable angina compared with those with stable angina (18). Miwa et al (27) showed that patients with coronary spastic angina had a higher serum thioredoxin level associated with a lower serum level of antioxidant vitamin E.
These findings suggest that coronary artery disease status may be determined by oxidative stress activity rather than the degree of coronary stenosis.
Ischemia-reperfusion injury of myocardium and predictive value of oxidative stress in recurrent cardiac events
Oxidative stress plays a key role in ischemia-reperfusion injury (2,5,28,29) and subsequent cardiac repair. Interruption of blood flow in the coronary arteries causes ischemia of adjacent tissues, leading to cell injury, which may result in cell necrosis and apoptosis. The duration of ischemia determines the extent of damage to the myocardium and related tissues (30). During ischemia, cellular defenses against oxidative injury are impaired, with lower activities of antioxidants such as superoxide dismutase and glutathione peroxidase. Furthermore, greater amounts of ROS are produced, for example, by xanthine dehydrogenase, which is converted to xanthine oxidase, a potent generator of O2− and hydrogen peroxide (31).
Timely blood supply restoration, especially in acute coronary artery occlusion, is essential for myocardial salvage. Nevertheless, abrupt blood flow restoration (thrombolysis, percutaneous coronary intervention or spontaneous) in ischemic tissue causes a precipitous increase in ROS levels, which may result in paradoxical tissue damage rather than improvement. ROS- and cytokine-initiated apoptosis after reperfusion participate in tissue damage and heart remodelling in the ensuing postinfarction period (32).
Oxidative stress contributes to the occurrence of cardiac events after reperfusion therapy in ACS. In a study by Feng et al (22), oxidative stress marker-plasma AOPP concentration correlated positively with an increased incidence of major cardiac events in patients treated with percutaneous coronary intervention for ST-segment elevation myocardial infarction during a six-month follow-up. Naruko et al (33) showed a correlation between persistently high levels of oxidized LDL in patients after myocardial infarction who underwent primary coronary stenting and stent restenosis (>50% diameter stenosis) after a six-month follow-up.
Results of the study by Nagayoshi et al (34), who studied the dynamics of urinary 8-hydroxy-2′-deoxyguanosine related to creatinine levels in patients with acute myocardial infarction, support this theory. In patients who did not experience subsequent cardiac events, 8-hydroxy-2′-deoxyguanosine/creatinine levels decreased to normal by 24 h after reperfusion therapy. However, in the cardiac event group, the levels at 24 h remained higher than those in the noncardiac event group. Hokamaki et al (18) demonstrated that after treatment (not specified) of unstable angina, recurrent angina attacks at rest occurred more frequently in patients with high thioredoxin levels than in patients with low thioredoxin levels.
Long-term predictive value of oxidative stress
There is a correlation among oxidative stress, ventricular remodelling and progressive dilation leading to end-stage heart failure. The remodelling process in postischemic myocardium is the result of multiple underlying structural and signalling changes comprising myocardial cell contractile dysfunction, necrosis, apoptosis, inflammation, microvascular dysfunction and ECM changes. Following myocardial infarction, ROS-activated matrix metalloproteinases and fibroblast proliferation lead to structural and functional rearrangement of the ECM. The migration of cytokine-attracted neutrophils and macrophages producing additional free radicals is facilitated. The neuroendocrine system, ROS and inflammatory cytokines are important regulators of the remodelling process (11,35). The extent of ischemia-reperfusion damage may be more widespread than the area primarily affected, with apoptosis, necrosis, interstitial fibrosis and remodelling occuring not only at the site of infarction, but also in remote areas of the myocardium (8). Heart failure under both acute and chronic conditions is associated with increased levels of oxidative markers (eg, malonyldialdehyde, glutathione peroxidase [36], thioredoxin [37] or superoxide dismutase [38]). Mitochondria damaged by ischemia-reperfusion injury are one of the sources of persistently elevated ROS levels (39,40). Increased renin-angiotension-aldosterone system activity stimulates NADH/NADPH oxidase, as shown by Griendling et al (41) and Rajagopalan et al (42).
PERSPECTIVE: OXIDATIVE STRESS – A NEW PROGNOSTIC MARKER?
In the past two decades, numerous studies have demonstrated the importance of oxidative stress in the development of atherosclerosis and ischemia-reperfusion injury. Elevated concentrations of a variety of oxidative stress markers were linked with a more frequent occurrence of cardiac events. These findings could help assess risk stratification, diagnosis and prevention of ACS, both in patients with and without previous cardiac history. This topic needs to be further studied.
Footnotes
FUNDING: Third Faculty of Medicine, Charles University Prague, Research Project UNCE204010.
REFERENCES
- 1.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–66. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 2.Misra MK, Sarwat M, Bhakuni P, et al. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–19. [PubMed] [Google Scholar]
- 3.Duilio C, Ambrosio G, Kuppusamy P, et al. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H2649–57. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- 4.Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: The relevance to anesthesia practice. Anesth Analg. 2005;101:1275–87. doi: 10.1213/01.ANE.0000180999.81013.D0. [DOI] [PubMed] [Google Scholar]
- 5.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–90. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Cooke MS, Evans MD, Dizdaroglu M, et al. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 7.Varadarajan SG, An J, Novalija E, et al. Changes in [Na(+)](i), compartmental [Ca(2+)], and NADH with dysfunction after global ischemia in intact hearts. Am J Physiol Heart Circ Physiol. 2001;280:H280–293. doi: 10.1152/ajpheart.2001.280.1.H280. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y. Myocardial repair/remodelling following infarction: Roles of local factors. Cardiovasc Res. 2009;81:482–90. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azumi H, Inoue N, Ohashi Y, et al. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: Important role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2002;22:1838–44. doi: 10.1161/01.atv.0000037101.40667.62. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari R, Ceconi C, Curello S, et al. Oxygen free radicals and myocardial damage: Protective role of thiol-containing agents. Am J Med. 1991;91:95S–105S. doi: 10.1016/0002-9343(91)90291-5. [DOI] [PubMed] [Google Scholar]
- 11.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–64. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 12.Ito H. No-reflow phenomenon in patients with acute myocardial infarction: Its pathophysiology and clinical implications. Acta Med Okayama. 2009;63:161–8. doi: 10.18926/AMO/31817. [DOI] [PubMed] [Google Scholar]
- 13.Ohoi I, Sone K, Tobari H, et al. A simple chemiluminescence method for measuring oxygen-derived free radicals generated in oxygenated rat myocardium. Jpn J Pharmacol. 1993;61:101–7. doi: 10.1254/jjp.61.101. [DOI] [PubMed] [Google Scholar]
- 14.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–51. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuda M, Inoue N, Azumi H, et al. Expression of glutaredoxin in human coronary arteries: Its potential role in antioxidant protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1483–7. doi: 10.1161/hq0901.095550. [DOI] [PubMed] [Google Scholar]
- 16.Terashima M, Inoue N, Ohashi Y, et al. Relationship between coronary plaque formation and NAD(P)H oxidase-derived reactive oxygen species – comparison of intravascular ultrasound finding of atherosclerotic lesions with histochemical characteristics. Kobe J Med Sci. 2007;53:107–17. [PubMed] [Google Scholar]
- 17.Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–7. [PubMed] [Google Scholar]
- 18.Hokamaki J, Kawano H, Soejima H, et al. Plasma thioredoxin levels in patients with unstable angina. Int J Cardiol. 2005;99:225–31. doi: 10.1016/j.ijcard.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura H, Vaage J, Valen G, et al. Measurements of plasma glutaredoxin and thioredoxin in healthy volunteers and during open-heart surgery. Free Radic Biol Med. 1998;24:1176–86. doi: 10.1016/s0891-5849(97)00429-2. [DOI] [PubMed] [Google Scholar]
- 20.Iuliano L, Praticò D, Greco C, et al. Angioplasty increases coronary sinus F2-isoprostane formation: Evidence for in vivo oxidative stress during PTCA. J Am Coll Cardiol. 2001;37:76–80. doi: 10.1016/s0735-1097(00)01040-8. [DOI] [PubMed] [Google Scholar]
- 21.Delanty N, Reilly MP, Pratico D, et al. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95:2492–9. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Shen C, Ma G, et al. Prolonged pain to hospital time is associated with increased plasma advanced oxidation protein products and poor prognosis in patients with percutaneous coronary intervention for ST-elevation myocardial infarction. Heart Vessels. 2010;25:374–8. doi: 10.1007/s00380-009-1220-8. [DOI] [PubMed] [Google Scholar]
- 23.Sigala F, Kotsinas A, Savari P, et al. Oxidized LDL in human carotid plaques is related to symptomatic carotid disease and lesion instability. J Vasc Surg. 2010;52:704–13. doi: 10.1016/j.jvs.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopalan S, Meng XP, Ramasamy S, et al. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–9. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–60. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 26.Kaneda H, Taguchi J, Ogasawara K, et al. Increased level of advanced oxidation protein products in patients with coronary artery disease. Atherosclerosis. 2002;162:221–5. doi: 10.1016/s0021-9150(01)00706-7. [DOI] [PubMed] [Google Scholar]
- 27.Miwa K, Kishimoto C, Nakamura H, et al. Increased oxidative stress with elevated serum thioredoxin level in patients with coronary spastic angina. Clin Cardiol. 2003;26:177–81. doi: 10.1002/clc.4960260406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari R, Alfieri O, Curello S, et al. Occurrence of oxidative stress during reperfusion of the human heart. Circulation. 1990;81:201–11. doi: 10.1161/01.cir.81.1.201. [DOI] [PubMed] [Google Scholar]
- 29.Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 30.González-Flecha B, Cutrin JC, Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest. 1993;91:456–64. doi: 10.1172/JCI116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari R, Guardigli G, Mele D, et al. Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des. 2004;10:1699–711. doi: 10.2174/1381612043384718. [DOI] [PubMed] [Google Scholar]
- 32.Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res. 2002;55:438–455. doi: 10.1016/s0008-6363(02)00442-x. [DOI] [PubMed] [Google Scholar]
- 33.Naruko T, Ueda M, Ehara S, et al. Persistent high levels of plasma oxidized low-density lipoprotein after acute myocardial infarction predict stent restenosis. Arterioscler Thromb Vasc Biol. 2006;26:877–83. doi: 10.1161/01.ATV.0000209886.31510.7f. [DOI] [PubMed] [Google Scholar]
- 34.Nagayoshi Y, Kawano H, Hokamaki J, et al. Urinary 8-hydroxy-2′-deoxyguanosine levels increase after reperfusion in acute myocardial infarction and may predict subsequent cardiac events. Am J Cardiol. 2005;95:514–7. doi: 10.1016/j.amjcard.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 35.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 36.Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–6. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 37.Jekell A, Hossain A, Alehagen U, et al. Elevated circulating levels of thioredoxin and stress in chronic heart failure. Eur J Heart Fail. 2004;6:883–90. doi: 10.1016/j.ejheart.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.de Meirelles LR, Resende AeC, Matsuura C, et al. Platelet activation, oxidative stress and overexpression of inducible nitric oxide synthase in moderate heart failure. Clin Exp Pharmacol Physiol. 2011;38:705–10. doi: 10.1111/j.1440-1681.2011.05580.x. [DOI] [PubMed] [Google Scholar]
- 39.Ide T, Tsutsui H, Hayashidani S, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–35. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 40.Ide T, Tsutsui H, Kinugawa S, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–63. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 41.Griendling KK, Minieri CA, Ollerenshaw JD, et al. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 42.Rajagopalan S, Kurz S, Münzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]