Abstract
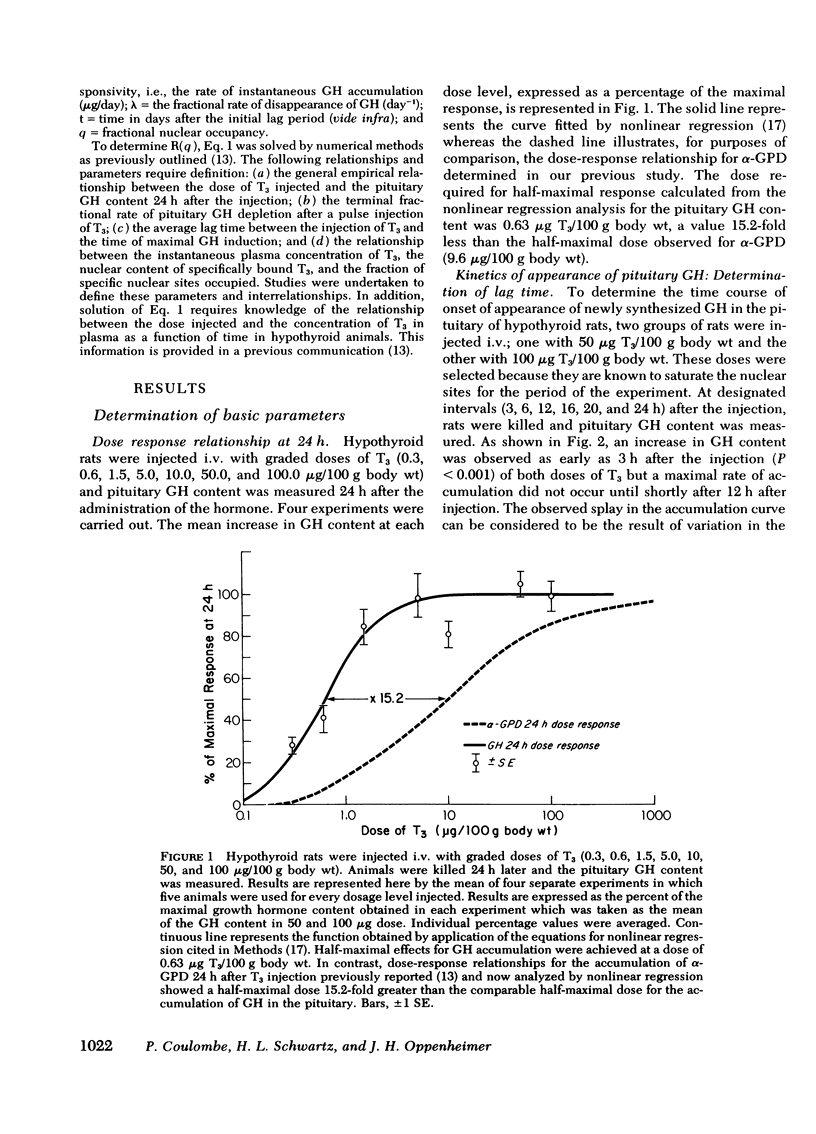
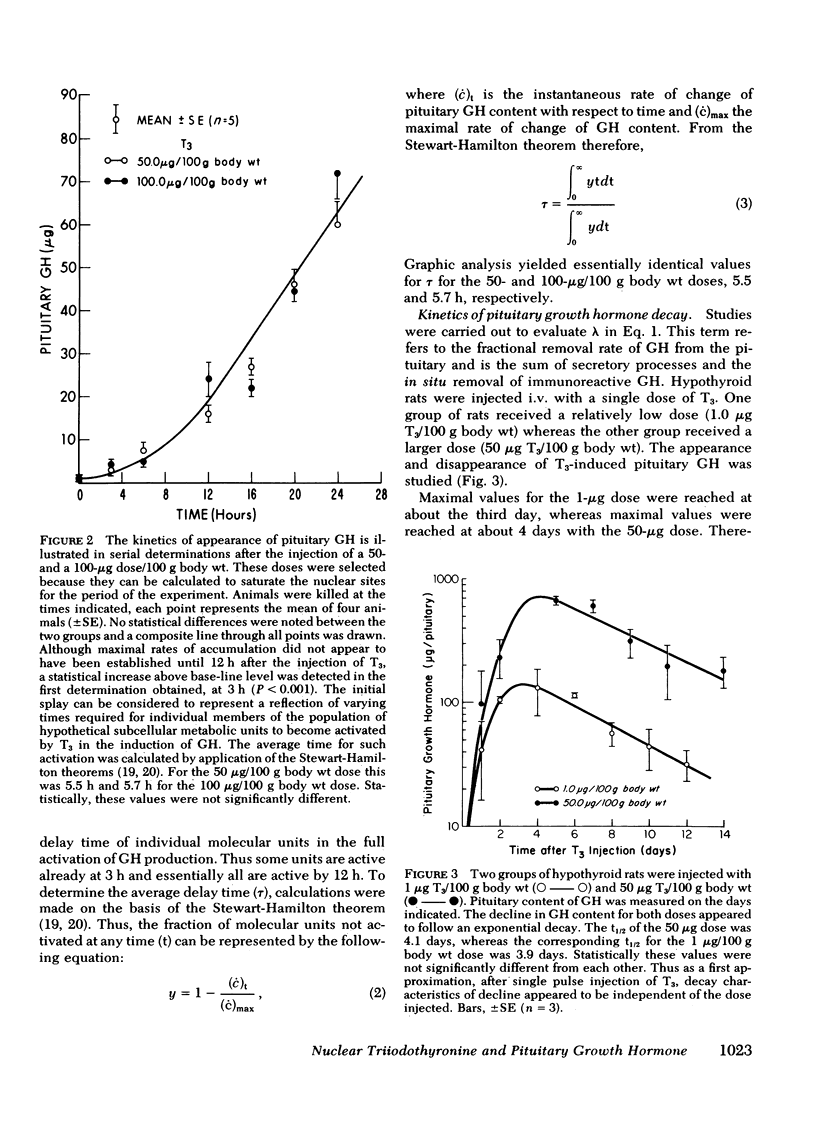
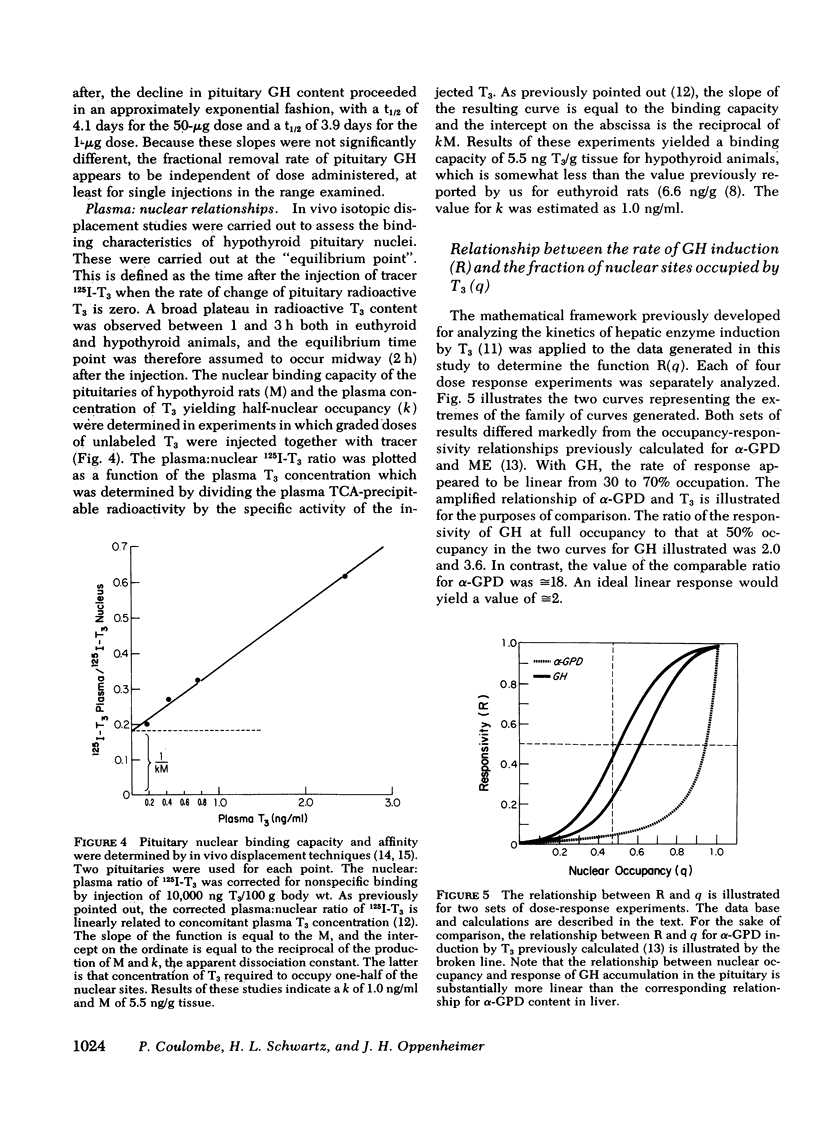
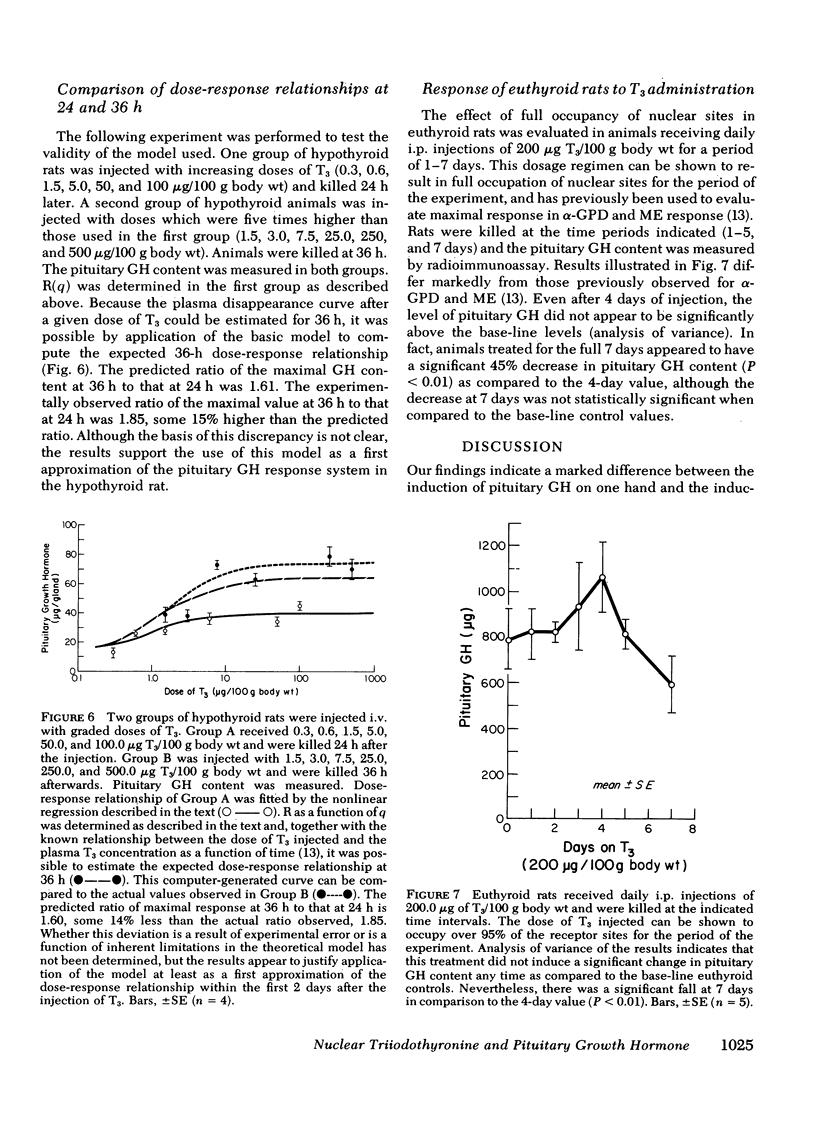
Studies were undertaken in hypothyroid rats in an effort to define the kinetics of growth hormone (GH) accumulation in response to i.v. pulse injections of triiodothyronine (T3) and to calculate the relationship between nuclear occupancy by T3 and the instantaneous rate of accumulation of pituitary GH. Results were contrasted to the findings in previous studies of the induction of hepatic mitochondrial α-glycerophosphate dehydrogenase (α-GPD) and malic enzyme (ME) by T3. The dose of T3 required to achieve half-maximal accumulation of GH in 24 h was 0.6 μg/100 g body wt, a value 15-fold less than the half-maximal dose for α-GPD and ME induction at a comparable time after injection. Although significant increase in pituitary GH were evident as early as 3 h after injection of maximally effective doses of T3, the rate of increase became linear only 12 h after injection. After achievement of peak values, the pituitary content of GH decayed with a similar terminal t½ of 3.9 days and 4.1 days in two groups of animals injected with a single dose of 1.0 and 50 μg T3/100 g body wt, respectively. In vivo isotopic displacement studies carried out at the equilibrium time point indicated that the pituitary nuclear binding capacity was 5.5 ng T3/g tissue and that the plasma concentration at which one-half of the nuclear sites are occupied is 1.0 ng/ml. Nuclear occupancy as a function of time was calculated from the estimated plasma T3 concentration after injection of the dose and the half-occupancy plasma concentration. These data were then analyzed by application of the mathematical model previously developed to ascertain the relationship between nuclear occupancy and the rate of hepatic enzyme induction. Results indicated that the pituitary nuclear occupancy-response relationship was generally linear, in marked contrast to the highly amplified relationship between nuclear occupancy and the response of ME and α-GPD to T3 in the liver. In supplementary experiments, euthyroid rats received daily injections of 200 μg of T3 for 7 days to keep nuclear sites nearly saturated for the duration of the experiment. No significant increase in the pituitary GH content above euthyroid base-line levels was noted. This also contrasts with the marked increase above euthyroid levels in α-GPD and ME observed in previous studies. Our findings suggest the existence of major differences between the specific mechanisms which lead to the induction of pituitary GH and the hepatic enzymes by T3.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burek C. L., Frohman L. A. Growth hormone synthesis by rat pituitaries in vitro: effect of age and sex. Endocrinology. 1970 Jun;86(6):1361–1367. doi: 10.1210/endo-86-6-1361. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Schwartz H. L., Silva E., Surks M. I., Oppenheimer J. H. Alpha-amanitin administration results in a temporary inhibition of hepatic enzyme induction by triiodothyronine: further evidence favoring a long-lived mediator of thyroid hormone action. Endocrinology. 1977 Jun;100(6):1621–1627. doi: 10.1210/endo-100-6-1621. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Silva E., Surks M. I., Oppenheimer J. H. Studies of a thyroid hormone and androgen dependent protein in rat liver cytosol. Acta Endocrinol (Copenh) 1977 Mar;84(3):548–558. doi: 10.1530/acta.0.0840548. [DOI] [PubMed] [Google Scholar]
- Hervas F., Morreale de Escobar G., Escobar Del Rey F. Rapid effects of single small doses of L-thyroxine and triiodo-L-thyronine on growth hormone, as studied in the rat by radioimmunoassy. Endocrinology. 1975 Jul;97(1):91–101. doi: 10.1210/endo-97-1-91. [DOI] [PubMed] [Google Scholar]
- Katz H. P., Youlton R., Kaplan S. L., Grumbach M. M. Growth and growth hormone. 3. Growth hormone release in children with primary hypothyroidism and thyrotoxicosis. J Clin Endocrinol Metab. 1969 Mar;29(3):346–351. doi: 10.1210/jcem-29-3-346. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Coulombe P., Schwartz H. L., Gutfeld N. W. Nonlinear (amplified) relationship between nuclear occupancy by triiodothyronine and the appearance rate of hepatic alpha-glycerophosphate dehydrogenase and malic enzyme in the rat. J Clin Invest. 1978 Apr;61(4):987–997. doi: 10.1172/JCI109024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I., Koerner D., Dillmann W. H. Nuclear receptors and the initiation of thyroid hormone action. Recent Prog Horm Res. 1976;32:529–565. doi: 10.1016/b978-0-12-571132-6.50029-4. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Nuclear binding capacity appears to limit the hepatic response to L-triiodothyronine (T3). Endocr Res Commun. 1975;2(4-5):309–325. doi: 10.1080/07435807509089004. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Silva E., Schwartz H. L., Surks M. I. Stimulation of hepatic mitochondrial alpha-glycerophosphate dehydrogenase and malic enzyme by L-triiodothyronine. Characteristics of the response with specific nuclear thyroid hormone binding sites fully saturated. J Clin Invest. 1977 Mar;59(3):517–527. doi: 10.1172/JCI108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Surks M. I., Schwartz H. L. Slow fractional removal of nonextractable iodine from rat tissue after injection of labeled L-thyroxine and 3,5,3'-triiodo-L-thyronine. A possible clue to the mechanism of initiation and persistence of hormonal action. J Clin Invest. 1972 Nov;51(11):2796–2807. doi: 10.1172/JCI107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake G. T., Birge C. A., Daughaday W. H. Alterations of radioimmunoassayable growth hormone and prolactin during hypothroidism. Endocrinology. 1973 Feb;92(2):487–493. doi: 10.1210/endo-92-2-487. [DOI] [PubMed] [Google Scholar]
- Rodbard D. Apparent positive cooperative effects in cyclic AMP and corticosterone production by isolated adrenal cells in response to ACTH analogues. Endocrinology. 1974 May;94(5):1427–1437. doi: 10.1210/endo-94-5-1427. [DOI] [PubMed] [Google Scholar]
- Roy A. K. Androgen-dependent synthesis of aplha-2u globulin in the rat: role of the pituitary gland. J Endocrinol. 1973 Feb;56(2):295–301. doi: 10.1677/joe.0.0560295. [DOI] [PubMed] [Google Scholar]
- SIMPSON M. E., ASLING C. W., EVANS H. M. Some endocrine influences on skeletal growth and differentiation. Yale J Biol Med. 1950 Sep;23(1):1–27. [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Shapiro L. E. Thyroid hormone stimulates de novo growth hormone synthesis in cultured GH1 cells: evidence for the accumulation of a rate limiting RNA species in the induction process. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3369–3373. doi: 10.1073/pnas.73.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Modulation of thyroid hormone nuclear receptor levels by 3,5,3'-triiodo-L-thyronine in GH1 cells. Evidence for two functional components of nuclear-bound receptor and relationship to the induction of growth hormone synthesis. J Biol Chem. 1977 Sep 10;252(17):6052–6060. [PubMed] [Google Scholar]
- Seo H., Vassart G., Brocas H., Refetoff S. Triiodothyronine stimulates specifically growth hormone mRNA in rat pituitary tumor cells. Proc Natl Acad Sci U S A. 1977 May;74(5):2054–2058. doi: 10.1073/pnas.74.5.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachura M. E., Frohman L. A. "Large" growth hormone: ribonucleic acid-associated precursor of other growth hormone forms in rat pituitary. Endocrinology. 1974 Mar;94(3):701–712. doi: 10.1210/endo-94-3-701. [DOI] [PubMed] [Google Scholar]
- Stachura M. E., Frohman L. A. Large growth hormone: evidence for the association of growth hormone with another protein moiety in the rat pituitary. Endocrinology. 1973 Jun;92(6):1708–1713. doi: 10.1210/endo-92-6-1708. [DOI] [PubMed] [Google Scholar]
- Surks M. I., DeFesi C. R. Determination of the cell number of each cell type in the anterior pituitary of euthyroid and hypothyroid rats. Endocrinology. 1977 Sep;101(3):946–958. doi: 10.1210/endo-101-3-946. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Oppenheimer J. H. A new radioimmunoassay for plasma L-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement. J Clin Invest. 1972 Dec;51(12):3104–3113. doi: 10.1172/JCI107137. [DOI] [PMC free article] [PubMed] [Google Scholar]


