Abstract
Patients with systemic lupus erythematosus have a significantly increased risk of cardiovascular events due to atherosclerosis. Traditional cardiac risk factors cannot fully explain this increased risk. Recent evidence strongly suggests that atherosclerotic plaque is largely driven by inflammation and an active immunological response, in contrast to the long-held belief that plaque is a passive accumulation of lipids in the arterial wall. Current approaches to the prevention of atherosclerosis in systemic lupus erythematosus involve targeting modifiable cardiac risk factors. Future preventive strategies may include therapies that counteract the immunologic responses that lead to plaque formation.
Keywords: apoA-1, atherosclerosis, HMG-CoA reductase inhibitors, homocysteine, oxidized LDL, proinflammatory HDL, statins, systemic lupus erythematosus
Premature atherosclerosis is a major comorbid condition in systemic lupus erythematosus (SLE). While typical features of SLE, such as nephritis and vasculitis, have been the traditional focus of treatment, the identification of comorbid conditions such as atherosclerosis has become more important as the treatments for SLE improve and patients live longer.
The increased risk of cardiovascular disease in SLE was first recognized in 1976 by Urowitz et al. who described a bimodal pattern of mortality in their Toronto SLE cohort [1]. Of the 11 deaths in their cohort, six occurred within 1 year of diagnosis, and were attributed to active SLE disease. Five patients died at a mean of 8.6 years, and all five experienced a recent myocardial infarction (MI), with four out of five deaths attributed to fatal MI [1]. This bimodal pattern of mortality due to cardiovascular disease has been confirmed in subsequent studies.
The overall prevalence of clinical coronary heart disease in SLE patients has ranged from 6 to 10% in various cohorts [2–4]. This risk is increased compared with the general population; for example, in a Swedish lupus population described in 1989, the risk of coronary artery disease in SLE patients was increased ninefold compared with the age-matched general population [5]. In the Toronto cohort, there was a fivefold increased risk of MI among SLE patients compared with the general population. Furthermore, the MIs occurred at an average age of 49 years in SLE patients compared with 65–74 years in the general population. Manzi et al. also found that women with SLE in the 35–44-year age group were over 50-times more likely to have a MI than women of a similar age in the Framingham Offspring Study [2].
The incidence of subclinical atherosclerosis is also increased in SLE. In a cross-sectional study, Roman et al. compared 197 lupus patients and 197 matched controls using carotid ultrasound, and found that plaque was present in 37% of SLE patients compared with 15% of controls (p < 0.001) [6]. In a short-term longitudinal follow-up study of the SLE patients in this cohort, atherosclerosis developed or progressed at an average rate of 10% per year. Further studies have reflected similar prevalences of subclinical atherosclerosis in SLE [7]. Manzi and colleagues found subclinical carotid atherosclerosis in 40% of their cohort [8]. Asanuma et al. also found an increased prevalence of subclinical atherosclerosis when electron beam computerized tomography was used as the screening instrument, with coronary calcification present in 31% of SLE patients compared with 9% of controls [9]. When endothelial dysfunction, another marker of subclinical atherosclerosis, was used as a marker of atherosclerosis, 55% of SLE patients had impaired flow-mediated dilation, compared with 26.3% of control subjects [10].
What accounts for the increased risk of atherosclerosis seen in patients with SLE? Traditional cardiac risk factors defined by the Framingham studies, such as older age, high blood pressure and high cholesterol levels [11], do appear to play a role; however, these factors alone do not adequately explain the increased incidence of cardiovascular disease seen in patients with SLE, including increased risk for MI (increased relative risk: 10.1) and stroke (increased relative risk: 7.9) [12]. Thus, the etiology of the increased risk of atherosclerosis in SLE is likely multifactorial, resulting from a complex interplay between traditional cardiac risk factors and SLE-driven inflammation. To develop a fuller understanding of atherosclerosis in SLE, and to develop strategies for the prevention and treatment of cardiovascular complications, it is important to first have a complete understanding of the role that both traditional and nontraditional risk factors play in the pathogenesis of atherosclerosis in SLE.
Traditional risk factors & the pathogenesis of atherosclerosis in SLE
Although they do not fully explain the increase in atherosclerosis seen in SLE patients, both traditional cardiovascular risk factors defined by the Framingham Heart Studies [13] and SLE-specific risk factors have been identified in patients. Assessment of cardiovascular risk factors in the Hopkins Lupus Cohort reported in 1992, demonstrated that 53% of patients with SLE had at least three traditional risk factors [4]; however, in a risk assessment for coronary heart disease-related events using the Framingham risk assessment model, the mean 10-year risk of a cardiac event did not differ between 250 patients with SLE and 250 controls [14]. However, this study did reveal a higher prevalence of nontraditional cardiac risk factors in patients with SLE, including premature menopause, sedentary lifestyle and increased waist-to-hip ratio [14]. Further evidence of the contribution of nontraditional, or SLE-specific, cardiac risk factors was demonstrated in a Canadian cohort, which revealed that the relative risk of overall coronary artery disease in patients with SLE was at least sevenfold greater compared with predictions based on traditional Framingham risk factors [12]. Nevertheless, although they cannot fully account for the increased risk, traditional cardiac risk factors do contribute to increased atherosclerosis and cardiovascular events in SLE. In a London cohort, traditional cardiac risk factors did predict a higher risk of coronary disease in SLE patients under the age of 40 years [15]. In addition, most SLE cohort studies have identified at least one traditional cardiac risk factor as a significant predictor of cardiac events or subclinical atherosclerosis using multivariate modeling. Table 1 summarizes the various risk factors contributing to the development of accelerated atherosclerosis in patients with SLE.
Table 1. Traditional and nontraditional cardiac risk factors in patients with systemic lupus erythematosus.
| Risk factor | References showing positive association | References showing no association |
|---|---|---|
| Traditional risk factors | ||
| Dyslipidemia | [8,27] | [6,9] |
| Age | [2–4,6,7,9,16,27,206–208] | |
| Hypertension | [3,4,10,25,27] | |
| Diabetes mellitus | [3,207] | |
| Cigarette smoking | [25] | [6,9] |
| Menopausal status | [2] | |
| BMI | [27], association with increased IMT in children [26] | [6,9] |
| Homocysteine | [7,16] | |
| Nontraditional (SLE-specific) risk factors | ||
| Renal disease | [16,24,26] | [27] |
| SLE disease activity and duration | [6–8,16] | |
| Corticosteroid therapy | Inverse association: [6] High and low doses with increased IMT, moderate (0.15–0.4 mg/kg/day) doses with decreased IMT in children [26] and adults [27] | |
IMT: Intima-media thickness; SLE: Systemic lupus erythematosus.
SLE-specific risk factors
Disease activity & duration
The association between SLE disease activity and atherosclerosis has been poorly understood to date. Manzi et al. found an inverse relationship between SLE activity and plaque size, and noted that longer disease duration was independently associated with carotid plaque [8]. Von Feldt et al. found disease duration was significantly associated with coronary calcium scores in a cross-sectional cohort [16]. Similarly, Roman et al. found, in multivariate analysis, that longer disease duration and higher Systemic Lupus International Collaborative Clinics damage index were independent predictors of carotid plaque in both a cross-sectional [6] and a longitudinal study [7].
Renal disease
The prevalence of cardiovascular morbidity and mortality is higher in patients in the general population with chronic kidney disease, and is even more strongly associated with end-stage renal disease [17]. Renal disease also appears to be a risk factor for atherosclerosis in patients with SLE. Factors that may contribute to this increased risk include hypertension [18] and dyslipidemia [19], both of which are frequently seen in patients with proteinuria. Patients with proteinuria also have an increased risk of thrombosis [20,21]. Both proteinuria [22,23] and elevated serum creatinine [24,25] have been associated with early atherosclerosis in patients with SLE, and a history of nephritis has been associated with subclinical atherosclerosis in some [16,24,26], but not all studies [6,27].
Glucocorticoid therapy
Glucocorticoid usage has been associated with atherosclerosis in SLE patients [27]. Both longer duration of corticosteroid treatment [8,28] and a higher accumulated corticosteroid dose [8,24,29] have been associated with a higher incidence of atherosclerosis in various cohorts of patients with SLE, and may also impact traditional cardiac risk factors such as hypertension, obesity and diabetes. In addition, prednisone doses >10 mg/day have been shown to independently predict hypercholesterolemia in SLE [4]. However, conflicting data exists regarding the overall risk of glucocorticoid therapy; Roman et al. found that former or current use of prednisone and average dose of prednisone was significantly less in patients with carotid plaque, leading to the assumption that the anti-inflammatory effects of glucocorticoids may be atheroprotective [6].
Novel biomarkers/‘nontraditional’ cardiac risk factors
There are several novel biomarkers that have been implicated in the pathogenesis of atherosclerosis in SLE. Before discussing these novel biomarkers, however, it may be helpful to understand the relationship between inflammation and the development of atherosclerotic plaques in SLE.
Inflammation & the pathogenesis of atherosclerosis
For many years, atherosclerosis was regarded as a passive accumulation of lipids in the vessel wall. Recently, however, it has been realized that inflammation plays a role not only in the development of the atherosclerotic lesion, but also in the acute rupture of plaques that occurs during acute myocardial ischemic events [30,31]. As in the pathogenesis of SLE itself, the interplay of multiple inflammatory mediators, including leukocytes, cytokines, chemokines, adhesion molecules, complement and antibodies, results in the formation of atherosclerotic plaques [32]. To understand the role of inflammation in the development of atherosclerosis in SLE, it is important to first understand the development of the atherosclerotic plaque.
Recruitment of inflammatory cells to the arterial wall
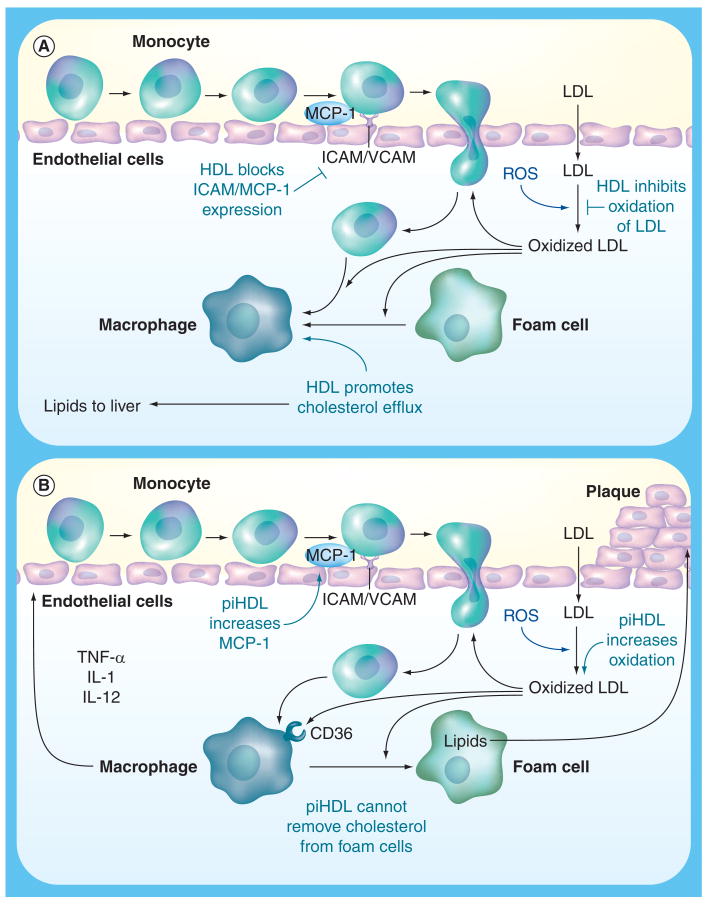
Atherosclerotic lesions begin with the recruitment of inflammatory cells such as monocytes and T cells to the endothelial wall. First, the vascular endothelial cells are stimulated to express leukocyte adhesion molecules, including E-selectin, vascular cell adhesion molecule-1 (VCAM-1) and inter-cellular adhesion molecule-1 (ICAM-1) [32]. These cell-surface proteins are upregulated during periods of inflammation, and can be induced by proinflammatory cytokines such as TNF-α and IL-1 [32]. VCAM-1 is also induced when endothelial cells are exposed to other inflammatory signals, such as the lipopolysaccharides of Gram-negative bacteria, lysophosphatidylcholine, and oxidized phospholipids such as oxidized low-density lipoprotein (OxLDL) [33,34]. High-density lipoproteins (HDL) inhibit the expression of adhesion molecules (Figure 1) [35,36].
Figure 1. Effects of high-density lipoprotein and proinflammatory high-density lipoprotein on atherosclerosis initiation and progression.
(A) Illustration of the interaction between LDL, the entrance of monocytes into the artery wall, formation of oxidized LDL (OxLDL), and the engulfment of OxLDL by macrophages to form foam cells. HDL interrupts this atherosclerotic process by reverse cholesterol transport of oxidized lipids from foam cells, by blocking endothelial cell activation, and by prevention of oxidation of LDL via antioxidative enzymes in the normal HDL particle such as paraoxonase.
(B) Proinflammatory, pro-oxidant HDL cannot carry out many of the protective functions of normal functioning HDL, leading to the formation of OxLDL, the release of chemokines and cytokines, the engulfment of OxLDL by CD36 receptors on macrophages to form foam cells and ultimately atherosclerotic plaque.
HDL: High-density lipoprotein; LDL: Low-density lipoprotein; MCP-1: Monocyte chemotactic protein-1; piHDL: Proinflammatory high-density lipoprotein; ROS: Reactive oxygen species.
The importance of these adhesion molecules in the development of atherosclerosis is highlighted by the fact that atherosclerosis-prone apoE-deficient mice who are also deficient in E-selectin develop fewer plaque lesions [37]. Also, soluble levels of VCAM-1 can be detected in the systemic circulation, and elevated levels of this adhesion molecule have been found in humans with coronary artery disease [38,39]. However, in one cross-sectional carotid ultrasound study of SLE patients neither levels of soluble VCAM nor ICAM were significantly associated with carotid plaque [6].
After leukocytes adhere to the cell surface, they migrate through the endothelium and into the intima [32]. This transmigration is influenced by several factors; first, several chemotactic proteins such as monocyte chemotactic protein-1 (MCP-1) are produced by the endothelial and smooth cell layers [40]. The expression of MCP-1 in smooth muscle cells and endothelial cells can be upregulated by cytokines such as TNF-α and IL-1 and by OxLDL (Figure 1) [40,41]. Conversely, normal HDL inhibits the expression of MCP-1 [42]. The importance of MCP-1 in the development of the atherosclerotic plaque is emphasized by the fact that elevated circulating levels of MCP-1 are positively related to increased carotid artery intima-media thickness (IMT) in humans [43]. Also, in low density lipoprotein receptor (LDLR)-/- mice, knockout of MCP-1 reduces atherosclerosis induced by high fat diets [44].
OxLDLs & the development of foam cells
Next, low-density lipoproteins (LDLs) are transported into artery walls, where they become trapped and bound in the extracellular matrix of the subendothelial space [45]. These trapped LDLs are then seeded with reactive oxygen species produced by nearby artery wall cells, resulting in the formation of proinflammatory OxLDL [45]. When endothelial cells are exposed to these proinflammatory OxLDL, they release cytokines such as MCP-1, M-CSF and GRO, resulting in monocyte binding, chemotaxis and differentiation into macrophages [46]. The OxLDLs are phagocytized by infiltrating monocytes/macrophages, which then become the foam cells around which atherosclerotic lesions are built (Figure 1) [47]. Elevated levels of circulating OxLDL are strongly associated with documented coronary artery disease in the general population [48]. Anti-OxLDL antibodies have been described in up to 80% of SLE patients with antiphospholipid antibody syndrome [49,50]. Some studies have demonstrated that anti-OxLDL are more common in SLE patients with a history of cardiovascular disease than in SLE controls or normal subjects [29]. In two other studies, however, anti-OxLDL and arterial disease were not associated [51,52]. Titers of anti-OxLDL have also been correlated with disease activity in SLE [53].
Next, monocytes and T cells infiltrate the margin of the plaque formed by foam cells [47], and smooth muscle cells from the media of the artery are stimulated to grow [54]. These muscle cells encroach on the lumen of the vessel and ultimately lead to fibrosis, which renders the plaques brittle. The occlusion that results in MI can occur when one of these plaques ruptures or when platelets aggregate in the narrowed area of the artery [54].
Normal HDL clears OxLDL from the endothelium
There are many mechanisms designed to clear OxLDL from the subendothelial space, such as macrophage engulfment using scavenger receptors [55–57], and enhanced reverse cholesterol transport mediated by HDL [58,59]. In addition, both HDL and its major apolipoprotein constituent, apoA-1, have been shown to both prevent and reverse LDL oxidation (Figure 1) [60–63].
Role of cytokines in atherosclerosis
T cells, primarily of the Th1 subtype, are also abundant in atherosclerotic lesions, and may play a role in the formation of plaque through the cascade of cytokines that is initiated by their activation [64]. Vascular endothelial and smooth muscle cells are important targets for inflammatory cytokines and these cells can produce additional cytokines when stimulated [30]. At least two stimuli for Th1 differentiation are present in the atherosclerotic plaque. IL-12 is expressed by macrophages, smooth muscle cells and endothelial cells, and is an important stimulus for Th1 differentiation [65]. Elevated levels of IL-12 have been found in atherosclerotic plaques [65], and the inhibition of IL-12 using a vaccination technique that fully blocks the action of IL-12 has been shown to decrease atherosclerosis in mice [66]. IL-12 production is upregulated in monocytes exposed to OxLDL [65].
IFN-γ has also been detected in human plaques [32]. It is a powerful growth inhibitor for smooth muscle cells, endothelial cells and collagen production, and thus promotes plaque instability [67]. In addition, IFN-γ induces the expression of secretory phospholipase A2, leading to the production of inflammatory lipid mediators such as lysophosphatidylcholine, platelet-activating factor and eicosanoids [68]. IFN-γ also improves the efficiency of antigen presentation, and leads to increased synthesis of TNF-α and IL-1 [69]. All of these actions contribute to the formation of the atherosclerotic plaque, and indeed, in atherosclerosis-prone apoE-knockout mice also lacking IFN-γ, atherosclerosis is decreased by nearly 60% [70,71]. The administration of IFN-γ also accelerates atherosclerosis in apoE-knockout mice. Increased levels of both IFN-γ and IL-12 have been found in humans with both unstable and stable angina compared with controls [72].
As noted, TNF-α and IL-1 are also present in human atherosclerotic lesions. Like IFN-γ, they also affect smooth muscle proliferation. TNF-α and IL-1 induce local inflammation in blood vessels by stimulating the activation of macrophages [73], inducing the secretion of matrix metalloproteinases [74] and promoting the secretion of cell surface adhesion molecules [32]. TNF-α can also upregulate the expression of cell surface adhesion proteins; normal functioning HDL can inhibit this upregulation [75]. TNF-α and IL-1 can also inhibit lipoprotein lipase, an enzyme important in the metabolism of triglycerides and very LDL [76,77]. In addition, TNF-α and IL-1 enhance production of M-CSF, GM-CSF and G-CSF by smooth muscle cells, endothelial cells and monocytes. These mediators activate monocytes and stimulate their transformation into macrophages and foam cells [78]. Inhibition of TNF-α decreased the progression of atherosclerosis in apoE-knockout mice [79]. Elevated levels of TNF-α may also play a role in the increased risk of atherosclerosis in the general population [80]. TNF-α has been linked to vascular injury in both acute and chronic inflammatory conditions. TNF-α has been identified in human endothelial and smooth muscle cells in all stages of atherosclerosis, from early intima thickening to established occlusive atherosclerosis [81,82].
In addition to TNF-α and IL-1, the proinflammatory cytokine pathway also involves the expression of IL-6 [32]. Circulating IL-6 is another cytokine that is a strong independent marker of increased mortality in unstable coronary artery disease [83]. IL-6 stimulates hepatocytes to produce C-reactive protein (CRP) and other markers of inflammation [84]. CRP is not only a marker of acute phase reaction, but may also have a direct effect on leukocyte recruitment and apoptosis in vessel walls [85,86]. IL-6 is also required for short-term regulation of paraoxonase, an antioxidant enzyme present in HDL [87]. LDL-derived phospholipids such as oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine induce the expression of IL-6, which in turn downregulates paraoxonase mRNA levels [83]. Patients with unstable coronary syndromes have elevated levels of both IL-6 and CRP [88,89]. In addition, IL-6 has been described as an independent predictor of endothelial dysfunction in rheumatoid arthritis patients [90].
By contrast, TGF-β likely plays a protective role against atherosclerotic plaque formation, as discussed in several publications [39,70–76]. Thus, the balance of proinflammatory and anti-inflammatory cytokines and their interactions with inflammatory cells and lipid components contributes to the formation and maintenance of the atherosclerotic plaque.
Antiphospholipid antibodies
In animal studies, the role of antiphospholipid antibodies in the development of atherosclerosis has been inconsistent. Some have reported an association between antiphospholipid antibodies and accelerated atherosclerosis [91,92], while others have suggested that antiphospholipid antibodies actually play a protective role in the pathogenesis of atherosclerosis [93,94]. Patients with primary antiphospholipid syndrome have been shown to have thicker carotid artery intima-media at the carotid bifurcation and internal carotid artery compared with controls, especially those over 40 years of age [95]. In healthy men, elevated antiphospholipid antibodies have been correlated with an increased risk of future MI [96,97], and in renal transplant patients, the presence of antiphospholipid antibodies has been associated with a relative risk for an atherosclerotic event of 2.82 compared with those without antiphospholipid antibodies [98]. By contrast, although approximately half of patients with SLE possess antiphospholipid antibodies [91], reports of an association between antiphospholipid antibodies and atherosclerosis in cohorts of patients with SLE have been inconsistent. In a study of a diverse cohort of patients with SLE, antiphospholipid antibodies were found to be an independent predictor of cardiovascular, cerebrovascular or peripheral vascular events [99]. In the Hopkins Lupus cohort, 10% of patients with SLE developed MI, and patients with a positive lupus anticoagulant were more likely to develop an MI compared with controls; however, this association was not consistent with presence of anticardiolipin antibodies or evidence of subclinical atherosclerosis [100]. Similarly, in another study, while a positive association between coronary calcification scores and antiphospholipid antibodies was reported in univariate analysis, this association was no longer significant after adjustment for age and sex [9]. Finally, a significant association between antiphospholipid antibodies and atherosclerosis was not found in three large cohorts of patients with SLE [6,8,27]. The inconsistent results of these studies have raised questions about the exact nature of the effect of antiphospholipid antibodies on the risk of atherosclerotic events. One study that sought to confirm in vitro evidence of a role for antiphospholipid-mediated endothelium perturbation in antiphospholipid syndrome-associated vasculopathy reported that patients with antiphospholipid antibody syndrome did not differ in plasma levels of circulating endothelial cells and flow-mediated vasodilation, suggesting that antiphospholipid antibodies alone are not able to support a full-blown endothelial perturbation in vivo [101]. These findings suggest a ‘two-hit hypotheses’ in which antiphospholipid antibodies may increase the risk of thrombotic events by inducing a threshold endothelial perturbation; however, other thrombophilic conditions are necessary to trigger clot formation. Further studies are needed to identify the contribution of antiphospholipid antibodies to the development of atherosclerosis in patients with SLE.
Innate immunity in atherosclerosis
In addition to the role the adaptive immune response plays in the pathogenesis of atherosclerosis, there is accumulating evidence that innate immunity also plays a role in the formation of plaques. In contrast to adaptive immunity, the components of innate immunity are essentially present at birth, and allow for immediate host defenses until adaptive responses mature. The receptors of innate immunity are known as pattern recognition receptors; these receptors bind to preserved motifs on various pathogens that are termed pathogen-associated molecular patterns. Toll-like receptors are one type of pattern recognition receptors that respond to various pathogen-associated molecular patterns by activating their intracellular signaling pathway, leading to the upregulation of immune responsive genes [102]. The ligands for Toll-like receptors can include microbial ligands, which may explain some of the connections that have been postulated to exist between infectious organisms such as Chlamydia pneumoniae and the development of atherosclerosis [103].
Endogenous ligands can also trigger TLR signaling in a manner similar to microbial ligands. For example, minimally OxLDL interacts with TLR4 and with the scavenger receptors CD14 and CD36 [104]. When OxLDL binds to the CD14 receptor on macrophages, there is an inhibition of phagocytosis of apoptotic cells, and enhanced expression of the scavenger receptor CD36, which leads to increased uptake of OxLDL. Both of these effects are thought to be proinflammatory and proatherogenic [104].
Activation of TLR-7 and -9, resulting in the upregulation of IFN-α, has also recently been shown to play a major role in lupus disease activity [105]. This pathway may also have implications in atherogenesis, as IFN-α plays a crucial role in premature vascular damage in SLE by altering the balance between endothelial cell apoptosis and vascular repair [106]. High IFN-α levels have been associated with endothelial dysfunction in patients with SLE [107].
Novel biomarkers of atherosclerosis in SLE
Antibodies against apoA-I
As noted, apoA-I is the major apolipoprotein component of HDL. Reduced levels of apoA-I have been found in rheumatoid arthritis patients [108], and in SLE patients with IgG anticardiolipin antibodies [109]. In the general population, antibodies to apoA-I have been found in up to 21% of patients with acute coronary syndromes who have no other features of autoimmune disease [110]. Antibodies to apoA-I have also been described in SLE. In one study, antibodies to apoA-I were found in 32.5% of patients with SLE and 22.9% of patients with primary antiphospholipid syndrome [111], and have been associated with increased disease activity [112]. It is unclear, however, how the presence of these antibodies affects the function of apoA-I in either SLE or acute coronary syndrome patients.
Homocysteine
Homocysteine is another predictor of atherosclerosis in the general population [113]. Homocysteine is a metabolite in methionine production, and may play a direct role in the pathogenesis of SLE through its toxic effects on the endothelium [114]. Homocysteine is also prothrombotic [115] and decreases the availability of nitric oxide [116]. High levels stimulate monocytes to secrete MCP-1 and IL-8 [117]. The thiolactone metabolite of homocysteine combines with LDL to enhance foam cell formation in vessel walls [118]. The molecule releases free oxygen radicals that can damage tissue [119], and has several prothrombotic actions on platelets and endothelial cells [120]. Hyperhomocysteinemia can result from genetic and/or dietary factors. As previously noted, population studies have identified an association between high homocysteine levels and atherosclerosis in the general population [118]. Petri has prospectively demonstrated that elevated homocysteine levels may also be a risk factor for the later development of coronary artery disease in SLE patients [28]. In several studies, elevated levels of homocysteine have also correlated with atherosclerosis in SLE [14,16,28,29,121]. In other recent studies of SLE, however, homocysteine has not correlated with evidence of plaque on carotid ultrasound [6,9,23].
Adipocytokines
White adipose tissue, traditionally viewed as a simple energy storage site, has more recently been recognized as an endocrine organ that secretes adipokines such as leptin and adiponectin. These adipokines are responsible for regulating energy homeostasis and metabolism. Leptin is an anorectic peptide that functions as a hypothalamic modulator of food intake, bodyweight and fat stores [122]. Obese patients develop resistance to leptin similar to insulin resistance in Type 2 diabetes [123], and high circulating leptin levels are seen in overweight individuals [124]. Hyperleptinemia in the general population also associates with hypertension [125], metabolic syndrome [124] and atherosclerosis [124]. In addition, leptin has been linked to increased oxidative stress. Elevated leptin induces oxidative stress in endothelial cells [126] and cardiomyocytes [127]. Serum levels of leptin are correlated with OxLDL in postmenopausal women and decreased leptin after weight loss is predictive of reduced OxLDL levels [128]. Several small cohort studies have demonstrated elevated leptin levels in adult [129–131] and pediatric [132] SLE patients. Conversely, adiponectin is the most abundant adipocytokine in human plasma, and levels are inversely correlated with adipose tissue mass [133]. Adiponectin levels are reduced in Type 2 diabetes and cardiovascular disease [134].
In our cohort, leptin levels were examined in 244 SLE subjects. Mean leptin levels were significantly higher in the 40 patients with plaque than in those without plaque, and also weakly correlated with carotid IMT [135]. In another cohort, adiponectin levels were significantly and independently associated with carotid plaque in SLE [136]. However, Chung et al. found no significant relationship between leptin or adiponectin levels and coronary calcification measured by electron beam computerized tomography in 109 SLE patients and 78 control subjects [137].
Proinflammatory HDL
Although quantities of HDL partially determine atherosclerotic risk (low levels are associated with increased risk), HDL function is equally significant [73]. For example, during the acute phase response HDLs can be converted from their usual anti-inflammatory state to proinflammatory, and can actually cause increased oxidation of LDL [138]. This acute phase response can also become chronic, and may be a mechanism for HDL dysfunction in SLE [139]. Indeed, our group has found that HDL function is abnormal in many women with SLE; 45% of women with SLE, compared with 20% of rheumatoid arthritis patients and 4% of controls, had proinflammatory HDL (piHDL) that was not only unable to prevent oxidation of LDL but caused increased levels of oxidation [140]. In this study, four out of four SLE patients with a history of documented atherosclerosis had piHDL, further suggesting that HDL plays an important role in the pathogenesis of atherosclerosis. Subsequent studies have indicated that 85% of SLE women with plaque have piHDL, indicating that piHDL may be a biomarker of risk for atherosclerosis in SLE [27].
Potential for future intervention: existing therapies
Currently, no randomized clinical trials for the prevention of atherosclerosis in SLE exist to guide clinicians; therefore, the best recommendations for therapy are based on prevention guidelines for the general population. Some experts have advocated that SLE, like diabetes, should be considered a coronary heart disease equivalent, and targets for blood pressure and lipid levels should be adjusted accordingly [141]. Special attention should be paid to all modifiable risk factors; in addition, control of inflammation and disease activity may also be desirable from a cardiac risk modification standpoint.
Smoking cessation
Smoking is a well-established modifiable risk factor for coronary carotid atherosclerosis [142,143], and smoking cessation is recommended for patients with SLE [144].
Statins
Statins are competitive inhibitors of HMG-CoA reductase, the rate-limiting step in cholesterol biosynthesis [145], and are now used widely to reduce cardiovascular morbidity [146–148]. Statins have a variety of direct anti-inflammatory and immunomodulatory effects. For example, statins have been demonstrated to decrease the secretion of proinflammatory cytokines and chemokines such as IL-6, IL-8, TNF-α and MCP-1 [149–151], and have been found in several in vitro and in vivo studies to both diminish secretion of proatherogenic Th1 subtype cytokines such as IFN-γ, IL-2 and IL-12 [150,152], and increase secretion of antiatherogenic Th2 cytokines such as IL-4 and IL-10 [153–155]. These findings, however, have not been consistently demonstrated in all studies [150,156]. Statins have also been shown to directly inhibit IFN-γ-induced MHC class II expression in both macrophages [157] and endothelial cells [158], thus acting as a repressor of MHC-II-mediated T-cell activation. Statins also downregulate molecules involved in adhesion (e.g., ICAM-1, VCAM-1 and selectins) [159,160] and costimulation (e.g., CD40L) [161].
There is an abundance of data to support the use of statins in primary and secondary prevention of atherosclerosis in the general population [146,162,163]. Several studies have also examined the efficacy of statins in prevention of atherosclerosis in rheumatic diseases. In one study using a rat model of adjuvant-induced arthritis, fluvastatin reversed aortic endothelial dysfunction, although it did not affect the severity of arthritis or serum cholesterol concentrations [164]. The statins also decreased reactive oxygen species production in the aorta [164]. Another study examined the effect of statins in a mouse model of SLE and atherosclerosis, the gld.apoE-/- mouse [165]. Although simvastatin therapy did not alter cholesterol levels, it did decrease the atherosclerotic lesion area in both gld.apoE-/- and apoE-/- mice. In addition, simvastatin reduced lymphadenopathy, renal disease and proinflammatory cytokine production in the double-knockout mouse [165]. Although further studies are necessary, these findings raise the possibility that statins may be beneficial not only in reducing the increased atherosclerosis of rheumatic disease, but also the disease-related inflammation.
There are also some data to support the use of statins in patients with SLE. In a trial of 64 women with SLE, atorvastatin 20 mg daily for 8 weeks improved endothelium-dependent vasodilation, even after accounting for the presence of traditional cardiac risk factors [166]. In a 2-year randomized-controlled trial of atorvastatin in 200 women with SLE, however, statins did not significantly prevent progression of coronary calcium, IMT or disease activity [167]. Similarly, in a trial of 33 post-renal transplant lupus patients, those randomized to fluvastatin therapy had a 73% reduction in cardiac events, although this difference did not quite reach statistical significance (p = 0.06) [168]. Many trials that have demonstrated a preventive effect of statins in the general population have larger sample sizes and a longer follow-up duration [169], so it is possible that increased sample sizes and study lengths might have resulted in positive studies. Further investigations are needed to clarify the potential role of statins in the prevention of atherosclerosis in rheumatic disease populations. Until further studies are conducted to determine the safety and efficacy of statin therapy in a broader population of patients with SLE, statin therapy should be limited to published guidelines such as the National Cholesterol Education Panel [170].
Hypertension
Similar to the recommendations for management of dyslipidemia, patients with SLE should be treated to the target blood pressure levels recommended for those with other high-risk comorbid conditions such as diabetes (i.e., systolic blood pressure <130 mmHg; diastolic blood pressure <80 mmHg) [171], with a minimally acceptable blood pressure of 140/90 mmHg. Angiotensin-converting enzyme inhibitors are generally the drug of choice in patients with renal disease [172]; however, difficulty in recruitment to prevention trials has thus far prevented investigators from prospectively establishing the most atheroprotective medication regimen in patients with SLE [173].
Antimalarial therapy
Hydroxychloroquine is thought to be cardioprotective [174], and in fact, Selzer et al. noted that non-use of hydroxychloroquine was associated with higher aortic stiffness in SLE patients measured by ultrasound [175]. Roman et al. also found that patients with carotid artery plaque used less hydroxychloroquine [6]. In addition, antimalarials have been shown to lower total cholesterol in patients receiving steroids, and may minimize steroid induced hypercholesterolemia [176]. Patients with SLE taking an antimalarial agent have also been shown to have lower fasting blood glucose conentrations – a risk factor for premature atherosclerosis – compared with controls [177], and one study demonstrated that hydroxychloroquine prolongs the half-life of the active insulin-receptor complex via inhibition of the insulin-receptor dissociation, thus augmenting insulin-stimulated responses [178]. In addition, beneficial effects of hydroxychloroquine on thrombosis formation have also been described. Hydroxychloroquine has been shown in animal studies to reduce thrombosis induced by antiphospholipid antibody exposure [179] and to reverse platelet aggregation [180]. Multiple retrospective cohort studies have shown a reduced incidence of thrombotic events [181–184] and improved overall survival in patients with SLE treated with antimalarial agents [183,185]. The recent understanding that one mechanism of action of hydroxychloroquine is the antagonism of TLR-7 and -9 signaling is also intriguing, given the postulated roles of IFN-α in endothelial dysfunction and abnormal vascular repair [186]. Prospective studies demonstrating a cardioprotective effect of hydroxychloroquine in patients with SLE are needed.
Glucocorticoids
As discussed previously, glucocorticoid usage has been associated with atherosclerosis in SLE patients [27], although it is unclear whether steroid use is atheroprotective or contributes to added cardiovascular disease risk in SLE patients. In a pediatric lupus cohort, moderate doses of prednisone (0.15–0.4 mg/kg/day) were associated with decreased carotid artery IMT, while high- and low-dose prednisone were associated with increased IMT [26], suggesting that a narrow therapeutic window for the atheroprotective effects of glucocorticoid therapy may exist.
Future directions
B-cell-directed therapies
Several studies have suggested a protective role for activated B cells in the formation of atherosclerotic plaques; for example, both splenectomy [187] and transfer of B-cell-deficient bone marrow into lethally irradiated atherosclerosis prone mice [188] resulted in an increase in atherosclerotic lesion development. This raises some concerns that the B-cell-depleting therapies that are on the horizon as treatments of SLE could have the unintended consequence of increasing atherosclerosis. However, a recent study by Ait-Oufella et al. demonstrated that B-cell depletion with an anti-CD20 specific monoclonal antibody significantly reduced atherosclerosis in both the apoE-/- and the LDLR-/- atherosclerosis-prone mouse models [189]. This work complements a recent human study that demonstrates improvement in proatherogenic lipid profiles in SLE patients treated with B-cell-depletion therapy [190]. Further studies will be required to determine the effects of B-cell-targeted therapies on the development of atherosclerosis in patients with SLE.
Mimetic peptides in rheumatic diseases
Oxidized lipids represent another potential new target for therapy, not only in the prevention of atherosclerosis in SLE, but also in the treatment of inflammatory disease manifestations. There is great interest in the therapeutic potential of specific peptides derived from HDL-related proteins in the prevention of atherosclerosis. Several peptide fragments from apoA-I, such as the 4-F peptide, have been selected for their ability to prevent the inflammation induced by oxidized lipids [191].
Animal models suggest that a synergistic combination of therapies, such as statins and apoA-I mimetic peptides, may be a successful strategy to reverse oxidized lipids and possibly atherosclerosis [192,193]. Oral administration of D-4F plus pravastatin regresses established lesions in apoE-null mice and renders HDL anti-inflammatory in monkeys, suggesting a combination treatment strategy may be particularly effective in treating human atherosclerosis [194]. D-4F is currently in clinical trials in humans, and a Phase I trial recently demonstrated safety and tolerability, as well as an ability to improve the HDL inflammatory index [195]. It is possible that previously unexpected negative trial results, such as the Lupus Atherosclerosis Prevention Study (LAPS) trial of statins in SLE, may be partially explained by the inability of a single agent to reverse oxidized lipids [167]. The future of therapy in rheumatic diseases may well entail combination therapy, with agents to inhibit generalized inflammation and disease activity, and other agents to target oxidized lipids.
Owing to the anti-inflammatory nature of these peptides, it may not be surprising that there have also been implications that apoA-1 mimetics may be of use in treating other inflammatory manifestations of disease. A recent study with the L-4F peptide plus pravastatin in a murine SLE model (apoE-/-Fas-/-C57BL/6) resulted in significant reductions in proteinuria, glomerulonephritis and osteopenia. Aortic lesion size was increased in treated mice; however, there was evidence of anti-inflammatory plaque remodeling, with decreases in macrophage infiltration and proatherogenic chemokines, and increased smooth muscle content [196]. Future work will determine if these mimetic peptides are useful for preventing atherosclerosis and/or other complications in patients with SLE.
Discovery of novel targets for atherosclerosis prevention in SLE
Future studies into the mechanisms behind increased atherosclerosis in SLE may identify new pathways that can be targeted for therapy. For example, recent work by Denny et al. described an abnormal phenotype and functionality in endothelial progenitor cells and myelomonocytic circulating angiogenic cells in SLE subjects, indicative of endothelial dysfunction [106]. These abnormalities were triggered by IFN-α, and neutralization of interferon pathways restored a normal endothelial progenitor cells/circulating angiogenic cells phenotype [106]. At least two monoclonal antibodies against IFN-α are currently entering clinical trials in SLE patients; if efficacious, future studies should examine their effectiveness in preventing atherosclerosis.
Our group recently reported that PDGF receptor-β (PDGFRβ) is upregulated in monocytes from SLE patients with piHDL and carotid artery plaque [197]. Both monocyte chemotaxis and TNF-α secretion were significantly increased when cells were treated with piHDL in vitro compared with normal HDL. Notably, piHDL-driven chemotaxis and TNF-α levels were reduced to levels observed in cells treated with normal HDL with concomitant treatment using the small-molecule PDGFRβ kinase inhibitor imatinib (Gleevec®, Novartis, Basel, Switzerland). Cells treated with normal HDL and imatinib did not behave differently to normal HDL-treated cells alone, suggesting that piHDL dysregulates the PDGFR signaling axis in monocytes and a therapeutic intervention in this signaling pathway could neutralize atherogenic cells. Although chronic imatinib treatment for subjects with atherosclerosis would not be recommended due to multiple side effects, including the controversial possibility of cardiotoxicity [198–202], mechanistic studies into atherosclerosis-specific dysregulated signaling pathways driving monocyte and T cells could lead to novel, safe molecular targets for the treatment of accelerated preclinical atherosclerosis in autoimmune diseases.
Expert commentary & five-year view
It is now well recognized that patients with SLE are at an increased risk of cardiovascular disease. Cardiovascular risk in patients with SLE is multifactorial, comprising both an increased incidence of many traditional cardiovascular risk factors and the occurrence of SLE-specific factors, including disease activity and duration, and drug therapy. Management of both traditional and SLE-specific risk factors is important to effectively prevent and treat cardiovascular disease in patients with SLE. Although traditional cardiac risk factors cannot fully account for the increased risk of atherosclerosis in SLE, they do contribute, and at this time, they provide our best strategy for modifying cardiovascular risk in our patients. Data from several cohorts suggest that control of traditional risk factors has not been optimized in the SLE patient population; for example, the new quality indicators for SLE recommend that SLE subjects be screened annually for cardiac risk factors [203]; however, in a Boston cohort, only 26% of the patients had four cardiac risk factors assessed annually [204]. Similarly, in the Systemic Lupus International Collaborative Clinics cohort, hypercholesterolemia was not treated in up to two-thirds of patients [205]. In the future, long-term, well-controlled trials will provide evidence to support the use of traditional preventive strategies, as well as increased understanding of the role of current immunosuppressive and future therapies for combating atherosclerosis in SLE.
Inflammation is associated with the increased development of atherosclerosis in patients with SLE. It is unclear, however, if targeting disease activity with anti-inflammatory therapies will be adequate to decrease the incidence of cardiovascular disease in patients with SLE. Unanswered questions include how existing and developing therapies for SLE will affect future cardiovascular risk, and whether traditional and novel biomarkers of cardiovascular risk in SLE patients can be used to monitor response to these therapies. Advances in the next 5–10 years may make clear the best strategies for preventing atherosclerosis in our SLE patient population.
Key issues.
Individuals with systemic lupus erythematosus (SLE) have a significantly increased risk for developing cardiovascular disease (CVD) at a younger age.
Increased incidence of CVD in SLE is due to a combination of traditional (Framingham) and SLE-specific risk factors.
The longer a patient has had SLE, the higher the risk of developing CVD; it is unclear at this time whether long-term and/or high glucocorticoid use (as SLE therapy) promotes or protects against full CVD.
Novel biomarkers for accelerated CVD in SLE identified in recent years include elevated homocysteine and leptin levels, in addition to dysfunctional high-density lipoprotein.
Current therapeutic approaches to prevent CVD in SLE include following recommended national guidelines to target modifiable traditional cardiac risk factors such as hypertension, dyslipidemia, BMI, diabetes and tobacco use.
Future novel therapeutic approaches to counteract accelerated CVD specifically in SLE may include ApoA-I mimetic peptides and B-cell depletion therapy, as well as delineating dysregulated pathways in immune cells that directly contribute to atherosclerosis initiation and progression then targeting molecules in these pathways with small molecule inhibitors.
Acknowledgments
Supported by grants from the Arthritis Foundation, NIH/NIAMS 1K23AR053864-01A1 and Rheuminations.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60(2):221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145(5):408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Urowitz MB. Morbidity in systemic lupus erythematosus. J Rheumatol. 1987;14(Suppl. 13):S223–S226. [PubMed] [Google Scholar]
- 4.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93(5):513–519. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 5.Jonsson H, Nived O, Sturfelt G. Outcome in systemic lupus erythematosus: a prospective study of patients from a defined population. Medicine (Baltimore) 1989;68(3):141–150. [PubMed] [Google Scholar]
- 6•.Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2399–2406. doi: 10.1056/NEJMoa035471. Describes the incidence of subclinical atherosclerosis in a large cross-sectional cohort of systemic lupus erythematosus (SLE) and control subjects, and describes risk factors associated with the presence of carotid plaque. [DOI] [PubMed] [Google Scholar]
- 7•.Roman MJ, Crow MK, Lockshin MD, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56(10):3412–3419. doi: 10.1002/art.22924. Describes the longitudinal progression of subclinical atherosclerosis in a cohort of SLE and control subjects and describes risk factors associated with the presence of carotid plaque progression, including homocysteine. [DOI] [PubMed] [Google Scholar]
- 8.Manzi S, Selzer F, Sutton-Tyrrell K, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42(1):51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 10.El-Magadmi M, Bodill H, Ahmad Y, et al. Systemic lupus erythematosus: an independent risk factor for endothelial dysfunction in women. Circulation. 2004;110(4):399–404. doi: 10.1161/01.CIR.0000136807.78534.50. [DOI] [PubMed] [Google Scholar]
- 11.Salel AF, Fong A, Zelis BS, Miller RR, Borhani NO, Mason DT. Accuracy of numerical coronary profile Correlation of risk factors with arteriographically documented severity of atherosclerosis. N Engl J Med. 1977;296(25):1447–1450. doi: 10.1056/NEJM197706232962507. [DOI] [PubMed] [Google Scholar]
- 12•.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. Confirmed that traditional Framingham risk factors do not fully explain the high incidence of atherosclerosis in SLE. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Schwartz MJ, McNamara PM. Blood pressure and risk of coronary heart disease: the Framingham study. Dis Chest. 1969;56(1):43–52. doi: 10.1378/chest.56.1.43. [DOI] [PubMed] [Google Scholar]
- 14.Bruce IN, Urowitz MB, Gladman DD, Ibanez D, Steiner G. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 2003;48(11):3159–3167. doi: 10.1002/art.11296. [DOI] [PubMed] [Google Scholar]
- 15.Bessant R, Hingorani A, Patel L, MacGregor A, Isenberg DA, Rahman A. Risk of coronary heart disease and stroke in a large British cohort of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2004;43(7):924–929. doi: 10.1093/rheumatology/keh213. [DOI] [PubMed] [Google Scholar]
- 16.Von Feldt JM, Scalzi LV, Cucchiara AJ, et al. Homocysteine levels and disease duration independently correlate with coronary artery calcification in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(7):2220–2227. doi: 10.1002/art.21967. [DOI] [PubMed] [Google Scholar]
- 17.Drueke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6(12):723–735. doi: 10.1038/nrneph.2010.143. [DOI] [PubMed] [Google Scholar]
- 18.Mak A, Mok CC, Chu WP, To CH, Wong SN, Au TC. Renal damage in systemic lupus erythematosus: a comparative analysis of different age groups. Lupus. 2007;16(1):28–34. doi: 10.1177/0961203306074469. [DOI] [PubMed] [Google Scholar]
- 19.Leong KH, Koh ET, Feng PH, Boey ML. Lipid profiles in patients with systemic lupus erythematosus. J Rheumatol. 1994;21(7):1264–1267. [PubMed] [Google Scholar]
- 20.Nickolas TL, Radhakrishnan J, Appel GB. Hyperlipidemia and thrombotic complications in patients with membranous nephropathy. Semin Nephrol. 2003;23(4):406–411. doi: 10.1016/s0270-9295(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 21.Ordonez JD, Hiatt RA, Killebrew EJ, Fireman BH. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int. 1993;44(3):638–642. doi: 10.1038/ki.1993.292. [DOI] [PubMed] [Google Scholar]
- 22.Theodoridou A, Bento L, D'Cruz DP, Khamashta MA, Hughes GR. Prevalence and associations of an abnormal ankle-brachial index in systemic lupus erythematosus: a pilot study. Ann Rheum Dis. 2003;62(12):1199–1203. doi: 10.1136/ard.2002.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manger K, Kusus M, Forster C, et al. Factors associated with coronary artery calcification in young female patients with SLE. Ann Rheum Dis. 2003;62(9):846–850. doi: 10.1136/ard.62.9.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doria A, Shoenfeld Y, Wu R, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62(11):1071–1077. doi: 10.1136/ard.62.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selzer F, Sutton-Tyrrell K, Fitzgerald SG, et al. Comparison of risk factors for vascular disease in the carotid artery and aorta in women with systemic lupus erythematosus. Arthritis Rheum. 2004;50(1):151–159. doi: 10.1002/art.11418. [DOI] [PubMed] [Google Scholar]
- 26•.Schanberg LE, Sandborg C, Barnhart HX, et al. Premature atherosclerosis in pediatric systemic lupus erythematosus: risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum. 2009;60(5):1496–1507. doi: 10.1002/art.24469. Cross-sectional cohort study describing the risks associated with increased intima-media thickness in a pediatric lupus population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.McMahon M, Grossman J, Skaggs B, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 2009;60(8):2428–2437. doi: 10.1002/art.24677. Describes the association between subclinical atherosclerosis and a novel biomarker, proinflammatory high-density lipoprotein, in a cross-sectional cohort of SLE subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus. 2000;9(3):170–175. doi: 10.1191/096120300678828226. [DOI] [PubMed] [Google Scholar]
- 29.Svenungsson E, Jensen-Urstad K, Heimburger M, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104(16):1887–1893. doi: 10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]
- 30•.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. Review of the role of inflammation in the pathogenesis of the atherosclerotic plaque. [DOI] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(12):1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 33.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90(3):1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson AD, Leitinger N, Navab M, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272(21):13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 35.Calabresi L, Franceschini G, Sirtori CR, et al. Inhibition of VCAM-1 expression in endothelial cells by reconstituted high density lipoproteins. Biochem Biophys Res Commun. 1997;238(1):61–65. doi: 10.1006/bbrc.1997.7236. [DOI] [PubMed] [Google Scholar]
- 36.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15(11):1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 37.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102(1):145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 39.Mehra VC, Ramgolam VS, Bender JR. Cytokines and cardiovascular disease. J Leukoc Biol. 2005;78(4):805–818. doi: 10.1189/jlb.0405182. [DOI] [PubMed] [Google Scholar]
- 40.Wang JM, Sica A, Peri G, et al. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb. 1991;11(5):1166–1174. doi: 10.1161/01.atv.11.5.1166. [DOI] [PubMed] [Google Scholar]
- 41.Torzewski J, Oldroyd R, Lachmann P, Fitzsimmons C, Proudfoot D, Bowyer D. Complement-induced release of monocyte chemotactic protein-1 from human smooth muscle cells. A possible initiating event in atherosclerotic lesion formation. Arterioscler Thromb Vasc Biol. 1996;16(5):673–677. doi: 10.1161/01.atv.16.5.673. [DOI] [PubMed] [Google Scholar]
- 42.Barter PJ, Baker PW, Rye KA. Effect of high-density lipoproteins on the expression of adhesion molecules in endothelial cells. Curr Opin Lipidol. 2002;13(3):285–288. doi: 10.1097/00041433-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Larsson PT, Hallerstam S, Rosfors S, Wallen NH. Circulating markers of inflammation are related to carotid artery atherosclerosis. Int Angiol. 2005;24(1):43–51. [PubMed] [Google Scholar]
- 44.Gu L, Okada Y, Clinton SK, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2(2):275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 45.Camejo G, Olofsson SO, Lopez F, Carlsson P, Bondjers G. Identification of apo B-100 segments mediating the interaction of low density lipoproteins with arterial proteoglycans. Arteriosclerosis. 1988;8(4):368–377. doi: 10.1161/01.atv.8.4.368. [DOI] [PubMed] [Google Scholar]
- 46.Navab M, Hama SY, Cooke CJ, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41(9):1481–1494. [PubMed] [Google Scholar]
- 47.Navab M, Berliner JA, Watson AD, et al. The yin and yang of oxidation in the development of the fatty streak A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol. 1996;16(7):831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- 48.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353(1):46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 49.Vaarala O, Alfthan G, Jauhiainen M, Leirisalo-Repo M, Aho K, Palosuo T. Crossreaction between antibodies to oxidised low-density lipoprotein and to cardiolipin in systemic lupus erythematosus. Lancet. 1993;341(8850):923–925. doi: 10.1016/0140-6736(93)91213-6. [DOI] [PubMed] [Google Scholar]
- 50.Aho K, Vaarala O, Tenkanen L, et al. Antibodies binding to anionic phospholipids but not to oxidized low-density lipoprotein are associated with thrombosis in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1996;14(5):499–506. [PubMed] [Google Scholar]
- 51.Romero FI, Amengual O, Atsumi T, Khamashta MA, Tinahones FJ, Hughes GR. Arterial disease in lupus and secondary antiphospholipid syndrome: association with anti-β2-glycoprotein I antibodies but not with antibodies against oxidized low-density lipoprotein. Br J Rheumatol. 1998;37(8):883–888. doi: 10.1093/rheumatology/37.8.883. [DOI] [PubMed] [Google Scholar]
- 52.Hayem G, Nicaise-Roland P, Palazzo E, et al. Anti-oxidized low-density-lipoprotein (OxLDL) antibodies in systemic lupus erythematosus with and without antiphospholipid syndrome. Lupus. 2001;10(5):346–351. doi: 10.1191/096120301667475689. [DOI] [PubMed] [Google Scholar]
- 53.Gomez-Zumaquero JM, Tinahones FJ, De Ramon E, Camps M, Garrido L, Soriguer FJ. Association of biological markers of activity of systemic lupus erythematosus with levels of anti-oxidized low-density lipoprotein antibodies. Rheumatology (Oxford) 2004;43(4):510–513. doi: 10.1093/rheumatology/keh109. [DOI] [PubMed] [Google Scholar]
- 54.Navab M, Fogelman AM, Berliner JA, et al. Pathogenesis of atherosclerosis. Am J Cardiol. 1995;76(9):18C–23C. doi: 10.1016/s0002-9149(99)80466-4. [DOI] [PubMed] [Google Scholar]
- 55.Podrez EA, Poliakov E, Shen Z, et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277(41):38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 56.Podrez EA, Poliakov E, Shen Z, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277(41):38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 57.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M. Oxidized lipids in atherogenesis: formation, destruction and action. Thromb Haemost. 1997;78(1):195–199. [PubMed] [Google Scholar]
- 58.Remaley AT, Thomas F, Stonik JA, et al. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res. 2003;44(4):828–836. doi: 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108(6):661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 60.Parthasarathy S, Barnett J, Fong LG. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1990;1044(2):275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- 61.Ohta T, Takata K, Horiuchi S, Morino Y, Matsuda I. Protective effect of lipoproteins containing apoprotein A-I on Cu2+-catalyzed oxidation of human low density lipoprotein. FEBS Lett. 1989;257(2):435–438. doi: 10.1016/0014-5793(89)81590-x. [DOI] [PubMed] [Google Scholar]
- 62.Watson AD, Berliner JA, Hama SY, et al. Protective effect of high density lipoprotein associated paraoxonase Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96(6):2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Navab M, Hama SY, Anantharamaiah GM, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41(9):1495–1508. [PubMed] [Google Scholar]
- 64.Frostegard J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145(1):33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 65.Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97(9):2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hauer AD, Uyttenhove C, de Vos P, et al. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112(7):1054–1062. doi: 10.1161/CIRCULATIONAHA.104.533463. [DOI] [PubMed] [Google Scholar]
- 67.Hansson GK, Jonasson L, Holm J, Clowes MM, Clowes AW. γ-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988;63(4):712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- 68.Peilot H, Rosengren B, Bondjers G, Hurt-Camejo E. Interferon-γ induces secretory group IIA phospholipase A2 in human arterial smooth muscle cells. Involvement of cell differentiation, STAT-3 activation, and modulation by other cytokines. J Biol Chem. 2000;275(30):22895–22904. doi: 10.1074/jbc.M002783200. [DOI] [PubMed] [Google Scholar]
- 69.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 70.Whitman SC, Ravisankar P, Daugherty A. IFN-γ deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E-/- mice. J Interferon Cytokine Res. 2002;22(6):661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 71.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-γ potentiates atherosclerosis in apoE knock-out mice. J Clin Invest. 1997;99(11):2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fernandes JL, Mamoni RL, Orford JL, et al. Increased Th1 activity in patients with coronary artery disease. Cytokine. 2004;26(3):131–137. doi: 10.1016/j.cyto.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 74.Saren P, Welgus HG, Kovanen PT. TNF-α and IL-1β selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157(9):4159–4165. [PubMed] [Google Scholar]
- 75.Wadham C, Albanese N, Roberts J, et al. High-density lipoproteins neutralize C-reactive protein proinflammatory activity. Circulation. 2004;109(17):2116–2122. doi: 10.1161/01.CIR.0000127419.45975.26. [DOI] [PubMed] [Google Scholar]
- 76.Semb H, Peterson J, Tavernier J, Olivecrona T. Multiple effects of tumor necrosis factor on lipoprotein lipase in vivo. J Biol Chem. 1987;262(17):8390–8394. [PubMed] [Google Scholar]
- 77.Ehnholm C, Aho K, Huttunen JK, et al. Effect of interferon on plasma lipoproteins and on the activity of postheparin plasma lipases. Arteriosclerosis. 1982;2(1):68–73. doi: 10.1161/01.atv.2.1.68. [DOI] [PubMed] [Google Scholar]
- 78.Filonzi EL, Zoellner H, Stanton H, Hamilton JA. Cytokine regulation of granulocyte-macrophage colony stimulating factor and macrophage colony-stimulating factor production in human arterial smooth muscle cells. Atherosclerosis. 1993;99(2):241–252. doi: 10.1016/0021-9150(93)90026-q. [DOI] [PubMed] [Google Scholar]
- 79.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 80.Barath P, Cao J, Forrester JS. Low density lipoprotein activates monocytes to express tumor necrosis factor. FEBS Lett. 1990;277(1–2):180–184. doi: 10.1016/0014-5793(90)80838-a. [DOI] [PubMed] [Google Scholar]
- 81.Haddy N, Sass C, Droesch S, et al. IL-6, TNF-α and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis. 2003;170(2):277–283. doi: 10.1016/s0021-9150(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 82.Ponthieux A, Herbeth B, Droesch S, Haddy N, Lambert D, Visvikis S. Biological determinants of serum ICAM-1, E-selectin, P-selectin and L-selectin levels in healthy subjects: the Stanislas study. Atherosclerosis. 2004;172(2):299–308. doi: 10.1016/j.atherosclerosis.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA. 2001;286(17):2107–2113. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 84.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 85.Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103(21):2531–2534. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- 86.Rovere P, Peri G, Fazzini F, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96(13):4300–4306. [PubMed] [Google Scholar]
- 87.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation. 2001;103(18):2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 88.Biasucci LM, Vitelli A, Liuzzo G, et al. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;94(5):874–877. doi: 10.1161/01.cir.94.5.874. [DOI] [PubMed] [Google Scholar]
- 89.Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331(7):417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 90.Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther. 2005;7(3):R634–R643. doi: 10.1186/ar1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.George J, Harats D, Gilburd B, et al. Adoptive transfer of β2-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation. 2000;102(15):1822–1827. doi: 10.1161/01.cir.102.15.1822. [DOI] [PubMed] [Google Scholar]
- 92.George J, Yacov N, Breitbart E, et al. Suppression of early atherosclerosis in LDL-receptor deficient mice by oral tolerance with β 2-glycoprotein I. Cardiovasc Res. 2004;62(3):603–609. doi: 10.1016/j.cardiores.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 93.Freigang S, Horkko S, Miller E, Witztum JL, Palinski W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol. 1998;18(12):1972–1982. doi: 10.1161/01.atv.18.12.1972. [DOI] [PubMed] [Google Scholar]
- 94.Nicolo D, Goldman BI, Monestier M. Reduction of atherosclerosis in low-density lipoprotein receptor-deficient mice by passive administration of antiphospholipid antibody. Arthritis Rheum. 2003;48(10):2974–2978. doi: 10.1002/art.11255. [DOI] [PubMed] [Google Scholar]
- 95.Ames PR, Margarita A, Sokoll KB, Weston M, Brancaccio V. Premature atherosclerosis in primary antiphospholipid syndrome: preliminary data. Ann Rheum Dis. 2005;64(2):315–317. doi: 10.1136/ard.2004.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu R, Nityanand S, Berglund L, Lithell H, Holm G, Lefvert AK. Antibodies against cardiolipin and oxidatively modified LDL in 50-year-old men predict myocardial infarction. Arterioscler Thromb Vasc Biol. 1997;17(11):3159–3163. doi: 10.1161/01.atv.17.11.3159. [DOI] [PubMed] [Google Scholar]
- 97.Vaarala O, Manttari M, Manninen V, et al. Anti-cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middle-aged men. Circulation. 1995;91(1):23–27. doi: 10.1161/01.cir.91.1.23. [DOI] [PubMed] [Google Scholar]
- 98.Ducloux D, Bourrinet E, Motte G, Chalopin JM. Antiphospholipid antibodies as a risk factor for atherosclerotic events in renal transplant recipients. Kidney Int. 2003;64(3):1065–1070. doi: 10.1046/j.1523-1755.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 99.Toloza SM, Uribe AG, McGwin G, Jr, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50(12):3947–3957. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- 100.Petri M. The lupus anticoagulant is a risk factor for myocardial infarction (but not atherosclerosis): Hopkins Lupus Cohort. Thromb Res. 2004;114(5–6):593–595. doi: 10.1016/j.thromres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 101.Meroni PL, Raschi E, Testoni C, Borghi MO. Endothelial cell activation by antiphospholipid antibodies. Clin Immunol. 2004;112(2):169–174. doi: 10.1016/j.clim.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 102.Pisetsky DS. The role of innate immunity in the induction of autoimmunity. Autoimmun Rev. 2008;8(1):69–72. doi: 10.1016/j.autrev.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 103.Campbell LA, Yaraei K, Van Lenten B, et al. The acute phase reactant response to respiratory infection with Chlamydia pneumoniae: implications for the pathogenesis of atherosclerosis. Microbes Infect. 2010;12(8–9):598–606. doi: 10.1016/j.micinf.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278(3):1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 105.Avalos AM, Busconi L, Marshak-Rothstein A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity. 2010;43(1):76–83. doi: 10.3109/08916930903374618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Denny MF, Thacker S, Mehta H, et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110(8):2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee PY, Li Y, Richards HB, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56(11):3759–3769. doi: 10.1002/art.23035. [DOI] [PubMed] [Google Scholar]
- 108.Park YB, Lee SK, Lee WK, et al. Lipid profiles in untreated patients with rheumatoid arthritis. J Rheumatol. 1999;26(8):1701–1704. [PubMed] [Google Scholar]
- 109.Delgado Alves J, Kumar S, Isenberg DA. Cross-reactivity between anti-cardiolipin, anti-high-density lipoprotein and anti-apolipoprotein A-I IgG antibodies in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology (Oxford) 2003;42(7):893–899. doi: 10.1093/rheumatology/keg248. [DOI] [PubMed] [Google Scholar]
- 110.Vuilleumier N, Reber G, James R, et al. Presence of autoantibodies to apolipoprotein A-1 in patients with acute coronary syndrome further links autoimmunity to cardiovascular disease. J Autoimmun. 2004;23(4):353–360. doi: 10.1016/j.jaut.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Dinu AR, Merrill JT, Shen C, Antonov IV, Myones BL, Lahita RG. Frequency of antibodies to the cholesterol transport protein apolipoprotein A1 in patients with SLE. Lupus. 1998;7(5):355–360. doi: 10.1191/096120398678920262. [DOI] [PubMed] [Google Scholar]
- 112.O'Neill SG, Giles I, Lambrianides A, et al. Antibodies to apolipoprotein A-I, high-density lipoprotein, and C-reactive protein are associated with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):845–854. doi: 10.1002/art.27286. [DOI] [PubMed] [Google Scholar]
- 113.Malinow MR, Nieto FJ, Szklo M, Chambless LE, Bond G. Carotid artery intimal-medial wall thickening and plasma homocyst(e)ine in asymptomatic adults. The Atherosclerosis Risk in Communities Study. Circulation. 1993;87(4):1107–1113. doi: 10.1161/01.cir.87.4.1107. [DOI] [PubMed] [Google Scholar]
- 114.Wall RT, Harlan JM, Harker LA, Striker GE. Homocysteine-induced endothelial cell injury in vitro: a model for the study of vascular injury. Thromb Res. 1980;18(1–2):113–121. doi: 10.1016/0049-3848(80)90175-9. [DOI] [PubMed] [Google Scholar]
- 115.Hajjar KA. Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J Clin Invest. 1993;91(6):2873–2879. doi: 10.1172/JCI116532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Upchurch GR, Jr, Welch GN, Fabian AJ, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272(27):17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 117.Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103(22):2717–2723. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]
- 118.McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2(4):386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 119.Stamler JS, Osborne JA, Jaraki O, et al. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. 1993;91(1):308–318. doi: 10.1172/JCI116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woo KS, Chook P, Lolin YI, et al. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997;96(8):2542–2544. doi: 10.1161/01.cir.96.8.2542. [DOI] [PubMed] [Google Scholar]
- 121.Refai TM, Al-Salem IH, Nkansa-Dwamena D, Al-Salem MH. Hyperhomocysteinaemia and risk of thrombosis in systemic lupus erythematosus patients. Clin Rheumatol. 2002;21(6):457–461. doi: 10.1007/s100670200115. [DOI] [PubMed] [Google Scholar]
- 122.Otero M, Lago R, Gomez R, et al. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford) 2006;45(8):944–950. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- 123.Bjorbaek C, Elmquist JK, Michl P, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139(8):3485–3491. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 124.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189(1):47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 125.Beltowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24(5):789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 126.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13(10):1231–1238. [PubMed] [Google Scholar]
- 127.Dong F, Zhang X, Ren J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH oxidase pathway. Hypertension. 2006;47(2):222–229. doi: 10.1161/01.HYP.0000198555.51645.f1. [DOI] [PubMed] [Google Scholar]
- 128.Porreca E, Di Febbo C, Moretta V, et al. Circulating leptin is associated with oxidized LDL in postmenopausal women. Atherosclerosis. 2004;175(1):139–143. doi: 10.1016/j.atherosclerosis.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 129.Garcia-Gonzalez A, Gonzalez-Lopez L, Valera-Gonzalez IC, et al. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol Int. 2002;22(4):138–141. doi: 10.1007/s00296-002-0216-9. [DOI] [PubMed] [Google Scholar]
- 130.Wislowska M, Rok M, Stepien K, Kuklo-Kowalska A. Serum leptin in systemic lupus erythematosus. Rheumatol Int. 2008;28(5):467–473. doi: 10.1007/s00296-008-0526-7. [DOI] [PubMed] [Google Scholar]
- 131.Sada KE, Yamasaki Y, Maruyama M, et al. Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J Rheumatol. 2006;33(8):1545–1552. [PubMed] [Google Scholar]
- 132.Al M, Ng L, Tyrrell P, Bargman J, Bradley T, Silverman E. Adipokines as novel biomarkers in paediatric systemic lupus erythematosus. Rheumatology (Oxford) 2009;48(5):497–501. doi: 10.1093/rheumatology/kep030. [DOI] [PubMed] [Google Scholar]
- 133.Otero M, Lago R, Gomez R, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(9):1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anderson PD, Mehta NN, Wolfe ML, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92(6):2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 135.McMahon M, Anderson M, Grossman J, et al. Plasma leptin levels are associated with carotid artery plaque and intima-media thickness (IMT) in women with SLE and a matched population of healthy women. Arthritis Rheum. 2007;56:S796. [Google Scholar]
- 136.Reynolds HR, Buyon J, Kim M, et al. Association of plasma soluble E-selectin and adiponectin with carotid plaque in patients with systemic lupus erythematosus. Atherosclerosis. 2010;210(2):569–574. doi: 10.1016/j.atherosclerosis.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chung C, Long A, Solus J, et al. Adipocytokines in systemic lupus erythematosus: relationship to inflammation, insulin resistance and coronary atherosclerosis. Lupus. 2009;18(9):799–806. doi: 10.1177/0961203309103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96(6):2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 140.McMahon M, Grossman J, Fitzgerald J, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54(8):2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 141.Bruce IN. ‘Not only…but also’: factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatology (Oxford) 2005;44(12):1492–1502. doi: 10.1093/rheumatology/kei142. [DOI] [PubMed] [Google Scholar]
- 142.Homer D, Ingall TJ, Baker HL, Jr, O'Fallon WM, Kottke BA, Whisnant JP. Serum lipids and lipoproteins are less powerful predictors of extracranial carotid artery atherosclerosis than are cigarette smoking and hypertension. Mayo Clin Proc. 1991;66(3):259–267. doi: 10.1016/s0025-6196(12)61007-6. [DOI] [PubMed] [Google Scholar]
- 143.Whisnant JP, Homer D, Ingall TJ, Baker HL, Jr, O'Fallon WM, Wievers DO. Duration of cigarette smoking is the strongest predictor of severe extracranial carotid artery atherosclerosis. Stroke. 1990;21(5):707–714. doi: 10.1161/01.str.21.5.707. [DOI] [PubMed] [Google Scholar]
- 144.Wajed J, Ahmad Y, Durrington PN, Bruce IN. Prevention of cardiovascular disease in systemic lupus erythematosus – proposed guidelines for risk factor management. Rheumatology (Oxford) 2004;43(1):7–12. doi: 10.1093/rheumatology/keg436. [DOI] [PubMed] [Google Scholar]
- 145.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 146.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 147.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. 1995. Atheroscler Suppl. 2004;5(3):S91–S97. doi: 10.1016/j.atherosclerosissup.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 148.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 149.Chen CH, Jiang W, Via DP, et al. Oxidized low-density lipoproteins inhibit endothelial cell proliferation by suppressing basic fibroblast growth factor expression. Circulation. 2000;101(2):171–177. doi: 10.1161/01.cir.101.2.171. [DOI] [PubMed] [Google Scholar]
- 150.Leung BP, Sattar N, Crilly A, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170(3):1524–1530. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 151.Xu ZM, Zhao SP, Li QZ, Nie S, Zhou HN. Atorvastatin reduces plasma MCP-1 in patients with acute coronary syndrome. Clin Chim Acta. 2003;338(1–2):17–24. doi: 10.1016/s0009-8981(03)00321-8. [DOI] [PubMed] [Google Scholar]
- 152.Aktas O, Waiczies S, Smorodchenko A, et al. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197(6):725–733. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 154.Neuhaus O, Strasser-Fuchs S, Fazekas F, et al. Statins as immunomodulators: comparison with interferon-β 1b in MS. Neurology. 2002;59(7):990–997. doi: 10.1212/wnl.59.7.990. [DOI] [PubMed] [Google Scholar]
- 155.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, et al. Inhibition of hydroxymethylglutaryl-coenzyme A reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93(10):948–956. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 156.Cherfan P, Tompa A, Wikby A, Lofgren S, Jonasson L. Effects of simvastatin on human T cells in vivo. Atherosclerosis. 2007;193(1):186–192. doi: 10.1016/j.atherosclerosis.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 157.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6(12):1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 158.Kwak B, Mulhaupt F, Veillard N, Pelli G, Mach F. The HMG-CoA reductase inhibitor simvastatin inhibits IFN-γ induced MHC class II expression in human vascular endothelial cells. Swiss Med Wkly. 2001;131(3–4):41–46. doi: 10.4414/smw.2001.06144. [DOI] [PubMed] [Google Scholar]
- 159.Rasmussen LM, Hansen PR, Nabipour MT, Olesen P, Kristiansen MT, Ledet T. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem J. 2001;360(Pt 2):363–370. doi: 10.1042/0264-6021:3600363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chung HK, Lee IK, Kang H, et al. Statin inhibits interferon-γ-induced expression of intercellular adhesion molecule-1 (ICAM-1) in vascular endothelial and smooth muscle cells. Exp Mol Med. 2002;34(6):451–461. doi: 10.1038/emm.2002.63. [DOI] [PubMed] [Google Scholar]
- 161.Schonbeck U, Gerdes N, Varo N, et al. Oxidized low-density lipoprotein augments and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors limit CD40 and CD40L expression in human vascular cells. Circulation. 2002;106(23):2888–2893. doi: 10.1161/01.cir.0000043029.52803.7b. [DOI] [PubMed] [Google Scholar]
- 162.Salonen R, Nyyssonen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92(7):1758–1764. doi: 10.1161/01.cir.92.7.1758. [DOI] [PubMed] [Google Scholar]
- 163.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 164.Haruna Y, Morita Y, Yada T, Satoh M, Fox DA, Kashihara N. Fluvastatin reverses endothelial dysfunction and increased vascular oxidative stress in rat adjuvant-induced arthritis. Arthritis Rheum. 2007;56(6):1827–1835. doi: 10.1002/art.22632. [DOI] [PubMed] [Google Scholar]
- 165.Aprahamian T, Bonegio R, Rizzo J, et al. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 2006;177(5):3028–3034. doi: 10.4049/jimmunol.177.5.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ferreira GA, Navarro TP, Telles RW, Andrade LE, Sato EI. Atorvastatin therapy improves endothelial-dependent vasodilation in patients with systemic lupus erythematosus: an 8 weeks controlled trial. Rheumatology (Oxford) 2007;46(10):1560–1565. doi: 10.1093/rheumatology/kem186. [DOI] [PubMed] [Google Scholar]
- 167.Petri M, Kiani A, Post W, Madger L. Lupus atherosclerosis prevention study (LAPS): a randomized double blind placebo controlled trial of atorvastatin versus placebo. Arthritis Rheum. 2006;54(9):S520. [Google Scholar]
- 168.Norby GE, Holme I, Fellstrom B, et al. Effect of fluvastatin on cardiac outcomes in kidney transplant patients with systemic lupus erythematosus: a randomized placebo-controlled study. Arthritis Rheum. 2009;60(4):1060–1064. doi: 10.1002/art.24379. [DOI] [PubMed] [Google Scholar]
- 169.Kang S, Wu Y, Li X. Effects of statin therapy on the progression of carotid atherosclerosis: a systematic review and meta-analysis. Atherosclerosis. 2004;177(2):433–442. doi: 10.1016/j.atherosclerosis.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 170.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III Update: adjustments and options. Am J Cardiol. 2005;96(4A):53–59. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 171.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 172.Duran-Barragan S, McGwin G, Jr, Vila LM, Reveille JD, Alarcon GS. Angiotensin-converting enzyme inhibitors delay the occurrence of renal involvement and are associated with a decreased risk of disease activity in patients with systemic lupus erythematosus – results from LUMINA (LIX): a multiethnic US cohort. Rheumatology (Oxford) 2008;47(7):1093–1096. doi: 10.1093/rheumatology/ken208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Costenbader KH, Karlson EW, Gall V, et al. Barriers to a trial of atherosclerosis prevention in systemic lupus erythematosus. Arthritis Rheum. 2005;53(5):718–723. doi: 10.1002/art.21441. [DOI] [PubMed] [Google Scholar]
- 174.Chen HW, Leonard DA. Chloroquine inhibits cyclization of squalene oxide to lanosterol in mammalian cells. J Biol Chem. 1984;259(13):8156–8162. [PubMed] [Google Scholar]
- 175.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37(4):1075–1082. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 176.Rahman P, Gladman DD, Urowitz MB, Yuen K, Hallett D, Bruce IN. The cholesterol lowering effect of antimalarial drugs is enhanced in patients with lupus taking corticosteroid drugs. J Rheumatol. 1999;26(2):325–330. [PubMed] [Google Scholar]
- 177.Petri M. Hydroxychloroquine use in the Baltimore Lupus Cohort: effects on lipids, glucose and thrombosis. Lupus. 1996;5(Suppl. 1):S16–S22. [PubMed] [Google Scholar]
- 178.Bevan AP, Krook A, Tikerpae J, Seabright PJ, Siddle K, Smith GD. Chloroquine extends the lifetime of the activated insulin receptor complex in endosomes. J Biol Chem. 1997;272(43):26833–26840. doi: 10.1074/jbc.272.43.26833. [DOI] [PubMed] [Google Scholar]
- 179.Edwards MH, Pierangeli S, Liu X, Barker JH, Anderson G, Harris EN. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation. 1997;96(12):4380–4384. doi: 10.1161/01.cir.96.12.4380. [DOI] [PubMed] [Google Scholar]
- 180.Espinola RG, Pierangeli SS, Gharavi AE, Harris EN. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb Haemost. 2002;87(3):518–522. [PubMed] [Google Scholar]
- 181.Wallace DJ. Does hydroxychloroquine sulfate prevent clot formation in systemic lupus erythematosus? Arthritis Rheum. 1987;30(12):1435–1436. doi: 10.1002/art.1780301219. [DOI] [PubMed] [Google Scholar]
- 182.Jung H, Bobba R, Su J, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 183.Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15(9):577–583. doi: 10.1177/0961203306071872. [DOI] [PubMed] [Google Scholar]
- 184.Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis. 2009;68(2):238–241. doi: 10.1136/ard.2008.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alarcon GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Ann Rheum Dis. 2007;66(9):1168–1172. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun S, Rao NL, Venable J, Thurmond R, Karlsson L. TLR7/9 antagonists as therapeutics for immune-mediated inflammatory disorders. Inflamm Allergy Drug Targets. 2007;6(4):223–235. doi: 10.2174/187152807783334300. [DOI] [PubMed] [Google Scholar]
- 187.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109(6):745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22(11):1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 189.Ait-Oufella H, Herbin O, Bouaziz JD, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207(8):1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pego-Reigosa JM, Lu TY, Fontanillo MF, del Campo-Perez V, Rahman A, Isenberg DA. Long-term improvement of lipid profile in patients with refractory systemic lupus erythematosus treated with B-cell depletion therapy: a retrospective observational study. Rheumatology (Oxford) 2010;49(4):691–696. doi: 10.1093/rheumatology/kep446. [DOI] [PubMed] [Google Scholar]
- 191.Navab M, Anantharamaiah GM, Reddy ST, et al. Oral D-4F causes formation of pre-β high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109(25):3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 192.Charles-Schoeman C, Banquerigo ML, Hama S, et al. Treatment with an apolipoprotein A-1 mimetic peptide in combination with pravastatin inhibits collagen-induced arthritis. Clin Immunol. 2008;127(2):234–244. doi: 10.1016/j.clim.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 193.Navab M, Anantharamaiah GM, Reddy ST, et al. An oral apoJ peptide renders HDL antiinflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2005;25(9):1932–1937. doi: 10.1161/01.ATV.0000174589.70190.e2. [DOI] [PubMed] [Google Scholar]
- 194.Navab M, Anantharamaiah GM, Hama S, et al. D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2005;25(7):1426–1432. doi: 10.1161/01.ATV.0000167412.98221.1a. [DOI] [PubMed] [Google Scholar]
- 195.Bloedon LT, Dunbar R, Duffy D, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49(6):1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Woo JM, Lin Z, Navab M, et al. Treatment with apolipoprotein A-1 mimetic peptide reduces lupus-like manifestations in a murine lupus model of accelerated atherosclerosis. Arthritis Res Ther. 2010;12(3):R93. doi: 10.1186/ar3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Skaggs BJ, Hahn BH, Sahakian L, Grossman J, McMahon M. Dysfunctional, pro-inflammatory HDL directly upregulates monocyte PDGFRβ, chemotaxis and TNF-α production. Clin Immunol. 2010;137(1):147–156. doi: 10.1016/j.clim.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Quintas-Cardama A, Cortes JE, Kantarjian H. Practical management of toxicities associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Clin Lymphoma Myeloma. 2008;8(Suppl. 3):S82–S88. doi: 10.3816/CLM.2008.s.003. [DOI] [PubMed] [Google Scholar]
- 199.Kerkela R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 200.Distler JH, Distler O. Cardiotoxicity of imatinib mesylate: an extremely rare phenomenon or a major side effect? Ann Rheum Dis. 2007;66(6):836. doi: 10.1136/ard.2006.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Garcia-Alvarez A, Sitges M, Garcia-Albeniz X, Sionis A, Loma-Osorio P, Bosch X. Atypical cardiac manifestation of hypereosinophilic syndrome and reversible cardiotoxicity to imatinib. Int J Cardiol. 2010;139(2):e29–e31. doi: 10.1016/j.ijcard.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 202.Wolf A, Couttet P, Dong M, et al. Imatinib does not induce cardiotoxicity at clinically relevant concentrations in preclinical studies. Leuk Res. 2010;34(9):1180–1188. doi: 10.1016/j.leukres.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 203.Yazdany J, Panopalis P, Gillis JZ, et al. A quality indicator set for systemic lupus erythematosus. Arthritis Rheum. 2009;61(3):370–377. doi: 10.1002/art.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Demas KL, Keenan BT, Solomon DH, Yazdany J, Costenbader KH. Osteoporosis and cardiovascular disease care in systemic lupus erythematosus according to new quality indicators. Semin Arthritis Rheum. 2010;40(3):193–200. doi: 10.1016/j.semarthrit.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Urowitz MB, Gladman D, Ibanez D, et al. Accumulation of coronary artery disease risk factors over three years: data from an international inception cohort. Arthritis Rheum. 2008;59(2):176–180. doi: 10.1002/art.23353. [DOI] [PubMed] [Google Scholar]
- 206.Wolak T, Todosoui E, Szendro G, et al. Duplex study of the carotid and femoral arteries of patients with systemic lupus erythematosus: a controlled study. J Rheumatol. 2004;31(5):909–914. [PubMed] [Google Scholar]
- 207.Gustafsson J, Gunnarsson I, Borjesson O, et al. Predictors of the first cardiovascular event in patients with systemic lupus erythematosus – a prospective cohort study. Arthritis Res Ther. 2009;11(6):R186. doi: 10.1186/ar2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kiani AN, Magder L, Petri M. Coronary calcium in systemic lupus erythematosus is associated with traditional cardiovascular risk factors, but not with disease activity. J Rheumatol. 2008;35(7):1300–1306. [PubMed] [Google Scholar]