Abstract
This article reviews background on proteases and their functions, their physiological significance in skin, and the potential implications of incorporating specific proteases and protease blends into dermatological products, including skin care formulations. The history of protease blend formulations used in wound model studies and for other disorders is reviewed. In vitro data with use of a specific 3-protease blend with evaluation of the impact on various skin proteins and peptides is also discussed in this article.
Background on Proteases
Found in many living organisms including plants and animals, proteases are a diverse group of enzymes that provide vital physiological activity in a multitude of reactions involved in many organ systems.1-5 Also referred to as peptidases and proteinases, the activities of proteases in humans include the gastrointestinal tract (i.e., digestion of ingested food proteins), hematological and immunological systems (blood-clotting, complement system, apoptosis cascades, activation of cathelicidin antimicrobial peptide pathway), and epidermal barrier function (i.e., permeability, desquamation). Depending on the amino acid sequence of a given protein, proteases can induce limited proteolysis with cleavage of a specific bond or can cause unlimited proteolysis with conversion of a protein into its component amino acids.1-5
The resultant effects induced by a protease enzyme can be (1) to inhibit the function of a physiological protein (i.e., cytokine, chemokine, enzyme) through its breakdown into inactive components, (2) to convert an inactive precursor protein into an active physiological peptide, and (3) to serve as a signaling agent to transactivate the upregulation of a specific process or pathway.1-5 Ultimately, both endogenous proteases and protease inhibitors play vital roles that are integral to maintaining the functional and structural integrity of various organ systems and their physiological functions, including skin. Abnormalities in protease function and/or activity may also be operative in certain disease states (i.e., rosacea, atopic dermatitis, psoriasis, immunobullous diseases).4-6
Proteases and Human Skin
A multitude of proteases and protease inhibitors are involved in modulating many physiological activities in human skin, with normal desquamation of upper stratum corneum (SC) corneocytes being a major process involved in maintaining healthy skin. Desquamation of a youthful and healthy epidermis incorporates a “one-to-one coupling” of keratinocyte formation (basal layer) and corneocyte desquamation.7 In order for desquamation to occur without impairment of SC permeability barrier function, epidermal differentiation requires a system of proteolytic enzymes that is both “timely and spatially well-coordinated” to allow for physiological corneocyte detachment.5 The optimal function of these enzymes requires a proper magnitude and gradient of SC/epidermal hydration.8-10 Epidermal barrier functions and physiological desquamation are dependent on the interactions of several proteases and protease inhibitors, which require an adequate water content and gradient for optimal functioning to occur. As human skin ages, the rate of both desquamation and epidermal cell replacement decrease significantly over time. Uncoupling of “lost cells” (desquamated corneocytes) versus “replacement cells” (new epidermal cells produced by the basal layer) is at least partially related to reduced endogenous protease activity and occurs in association with SC permeability barrier dysfunction and xerotic skin.7
Proteases are currently classified into six broad groups based on their catalytic domain. These are serine proteases, cysteine proteases, aspartate proteases, threonine proteases, glutamic acid proteases, and metalloproteases (also referred to as metalloproteinases).5 Proteases in at least the first three categories are believed to be involved in the desquamation process.3 The individual endogenous protease enzyme families, specific enzymes in each group, and their activities are beyond the scope of this manuscript and are reviewed in more detail by Meyer-Hoffert and Schroder.5
History of cutaneous application of proteases. Proteases (i.e., collagenases) have commonly been used for several years in dermatological products applied to debride necrotic and escharotic wounds, such as decubiti and skin ulcers.11 This common application may give some clinicians the impression that all proteases are caustic and highly degradative to skin tissue, which is not accurate. Over time, the diversity of proteases and their biological sources, their various properties in skin, chemical modifications of specific proteases to improve stabilization, adjustments in protease concentrations, advances in vehicles to provide better delivery and reduce irritancy, and evaluation of clinical applications have led to the development of protease formulations that appear to be applicable for dermatological uses other than wound debridement. Such applications have been designed to achieve skin cleansing, moisturization, improvements in skin surface characteristics and appearance, restoration and maintenance of SC permeability barrier function, and amelioration of cutaneous inflammation associated with an overstressed or compromised SC (Table 1).7,12-16
TABLE 1.
Cutaneous use of protease formulations: Implications and outcomes
| SKIN CLEANSERS12 |
| Proteases incorporated to degrade protein in “skin dirt” comprised primarily of desquamated corneocytes (i.e., keratin) and sweat proteins. Degraded protein fragments are more amenable to removal by detergent component of cleansers. |
| Low specificity proteases optimal for cleansing due to multiple protein types on skin surface |
| Chemical modifications of proteases allowing for improved stabilization have led to advances which improve the ability to incorporate these agents into skin cleanser formulations. Such modifications may also reduce cutaneous irritancy. |
| LEAVE-ON FORMULATIONS13 |
| Proteases shown to reduce visible skin scaling and dryness |
| Induction of desquamation by various proteases including those from plant and bacterial sources |
| Broad-specificity proteases may be optimal for promotion of desquamation (generalized proteolysis effect). |
| Some proteases may induce desmolysis (corneodesmosome degradation). |
| Proteases applied topically may improve efficacy of conventional moisturizers (visual dryness reduction). |
| Enzyme encapsulation technologies increase stability by circumventing protease lysis and denaturation by high water content of lotions/creams. |
| Augmented reduction in mean dryness score shown with addition of protease as compared to base moisturizer (control without protease) |
| Topically applied proteases shown to rapidly reduce visual skin scaling and visible signs of xerosis |
| Rational to incorporate together specific proteases using a stable technology that releases and delivers the protease ingredient or blend of proteases with a well-designed moisturizer/barrier repair topical formulation |
Rationale for Incorporation of Proteases into Skin Care Formulations
The epidermal permeability barrier, which primarily involves the SC, is compromised by a variety of exogenous and endogenous factors.7-10,17-19 Exogenous factors include changes in humidity, poor skin care (i.e., harsh cleansers, astringents, aggressive exfoliation), and certain topical (i.e., retinoids, benzoyl peroxide, topical corticosteroids, some vehicle formulations) and systemic medications (HMG-CoA reductase inhibitors [“statins”]).8 Endogenous factors include underlying inherent disorders, such as atopic skin, xerosis without atopy, ichthyoses, facial skin of rosacea, psoriatic skin, photodamaged skin, and aged skin.8
SC permeability barrier compromise which exceeds the capacity of self-repair, referred to as an overstressed SC, leads to increased transepidermal water loss (TEWL), decreased epidermal hydration, and impaired desquamation resulting in clinically evident flaky, scaly, dry skin that is less pliable (“stiff”) and prone to micro- and macrofissuring.8,9 Impaired desquamation causes incomplete separation of individual corneocytes, which leads to “corneocyte clumping,” which translates clinically to visible scales and flakes on the skin surface.
In experimental studies, acute barrier disruption is induced to simulate what may adversely affect the SC permeability barrier and trigger an increase in TEWL. This is carried out by a variety of methods in both human and animal skin, including acetone-treated skin, surfactant-treated skin (i.e., sodium lauryl sulfate [SLS]), tape-stripping techniques, and a variety of other methods used to evaluate specific parameters of epidermal function and/or integrity (i.e., Raman confocal spectroscopy).20-24 It has been shown that epidermal messenger ribonucleic acid (mRNA) levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, and IL-6 increase as an immediate response to SC permeability impairment and increased TEWL, suggesting that these “jump-start cytokines” play an integral role in initiating epidermal barrier repair, inflammatory responses, and keratinocyte proliferation.25,26 If SC permeability barrier dysfunction remains unchecked, epidermal hyperplasia ensues, which may be evident visibly as marked scaling and hyperkeratosis.8,17,19 The immediate increase of IL-1a is likely from a “stored pool” that is pre-formed in order to be immediately available in response to epidermal barrier disruption, with TNF-α and IL-6 also present as components of the initial response to the increase in TEWL and decrease in SC hydration.25
When the SC is disrupted, the ensuing increase in TEWL adversely affects the physiological SC water content and gradient, which results in suboptimal function of proteolytic enzymes involved in normal desquamation of corneocytes.8,9 When TEWL increases and SC water content decreases, the “biosensor” functions of the SC respond by signaling the recruitment of innate repair mechanisms that work “actively” to restore SC permeability barrier function.8,9,17-19 An important immediate repair response inherent to human skin is the increase in the release of IL-1, TNF-α, and IL-6. This jump-start cytokine profile functions to trigger repair of the SC permeability barrier to reverse the increase in TEWL and restore physiological SC water content and gradient.25 However, in xerotic and/or eczematous skin affected by chronic SC barrier disruption, these same cytokines can promote inflammation and epidermal hyperplasia that can induce, worsen, and/or prolong the underlying skin disorder. Therefore, these same cytokines may be beneficial or problematic depending on the clinical scenario and whether or not permeability barrier function is restored by innate SC repair mechanisms and/or incorporation of proper cleansing and moisturizer use.4,7,8,12,13
How May Proteases Augment the Beneficial Outcomes Achieved with Skin Care Products?
It is important to recognize that use of proteases in skin care formulations is not intended to “shut off” the physiological effects of specific endogenous proteins, such as cytokines, growth factors, inhibitor proteins, and other enzymes, which signal pathways and/or directly modify host tissues (i.e., metalloproteases).14-16 Rather, the type and concentration of protease(s) used are designed to soften the effects of pro-inflammatory endogenous proteins that may be detrimental when overexpressed or overactive, to not degrade certain key beneficial proteins, and in some cases to provide some advantageous protective effects on some endogenous proteins.14-16 Therefore, products containing proteases may warrant the use of different protease blends to achieve specific effects, offer the advantage of being nonsteroidal, and, if properly stabilized, can be incorporated into vehicles with effective moisturization and barrier restoration qualities and favorable skin tolerability. The inclusion of a specific protease or protease blend may potentially augment the moisturization/barrier repair effects compared to conventional skin care products (i.e., cleanser, moisturizer) and may provide adjunctive benefit in support of the therapeutic regimen.4,7,8,12,13
The Development of Protease Skin Care Formulations
In developing skin care products (cleansing wash, moisturizing lotion), how does one determine which proteases to include? The decision to incorporate a blend of three plant-derived proteases (3-protease blend) in skin care products was based on multiple considerations with direction provided by in vitro and in silico studies using protease technology in acute and chronic wound models. In vitro assays were completed with chronic wound fluid (CWF) and with purified protein solutions.14-16 The results of these studies were consistent using enzyme-linked immunosorbent assay (ELISA) and Searchlight microarray analysis with results depicted in Table 2.
TABLE 2.
Results of protease effects on key endogenous physiological proteins/peptides using a designated protease blend14–16
| KEY PROTEINS | MODULATORY EFFECTS* |
|---|---|
| TNF-α | Rapid reduction in monomeric and trimeric forms |
| IL-6/IL-6R | Sustained reduction |
| Substance P (neuropeptide) | Rapid reduction |
| Metalloproteinases (MMPs) | Varies degrees of reduction in several MMPs including MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, and MMP-9. Some MMPs with rapid reduction (i.e., MMP-1, MMP-9). Some MMPs with no reduction (i.e., MMP-10, MMP-13) |
| IL-1 (IL-1 alpha, IL-1 beta) | No reduction |
| PDGF | No reduction |
Note that the magnitude and time course of reduction (degradation) of a given protein in these assays does not necessarily correlate with the magnitude/course of clinical effect and does not imply complete elimination of in vivo protein activity; clinical studies are needed to capture effects in patients with various skin conditions (i.e., normal skin, xerosis, atopic skin/eczematous disorders, psoriasis, rosacea, corticosteroid- treated skin, geriatric skin, etc.)
TNF=tumor necrosis factor; IL=interleukin; R=receptor; PDGF=platelet-derived growth factor
Have protease-containing skin care formulations been used in any studies of patients with inflammatory dermatoses? A protease blend formulation has been shown to relieve pruritus in post-burn itch subjects with encouraging results, a response possibly related to ameliorating the effects of substance P and potentially other neuropeptides.15,27,28 Application to human skin of a designated protease blend has been published in a case report of a patient with contact dermatitis.15,29 Other protease-containing topical products have also been evaluated.13,30 A small observational pilot study assessed the use of an antimicrobial skin cleanser (EltaMD Relief Exfoliating Gel, EltaMD, Carrollton, Texas) and a protease blend lotion (EltaMD Relief Psoriasis Treatment, EltaMD) in patients with psoriasis (N=8).30 The protease blend lotion used in this psoriasis trial has been shown to exhibit a similar profile of in vitro endogenous protein activity as the 3-protease blend used in a moisturizing lotion formulated for skin care, although there are some differences between the lotions in both the ingredient blend and concentrations used.30,31
In the aforementioned six-week pilot evaluation completed in subjects with psoriasis, significant reductions in disease severity ratings (i.e., Psoriasis Area Severity Index [PASI], Investigator Global Assessment [IGA]) at Week 3 and Week 6 compared to baseline were shown with application of the protease-containing lotion twice daily and use of the non-protease-containing antimicrobial cleanser.30 In addition, decreases in erythema, scaling, induration, and pruritus were noted at target lesion sites treated with the protease-containing lotion-antimicrobial cleanser regimen as compared to an untreated comparison site. Importantly, in the psoriasis study, subjects were permitted to apply the protease-containing lotion up to five times a day if they felt it was needed. Additional well-designed clinical studies are needed to further define optimal use and clinical outcomes with protease-containing topical formulations. Results need to be evaluated with each protease-containing topical formulation as differences in both the proteases and excipients used can have direct impact on efficacy and tolerability. Further advances in skin sampling techniques from patients will provide improved ability to evaluate clinical outcomes correlated with micro-environmental changes in both normal and compromised skin treated with protease-containing topical formulations.15
In Vitro Assessment of a Designated “Three-Protease Blend” Used in New Cleansing Wash and Moisturizing Lotion Formulations
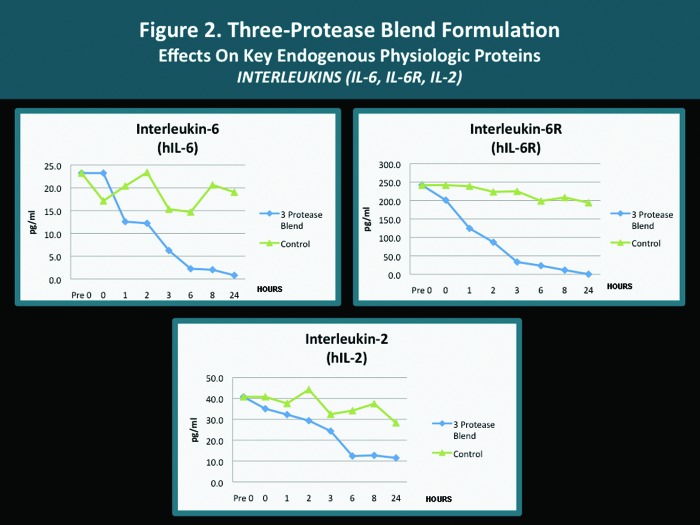
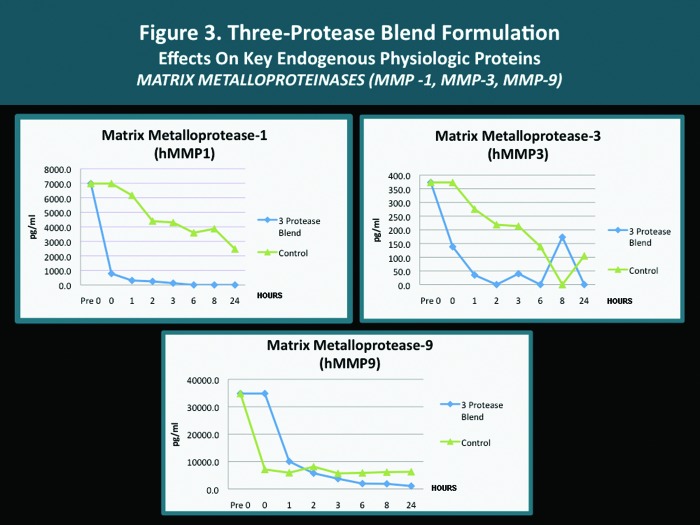
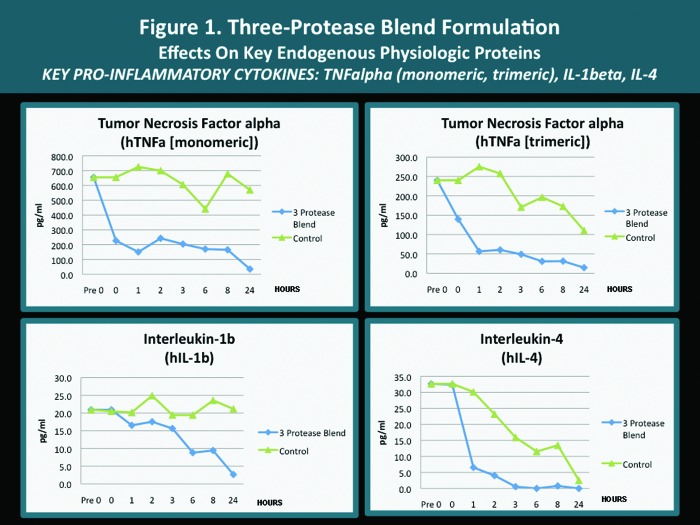
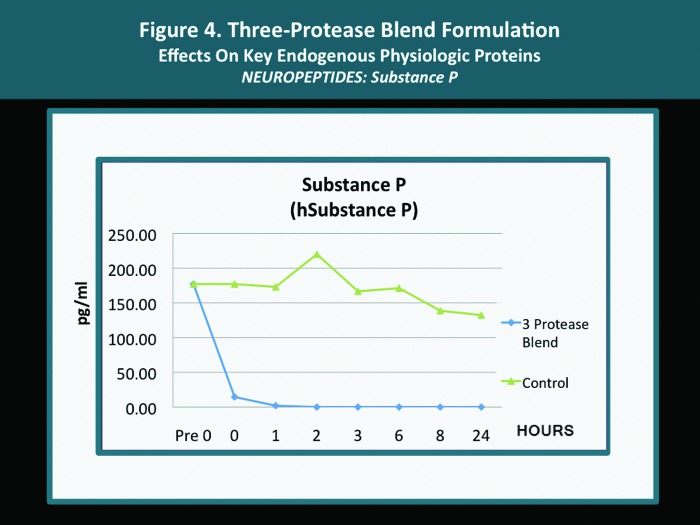
A pilot in vitro study was completed with several endogenous proteins incubated with the same 3-protease blend (bromelain, ficin, actinidin) that is contained in both the cleansing wash and the moisturizing lotion. The analyses were carried out according to standardized methodology as mandated by established research protocol with quantification performed using the Searchlight microarray assaying technique.31 The Searchlight technique has been shown to correlate with ELISA results, with the advantages of being faster and requiring a smaller sample size.14,15 The outcomes observed with individual key endogenous proteins (all of which are known to be contributors to cutaneous inflammation) after their exposure in vitro to the 3-protease blend are depicted in Figures 1 to 4. The potential clinical relevance of the reduction of individual endogenous physiological proteins/peptides is described in Table 3 and is based on the published activity profiles and data reported with these endogenous proteins/peptides.32
TABLE 3.
Potential clinical relevance of reductions in key endogenous physiological proteins after exposure in vitro to a specified 3-protease blend14–16,30–32
| ENDOGENOUS PROTEIN | EFFECT OF 3-PROTEASE FORMULATION* | POTENTIAL CLINICAL RELEVANCE (TOPICAL APPLICATION) |
|---|---|---|
| TNF alpha (monomeric, trimeric) | Reduction | Expressed by multiple cell types and involved in several pro-inflammatory and proliferative processes; works in concert with IL-1; increase in dendritic cells involved in immune response. Reduction may soften the intensity of inflammation induced by impairment of SC and in association with several dermatoses. |
| IL-1 (IL-1 beta) | Reduction | Increased early with SC permeability barrier damage (increased TEWL) and several dermatological disorders; pro-inflammatory via signaling release of other cytokines, growth factors, and recruitment of T cells; upregulates adhesion molecules; enhances epidermal a differentiation; works in concert with TNF-α. Reduction may soften the magnitude of inflammation induced by impairment of SC and in association with several dermatoses. |
| IL-6/IL-6R | Reduction | An early pro-inflammatory cytokine which contributes to amplification of inflammation (with contribution from TNF-α and IL-1); modulates the activation, differentiation, and growth of T cells; functional regulation of T, NK, and B cells. Reduction may soften early inflammation and amplification loop through reduction of IL-6, IL-1, and TNF-α. |
| IL-4 | Reduction | Promotes proliferation of activated mature T cells and cytotoxic properties; suppresses Th1 cytokine pattern; upregulated in atopic dermatitis (Th2 pattern); enhances IgE receptor expression, mast cell growth, and contributes to IgE production. Reduction may ameliorate inflammation patterns associated with atopic skin. |
| IL-2 | Reduction | “T cell growth factor” essential for developing immune response, the proliferation and clonal expansion of activated T cells, and regulation of T cell function in Th1-mediated diseases (i.e., psoriasis). Reduction may diminish the intensity of inflammation associated with response patterns observed with inflammatory skin changes and disease states. |
| MMPs | Reduction (some MMPs) | MMPs modulate/degrade dermal matrix; some induce other inflammatory cascades; increased or involved in several skin disorders (i.e., rosacea, acne) |
| MMP-1 (collagenase) | Reduction | Degradation of fibrillar collagen (collagenolysis) |
| MMP-3 (gelatinase) | Reduction | Degradation of type IV collagen and gelatin |
| MMP-9 (stromelysin) | Reduction | Degradation of several extracellular matrix proteins (not fibrillar collagen). Reduction may soften degradation of dermal matrix and inflammatory pathways signaled by specific MMPs. |
| Substance P | Reduction | Neuropeptide involved in mediation of nocioception and pain; involved in axon reflex- mediated vasodilation, wheal-flare reaction, and neurogenic inflammation; increased levels in association with eczema (atopic dermatitis and pruritus. Reduction can decrease symptomatology and edema associated with inflammatory skin disorders. |
3-protease blend: bromelain, ficin, actinidin
TNF=tumor necrosis factor; IL=interleukin; MMPs=matrix metalloproteinases
The results of this analysis are consistent overall with previous in vitro results evaluating protease technology with CWF, demonstrating softening of the activity of several pro-inflammatory endogenous proteins and apparent protection of other key proteins.14-16 This in vitro study suggests that the 3-protease blend used in a designated proprietary cleansing wash and moisturizing lotion may assist in ameliorating cutaneous inflammation observed in association with SC permeability barrier impairment secondary to xerotic skin changes and several inflammatory dermatoses.
Other Considerations
The potential for proteases to induce allergic sensitization (i.e., IgE-mediated hypersensitivity) has been discussed in the literature primarily with occupational exposure (i.e., inhalant exposure) and possible relevance to food allergy.33-37 As with all topical products, allergic contact dermatitis is a potential risk so this should be considered if a suspected case arises.33 Although IgE-mediated hypersensitivity has been reported with systemic exposure to some proteases, there is not a definitive correlation that this creates a risk with topical exposure. One study has shown that actinidin, a fruit-derived protease found in kiwifruit, was not shown to be the major allergen in a well-characterized population of individuals with established kiwifruit allergy.38 Additional studies are warranted, especially if cases of cutaneous hypersensitivity emerge with more prolonged use. As the concentrations of proteases in recently developed skin care formulations are relatively low, it is anticipated that cutaneous sensitization will not likely emerge as a major concern; however, clinical vigilance is always prudent, especially with newer products brought to the marketplace.
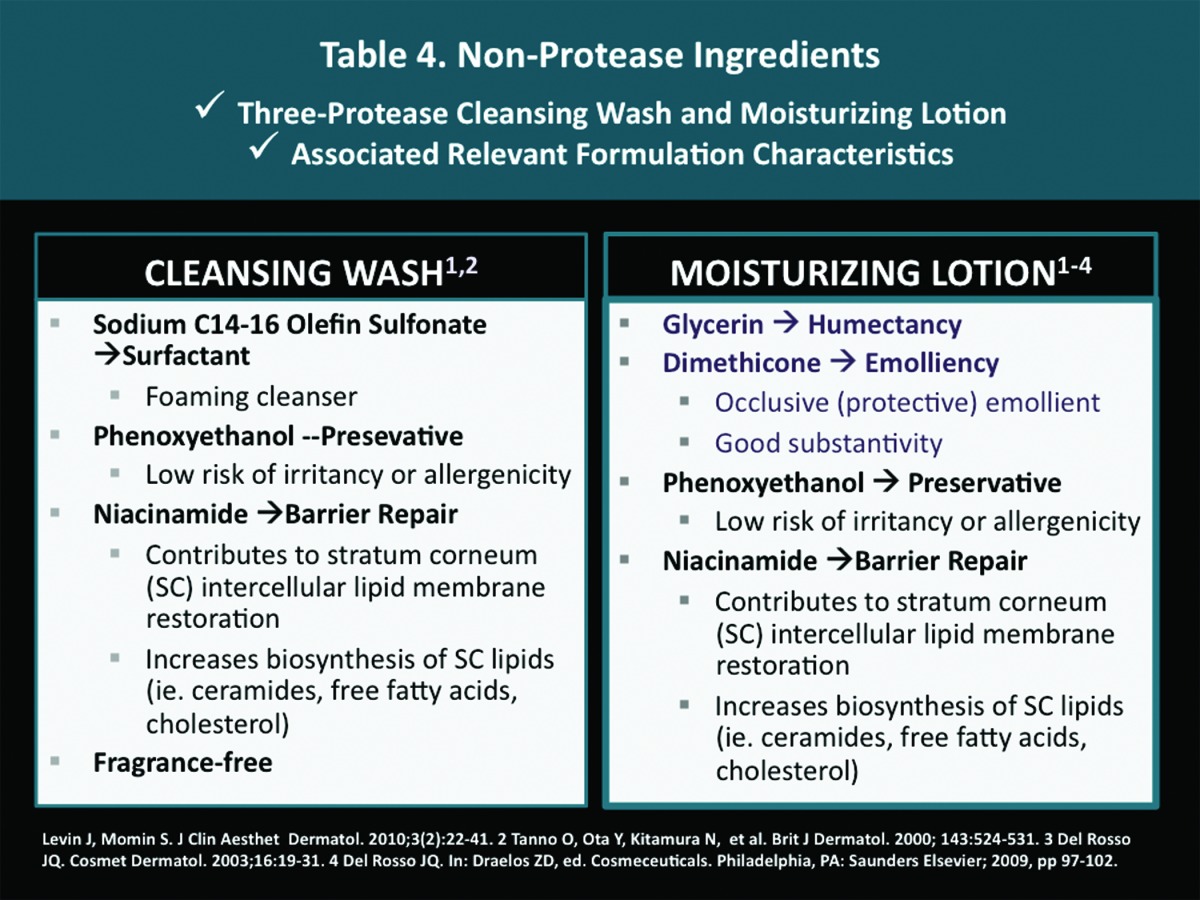
Characteristics of the Non-Protease Ingredients Used in the Vehicle Formulation
In addition to the effects induced by the three proteases, other ingredients incorporated into cleanser and moisturizer formulations play significant roles in contributing to effective gentle cleansing, and SC hydration, along with SC permeability barrier restoration. When a skin care product will be used on facial skin, non-comedogenicity is also important. An illustrative example of these principles is shown in Table 4, which depicts the non-protease ingredients and vehicle characteristics of a 3-protease blend cleansing wash and moisturizing lotion. Clinical experience and additional studies should expand our understanding of clinical responses that may be achieved by topical products that incorporate proteases. However, it is important to note that vehicle technology and other ingredients used in the formulation also contribute markedly to clinical efficacy, skin tolerability, safety, and patient satisfaction.
 |
Conclusion
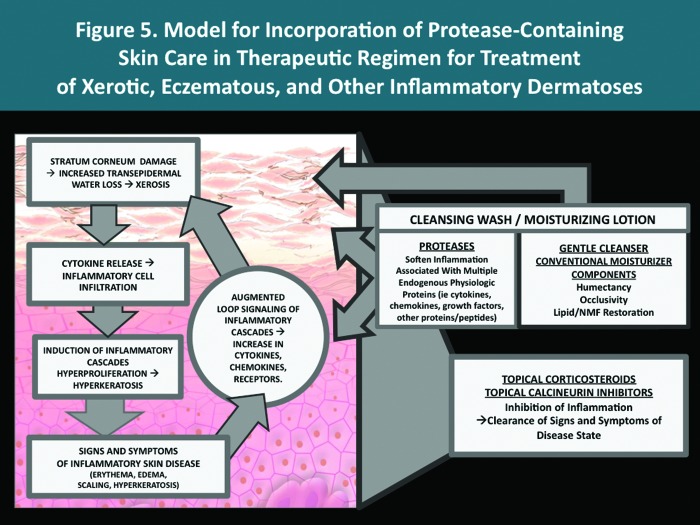
Endogenous proteases comprise several families with diverse activities and play a major role in the modulation of many physiological functions in several organ systems, including skin. Over time, advances in assessing the sources and properties of various proteases, and improved stabilization in topical vehicles, is likely to stimulate the development of newer products designed to achieve specific outcomes. Thus far, two new topical formulations, a cleansing wash and a moisturizing lotion, have been developed that incorporate a specified 3-protease blend along with other conventional ingredients included to optimize the overall performance characteristics of the products. In vitro evidence suggests that the 3-protease blend may decrease skin inflammation by softening the effects of certain key physiological proteins, such as cytokines, metalloproteinases, and the neuropeptide substance P. The goal of those developing formulations that incorporate protease technology is for these products to serve a rational adjunctive role that supports the medical therapeutic regimen, through gentle cleansing, moisturization, and contribution to the amelioration of inflammatory pathways common to many dermatoses. A therapeutic model for incorporation of protease-containing skin care products is depicted in Figure 5. Additional clinical experience and studies will help to further define the clinical profiles of protease-containing topical products.
Footnotes
Disclosure:The content of this manuscript was written solely by the author. Dr. Del Rosso has served as a consultant, advisory board participant, clinical investigator, and/or speaker for Allergan, Bayer Healthcare (Dermatology), Eisai, Galderma, Medicis (a division of Valeant), Obagi Medical Products, Onset Dermatologics, PharmaDerm, Primus, Promius, Quinnova, Ranbaxy, Taro Pharmaceuticals, TriaBeauty, Unilever, Valeant, and Warner-Chilcott. Dr. Del Rosso has served as a consultant to Taro Pharamaceuticals related to protease technology and skin care formulations. Information included in this manuscript was presented by the author in posters at Maui Dermatology in Maui, Hawaii, and Winter Clinical Dermatology, in Kauai, Hawaii, in January 2013.
References
- 1.Barrett AJ, Rawlings ND, Woessner JF. Academic Press: 2003. The Handbook of Proteolytic Enzymes, 2nd ed. [Google Scholar]
- 2.Suzuki Y, Nomura J, Koyama J, et al. The role of proteases in stratum corneum: involvement of stratum corneum desquamation. Arch Dermatol Res. 1994;286:249–253. doi: 10.1007/BF00387596. [DOI] [PubMed] [Google Scholar]
- 3.Bernard D, Mehul B, Schmidt R. Update on desquamation and first evidence for the presence of the endoglycosidase herapinase 1 in the human stratum coneum. In: Marks R, Leveque JL, Voegeli R, editors. The Essential Stratum Corneum. London, United Kingdom: Martin-Dunitz; 2002. pp. 17–24. [Google Scholar]
- 4.Rawlings AV, Voegeli R. Stratum corneum proteases and dry skin conditions. Cell Tissue Res. doi: 10.1007/s00441-012-1501-x. 2012 Oct 9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Meyer-Hoffert U, Schroder JM. Epidermal proteases in the pathogenesis of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):16–23. doi: 10.1038/jidsymp.2011.2. [DOI] [PubMed] [Google Scholar]
- 6.Ogg GS, Winsey S, Wakelin S, Wajnarowska F. Immunobollous diseases. In: Bos JD, editor. Skin Immune System: Cutaneous Immunology and Clinical Immunodermatology, 3rd ed. Boca Raton, Florida: CRC Press; 2005. pp. 516–517. [Google Scholar]
- 7.Smith WP, Bishop MA, Norton SJ. Cosmetic benefits derived from the topical application of acid proteases. Skin Moisturization, 2nd ed. In: Rawlings AV, Leyden JJ, editors. New York: Informa Healthcare; 2009. pp. 397–410. [Google Scholar]
- 8.Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease affected skin. J Clin AesthetDermatol. 2011;4(9):22–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004;17:6–15. doi: 10.1111/j.1396-0296.2004.04s1001.x. [DOI] [PubMed] [Google Scholar]
- 10.Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103:731–740. doi: 10.1111/1523-1747.ep12398620. [DOI] [PubMed] [Google Scholar]
- 11.Raymundo J, Gray M. Enzymatic wound debridement. J Wound Ostomy ContinenceNurs. 2008;35(3):273–280. doi: 10.1097/01.WON.0000319125.21854.78. [DOI] [PubMed] [Google Scholar]
- 12.Masunaga T. Enzymes in cleansers. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization, 1st ed. New York: Marcel Dekker Inc.; 2002. pp. 385–403. [Google Scholar]
- 13.Pocalyko DJ, Chandar P, Harding C, et al. The efficacy, stability, and safety of topically applied protease in treating xerotic skin. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization, 1st ed. New York: Marcel Dekker Inc.; 2002. pp. 365–384. [Google Scholar]
- 14.Baskovich B, Sampson EM, Schultz GS, et al. Wound dressing components degrade proteins detrimental to wound healing. Int Wound J. 2008;5:543–551. doi: 10.1111/j.1742-481X.2007.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parnell LKS. Protein degradation and protection observed in the presence of novel wound dressing components. J Funct Biomater. 2012;2:338–354. doi: 10.3390/jfb2040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parnell L. Protein degradation and protection by wound dressing components. Eur J Wounds Burn Management. 2010;2(2):1–8. [Google Scholar]
- 17.Elias PM. Defensive functions of the stratum corneum: integrative aspects. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 5–14. [Google Scholar]
- 18.Kligman AM. A brief history of how the dead stratum corneum became alive. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 15–24. [Google Scholar]
- 19.Elias PM. The epidermal permeability barrier: from Saran wrap to biosensor. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 25–31. [Google Scholar]
- 20.Nole G. Clinical testing of moisturizers. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization, 1st ed. New York: Marcel Dekker Inc.; 2002. pp. 365–398. [Google Scholar]
- 21.Grove GL, Zerweck C, Pierce E. In: Skin Moisturization, 1st ed. Leyden JJ, Rawlings AV, editors. New York: Marcel Dekker Inc.; 2002. pp. 499–528. [Google Scholar]
- 22.Pierard-Franchimont C, Paye M. Laboratory-based ex vivo assessment of stratum corneum function. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization, 1st ed. New York: Marcel Dekker Inc.; 2002. pp. 529–546. [Google Scholar]
- 23.Crowther JM, Matts PJ. Measuring water gradients using confocal Raman microspectroscopy. In: Rawlings AV, Leyden JJ, editors. Skin Moisturization, 2nd ed. New York: Informa Healthcare USA; 2009. pp. 441–461. [Google Scholar]
- 24.Imhof RE, Xiao P, De Jesus MEP, et al. New developments in skin barrier mesasurements. In: Rawlings AV, Leyden JJ, editors. Skin Moisturization, 2nd ed. New York: Informa Healthcare USA; 2009. pp. 463–479. [Google Scholar]
- 25.Biao L, Elias PM, Feingold KR. The role of the primary cytokines, TNF, IL-1, and IL-6, in permeability barrier homeostasis. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 305–318. [Google Scholar]
- 26.Barland CO, Elias PM. Ruby Ghadialy. The aged epidermal permeability barrier: basis for functional abnormalities. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 535–552. [Google Scholar]
- 27.Nedelec B, Rachelska G, Parnell LKS, LaSalle L. Double-blind, randomized, pilot study assessing the resolution of post-burn pruritus. J Burn Care Res. 2012;33(3):398–406. doi: 10.1097/BCR.0b013e318233592e. [DOI] [PubMed] [Google Scholar]
- 28.Parnell LK, Nedelec B, Rachelska G, et al. Assessment of pruritus characteristics and impact on burn survivors. J Burn Care Res. 2012;33(3):407–418. doi: 10.1097/BCR.0b013e318239d206. [DOI] [PubMed] [Google Scholar]
- 29.Barnett L, Parnell LK. Contact dermatitis treated with new topical products: a case study. Ostomy Wound Manage. 2001;47(9):47–53. [PubMed] [Google Scholar]
- 30. Data on file. Swiss-American Products Inc., Carrollton, Texas; 2012.
- 31. Data on file. Taro Pharmaceuticals USA Hawthorne, New York; 2012. [Google Scholar]
- 32.Steinhoff M, Luger TA. The skin cytokine network. In: Bos JD, editor. Skin Immune System: Cutaneous Immunology and Clinical Immunodermatology, 3rd ed. Boca Raton, Florida: CRC Press; 2005. pp. 349–372. [Google Scholar]
- 33.van Kampen V, Merget R, Bruning T. Occupational allergies to bromelain. Pneumologie. 2007;61(3):159–161. doi: 10.1055/s-2006-955001. [DOI] [PubMed] [Google Scholar]
- 34.Secor ER, Jr, Carson WF, 4th, Cloutier MM, et al. Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell Immunol. 2005;237(1):68–75. doi: 10.1016/j.cellimm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nettis E, Napoli G, Ferrannini A, et al. IgE-mediated allergy to bromelain. Allergy. 2001;56(3):257–258. doi: 10.1034/j.1398-9995.2001.056003257.x. [DOI] [PubMed] [Google Scholar]
- 36.Hemmer W, Focke M, Gotz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34(8):1251–1258. doi: 10.1111/j.1365-2222.2004.02046.x. [DOI] [PubMed] [Google Scholar]
- 37.Grozdanovic M, Popovic M, Polovic N, et al. Evaluation of IgE reactivity of active and thermally inactivated actinidin, a biomarker of kiwifruit allergy. Food Chem Toxicol. 2012;50(3-4):1013–1018. doi: 10.1016/j.fct.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 38.Lucas JS, Nieuwenhuizen NJ, Atkinson RG, et al. Kiwifruit allergy: actinidin is not a major allergen in the United Kingdom. Clin Exp Allergy. 2007;37(9):1340–1348. doi: 10.1111/j.1365-2222.2007.02776.x. [DOI] [PubMed] [Google Scholar]