Abstract
To investigate the efficacy and safety of the addition of the microdroplet technique to conventional injections for the treatment of crow’s feet with incobotulinumtoxin A, four women with moderate-to-severe crow’s feet at maximum expression received three standard intradermal injections of incobotulinumtoxin A on both sides of the face (4U [0.1mL] at each injection point) plus six microdroplet injections of incobotulinumtoxin A (0.5U [0.025mL] per point) into the lateral canthal area on the left side of the face and an equal quantity of saline at the same injection points on the right side of the face. Investigators and subjects independently evaluated the degree of rhytide improvement both at rest and maximum expression after 28 and 84 days. Investigator and subject ratings for wrinkle severity at maximum expression were improved to a greater degree on the side of the face treated with additional microdroplet incobotulinumtoxin A in all subjects, except one who was over 65 years old. All women preferred the results on the left side of their face. Treatment was well tolerated. The addition of the microdroplet technique to standard practice for injecting incobotulinumtoxin A is a promising procedure for the treatment of crow’s feet in women with moderate-to-severe wrinkles.
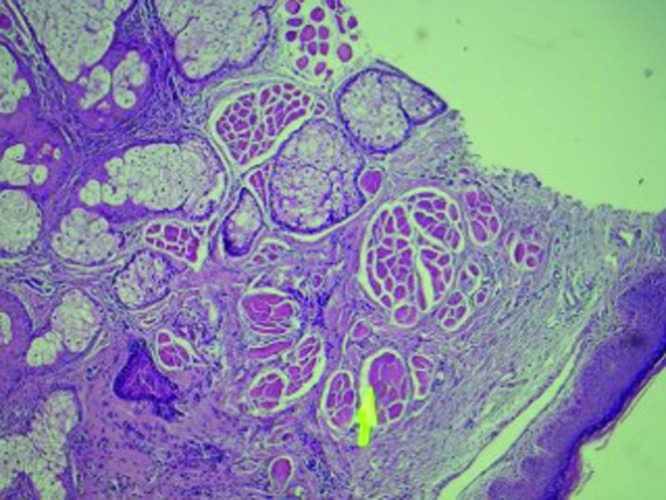
The microdroplet technique for injection of botulinum neurotoxin has been developed to Thesupplement traditional injection procedures. It microdroplet technique for injection oftargets only the superficial muscle fibers where they insert into the dermis (Figure 1) with a series of small, highly diluted microdroplet injections. In this manner, a smooth transition between treated and nontreated areas is achieved for a more harmonious effect. The more precise placement of small amounts of neurotoxin into superficial fibers is also less likely to lead to diffusion of neurotoxin into untargeted muscle and unwanted side effects. The microdroplet technique may therefore allow areas of the face to be treated that are generally avoided with conventional injection techniques.
Figure 1.
Histology slide demonstrating superficial facial muscle fibers inserting into the dermis
In a preliminary assessment of this technique, the authors present a case report of four subjects in whom the microdroplet technique in addition to standard injections was compared with a standard injection technique alone. Incobotulinumtoxin A (Xeomin®/Xeomeen®/Bocouture®/XEOMIN Cosmetic™; botulinum toxin type A free from complexing proteins [150 kDa]/NT 201, Merz Pharmaceuticals GmbH, Frankfurt, Germany) was chosen as the neurotoxin for injection because of its comparable efficacy to other botulinum toxins and reduced likelihood of neutralizing antibody development.1-4
METHODS
Women aged 18 to 65 years with moderate-to-severe lateral canthal lines (crow's feet) at maximum expression (Merz scale5 severity score 3 to 4, Table 1) and in a stable medical condition were recruited. Typical exclusion criteria for botulinum neurotoxin trials were fulfilled.3 Informed consent was obtained from each subject.
TABLE 1.
Investigator and subject assessment of crow’s feet severity at rest and maximum expression using the Merz scale and patient assessment scale, respectively
| AT REST | AT MAXIMUM EXPRESSION | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAY 0 | DAY 28 | DAY 84 | DAY 0 | DAY 28 | DAY 84 | |||||||
| R | L | R | L | R | L | R | L | R | L | R | L | |
| INVESTIGATOR RATING | ||||||||||||
| Patient 1 | 3 | 3 | 1 | 1 | 2 | 1 | 4 | 4 | 2 | 1 | 3 | 2 |
| Patient 2 | 3 | 3 | 2 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 3 | 2 |
| Patient 3 | 2 | 2 | 1 | 1 | 1 | 1 | 4 | 4 | 2 | 2 | 2 | 1 |
| Patient 4 | 4 | 4 | 3 | 3 | 3 | 3 | 4 | 4 | 3 | 3 | 3 | 3 |
| SUBJECT RATING | ||||||||||||
| Patient 1 | 3 | 3 | 1 | 1 | 2 | 1 | 4 | 4 | 2 | 1 | 3 | 2 |
| Patient 2 | 3 | 3 | 2 | 1 | 2 | 1 | 3 | 3 | 2 | 1 | 3 | 2 |
| Patient 3 | 2 | 2 | 1 | 1 | 1 | 1 | 4 | 4 | 1 | 0 | 1 | 1 |
| Patient 4 | 4 | 4 | 3 | 3 | 3 | 3 | 4 | 4 | 3 | 3 | 3 | 3 |
Abbreviations: R=right side of face (standard injection + saline microdroplet). L=left side of face (standard injection + incobotulinumtoxin A microdroplet). Merz scale grades: 0=no wrinkles, 1=very fine wrinkles; 2=fine wrinkles; 3=moderate wrinkles; 4=severe wrinkles. At rest patient- assessment scale ratings: 0=no visible lines; 1=very fine visible lines; 2=fine visible lines; 3=moderate visible lines; 4=severe visible lines. At maximum expression patient-assessment scale ratings: 0=no muscle action achievable; 1=some very fine muscle action; 2=fine muscle action; 3=moderate muscle action; 4=strong muscle action.
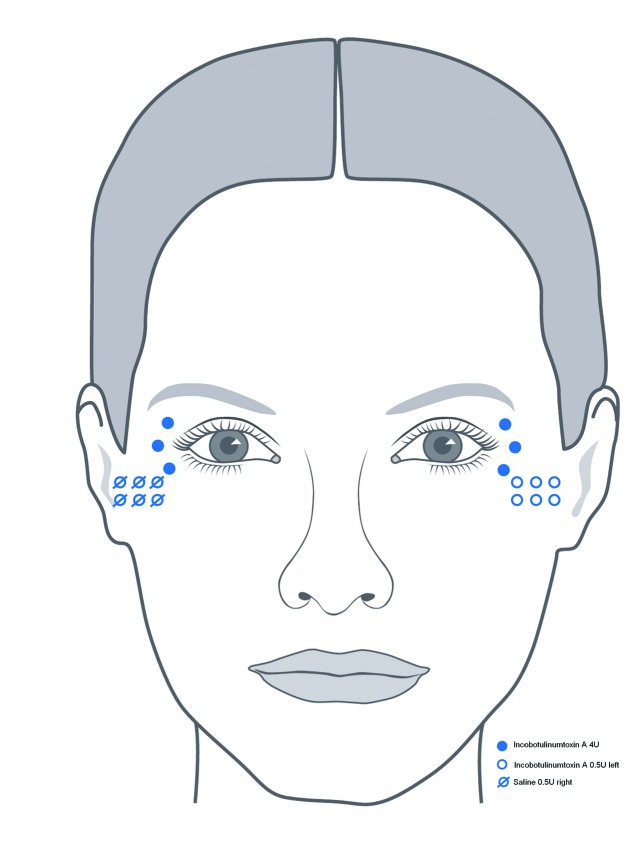
Conventional injection technique. Using a split-face design, the women received intradermal injections of incobotulinumtoxin A into three defined points in the crow’s feet area on both sides of the face (4U at each point). Injection points were approximately 1cm apart and 1cm outside the bony orbital rim.
Microdroplet technique. In the same injection session, subjects also received an additional 3U of incobotulinumtoxin A with the microdroplet technique at six injection points in the left lower crow’s feet area (0.5U [0.025mL] at each point) and 3U of saline at the same six injection points on the contralateral side (0.025mL at each point) (Figure 2). The microdroplet technique only targets the most superficial muscle fibers inserting into the dermis. To achieve this, the needle is advanced very slowly with the bevel parallel to the skin and pressure applied to the plunger until a small bleb appears in the skin.
Figure 2.
Illustration of standard and microdroplet technique injection points
Dilution. Each vial of 50 LD50 units of incobot-ulinumtoxin A was reconstituted with 1.25mL preservative-free sterile saline (0.9% isotonic sodium chloride) to give a final concentration of 1U per 0.025mL. For the microdroplet technique, this solution was further diluted with 0.025mL preservative-free saline directly in the syringe to give a total volume of 0.05mL. Injection of 0.025mL of this solution was equivalent to 0.5U incobotulinumtoxin A.
Study endpoint. Efficacy assessments were made by both investigators and subjects at Days 28 and 84. The primary endpoint was an improvement in physician-rated crow’s feet severity (1 point on the Merz scale) from Day 0 to Day 28 both at rest and maximum expression.
RESULTS
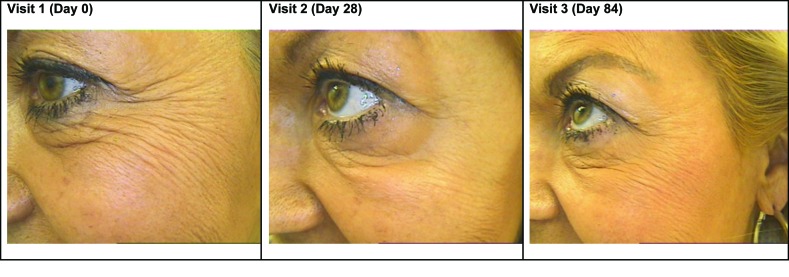
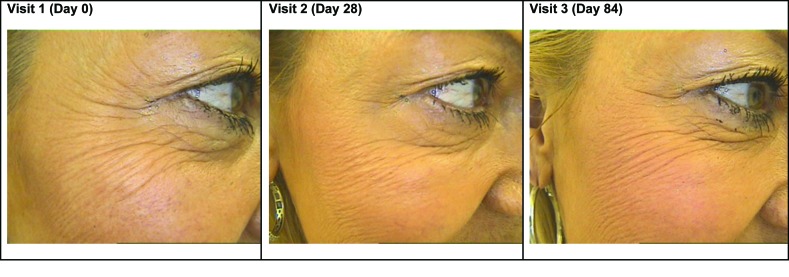
The four women in this study were aged 44, 48, 60, and 65 years. At baseline, three had moderate-to-severe wrinkles at rest and all had moderate-to-severe wrinkles at maximum expression. At Day 28, an improvement in physician-rated crow’s feet severity of ≥1 point was achieved in all women (Table 1). Figure 3 illustrates the beneficial effect of the addition of the microdroplet technique at both assessment visits. At Day 84, investigators reported less severe wrinkles at maximum expression with microdroplet plus standard injection compared with standard injection alone, with the exception of Patient 4 (65 years old).
Figure 3.
Before and after photos of Patient 1 illustrating the left and right sides of the face at Visit 1 (Day 0), Visit 2 (Day 28), and Visit 3 (Day 84). The crow’s feet area on the left side of the face was injected with the conventional three-point injection plus the microdroplet technique. The right side of the face received conventional injection only. A comparison of the two sides of the face clearly shows the benefit of the addition of the microdroplet technique.
Subject assessment of wrinkle severity was also improved compared with baseline. On the side of the face treated with incobotulinumtoxin A microdroplet plus standard injection, these improvements were maintained in all women at Day 84 (Table 1). All women also reported muscle action below baseline levels on the left side of the face at Day 84.
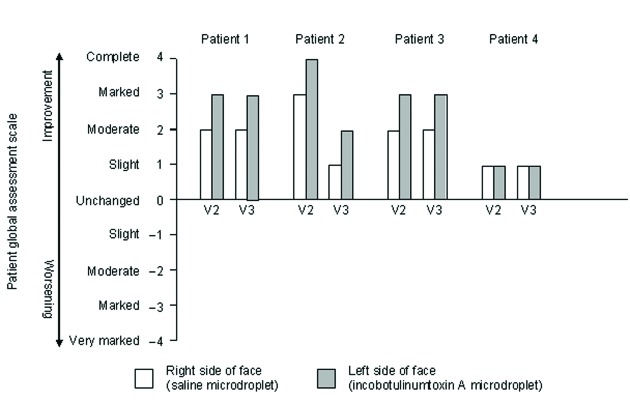
At Day 28, two subjects reported marked improvement and one subject reported complete improvement in the appearance of their crow’s feet compared with baseline (Figure 4). Patient 4 reported a slight improvement. At Day 84, lines continued to be assessed as improved compared with baseline. All patients preferred the cosmetic outcome on the left side of their face. No adverse events related to the microdroplet technique were reported.
Figure 4.
Subject response to the question: “How would you rate the change in the appearance of your crow’s feet compared with the situation immediately before injection?” using Patient Global Assessment scale rating responses. V2=visit 2; V3=visit 3.
DISCUSSION
The microdroplet technique is a new procedure that has been developed for use in conjunction with standard injections. Preliminary results reported here in four women of varying ages show that the beneficial effects of incobotulinumtoxin A for the treatment of crow’s feet are enhanced by the addition of this technique. All women preferred the side of their face treated with the additional microdroplet technique.
Crow’s feet are caused by the activity of the lateral orbicularis oculi muscle. The cumulative effect of the microdroplet injections is selective weakening of this muscle over the whole lateral canthal area, which results in less skin tension and as a consequence fewer superficial wrinkles. As the orbicularis oculi muscle is diffusely innervated, multiple injections are often required to weaken the muscle effectively, which may make it particularly suitable to additional treatment with the microdroplet technique.
In older patients, a greater proportion of rhytides may be due to loss of skin elasticity, which is not alleviated by botulinum toxin.6 It was thus not unexpected that the oldest patient in this study (65 years old) with severe visible lines at baseline demonstrated the least benefit from the addition of the microdroplet technique.
Precise localization of neurotoxin is required to produce the desired clinical results. Temporary disfigurement or functional impairment can occur if neurotoxin inadvertently diffuses into adjacent muscle. With the microdroplet technique, only the most superficial muscle fibers inserting into the dermis are targeted. Furthermore, the small dilution volumes allow more precise administration of the toxin. This makes it possible to extensively treat the whole lateral periorbital area with no affect on the zygomaticus muscles and therefore unwanted side effects, such as mid-face ptosis.
To the authors’ knowledge, this is the first report in the literature describing the use of the microdroplet technique for injection of botulinum toxin to correct facial rhytides. The results demonstrate that the addition of this technique to standard injections enhances the effects of incobotulinumtoxin A for the temporary resolution of crow’s feet. Although this is a limited set of data, it provides insight into the potential for this technique and should serve as a stimulus for larger studies to evaluate the benefits of the microdroplet technique for crow’s feet as well as rhytides in other facial areas.
Footnotes
DISCLOSURE:Drs. Imhof and Kuhne are speakers and advisors for Merz and have received financial support from Merz Pharmaceuticals to perform this clinical pilot study.
REFERENCES
- 1.Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/ Bocouture®. Drugs R D. 2010;10:67–73. doi: 10.2165/11584780-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattler G, Callander MJ, Grablowitz D, et al. Noninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg. 2010;36(Suppl 4):2146–2154. doi: 10.1111/j.1524-4725.2010.01706.x. [DOI] [PubMed] [Google Scholar]
- 3.Imhof M, Kiihne U. A phase III study of incobotulinumtoxinA in the treatment of glabellar frown lines. J Clin Aesthet Dermatol. 2011;4(10):28–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Hefter H, Hartmann C, Kahlen U, Moll M, Bigalke H. Prospective analysis of neutralizing antibody titres in secondary non-responders under continuous treatment with a botulinumtoxin type A preparation free of complexing proteins—a single cohort 4-year follow-up study. BMJ Open. 2012;2(4):pii: e000646. doi: 10.1136/bmjopen-2011-000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn TC, Carruthers A, Carruthers J, et al. Validated assessment scales for the upper face. Dermatol Surg. 2012;38(2 Spec No.):309–319. doi: 10.1111/j.1524-4725.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CM. Cosmetic use of botulinum toxin type A in the elderly. Clin Interv Aging. 2007;2:81–83. doi: 10.2147/ciia.2007.2.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]