Abstract
Background
Postlicensure data has identified a causal link between rotavirus vaccines and intussusception in some settings. As rotavirus vaccines are introduced globally, monitoring intussusception will be crucial for ensuring safety of the vaccine programs.
Methods
To obtain updated information on background rates and clinical management of intussusception, we reviewed studies of intussusception in children <18 years of age published since 2002. We assessed the incidence of intussusception by month of life among children <1 year of age, seasonality, method of diagnosis, treatment, and case-fatality.
Findings
We identified 82 studies from North America, Asia, Europe, Oceania, Africa, Eastern Mediterranean, and Central & South America that reported a total of 44,454 intussusception events. The mean incidence of intussusception was 74 per 100,000 (range: 9–328) among children <1 year of age, with peak incidence among infants 5–7 months of age. No seasonal patterns were observed. A radiographic modality was used to diagnose intussusception in over 95% of the cases in all regions except Africa where clinical findings or surgery were used in 65% of the cases. Surgical rates were substantially higher in Africa (77%) and Central and South America (86%) compared to other regions (13–29%). Case-fatality also was higher in Africa (9%) compared to other regions (<1%). The primary limitation of this review relates to the heterogeneity in intussusception surveillance across different regions.
Conclusion
This review of the intussusception literature from the past decade provides pertinent information that should facilitate implementation of intussusception surveillance for monitoring the postlicensure safety of rotavirus vaccines.
Background
Intussusception is the invagination of one segment of the intestine within a more distal segment [1], [2]. It is the most common cause of bowel obstruction in infants, occurring usually between 4 and 10 months of age [3]. In most infants, the intussusception involves the ileum invaginating through the ileocecal valve into the cecum. As the bowel intussuscepts, it pulls along its blood supply. If the intussusception is not relieved, the vascular supply of the bowel may be compromised, resulting in intestinal ischemia and possibly perforation. Untreated intussusception may be fatal.
In 1999, an association was found between a tetravalent rhesus reassortant rotavirus vaccine (RotaShield) and intussusception in US infants prompting the manufacturer to withdraw the vaccine from the market [4], [5]. Because of this association, the two rotavirus vaccines that are currently recommended by the World Health Organization [RotaTeq (RV5), Merck & Co.; Rotarix (RV1), GSK Biologicals] were evaluated in large safety trials which excluded a risk of intussusception similar to that found with RotaShield (1 excess case per 10,000 vaccinated infants) [5], [6]. However, postlicensure studies in some high and middle income settings have identified a low level risk of 1–2 excess cases of intussusception per 100,000 infants after both RV1 and RV5 [7], [8]. These vaccines have now been introduced in some 45 countries worldwide, primarily in middle and high income countries, but broader introductions in low income countries of Africa and Asia are expected in next 3–5 years [9].
The past experience with RotaShield and the identification of a lower level risk of intussusception after RV1 and RV5 highlights the importance of ongoing intussusception monitoring as these new vaccines are broadly adopted in other regions of the world. In 2002, the WHO published a comprehensive literature review of childhood intussusception globally between the years 1960–2002 [3]. In anticipation of the global introduction of rotavirus vaccines, the objective of this literature review was to describe the epidemiology, diagnosis, and clinical manifestations of intussusception between the years 2002–2012. This review of the epidemiology of intussusception based on contemporary studies published in the last decade should serve as a valuable resource for implementing surveillance for intussusception to monitor the safety of these vaccines as they are broadly rolled out across the world.
Methods
Search strategy and selection criteria
We followed the PRISMA guidelines to identify all intussusception studies published between January 2002 (when the last review was conducted) and June 2012. We included all peer-reviewed articles and selected meeting abstracts. We searched PubMed MEDLINE and Google Scholar using the following keywords: “intussusception” or “intestinal invagination.” We included all human studies of intussusception among children <18 years of age published in English. Because we were interested in understanding the epidemiology of all intussusception events among children, full-text articles were not reviewed if they were case reports, animal studies, follow-up evaluation of chronic or recurrent intussusception, reports of intussusception events secondary to other conditions (e.g., vascular or congenital malformations, tumors), or reports of surgical or radiological management of intussusception. We also excluded those studies which did not include children <1 year of age. We reviewed the reference lists of all included articles to identify additional sources of data, missing articles, or meeting abstracts. Where there were multiple sources of data from the same study population, we relied on the data from the most complete peer-reviewed publication.
Data Extraction and Management
Data from included studies were extracted onto a standardized table by JJ and MP. Reviewers were not blinded to study authors, affiliations, or journal name. Variables recorded from each article included, study locations (city & country), sample size, study period, age at diagnosis, month of presentation, diagnostic modality, treatment, death, and incidence.
Data Analysis
We grouped data according to seven geographic regions of the world: Africa, Asia, Central & South America, Eastern Mediterranean, Europe, North America, and Oceania. Because of heterogeneity in under 5 mortality rates (u5MR) in Asia, we grouped Asian countries into high u5MR and low/very low u5MR according to WHO classification [10]. We present data on incidence of intussusception among children<1 year of age. We averaged the annual rate when rates for multiple years were presented individually. Among countries reporting incidence of intussusception, we were also interested in determining incidence of intussusception by month during the first year of life. To determine a pooled estimate of intussusception incidence by month of age globally, we extrapolated the proportion of intussusception events by month of age from studies that either presented monthly incidence or number of cases to the remaining studies that only presented intussusception incidence among children <1 year of age. While only 5 studies reported intussusception incidence by month of age, an additional 17 studies presented data on number of cases by month of age.
For studies reporting data on seasonality, we pooled data by geographic region on number of intussusception cases by calendar month. We also present pooled data on diagnostic modality, treatment, and prevalence of death by geographic region. For diagnosis and treatment, we excluded cases where this information was missing or unspecified.
All analyses were done with Microsoft EXCEL (Microsoft Corp, 2007).
Results
We reviewed 2757 abstracts to identify a total of 113 potential full-text articles and 1 meeting abstract, from which 82 studies met the inclusion criteria for our analysis (Figure 1; Table 1) [11]–[91]. We excluded 31 articles due to non-English language (n = 8), duplicate data (n = 14), non-epidemiology study or review (6), included in previous review (2), or age <1 excluded (1). Included studies reported a total of 44,454 intussusception events from North America (n = 16,425), Asia: low or very low mortality (n = 14,382), Europe (n = 6,280), Oceania (2,761), Africa (2,895), Eastern Mediterranean (n = 644), and Central & South America (n = 538), Asia: high mortality (n = 356). Of these 82 studies, 44 (60%) were hospital-based studies, whereas 38 (40%) represented national or regional data on intussusception hospitalizations.
Figure 1. PRISMA flow diagram for the intussusception literature review.
Table 1. List of all included studies.
| Country | Study dates | National or Hospital based | No. of cases | Age, yrs | Ref. |
| Africa | 2895 | ||||
| Multiplea | 1993–2003 | International | 1069 | <1 | {Steele, 2012 #92} |
| Kenya | 2000–2003 | Hospital | 36 | <6 | {Kuremu, 2004 #55} |
| Kenya | 1992–1999 | Hospital | 29 | NR | {Muyembe, 2000 #65} |
| Ghana | 2004–2007 | Hospital | 44 | <13 | {Abantanga, 2008 #1} |
| Ghana | 2008–2009 | Hospital | 77 | <5 | {Enweronu-Laryea, 2012 #35} |
| Nigeria | 1980–1998 | Hospital | 80 | <12 | {Ameh, 2002 #3} |
| Nigeria | 1988–1998 | Hospital | 33 | <1 | {Ameh, 2002 #4} |
| Nigeria | 1989–1998 | Hospital | 89 | <1 | {Archibong, 2001 #5} |
| Nigeria | 1995–2001 | Hospital | 174 | <7 | {Bode, 2008 #12} |
| Nigeria | 1996–2005 | Hospital | 38 | <15 | {Ogundoyin, 2009 #69} |
| Nigeria | 1998–2003 | Hospital | 29 | <8 | {Edino, 2003 #30} |
| Nigeria | 1998–2007 | Hospital | 87 | <8 | {Ekenze, 2010 #31} |
| Nigeria | 2008–2009 | Hospital | 20 | <2 | {Ekenze, 2011 #32} |
| So. Africa | 1998–2003 | 9 Hospitals | 423 | <14 | {Moore, 2010 #63} |
| So. Africa | 1996–2001 | Hospital | 106 | NR | {Wiersma, 2004 #112} |
| Tanzania | 2000–2004 | Hospital | 28 | <10 | {Carneiro, 2004 #21} |
| Tunisia | 1984–2003 | National | 533 | <5 | {Chouikha, 2009 #27} |
| Asia–high u5MR | 356 | ||||
| Bangladesh | 2001–2006 | Regional | 4 | <2 | {Zaman, 2009 #109} |
| India | 2000–2003 | Hospital | 5 | <1 | {Bahl, 2009 #7} |
| India | 2001–2004 | Hospital | 31 | <5 | {Bhowmick, 2009 #8} |
| India | 1991–2000 | Hospital | 137 | <5 | {Raman, 2003 #75} |
| India | –– | Hospital | 179 | NR | {Ramachandran, 2008 #74} |
| Asia–low & very low u5MR | 14382 | ||||
| China | 2004–2009 | Hospital | 56 | <14 | {Zhang, 2011 #110} |
| Hong Kongb | 1997–2003 | National | 531 | <5 | {Hong Kong Intussusception Study, 2007 #44} |
| Hong Kongb | 1997–1999 | National | 190 | <5 | {Nelson, 2002 #67} |
| Japan | 2007–2008 | National | 2427 | <18 | {Takeuchi, 2012 #93} |
| Japan | 1978 – 2002 | Hospital | 91 | <5 | {Nakagomi, 2006 #66} |
| So. Korea | 2000–2002 | Regional | 408 | <5 | {Jo, 2009 #47} |
| Malaysia | 2000–2003 | Regional | 62 | <5 | {Giak, 2008 #40} |
| Singapore | 1997–2007 | National | 217 | <2 | {Tan, 2009 #94} |
| Taiwan | 1998–2007 | National | 8217 | <15 | {Chen, 2010 #24} |
| Thailand | 1992–2009 | Hospital | 737 | NR | {Kruatrachue, 2011 #54} |
| Thailand | 2001–2006 | 5 hospitals | 112 | <5 | {Khumjui, 2009 #51} |
| Thailand | 2000–2005 | Hospital | 94 | <14 | {Pruksananonda, 2007 #73} |
| Uzbekistan | 2004–2008 | Regional | 67 | <2 | {Latipov, 2011 #58} |
| Vietnam | 2002–2003 | Hospital | 640 | <2 | {Justice, 2007 #50} |
| Vietnam | 2002–2004 | Hospital | 533 | <2 | {Bines, 2006 #9} |
| Central & South Americas | 538 | ||||
| Latin America | n/a | International | 222 | <2 | {Abate, #111} |
| Chile | 2000–2001 | Regional | 95 | <2 | {O'Ryan, 2003 #71} |
| Panama | 1998–2002 | National | 103 | <1 | {Saez-Llorens, 2004 #80} |
| Trinidad | 2000–2007 | Hospital | 65 | <3 | {Tota-Maharaj, 2010 #98} |
| Venezuela | 1998–2001 | Regional | 53 | <1 | {Perez-Schael, 2003 #72} |
| Eastern Mediterranean | 644 | ||||
| Saudi Arabia | 1993 – 2000 | Hospital | 34 | <8 | {Al-Malki, 2005 #2} |
| Saudi Arabia | 1984–2000 | Hospital | 37 | <3 | {Crankson, 2003 #29} |
| Israel | 1990–2004 | Hospital | 316 | <5 | {Greenberg, 2008 #41} |
| Israel | 2990–2002 | Hospital | 148 | <5 | {Eshed, 2003 #36} |
| Jordan | 1979–2003 | Hospital | 109 | <14 | {Saleem, 2008 #81} |
| Europe | 6280 | ||||
| Austria | 1999–2006 | Hospital | 111 | <10 | {Saxena, 2007 #85} |
| Denmark | 1980–2001 | National | 1814 | <5 | {Fischer, 2004 #39} |
| Finland | 2001–2006 | National | 53 | <13 | {Lappalainen, 2012 #57} |
| Germany | 2006–2007 | Regional | 169 | <1 | {Wei, 2011 #102} |
| Germany | 2006–2007 | National | 1200 | <15 | {Jenke, 2011 #46} |
| Germany | 2005–2006 | Regional | 518 | <17 | {Kohl, 2010 #53} |
| Ireland | 1998–2010 | Hospital | 256 | <12 | {Tareen, 2011 #96} |
| Ireland | 1990–2000 | Hospital | 24 | <2.5 | {Hilal, 2002 #42} |
| Russia | 1994–2005 | Hospital | 280 | <14 | {Shapkina, 2006 #86} |
| Spain | unspecified | Hospital | 151 | <12 | {Rubi, 2002 #78} |
| Switzerland | 2003–2006 | National | 294 | <18 | {Buettcher, 2007 #17} |
| Turkey | 1991–2007 | Hospital | 105 | <15 | {Sonmez, 2012 #91} |
| Turkey | 1993–2003 | Hospital | 179 | NR | {Yalcin, 2009 #107} |
| UK/Ireland | 2008–2009 | National | 261 | <1 | {Samad, 2012 #83} |
| UK/Ireland | 1997 – 1998 | Hospital | 32 | <1.5 | {Willetts, 2001 #104} |
| England | 1993–1995 | National | 833 | <1 | {Gay, 1999 #113} |
| North America | 16425 | ||||
| Canada | 1993–2001 | Regional | 961 | <6 | {Somme, 2006 #90} |
| US | 2000–2009 | National | 10,836 | <1 | {Yen, 2012 #108} |
| US | 2001–2005 | Nationalc | 22 | <1 | {Eng, 2012 #34} |
| US | 2002–2012 | Hospital | 405 | <7 | {Fike, 2012 #38} |
| US | 1996–2007 | Regional | 188 | <17 | {Shekherdimian, 2011 #87} |
| US | 2002–2005 | Military hospitals | 293 | <5 | {Nylund, 2010 #68} |
| US | 2001–2006 | 3 Hospitals | 183 | <1 | {Cortese, 2009 #28} |
| US | 1993–2004 | National | 3463 | <1 | {Tate, 2008 #97} |
| US | 2001–2004 | Hospital | 26 | <14 | {Burjonrappa, 2007 #18} |
| Mexico | 1999–2001 | Regional | 48 | <1 | {Velazquez, 2004 #100} |
| Oceania | 2761 | ||||
| Australia | 2001–2009 | Regional | 197 | <2 | {Lloyd-Johnsen, 2012 #59} |
| Australia | 2002–2004 | Hospital | 51 | <2 | {Bines, 2006 #9} |
| Australia | 1994–2004 | Regional | 147 | <12 | {Blanch, 2007 #11} |
| Australia | 1993–2003 | Regional | 23 | <18 | {Webby, 2006 #101} |
| Australia | 1994–2000 | National | 1794 | <1 | {Justice, 2006 #49} |
| New Zealand | 1998–2007 | Hospital | 189 | <14 | {Kodikara, 2010 #52} |
| New Zealand | 1998–2003 | National | 277 | <3 | {Chen, 2005 #25} |
| New Zealand | 1987–1998 | Regional | 83 | NR | {Reid, 2001 #76} |
| Intercontinental/Global | 173 | ||||
| India, Brazil, Egypt Kenya | 2004–2006 | International | 173 | <10 | {Awasthi, 2009 #6} |
a: Botswana, Cote D'Ivoire, Ghana, Kenya, Nigeria, South Africa, Tanzania, Zambia, Zimbabwe.
b: these 2 studies had overlapping study years in same location but were included because data on age and clinical treatment were split.
c: authors randomly sampled a cohort of 100,000 infants from a national insurance claims database.
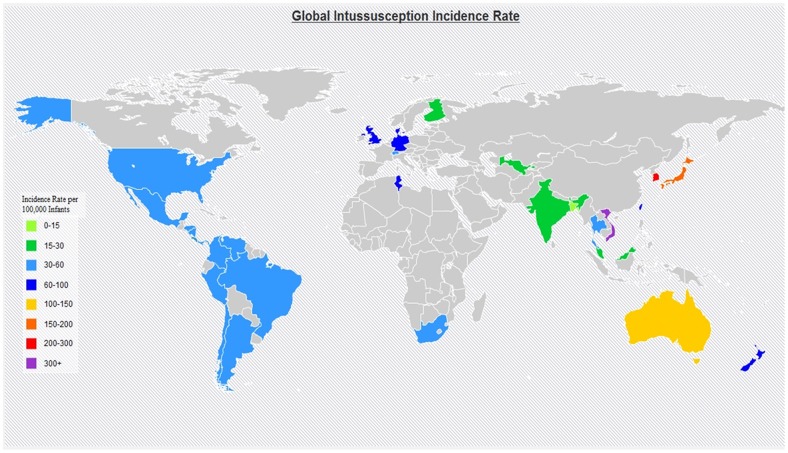
Five reports presented incidence data by month of age, 17 studies presented data on number of cases by month of age and the incidence among children <1 year of age, and 13 only reported aggregate intussusception incidence among children <1 year of age. Among studies (n = 35) reporting incidence of intussusception among children <1 year of age, the mean incidence was 74 per 100,000 infant years (range: 9–328) (Table 2). While incidence in a majority (83%) of the studies was <100 per 100,000, higher incidence was observed in some populations including: Australia (101), Hong Kong (108), Japan (185), Israel (219), Vietnam (302), and South Korea (328) (Figure 2). Incidence of intussusception was <20 per 100,000 in some populations from Finland (20), India (18), Malaysia (18), and Bangladesh (9).
Table 2. Intussusception incidence among children <1 year of age by region.
| Continent | Country | National/Regional/Hospital | Incidence per 100,000 <1 Yr. | Rates stratified by month of age |
| Africa | South Africa | National | 56a | No |
| Asia (high u5MR) | Bangladesh | Regional | 9 | No |
| India | Regional – Delhi | 18 | No | |
| Tunisia | National | 62 | No | |
| Asia-low/very low u5MR | Hong Kong | National | 108 | No |
| Japan | National | 185b | No | |
| South Korea | Regional (Jeonbuk Province) | 328c | No | |
| Malaysia | National | 18 | No | |
| Singapore | National | 51 | No | |
| Taiwan | National | 77 | No | |
| Thailand | National | 36 | No | |
| Uzbekistan | Regional – Bukhara | 23 | No | |
| Vietnam | Regional | 302 | No | |
| Central & South America | 11 countries | 11 countries | 51 | No |
| Chile | Regional – Santiago | 51 | No | |
| Panama | National | 30 | No | |
| Venezuela | Regional – Carabobo | 35 | No | |
| Eastern Mediterranean | Israel (Jewish) | Hospital | 219 | No |
| Israel (Bedouin) | Hospital | 75 | No | |
| Europe | Denmarkd | National | 66 | Yes |
| Finland | National | 20 | No | |
| Germanyd | Regional – Bavaria & NRW | 62 | Yes | |
| Germany | National | 60 | No | |
| Germany | Regional – Bavaria | 72 | No | |
| Switzerland | National | 38 | No | |
| Englandd | National | 66 | Yes | |
| North America | USd | National | 33 | Yes |
| US | National | 33 | No | |
| US | 3 hospitals | 49 | No | |
| USd | National | 34 | Yes | |
| Oceania | Australia | Regional – Melbourne | 27 | No |
| Hospital | 71 | No | ||
| Regional – Northern Territory | 65 | No | ||
| National | 101 | No | ||
| New Zealand | National | 65 | No | |
| ALL REGIONS (Average) | 74 | No |
a: rate for <2 year olds in the paper; we recalculated rate for <1 year of age based on presented data.
b: midpoint of the range presented in the study.
c: rates presented for <6 and 6–11 months in the paper; we recalculated rates for <1 based on these data.
d: rates by age in months for the most recent surveillance years 1995–1999 were obtained from figure using Digitize-It; mean incidence for <1 presented {Fischer, 2004 #39}.
Figure 2. Global map of intussusception incidence.
In countries where local, regional, and national studies were conducted, we preferentially mapped the national rates. For countries with more than one national study, average rates of the studies were used for the map. If no national rates were available, regional and/or local rates were used for the map.
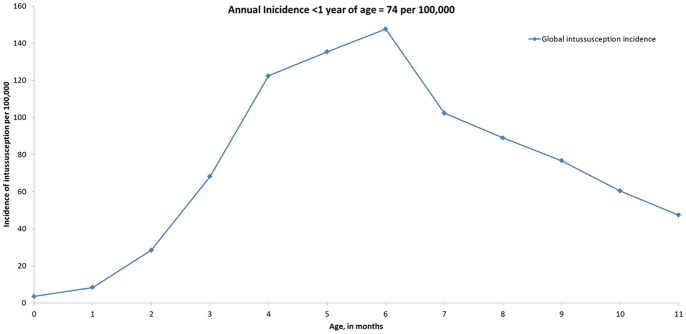
Five studies reported incidence of intussusception by month of age and 15 reported number of cases by month of age among children <1 year of age. We extrapolated the proportion of intussusception incidence by month of age relative to the mean incidence among children <1 year from the 5 studies to the 30 studies that only reported incidence among children <1 year (Figure 3). The age distribution of intussusception incidence and number of cases were similar, with lowest incidence among children 0–2 months of age (13–37 per 100,000) and peak incidence among those 4–7 months of age (97–126 per 100,000).
Figure 3. Incidence of intussusception by month of life during first year of life.
Intussusception incidence or number of cases by month of life from 22 studies extrapolated to remaining 13 studies that only presented data on rates for infants <1 year (as displayed in Table 2).
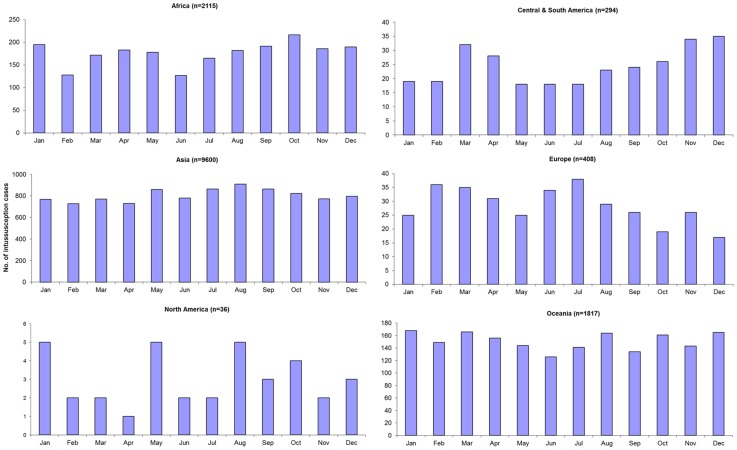
Distribution of intussusception cases by calendar month of year was reported in 21 studies (Figure 4). No particular seasonal pattern in intussusception was seen globally or in any of the regions except North America (Figure 5); however, only 1 small study of 36 cases provided data on seasonality of intussusception in North America [82].
Figure 4. Seasonality of intussusception globally.
Figure 5. Seasonality of intussusception, by WHO region.
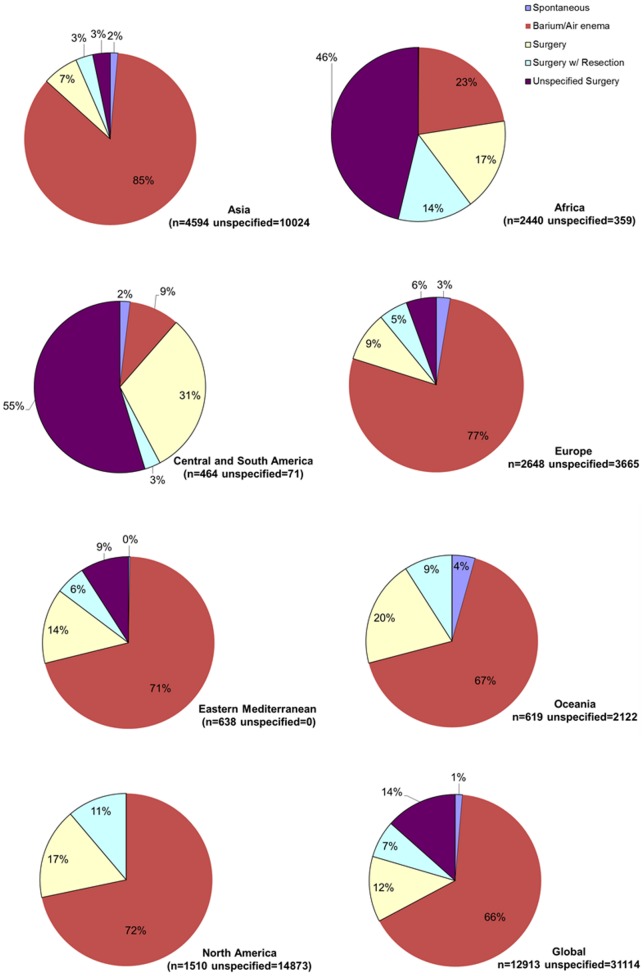
Diagnosis of intussusception varied substantially by region. Diagnosis of intussusception was made by a radiographic modality (e.g., air-contrast enema, ultrasound, or computed tomography) in 95%–100% of the cases from all regions of the world except Africa where intussusception was diagnosed by clinical findings or surgery in 65% of the case; however, data on method of diagnosing intussusception were limited in countries from Eastern Mediterranean, Americas, and Oceania (Figure 6). Treatment of intussusception also differed by region (Figure 7), with higher prevalence of surgery in Central/South America (86%) and Africa (77%) compared to Eastern Mediterranean (29%), Oceania (29%), North America (28%), Europe (20%), and Asia (13%).
Figure 6. Diagnosis of intussusception by WHO region.
Studies where diagnosis was unspecified were not included. Data from Central and South America were from one prospective research study that might not reflect clinical practice under routine conditions.
Figure 7. Treatment of intussusception by WHO region.
Studies where treatment was unspecified were not included.
The proportion of children with intussusception who died after presenting to the hospital was substantially higher in Africa (9.4%; range = 2–25) compared to Asia (0.2%; range = 0–6), Central and South America (0.6%; range = 0–1), Eastern Mediterranean (0.8%; range = 0–5), Europe (0.1%; range = 0–1), North America (0.4%; range = 0–1); and Oceania (0%; range 0–0.1%) (Table 3).
Table 3. Case-fatality among children hospitalized with intussusception.
| Country | No. of IS cases | Deaths | |
| No. | % | ||
| Total (Africa) | 1961 | 185 | 9.4% |
| Multi-country: Botswana, Cote D'Ivoire, Ghana, Kenya, Malawi, Nigeria, South Africa, Tanzania, Zambia, Zimbabwe | 863 | 108 | 12.5% |
| Kenya | 36 | 5 | 13.9% |
| 29 | 5 | 17.2% | |
| Ghana | 44 | 1 | 2.3% |
| Nigeria | 33 | 6 | 18.2% |
| 89 | 2 | 2.2% | |
| 174 | 21 | 12.1% | |
| 29 | 4 | 13.8% | |
| 87 | 7 | 8.0% | |
| 20 | 0 | 0.0% | |
| South Africa | 423 | 9 | 2.1% |
| 106 | 10 | 9.4% | |
| Tanzania | 28 | 7 | 25.0% |
| Total (Asia) | 4323 | 8 | 0.19% |
| China | 56 | 0 | 0.0% |
| India | 31 | 0 | 0.0% |
| 137 | 1 | 0.7% | |
| 179 | 0 | 0.0% | |
| Japan | 2427 | 2 | 0.1% |
| Korea | 408 | 1 | 0.2% |
| Malaysia | 62 | 0 | 0.0% |
| Singapore | 217 | 0 | 0.0% |
| Thailand | 112 | 0 | 0.0% |
| 94 | 0 | 0.0% | |
| Uzbekistan | 67 | 4 | 6.0% |
| Vietnam | 533 | 0 | 0.0% |
| Total (Central and South America) | 538 | 3 | 0.6% |
| Latin America | 222 | 2 | 0.9% |
| Chile | 95 | 0 | 0.0% |
| Panama | 103 | 1 | 1.0% |
| Trinidad & Tobago | 65 | 0 | 0.0% |
| Venezuela | 53 | 0 | 0.0% |
| Total (Eastern Mediterranean) | 644 | 5 | 0.8% |
| Saudi Arabia | 34 | 0 | 0.0% |
| 37 | 0 | 0.0% | |
| Israel | 316 | 0 | 0.0% |
| 148 | 0 | 0.0% | |
| Jordan | 109 | 5 | 4.6% |
| Total (Europe) | 2588 | 3 | 0.1% |
| Austria | 111 | 0 | 0.0% |
| Germany | 1189 | 0 | 0.0% |
| Ireland | 256 | 0 | 0.0% |
| 24 | 0 | 0.0% | |
| Russia | 280 | 0 | 0.0% |
| Spain | 151 | 1 | 0.7% |
| Turkey | 105 | 1 | 1.0% |
| 179 | 0 | 0.0% | |
| UK/Ireland | 261 | 1 | 0.4% |
| 32 | 0 | 0.0% | |
| Total (No. America) | 3860 | 16 | 0.4% |
| US | 188 | 0 | 0.0% |
| 183 | 2 | 1.1% | |
| 3463 | 14 | 0.4% | |
| 26 | 0 | 0.0% | |
| Total (Oceania) | 2738 | 1 | 0.0% |
| Australia | 51 | 0 | 0.0% |
| 197 | 0 | 0.0% | |
| 147 | 0 | 0.0% | |
| 1794 | 1 | 0.1% | |
| New Zealand | 189 | 0 | 0.0% |
| 277 | 0 | 0.0% | |
| 83 | 0 | 0.0% | |
Discussion
A key issue for rotavirus immunization programs in the postlicensure era is the need for safety monitoring with regard to intussusception [2], [5], [92]. Our review of the intussusception literature from the past decade provides pertinent information that should facilitate implementation of intussusception surveillance for monitoring the postlicensure safety of rotavirus vaccines.
First, we demonstrate that natural intussusception is a very rare condition in most regions of the world, particularly among infants <3–4 months of age when the first dose of rotavirus vaccine is typically administered. Because any potential risk with rotavirus vaccines is greatest after the first dose of vaccine which is typically recommended to be administered at 6–15 weeks of age, [5] the lower rates of intussusception in this age group suggests that timely administration of vaccine would minimize the attributable risk of vaccine [93]. That is, the higher rates of intussusception among infants older than 3–4 months indicates that any vaccine associated risk of intussusception could lead to more cases of intussusception associated with vaccine in settings where delays in vaccination are common. If vaccine is linked to a potential risk of intussusception, age stratified data on intussusception are necessary for interpreting these estimates of vaccine-associated risk and calculating the number of cases that are potentially attributable to vaccine after the implementation of a vaccine program [93]. The age-stratified data in this review will be a valuable resource for conducting benefit risk analyses, should a rotavirus vaccine program be linked to any potential risk of intussusception.
Second, the incidence of intussusception varies substantially by region, thus highlighting the importance of the need for regional baseline data when evaluating postlicensure trends of intussusception for assessing any potential vaccine-associated risk. Risk of intussusception after the current rotavirus vaccines has also varied by region. In Mexico and Australia, postlicensure studies have identified an increased low-level risk of intussusception after the first dose of both rotavirus vaccines, ranging from 1 to 2 cases of intussusception per 100,000 vaccinated infants [7], [8]. While an early study in the United States did not identify a risk of intussusception after RV5 [94], a recent study has shown that a low level risk of intussusception is likely to exist with both vaccines [95]. However, a similar level of risk was not observed in Brazil after RV1 [7]. While these differences in risk might be related to a chance finding, effect modification of risk related to an environmental or genetic factor that differs between the populations cannot be excluded. In our review, we observed a wide range in the incidence of intussusception globally. The cause of natural intussusception in a majority of infants is not known and has been previously reviewed. Differences in infant diet, breastfeeding, maternal antibody levels, prevalence of enteropathogens (e.g., respiratory adenovirus, rotavirus) might all contribute to the differences in background rates of intussusception [3], [96]. It remains unclear whether populations with higher background risk of intussusception also would carry a higher risk of vaccine-associated risk. However, any potential risk of intussusception associated with vaccine in populations with higher background risk would lead to higher number of excess cases that would be attributable to vaccine.
Third, the clinical management of intussusception in Africa differs markedly from other regions of the world where data exist, predominantly relying on clinical manifestations for diagnosis and surgery for treatment. The paucity of data on method of diagnosing intussusception from Eastern Mediterranean, Americas, and Oceania region limits the generalizability of this information in countries from these regions. In addition, limited published data are available on clinical management and outcome of intussusception in high mortality Asian countries but it would seem that surgical rates are likely to be higher than the lower mortality Asian countries. The differences in clinical management of intussusception in high mortality settings compared to low mortality settings emphasizes the need for augmenting surveillance practices depending on the location. For example, strengthening collaborations with surgeons to improve case-capture should be prioritized when monitoring the postlicensure safety of rotavirus vaccines in Africa. As rotavirus vaccines are more broadly introduced into immunization programs in Africa and Asia, these current data on the epidemiology and clinical management of intussusception should facilitate the future implementation of intussusception surveillance and inform benefit risk calculations for defining policy and decision making.
Studies assess the role of wild-type rotavirus infection as a cause of intussusception have led to conflicting findings with several studies suggesting that a significant association is unlikely [3]. The absence of any marked seasonal patterns in intussusception, particularly in settings of Europe, Oceania, and Central and South America where rotavirus disease is quite seasonal supports the contention that rotavirus is unlikely to be a prominent etiologic cause of natural intussusception. This has some implications for postlicensure monitoring because attempts have previously been made to assess whether rotavirus vaccination would have a protective effect against intussusception vis-à-vis protecting against wild-type rotavirus infection. Establishing whether vaccine confers protection against intussusception might be challenging if only a small etiologic fraction of naturally occurring intussusception is related to wild-type rotavirus infection [5], [97].
Our review provides background rates of intussusception against which rates of intussusception after the introduction of vaccine could be compared for assessing potential vaccine-associated risk. However, the paucity of background rates from most settings supports the need for relying on other analytic methods of safety monitoring after the introduction of rotavirus vaccine [2]. The self-controlled case-series method has now been successfully applied in several settings for assessing a potential link between vaccine and intussusception [7]. This method relies on active, hospital based surveillance of intussusception and does not require information on background rates of intussusception in the population under surveillance [98]. Our review provides important considerations when establishing surveillance for intussusception. First, in resource poor settings of Africa, specific attention should be placed on hospitals with surgery suites which are likely to manage most intussusception events. While background rates are not specifically needed for the case-series method, in settings where no background rates are available, some efforts could be made to establish population-based studies which would be necessary for determining the attributable risk of vaccine, should any increased risk be identified. Lastly, the varying incidence of intussusception during the first six months of life when rotavirus vaccines are administered strongly indicates the need for addressing confounding effects of age when determining whether a causal link exists between rotavirus vaccine and intussusception cases identified through surveillance.
Intussusception case-fatality was <1% in all developed countries of the world. However, the marked difference in case-fatality between Asia (<1%) and Africa (9%) was intriguing. This finding might reflect differences in healthcare infrastructure or delays in care, or perhaps might be related to a publication bias with a paucity of surveillance data in the poorest populations of Asia. Previous analyses of benefits versus risk of rotavirus vaccine have conservatively assumed intussusception case-fatality rate of 25% in Asia, which in light of this review is high and could be reconsidered for future benefit risk deliberations [93]. Even if case-fatality rates were to be higher than those identified in this review, some adjustment would be prudent for obtaining valid estimates of any potential risk associated with rotavirus vaccine in Asia.
Our review must be considered with some limitations. Because intussusception is a very rare condition, a fair amount of heterogeneity in surveillance practice is likely to exist across regions. In particular, establishing incidence of intussusception is rather challenging in many settings without established national or regional electronic records and thus published rates of intussusception might be prone to over or underestimation. The availability of a standardized case definition by the Brighton Collaboration reduces case misclassification but sensitivity of the case-definition might be reduced in resource poor settings where diagnostic modalities are not available and relying on clinical judgment and surgery is required for diagnosis [1]. The case-fatality from these hospital based surveillance studies in resource poor settings also certainly underestimates the true case-fatality because deaths are likely to occur out of hospital. While some 30 countries had introduced a rotavirus between 2006 and 2011, all but one of the studies included in this review [83] captured data before the introduction of a rotavirus vaccine.
In summary, this review of current epidemiology and clinical management of intussusception should prove to be a valuable resource for establishing intussusception surveillance and interpreting these surveillance data from various regions of the world where rotavirus vaccines are likely to be introduced in the next several years.
Ethics statement
Because these are publicly available non-identifiable data, an ethics statement was not required for this work.
Supporting Information
PRISMA Checklist for the preferred reporting list for the intussusception literature review.
(DOC)
Funding Statement
No current external funding sources for this study.
References
- 1. Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, et al. (2004) Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine 22: 569–574. [DOI] [PubMed] [Google Scholar]
- 2. Bines JE, Patel M, Parashar U (2009) Assessment of postlicensure safety of rotavirus vaccines, with emphasis on intussusception. J Infect Dis 200 Suppl 1S282–290. [DOI] [PubMed] [Google Scholar]
- 3. WHO (2002) Acute intussusception in infants and children. Incidence, clinical presentation and management: a global perspective. Geneva: World Health Organization. Document WHO/V & B/02 (19) 1–98. [Google Scholar]
- 4. Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, et al. (2001) Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 344: 564–572. [DOI] [PubMed] [Google Scholar]
- 5. Patel MM, Haber P, Baggs J, Zuber P, Bines JE, et al. (2009) Intussusception and rotavirus vaccination: a review of the available evidence. Expert Rev Vaccines 8: 1555–1564. [DOI] [PubMed] [Google Scholar]
- 6. WHO (2007) Rotavirus vaccines. Wkly Epidemiol Rec 82: 285–295. [PubMed] [Google Scholar]
- 7. Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, et al. (2011) Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 364: 2283–2292. [DOI] [PubMed] [Google Scholar]
- 8. Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, et al. (2011) Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine 29: 3061–3066. [DOI] [PubMed] [Google Scholar]
- 9. Patel MM, Glass R, Desai R, Tate JE, Parashar UD (2012) Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect Dis 12: 561–570. [DOI] [PubMed] [Google Scholar]
- 10. Okimoto S, Hyodo S, Yamamoto M, Nakamura K, Kobayashi M (2011) Association of viral isolates from stool samples with intussusception in children. Int J Infect Dis 15: e641–645. [DOI] [PubMed] [Google Scholar]
- 11. Abantanga FA, Amoah M, Adeyinka AO, Nimako B, Yankey KP (2008) Pneumatic reduction of intussusception in children at the Komfo Anokye Hospital, Kumasi, Ghana. East Afr Med J 85: 550–555. [DOI] [PubMed] [Google Scholar]
- 12. Ameh EA (2002) The morbidity and mortality of laparotomy for uncomplicated intussusception in children. West Afr J Med 21: 115–116. [PubMed] [Google Scholar]
- 13. Ameh EA (2002) The morbidity and mortality of right hemicolectomy for complicated intussusception in infants. Niger Postgrad Med J 9: 123–124. [PubMed] [Google Scholar]
- 14. Archibong AE, Usoro IN, Ikpi E, Inyang A (2001) Paediatric intussusception in Calabar, Nigeria. East Afr Med J 78: 19–21. [DOI] [PubMed] [Google Scholar]
- 15. Bode CO (2008) Presentation and management outcome of childhood intussusception in Lagos: a prospective study. Afr J Paediatr Surg 5: 24–28. [DOI] [PubMed] [Google Scholar]
- 16. Carneiro PM, Kisusi DM (2004) Intussusception in children seen at Muhimbili National Hospital, Dar es Salaam. East Afr Med J 81: 439–442. [DOI] [PubMed] [Google Scholar]
- 17. Chouikha A, Fodha I, Maazoun K, Ben Brahim M, Hidouri S, et al. (2009) Rotavirus infection and intussusception in Tunisian children: implications for use of attenuated rotavirus vaccines. J Pediatr Surg 44: 2133–2138. [DOI] [PubMed] [Google Scholar]
- 18. Edino ST, Ochicha O, Mohammed AZ, Anumah M (2003) Intussusception in Kano: a 5-year analysis of pattern, morbidity and mortality. Niger J Med 12: 221–224. [PubMed] [Google Scholar]
- 19. Ekenze SO, Anyanwu PA, Ezomike UO, Oguonu T (2010) Profile of pediatric abdominal surgical emergencies in a developing country. Int Surg 95: 319–324. [PubMed] [Google Scholar]
- 20. Ekenze SO, Mgbor SO (2011) Childhood intussusception: the implications of delayed presentation. Afr J Paediatr Surg 8: 15–18. [DOI] [PubMed] [Google Scholar]
- 21. Enweronu-Laryea CC, Sagoe KW, Glover-Addy H, Asmah RH, Mingle JA, et al. (2012) Prevalence of severe acute rotavirus gastroenteritis and intussusceptions in Ghanaian children under 5 years of age. J Infect Dev Ctries 6: 148–155. [DOI] [PubMed] [Google Scholar]
- 22. Kuremu RT (2004) Childhood intussusception at the Moi teaching and Referral Hospital Eldoret: management challenges in a rural setting. East Afr Med J 81: 443–446. [DOI] [PubMed] [Google Scholar]
- 23. Moore SW, Kirsten M, Muller EW, Numanoglu A, Chitnis M, et al. (2010) Retrospective surveillance of intussusception in South Africa, 1998–2003. J Infect Dis 202 Suppl: S156–161 [DOI] [PubMed] [Google Scholar]
- 24. Wiersma R, Hadley GP (2004) Minimizing surgery in complicated intussusceptions in the Third World. Pediatr Surg Int 20: 215–217. [DOI] [PubMed] [Google Scholar]
- 25. Muyembe VM, Suleman N (2000) Intestinal obstruction at a provincial hospital in Kenya. East Afr Med J 77: 440–443. [PubMed] [Google Scholar]
- 26. Ogundoyin OO, Afolabi AO, Ogunlana DI, Lawal TA, Yifieyeh AC (2009) Pattern and outcome of childhood intestinal obstruction at a tertiary hospital in Nigeria. Afr Health Sci 9: 170–173. [PMC free article] [PubMed] [Google Scholar]
- 27. Steele AD, Patel M, Cunliffe NA, Bresee JS, Borgstein E, et al. (2012) Workshop on intussusception in African countries–meeting report. Vaccine 30 Suppl 1A185–189. [DOI] [PubMed] [Google Scholar]
- 28. Bahl RS, M., Bhandari N, Taneja S, Mathur M, Parashar UD, et al. (2009) Population-based incidence of intussusception and a case-control study to examine the association of intussusception with natural rotavirus infection among indian children. J Infect Dis 200 Suppl 1S277–281. [DOI] [PubMed] [Google Scholar]
- 29. Bhowmick K, Kang G, Bose A, Chacko J, Boudville I, et al. (2009) Retrospective surveillance for intussusception in children aged less than five years in a South Indian tertiary-care hospital. J Health Popul Nutr 27: 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramachandran P, Gupta A, Vincent P, Sridharan S (2008) Air enema for intussusception: is predicting the outcome important? Pediatr Surg Int 24: 311–313. [DOI] [PubMed] [Google Scholar]
- 31. Raman T, Mukhopadhyaya A, Eapen CE, Aruldas V, Bose A, et al. (2003) Intussusception in southern Indian children: lack of association with diarrheal disease and oral polio vaccine immunization. Indian J Gastroenterol 22: 82–84. [PubMed] [Google Scholar]
- 32. Zaman K, Breiman RF, Yunus M, Arifeen SE, Mahmud A, et al. (2009) Intussusception surveillance in a rural demographic surveillance area in bangladesh. J Infect Dis 200 Suppl 1S271–276. [DOI] [PubMed] [Google Scholar]
- 33. Bines JE, Liem NT, Justice FA, Son TN, Kirkwood CD, et al. (2006) Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatr 149: 452–460. [DOI] [PubMed] [Google Scholar]
- 34. Chen SC, Wang JD, Hsu HY, Leong MM, Tok TS, et al. (2010) Epidemiology of childhood intussusception and determinants of recurrence and operation: analysis of national health insurance data between 1998 and 2007 in Taiwan. Pediatr Neonatol 51: 285–291. [DOI] [PubMed] [Google Scholar]
- 35. Giak CL, Singh HS, Nallusamy R, Leong TY, Ng TL, et al. (2008) Epidemiology of intussusception in Malaysia: a three-year review. Southeast Asian J Trop Med Public Health 39: 848–855. [PubMed] [Google Scholar]
- 36. Hong Kong Intussusception Study G (2007) Intussusception trends in Hong Kong children. Hong Kong Med J 13: 279–283. [PubMed] [Google Scholar]
- 37. Jo DS, Nyambat B, Kim JS, Jang YT, Ng TL, et al. (2009) Population-based incidence and burden of childhood intussusception in Jeonbuk Province, South Korea. Int J Infect Dis 13: e383–388. [DOI] [PubMed] [Google Scholar]
- 38. Justice FA, de Campo M, Liem NT, Son TN, Ninh TP, et al. (2007) Accuracy of ultrasonography for the diagnosis of intussusception in infants in Vietnam. Pediatr Radiol 37: 195–199. [DOI] [PubMed] [Google Scholar]
- 39. Khumjui C, Doung-ngern P, Sermgew T, Smitsuwan P, Jiraphongsa C (2009) Incidence of intussusception among children 0–5 years of age in Thailand, 2001–2006. Vaccine 27 Suppl 5F116–119. [DOI] [PubMed] [Google Scholar]
- 40. Kruatrachue A, Wongtapradit L, Nithipanya N, Ratanaprakarn W (2011) Result of air enema reduction in 737 cases of intussusception. J Med Assoc Thai 94 Suppl 3S22–26. [PubMed] [Google Scholar]
- 41. Latipov R, Khudoyorov R, Flem E (2011) Childhood intussusception in Uzbekistan: analysis of retrospective surveillance data. BMC Pediatr 11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakagomi T, Takahashi Y, Arisawa K, Nakagomi O (2006) A high incidence of intussusception in Japan as studied in a sentinel hospital over a 25-year period (1978–2002). Epidemiol Infect 134: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nelson EA, Tam JS, Glass RI, Parashar UD, Fok TF (2002) Incidence of rotavirus diarrhea and intussusception in Hong Kong using standardized hospital discharge data. Pediatr Infect Dis J 21: 701–703. [DOI] [PubMed] [Google Scholar]
- 44. Pruksananonda P, Athirakul K, Worawattanakul M, Varavithya W, Pisithpun A, et al. (2007) Intussusception in a private tertiary-care hospital, Bangkok, Thailand: a case series. Southeast Asian J Trop Med Public Health 38: 339–342. [PubMed] [Google Scholar]
- 45. Takeuchi M, Osamura T, Yasunaga H, Horiguchi H, Hashimoto H, et al. (2012) Intussusception among Japanese children: an epidemiologic study using an administrative database. BMC Pediatr 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tan N, Teoh YL, Phua KB, Quak SH, Lee BW, et al. (2009) An update of paediatric intussusception incidence in Singapore: 1997–2007, 11 years of intussusception surveillance. Ann Acad Med Singapore 38: 690–692. [PubMed] [Google Scholar]
- 47. Zhang Y, Bai YZ, Li SX, Liu SJ, Ren WD, et al. (2011) Sonographic findings predictive of the need for surgical management in pediatric patients with small bowel intussusceptions. Langenbecks Arch Surg 396: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 48.Abate H, Linhares AC, Venegas G, Vergara RF, Lopez P, et al. A multi-center study of intussusception in Latin America: first year results [abstract]. Presented at: ICP; August 15–20, 2004; Cancun, Mexico. [Google Scholar]
- 49. O'Ryan M, Lucero Y, Pena A, Valenzuela MT (2003) Two year review of intestinal intussusception in six large public hospitals of Santiago, Chile. Pediatr Infect Dis J 22: 717–721. [DOI] [PubMed] [Google Scholar]
- 50. Perez-Schael I, Escalona M, Salinas B, Materan M, Perez ME, et al. (2003) Intussusception-associated hospitalization among Venezuelan infants during 1998 through 2001: anticipating rotavirus vaccines. Pediatr Infect Dis J 22: 234–239. [DOI] [PubMed] [Google Scholar]
- 51. Saez-Llorens X, Guevara JN (2004) Intussusception and rotavirus vaccines: what is the background risk? Pediatr Infect Dis J 23: 363–365. [DOI] [PubMed] [Google Scholar]
- 52. Tota-Maharaj R, Rampersad B, Indalsingh R (2010) Barium enema reduction of intussusception in a developing country. West Indian Med J 59: 535–539. [PubMed] [Google Scholar]
- 53. Al-Malki TA (2005) Pediatric intussusception in a Saudi Arabian tertiary hospital. West Afr J Med 24: 309–310. [DOI] [PubMed] [Google Scholar]
- 54. Crankson SJ, Al-Rabeeah AA, Fischer JD, Al-Jadaan SA, Namshan MA (2003) Idiopathic intussusception in infancy and childhood. Saudi Med J 24 Suppl: S18–20 [PubMed] [Google Scholar]
- 55.Eshed I, Witzling M, Gorenstein A, Serour F (2003) [Reduction of intussusception by air enema in children–experience over a 13-year period]. Harefuah 142: 659–661, 720, 719. [PubMed] [Google Scholar]
- 56. Greenberg D, Givon-Lavi N, Newman N, Wheeler J, Cohen Z, et al. (2008) Intussusception in children in Southern Israel: disparity between 2 populations. Pediatr Infect Dis J 27: 236–240. [DOI] [PubMed] [Google Scholar]
- 57. Saleem MM, Al-Momani H, Abu Khalaf M (2008) Intussusception: Jordan University Hospital experience. Hepatogastroenterology 55: 1356–1359. [PubMed] [Google Scholar]
- 58. Buettcher M, Baer G, Bonhoeffer J, Schaad UB, Heininger U (2007) Three-year surveillance of intussusception in children in Switzerland. Pediatrics 120: 473–480. [DOI] [PubMed] [Google Scholar]
- 59. Fischer TK, Bihrmann K, Perch M, Koch A, Wohlfahrt J, et al. (2004) Intussusception in early childhood: a cohort study of 1.7 million children. Pediatrics 114: 782–785. [DOI] [PubMed] [Google Scholar]
- 60. Hilal A, MacMahon P, Cosgrove JF (2002) Outcome of acute intussusception in a regional paediatric centre. Ir Med J 95: 58–59. [PubMed] [Google Scholar]
- 61. Jenke AC, Klaassen-Mielke R, Zilbauer M, Heininger U, Trampisch H, et al. (2011) Intussusception: incidence and treatment-insights from the nationwide German surveillance. J Pediatr Gastroenterol Nutr 52: 446–451. [DOI] [PubMed] [Google Scholar]
- 62. Kohl LJ, Streng A, Grote V, Koletzko S, Liese JG (2010) Intussusception-associated hospitalisations in southern Germany. Eur J Pediatr 169: 1487–1493. [DOI] [PubMed] [Google Scholar]
- 63. Lappalainen S, Ylitalo S, Arola A, Halkosalo A, Rasanen S, et al. (2012) Simultaneous presence of human herpesvirus 6 and adenovirus infections in intestinal intussusception of young children. Acta Paediatr 101: 663–670. [DOI] [PubMed] [Google Scholar]
- 64. Rubi I, Vera R, Rubi SC, Torres EE, Luna A, et al. (2002) Air reduction of intussusception. Eur J Pediatr Surg 12: 387–390. [DOI] [PubMed] [Google Scholar]
- 65. Samad L, Marven S, El Bashir H, Sutcliffe AG, Cameron JC, et al. (2012) Prospective surveillance study of the management of intussusception in UK and Irish infants. Br J Surg 99: 411–415. [DOI] [PubMed] [Google Scholar]
- 66. Saxena AK, Seebacher U, Bernhardt C, Hollwarth ME (2007) Small bowel intussusceptions: issues and controversies related to pneumatic reduction and surgical approach. Acta Paediatr 96: 1651–1654. [DOI] [PubMed] [Google Scholar]
- 67. Shapkina AN, Shapkin VV, Nelubov IV, Pryanishena LT (2006) Intussusception in children: 11-year experience in Vladivostok. Pediatr Surg Int 22: 901–904. [DOI] [PubMed] [Google Scholar]
- 68. Sonmez K, Turkyilmaz Z, Demirogullari B, Karabulut R, Kale N, et al. (2012) Intussusception in children: Experience with 105 patients in a department of paediatric surgery, Turkey. S Afr J Surg 50: 37–39. [PubMed] [Google Scholar]
- 69. Tareen F, Ryan S, Avanzini S, Pena V, Mc Laughlin D, et al. (2011) Does the length of the history influence the outcome of pneumatic reduction of intussusception in children? Pediatr Surg Int 27: 587–589. [DOI] [PubMed] [Google Scholar]
- 70. Wei BS, Streng A, Kries R, Liese J, Wirth S, et al. (2011) Incidence of intussusception in early infancy: a capture-recapture estimate for Germany. Klin Padiatr 223: 419–423. [DOI] [PubMed] [Google Scholar]
- 71. Yalcin S, Ciftci AO, Karaagaoglu E, Tanyel FC, Senocak ME (2009) Presenting clinical features and outcome in intussusception. Indian J Pediatr 76: 401–405. [DOI] [PubMed] [Google Scholar]
- 72. Willetts IE, Kite P, Barclay GR, Banks RE, Rumley A, et al. (2001) Endotoxin, cytokines and lipid peroxides in children with intussusception. Br J Surg 88: 878–883. [DOI] [PubMed] [Google Scholar]
- 73. Gay N, Ramsay M, Waight P (1999) Rotavirus vaccination and intussusception. Lancet 354: 956. [DOI] [PubMed] [Google Scholar]
- 74. Burjonrappa SC (2007) Laparoscopic reduction of intussusception: an evolving therapeutic option. JSLS 11: 235–237. [PMC free article] [PubMed] [Google Scholar]
- 75. Cortese MM, Staat MA, Weinberg GA, Edwards K, Rice MA, et al. (2009) Underestimates of intussusception rates among US infants based on inpatient discharge data: implications for monitoring the safety of rotavirus vaccines. J Infect Dis 200 Suppl 1S264–270. [DOI] [PubMed] [Google Scholar]
- 76. Eng PM, Mast TC, Loughlin J, Clifford CR, Wong J, et al. (2012) Incidence of intussusception among infants in a large commercially insured population in the United States. Pediatr Infect Dis J 31: 287–291. [DOI] [PubMed] [Google Scholar]
- 77. Fike FB, Mortellaro VE, Holcomb GW 3rd, St Peter SD (2012) Predictors of failed enema reduction in childhood intussusception. J Pediatr Surg 47: 925–927. [DOI] [PubMed] [Google Scholar]
- 78. Nylund CM, Denson LA, Noel JM (2010) Bacterial enteritis as a risk factor for childhood intussusception: a retrospective cohort study. J Pediatr 156: 761–765. [DOI] [PubMed] [Google Scholar]
- 79. Shekherdimian S, Lee SL (2011) Management of pediatric intussusception in general hospitals: diagnosis, treatment, and differences based on age. World J Pediatr 7: 70–73. [DOI] [PubMed] [Google Scholar]
- 80. Somme S, To T, Langer JC (2006) Factors determining the need for operative reduction in children with intussusception: a population-based study. J Pediatr Surg 41: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 81. Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, et al. (2008) Trends in intussusception hospitalizations among US infants, 1993–2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics 121: e1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Velazquez FR, Luna G, Cedillo R, Torres J, Munoz O (2004) Natural rotavirus infection is not associated to intussusception in Mexican children. Pediatr Infect Dis J 23: S173–178. [DOI] [PubMed] [Google Scholar]
- 83. Yen C, Tate JE, Steiner CA, Cortese MM, Patel MM, et al. (2012) Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000–2009. J Infect Dis 206: 41–48. [DOI] [PubMed] [Google Scholar]
- 84. Blanch AJ, Perel SB, Acworth JP (2007) Paediatric intussusception: epidemiology and outcome. Emerg Med Australas 19: 45–50. [DOI] [PubMed] [Google Scholar]
- 85. Chen YE, Beasley S, Grimwood K, New Zealand Rotavirus Study G (2005) Intussusception and rotavirus associated hospitalisation in New Zealand. Arch Dis Child 90: 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Justice FA, Auldist AW, Bines JE (2006) Intussusception: trends in clinical presentation and management. J Gastroenterol Hepatol 21: 842–846. [DOI] [PubMed] [Google Scholar]
- 87. Kodikara H, Lynch A, Morreau P, Vogel S (2010) Ten-year review of intussusception at Starship Hospital: 1998–2007. N Z Med J 123: 32–40. [PubMed] [Google Scholar]
- 88. Lloyd-Johnsen C, Justice FA, Donath S, Bines JE (2012) Retrospective hospital based surveillance of intussusception in children in a sentinel paediatric hospital: benefits and pitfalls for use in post-marketing surveillance of rotavirus vaccines. Vaccine 30 Suppl 1A190–195. [DOI] [PubMed] [Google Scholar]
- 89. Reid R, Kulkarni M, Beasley S (2001) The potential for improvement in outcome of children with intussusception in the South Island. N Z Med J 114: 441–443. [PubMed] [Google Scholar]
- 90.Webby RJ, Bines JE, Barnes GL, Tindall H, Krause V, et al.. (2006) Intussusception in the Northern Territory: the incidence is low in Aboriginal and Torres Strait Islander children. J Paediatr Child Health 42: 235–239; discussion 227–238. [DOI] [PubMed] [Google Scholar]
- 91. Awasthi S, Agarwal GG, Mishra V, Nag VL, El Sayed HF, et al. (2009) Four-country surveillance of intestinal intussusception and diarrhoea in children. J Paediatr Child Health 45: 82–86. [DOI] [PubMed] [Google Scholar]
- 92. Tate JE, Steele AD, Bines JE, Zuber PL, Parashar UD (2012) Research priorities regarding rotavirus vaccine and intussusception: a meeting summary. Vaccine 30 Suppl 1A179–184. [DOI] [PubMed] [Google Scholar]
- 93. Patel MM, Clark AD, Sanderson CF, Tate J, Parashar UD (2012) Removing the age restrictions for rotavirus vaccination: a benefit-risk modeling analysis. PLoS Med 9: e1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shui IM, Baggs J, Patel M, Parashar U, Rett M, et al. (2012) Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA 307: 598–604. [DOI] [PubMed] [Google Scholar]
- 95. Haber P, Patel M, Pan Y, Baggs J, Haber M, et al. (2013) Intussusception After Rotavirus Vaccines Reported to US VAERS, 2006–2012. Pediatrics 131: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 96. Johnson B, Gargiullo P, Murphy TV, Parashar UD, Patel MM (2010) Sociodemographic and dietary risk factors for natural infant intussusception in the United States. J Pediatr Gastroenterol Nutr 51: 458–463. [DOI] [PubMed] [Google Scholar]
- 97. Simonsen L, Morens D, Elixhauser A, Gerber M, Van Raden M, et al. (2001) Effect of rotavirus vaccination programme on trends in admission of infants to hospital for intussusception. Lancet 358: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 98. Farrington CP (2004) Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine 22: 2064–2070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist for the preferred reporting list for the intussusception literature review.
(DOC)