Abstract
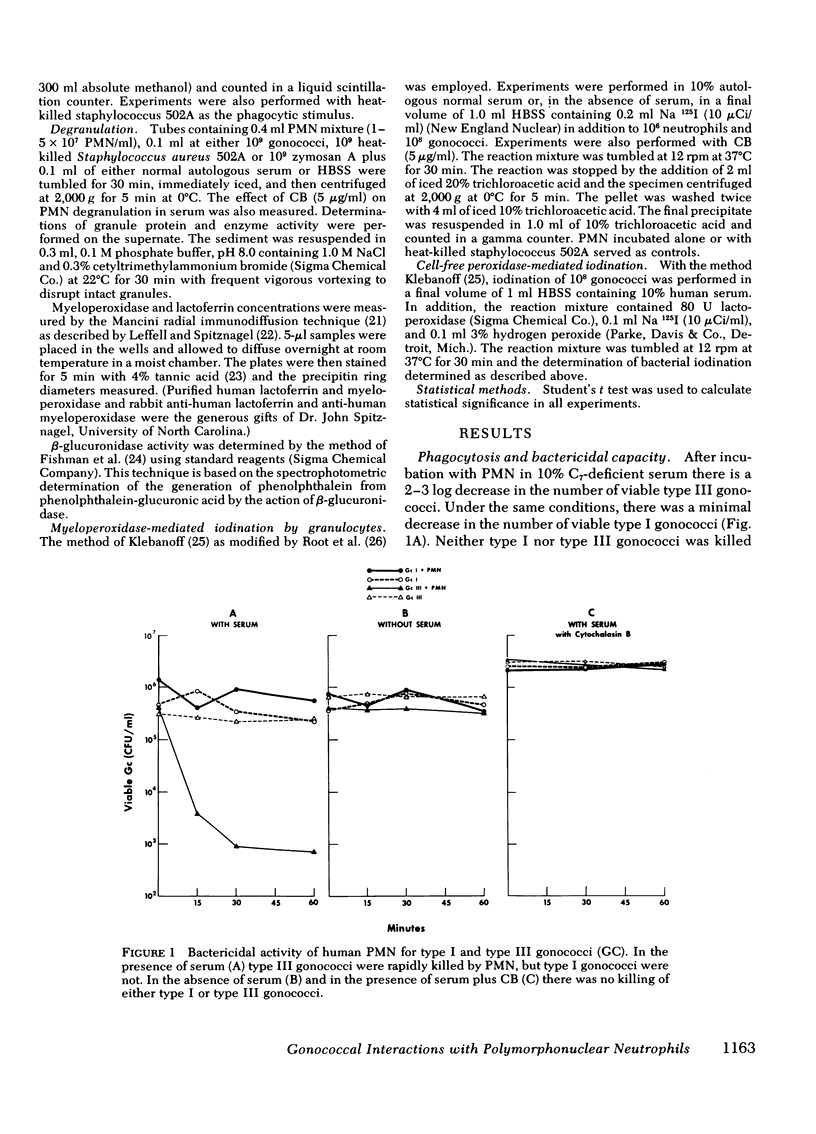
Gonococci are capable of attaching to the surface of polymorphonuclear leukocytes (PMN). In this location they resist phagocytosis and are not killed by PMN. To delineate the factors involved in the survival of these gonococci, we investigated the interaction of virulent gonococci, which adhere to cells and resist phagocytosis, and avirulent gonococci, which are phagocytized and killed by PMN. In the presence of serum, both virulent and avirulent gonococci associate equally well with PMN and stimulate increases in oxidative metabolism. In the absence of serum virulent gonococci attached to PMN and stimulated PMN oxidative metabolism to a greater extent than avirulent gonococci which did not attach to PMN (P = 0.0009). Therefore, the survival of virulent gonococci attached to the PMN surface is not a result of failure to activate oxidative and bactericidal mechanisms. Both virulent and avirulent gonococci stimulated equivalent PMN specific granule release as measured by the appearance of lactoferrin in the media. Phagocytosis of avirulent gonococci stimulated significantly greater beta-glucuronidase release (P = 0.01) and myeloperoxidase-mediated iodination of protein (P = 0.001) by PMN than attachment of virulent gonococci. In the absence of serum neither type of gonococci stimulated beta-glocuronidase release or protein iodination by PMN. Thus, virulent gonococci fail to stimulate primary granule release by PMN. To further assess the role of attachment versus ingestion on the survival of gonococci, PMN were treated with cytochalasin B to block ingestion. Cytochalasin B-treated PMN were unable to kill either virulent or avirulent gonococci despite normal degranulation stimulated by the latter. The failure of PMN to kill surface-attached gonococci appears to be a consequence of the failure of PMN to enclose the virulent gonococci within a phagosome. The phagocytic vacuole thus plays a critical role in normal PMN bactericidal activity by providing a closed space in which the proper concentration of substances may be achieved to generate microbicidal activity.
Full text
PDF


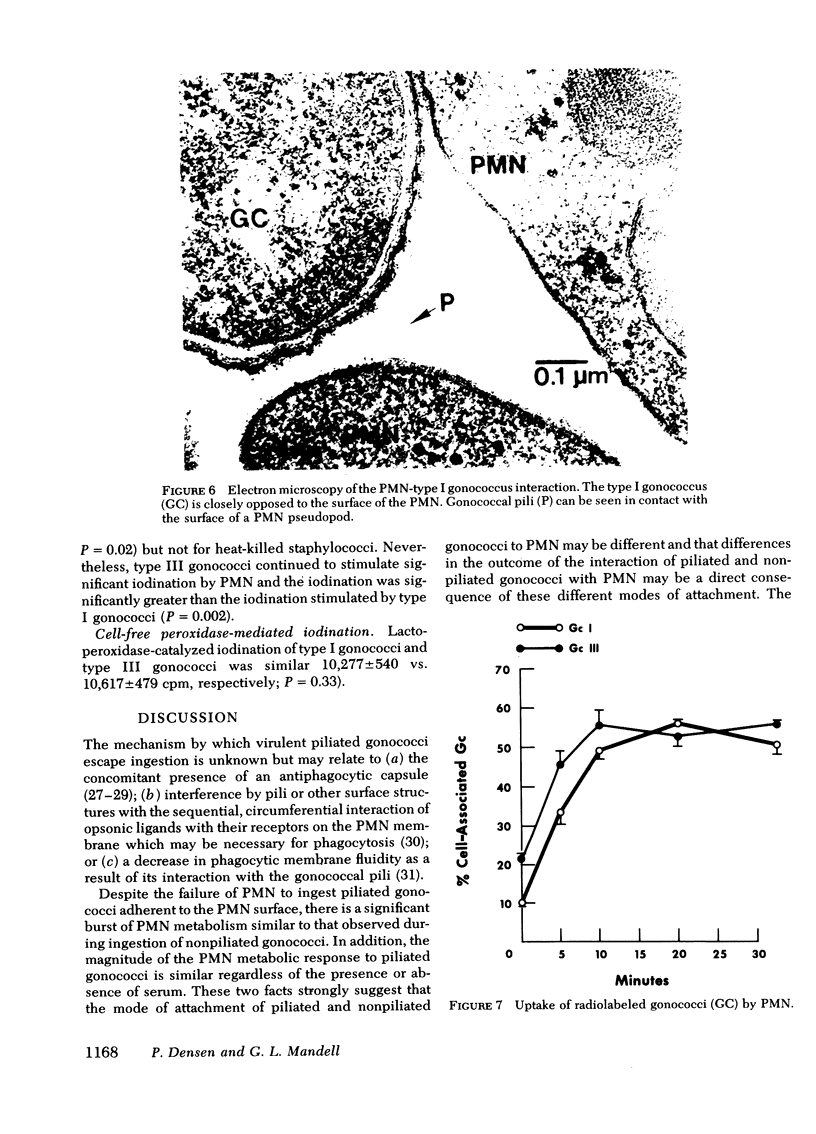
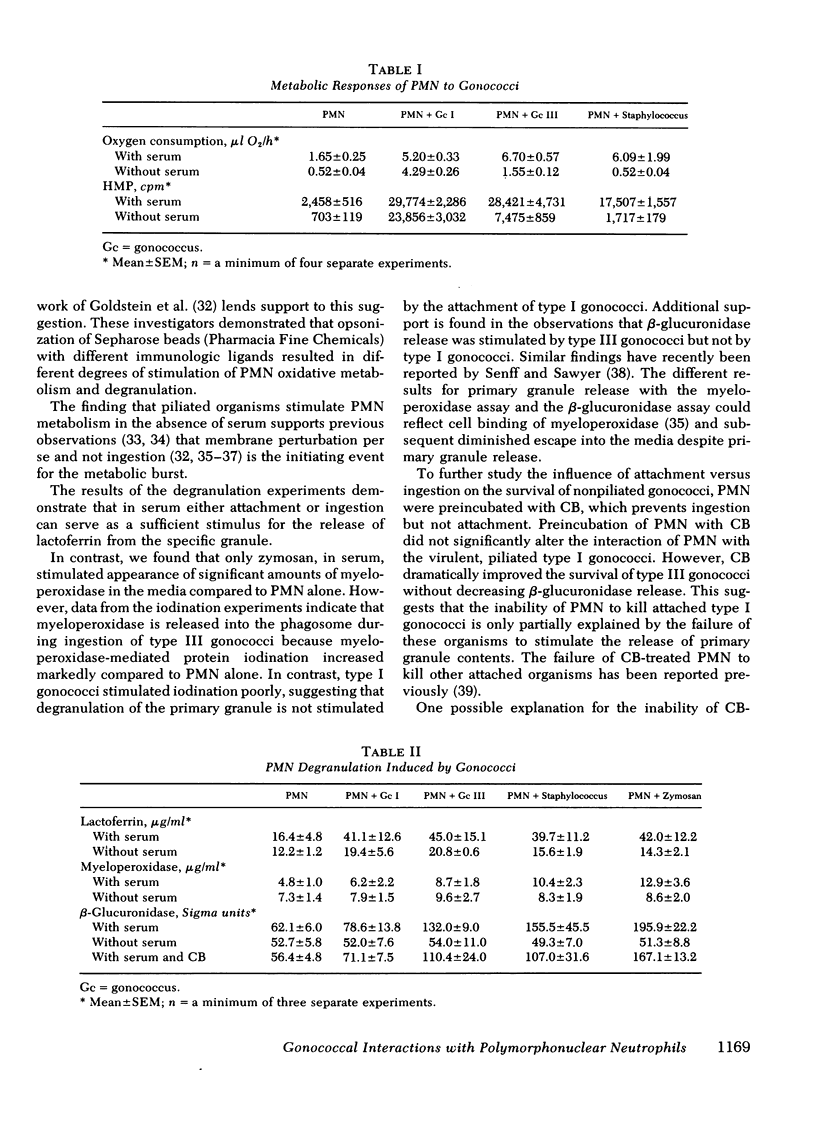


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman W. H., Kato K., Anstiss C. L., Green S. Human serum beta-glucuronidase; its measurement and some of its properties. Clin Chim Acta. 1967 Mar;15(3):435–447. doi: 10.1016/0009-8981(67)90008-3. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Radin A., Frosch M. Independent effects of IgG and complement upon human polymorphonuclear leukocyte function. J Immunol. 1976 Oct;117(4):1282–1287. [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendley J. W., Powell K. R., Rodewald R., Holzgrefe H. H., Lyles R. Demonstration of a capsule on Neisseria gonorrhoeae. N Engl J Med. 1977 Mar 17;296(11):608–611. doi: 10.1056/NEJM197703172961105. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- James J. F., Swanson J. The capsule of the gonococcus. J Exp Med. 1977 Apr 1;145(4):1082–1086. doi: 10.1084/jem.145.4.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Fate of human lactoferrin and myeloperoxidase in phagocytizing human neutrophils: effects of immunoglobulin G subclasses and immune complexes coated on latex beads. Infect Immun. 1975 Oct;12(4):813–820. doi: 10.1128/iai.12.4.813-820.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Intraphagosomal pH of human polymorphonuclear neutrophils. Proc Soc Exp Biol Med. 1970 Jun;134(2):447–449. doi: 10.3181/00379727-134-34810. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Rubin W., Hook E. W. The effect of an NADH oxidase inhibitor (hydrocortisone) on polymorphonuclear leukocyte bactericidal activity. J Clin Invest. 1970 Jul;49(7):1381–1388. doi: 10.1172/JCI106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Okuda K. Effects of cytochalasin B on the intrcellular bactericidal activity of human neutrophils. Antimicrob Agents Chemother. 1975 Jun;7(6):736–741. doi: 10.1128/aac.7.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. H., Graham J. A., Brooks G. F. Human deficiency of the eighth component of complement. The requirement of C8 for serum Neisseria gonorrhoeae bactericidal activity. J Clin Invest. 1976 Feb;57(2):283–290. doi: 10.1172/JCI108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. P., Sadoff J. C. Production of a capsule of Neisseria gonorrhoeae. Infect Immun. 1977 Feb;15(2):663–664. doi: 10.1128/iai.15.2.663-664.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Goldstein I. M., Kaplan H. B., Weissmann G. Dissociation of phagocytosis, metabolic stimulation and lysosomal enzyme release in human leukocytes. Agents Actions. 1976 Feb;6(1-3):256–259. doi: 10.1007/BF01972218. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senff L. M., Sawyer W. D. Release of enzymes from human leucocytes during incubation with Neisseria gonorrhoeae. Br J Vener Dis. 1977 Dec;53(6):360–363. doi: 10.1136/sti.53.6.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons P. Quantitation of plasma proteins in low concentrations using RID. Clin Chim Acta. 1971 Nov;35(1):53–57. doi: 10.1016/0009-8981(71)90292-0. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (first of three parts). N Engl J Med. 1974 Mar 28;290(13):717–723. doi: 10.1056/NEJM197403282901306. [DOI] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaka K., O'Brien P. J. Mechanisms of H2O2 formation by leukocytes. Evidence for a plasma membrane location. Arch Biochem Biophys. 1975 Aug;169(2):428–435. doi: 10.1016/0003-9861(75)90184-8. [DOI] [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- Zatti M., Rossi F. Relationship between glycolysis and respiration in surfactant-treated leucocytes. Biochim Biophys Acta. 1967 Nov 28;148(2):553–555. doi: 10.1016/0304-4165(67)90154-7. [DOI] [PubMed] [Google Scholar]