Abstract
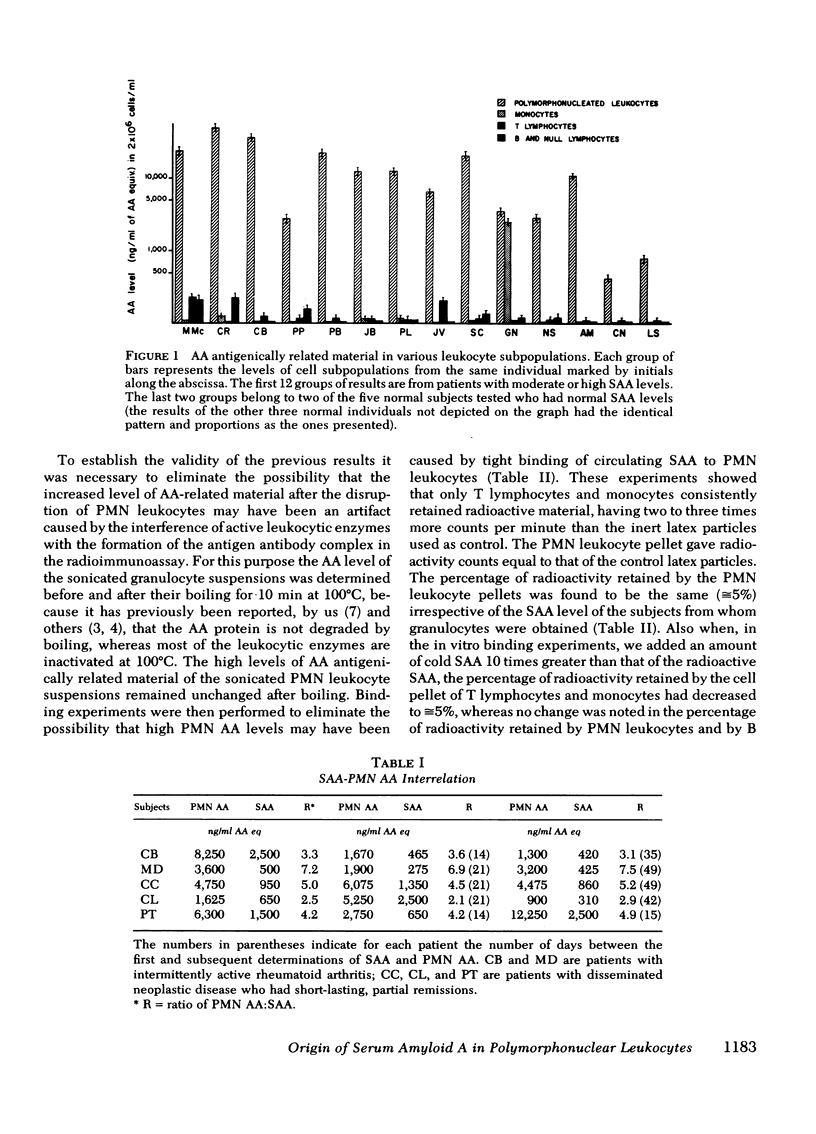
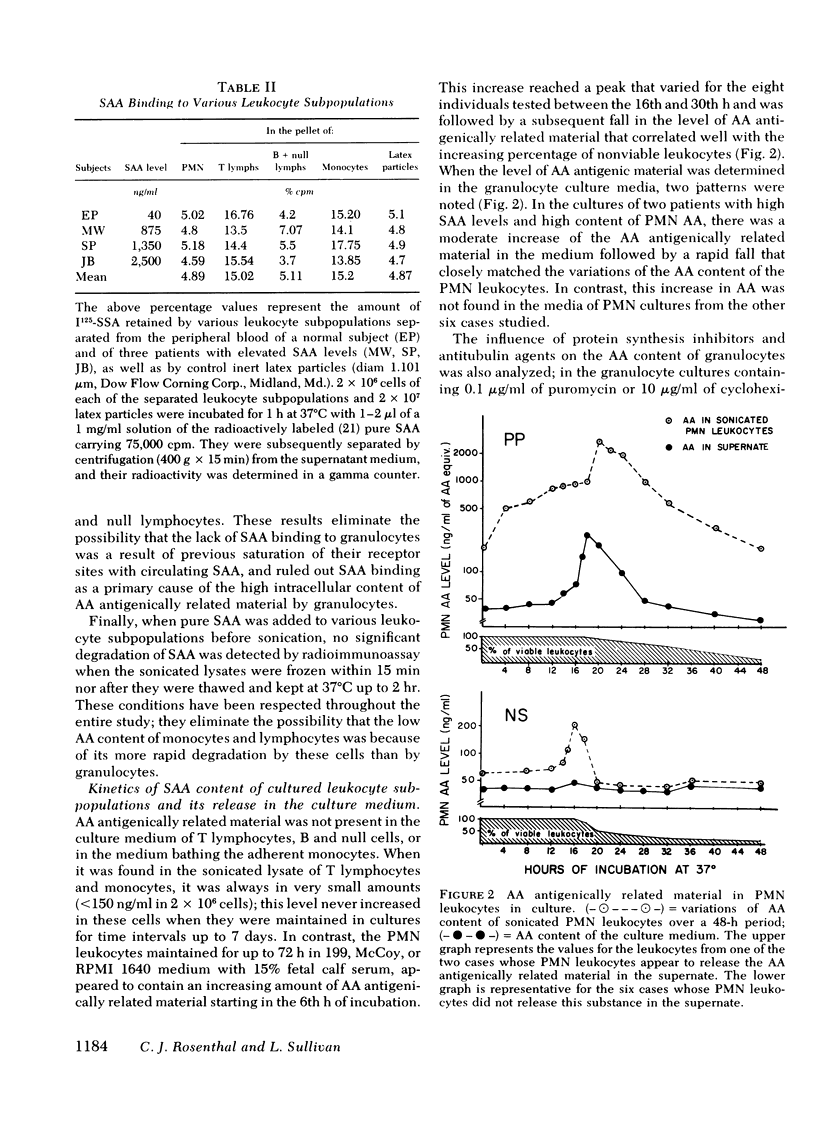
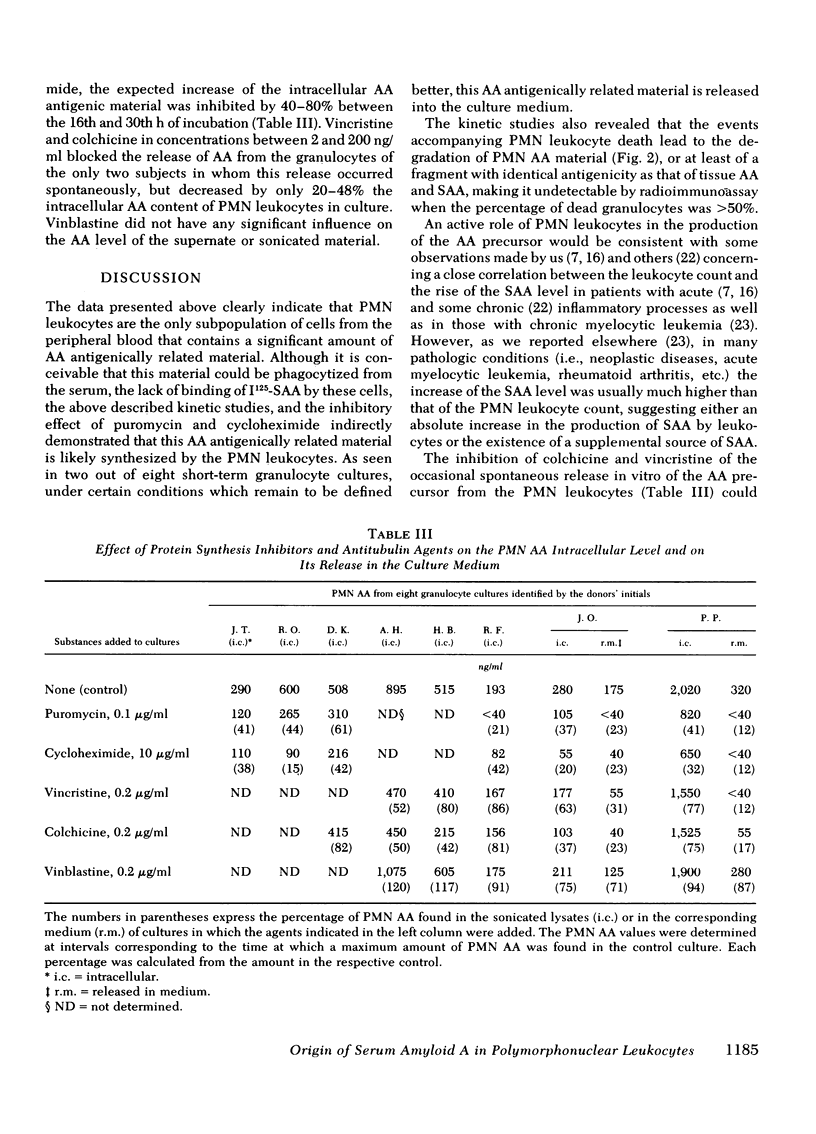
In this study the presence of an amyloid A, antigenically related material was determined in four subpopulations of human leukocytes. Monocytes, granulocytes, thymus-derived lymphocytes, and bone marrow-derived and null lymphocytes were isolated from the peripheral blood of five apparently normal subjects, two patients with secondary amyloidosis, three patients with acute infections, and seven patients with metastatic cancer. Mononuclear leukocytes, isolated from the interface of a Ficoll-Hypaque gradient, were separated into monocytes, thymus-derived lymphocytes, and bone marrow-derived plus null lymphocytes by glass adherence and depletion of sheep erythrocyte rosette-forming lymphocytes. Granulocytes were isolated by sedimentation in 2% methyl cellulose from the erythrocyte-rich pellet formed at the bottom of the Ficoll-Hypaque gradient. The four isolated leukocyte subpopulations were cultured and, at varying intervals, the amyloid A content of the culture medium and of sonicated, 2 × 106 cells was determined by radioimmunoassay. Our results indicated a 2-14 times greater amount of amyloid A-related material in the sonicated granulocytes compared with the individuals' serum amyloid A levels. The mononuclear subpopulations showed a low or negligible amyloid A content. The amount of amyloid A antigenic material was further found to increase in cultured granulocytes, reaching a peak value between the 16th and 30th h of culture. The granulocytes of only two out of eight individuals tested released amyloid A antigenically related material into the culture medium. This release was found to be blocked by the presence of colchicine, vincristine, puromycin, or cycloheximide in the culture medium. In contrast, only the presence of puromycin or cycloheximide was shown to significantly inhibit the intracellular increase of amyloid A in the cultured granulocytes. Thus, it appears that among the circulating blood cells, the granulocytes produce amyloid A antigenically related material and could release it under conditions that remain to be further defined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Natvig J. B., Michaelsen T. E., Husby G. Isolation and characterization of amyloid-related serum protein SAA as a low molecular weight protein. Scand J Immunol. 1975;4(4):397–401. doi: 10.1111/j.1365-3083.1975.tb02642.x. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid. 3. A protein related to the subunit structure of human amyloid fibrils. Proc Natl Acad Sci U S A. 1966 Feb;55(2):308–316. doi: 10.1073/pnas.55.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Scheinberg M. A., Shirahama T., Cathcart E. S., Skinner M. Kinetics of serum amyloid protein A in casein-induced murine amyloidosis. J Clin Invest. 1977 Mar;59(3):412–417. doi: 10.1172/JCI108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Skinner M., Lian J., Cohen A. S. "A" protein of amyloidosis. Isolation of a cross-reacting component from serum by affinity chromatography. Arthritis Rheum. 1975 Jul-Aug;18(4):315–322. doi: 10.1002/art.1780180404. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Page D. L. Amyloid, amyloidosis, and amyloidogenesis. Int Rev Exp Pathol. 1976;15:1–92. [PubMed] [Google Scholar]
- Goldfinger S. E. Colchicine for familial Mediterranean fever. N Engl J Med. 1972 Dec 21;287(25):1302–1302. doi: 10.1056/NEJM197212212872514. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Rosenthal C. J., Franklin E. C. Amyloid-related serum component (SAA)--studies in acute infections, medullary thyroid carcinoma, and postsurgery. Clin Immunol Immunopathol. 1976 Jul;6(1):83–93. doi: 10.1016/0090-1229(76)90063-5. [DOI] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. A serum component related to nonimmunoglobulin amyloid protein AS, a possible precursor of the fibrils. J Clin Invest. 1974 Apr;53(4):1054–1061. doi: 10.1172/JCI107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignaczak T. F., Sipe J. D., Linke F. P., Glenner G. G. Immunochemical studies on the nature of the serum component (SAA) related to secondary amyloidosis. J Lab Clin Med. 1977 May;89(5):1092–1104. [PubMed] [Google Scholar]
- Kedar I., Ravid M., Sohar E., Gafni J. Colchicine inhibition of casein-induced amyloidosis in mice. Isr J Med Sci. 1974 Jul;10(7):787–789. [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder E., Lehto V. P., Virtanen I., Stenman S., Natvig J. B. Localization of amyloid-related serum protein SAA-like material to intermediate (10 nm) filaments of cultured human embryonal fibroblasts. J Exp Med. 1977 Oct 1;146(4):1158–1163. doi: 10.1084/jem.146.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R. P., Sipe J. D., Pollock P. S., Ignaczak T. F., Glenner G. G. Isolation of a low-molecular-weight serum component antigenically related to an amyloid fibril protein of unknown origin. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1473–1476. doi: 10.1073/pnas.72.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam K. P., Anders R. F., Smith S. R., Russell D. A., Price M. A. Association of amyloidosis with erythema nodosum leprosum reactions and recurrent neutrophil leucocytosis in leprosy. Lancet. 1975 Sep 27;2(7935):572–573. doi: 10.1016/s0140-6736(75)90168-3. [DOI] [PubMed] [Google Scholar]
- Ravid M., Robson M., Kedar I. Prolonged colchicine treatment in four patients with amyloidosis. Ann Intern Med. 1977 Nov;87(5):568–570. doi: 10.7326/0003-4819-87-5-568. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C., Frangione B., Greenspan J. Isolation and partial characterization of SAA-an amyloid-related protein from human serum. J Immunol. 1976 May;116(5):1415–1418. [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Variation with age and disease of an amyloid A protein-related serum component. J Clin Invest. 1975 Apr;55(4):746–753. doi: 10.1172/JCI107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S. Casein-induced experimental amyloidosis. VI. A pathogenic role for b cells in the murine model. Immunology. 1976 Sep;31(3):443–453. [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. Blockage of amyloid induction by colchicine in an animal model. J Exp Med. 1974 Oct 1;140(4):1102–1107. doi: 10.1084/jem.140.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. Intralysosomal formation of amyloid fibrils. Am J Pathol. 1975 Oct;81(1):101–116. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Kíely J. M. Synthesis and secretion of a mitogenic protein by macrophages: description of a superinduction phenomenon. J Immunol. 1977 Sep;119(3):925–931. [PubMed] [Google Scholar]
- Watanabe S., Jaffe E., Pollock S., Sipe J., Glenner G. Amyloid AA protein. Cellular distribution and appearance. Am J Clin Pathol. 1977 Jun;67(6):540–544. doi: 10.1093/ajcp/67.6.540. [DOI] [PubMed] [Google Scholar]
- Yu D. T. Human lymphocyte subpopulation: early and late rosettes. J Immunol. 1975 Jul;115(1):91–93. [PubMed] [Google Scholar]



