Abstract
Background
To evaluate if plasma levels of midregional pro-adrenomedullin (MR-proADM) improve prediction of functional outcome in ischemic stroke.
Methods
In 168 consecutive ischemic stroke patients, plasma levels of MR-proADM were measured within 24 hours from symptom onset. Functional outcome was assessed by the modified Rankin Scale (mRS) at 90 days following stroke. Logistic regression, receiver operating characteristics (ROC) curve analysis, net reclassification improvement (NRI), and Kaplan-Meier survival analysis were applied.
Results
Plasma MR-proADM levels were found significantly higher in patients with unfavourable (mRS 3–6) compared to favourable (mRS 0–2) outcomes. MR-proADM levels were entered into a predictive model including the patients' age, National Institutes of Health Stroke Scale (NIHSS), and the use of recanalization therapy. The area under the ROC curve did not increase significantly. However, category-free NRI of 0.577 (p<0.001) indicated a significant improvement in reclassification of patients. Furthermore, MR-proADM levels significantly improved reclassification of patients in the prediction of outcome by the Stroke Prognostication using Age and NIHSS-100 (SPAN-100; NRI = 0.175; p = 0.04). Kaplan-Meier survival analysis showed a rising risk of death with increasing MR-proADM quintiles.
Conclusions
Plasma MR-proADM levels improve prediction of functional outcome in ischemic stroke when added to the patients' age, NIHSS on admission, and the use of recanalization therapy. Levels of MR-proADM in peripheral blood improve reclassification of patients when the SPAN-100 is used to predict the patients' functional outcome.
Introduction
Ischemic stroke is among the leading causes of death and disability and utilises a huge amount of health care expenses. Clinical criteria which predict worse functional outcome include increased age and higher National Institutes of Health Stroke Scale (NIHSS) on admission. [1] Early pharmacological recanalization improves outcome compared to placebo treatment. [2] A potential biomarker should provide predictive information in addition to established prognostic criteria. [3] Several proteins in peripheral blood which are related to an acute stress response have recently been shown to improve outcome prediction in ischemic stroke. [4]–[7] As derived from observations in patients with myocardial infarction and congestive heart failure (CHF), plasma midregional pro-adrenomedullin (MR-proADM) is an independent predictor of death. [8], [9] We hypothesized that MR-proADM would also reflect the acute stress response in ischemic stroke and could therefore be used to predict functional outcome. MR-proADM is a non-functional precursor of adrenomedullin. [10] This protein has been originally isolated from pheochromocytoma and is found in different organs and tissues including vascular smooth muscle cells and endothelium. [11]–[13] Thereby, it exerts vasodilating, vasoprotective and angiogenic effects. [14] Adrenomedullin is difficult to measure in peripheral blood because of complex formation and rapid clearance from the circulation. [15], [16] The more stable MR-proADM is secreted in equimolar amounts to adrenomedullin and can be reliably detected in human plasma. [17], [18].
Methods
Ethics statement
The study was approved by institutional review boards of the Medical University of Graz and Konventhospital Barmherzige Brueder Linz. Written informed consent was obtained from all participants. For patients with impaired consciousness or aphasia, written informed consent was obtained when these patients regained the ability to communicate.
Patients
Consecutive patients admitted between September 2010 and June 2012 to stroke units of the Departments of Neurology, Medical University of Graz and Konventhospital Barmherzige Brueder Linz, were considered for participation in this study. Patients with acute hemispheric, cerebellar or brainstem ischemia according to clinical examination and brain imaging (computerized tomography or magnetic resonance imaging) were eligible when they had a NIHSS [19] of more than 3 on admission and a modified Rankin Scale (mRS) [20] of 0 or 1 before symptom onset. Blood sampling for this study had to be performed within 24 h from symptom onset and before initiation of recanalization therapy (intravenous or intraarterial thrombolysis, endovascular thrombectomy). Subjects with minor stroke (NIHSS <3), transitory ischemic attack (TIA) or evidence for infectious disease on admission were not included. Patients were not eligible when they had major surgery or transfusion of blood components within one month prior to their stroke. Further exclusion criteria were applied as follows: acute renal failure, acute myocardial infarction, chronic hemodialysis, CHF New York Heart Association (NYHA) classes III and IV, active malignancy, immunosuppressive therapy.
Clinical variables and laboratory procedure
The NIHSS was obtained on admission by board certified neurologists. The mRS at day 90 following stroke was obtained during a routine follow-up visit or by telephone interviews with patients or their caregivers. [21] The Stroke Prognostication using Age and NIHSS (SPAN) was obtained by combining the patients' age in years and NIHSS on admission. [22] Individuals with SPAN >100 were considered SPAN-100 positive, and those with SPAN <100 were SPAN-100 negative. In a recent analysis, SPAN-100 positivity was associated with a significant lower odds of a composite favourable outcome (mRS <1, NIHSS <1, Barthel index >95, Glasgow Outcome Scale score 1) at three months following stroke after adjusting for thrombolytic treatment. [22] Stroke was classified according to the Oxfordshire Community Stroke Project (OCSP) [23] and the Causative Classification of Stroke System (CCS). [24] Cerebrovascular risk factors were identified as defined by preadmission history or the need for medication at discharge: hypertension, hypercholesterolaemia, and diabetes mellitus. Atrial fibrillation was diagnosed either by history, an electrocardiogram (ECG) on admission, or Holter-ECG during the hospital stay. Clinical care was performed according to guidelines of the European Stroke Organisation. Blood was drawn by venipuncture and collected into EDTA-coated tubes. Plasma was stored at −70°C for further analysis. Plasma MR-proADM was measured by a commercial chemoluminescence assay on a KRYPTOR® system (Thermo Scientific B.R.A.H.M.S, Hennigsdorf, Germany). [18] Measurements were performed blinded to all clinical data.
Statistical analysis
Student's t-test, Mann-Whitney's U-test, the Chi-square test or Fisher's exact test, and Spearman's rank order correlation were applied for two-group comparisons. Backwards elimination logistic regression was performed to generate predictive models for functional outcome at day 90 following stroke. Patients were dichotomized into favourable (mRS 0–2) and unfavourable (mRS 3–6) outcomes. From a previous small exploratory study, a sample size of 146 patients was derived to obtain significantly different MR-proADM levels between these patient groups with α = 0.05 and 80% power. To evaluate the added predictive ability of MR-proADM, discrimination of models was assessed by comparing areas under receiver operating characteristics (ROC) curves (AUC) [25] and category-free net reclassification improvement (NRI) [26] was applied. NRI offers incremental information over the comparison of AUCs of ROC curves. [27] Category-free NRI is not influenced by correct scaling of the model and offers the widest and most standardized application in quantification of improvement. [26] Based on outcome prediction by the use of the SPAN-100, categorial NRI was obtained by reclassification of patients according to their plasma MR-proADM quintiles. SPAN-100 negative patients in the upper three quintiles were reclassified upwards. SPAN-100 positive patients in the lower two quintiles were reclassified downwards. Z-statistics were calculated as described previously, [27] and p-value was obtained by GraphPad software. Comparison of ROC curves and Kaplan-Meier analysis were done with MedCalc 11.6.1. and sample size calculation with G*Power 3.1. [28] Other analyses were performed by IBM SPSS Statistics version 20 and R version 2.15.1.
Results
168 patients were included in the study, 85 men and 83 women at a mean age of 72.9 years (median 74; range 18–97), all of them Caucasians. Blood samples were collected within 12 hours in 138 (82.1%) and between 12 and 24 hours in 30 (17.9%) patients. 90 (53.6%) patients received revascularization therapy. Patients had a median NIHSS of 9 (range 4–25) on admission. No significant differences in the NIHSS were found between patients who received recanalization therapy and them who didn't. No significant differences in the NIHSS were found between men and women, and whether patients had blood sampling within 12 hours from symptom onset or afterwards. Stroke was classified according to the OCSP as follows: 19.6% total anterior circulation syndrome (TACS), 60.2% partial anterior circulation syndrome (PACS), 13.1% posterior circulation syndrome (POCS) and 7.1% lacunar syndrome (LACS). Causes of stroke according to the CCS were found as follows: 33.9% supra-aortic atherosclerosis, 43.5% cardio-aortic embolism, 7.1% small artery occlusion, 15.5% uncommon/undetermined causes. Cerebrovascular risk factors were found as follows: hypertension in 130 (77.4%), hypercholesterolemia in 86 (51.2%), diabetes mellitus in 40 (23.8%), and atrial fibrillation in 68 (40.5%) patients. Correlations of plasma MR-proADM were found with the patients' age (rS = 0.34; p<0.001), NIHSS on admission (rS = 0.18; p = 0.023) and mRS at day 90 (rS = 0.33; p<0.001). MR-proADM levels did not significantly differ between men and women, between patients with diabetes mellitus or without, and between patients with hypercholesterolemia or without. Higher median MR-proADM levels were found in patients with hypertension (0.77 vs. 0.64 nmol/l; p = 0.005), atrial fibrillation (0.85 vs. 0.70 nmol/l; p<0.001) and coronary heart disease (0.82 vs. 0.74 nmol/l; p = 0.041).
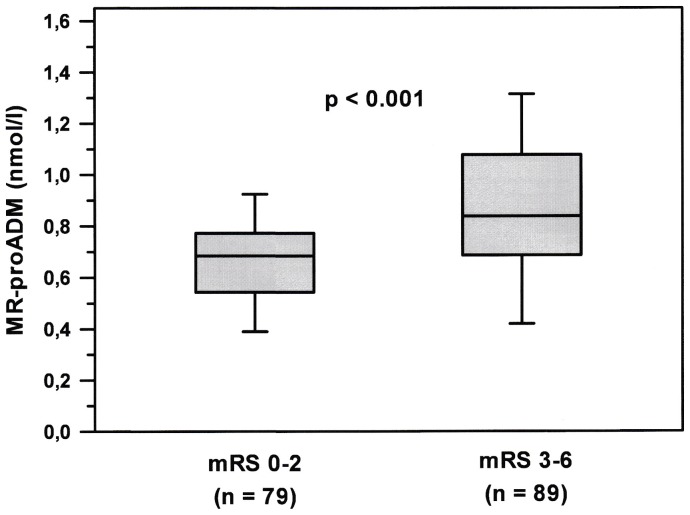
Patients had a median mRS of 3 at day 90. Patients with unfavourable outcome were significantly older, had a higher median NIHSS, a higher proportion of TACS and a higher prevalence of atrial fibrillation and cardio-aortic embolism as the cause of stroke (table 1). Plasma MR-proADM was found significantly higher in patients with unfavourable compared to favourable outcomes (median 0.84 vs. 0.68 nmol/l; p<0.001; figure 1). Predictive models were generated to assess the value of adding MR-proADM plasma levels to the patients' age, NIHSS on admission, and the use of recanalization therapy (table 2). The AUC of generated ROC curves did not increase significantly when plasma MR-proADM levels were added (0.803 and 0.819 for models 1 and 2, respectively; p = 0.204). Category-free NRI of 0.577 (p<0.001) indicated a significant improvement in reclassification of patients by adding MR-proADM levels to predict functional outcome.
Table 1. Demographic data and baseline clinical characteristics of patients.
| all patients | RS 0–2 | mRS 3–6 | p | |
| n | 168 | 79 | 89 | – |
| median age (range) | 74 years (18–97) | 71 years (18–97) | 79 years (60–89) | <0.001 |
| male : female | 85 : 83 | 43 : 36 | 42 : 47 | 0.434 |
| median NIHSS on admission | 9 (range 4–25) | 6 | 12 | <0.001 |
| recanalization therapy | 90 (53.6%) | 46 (58.2%) | 44 (49.4%) | 0.325 |
| hypertension | 130 (77.4%) | 59 (74.7%) | 71 (79.8%) | 0.547 |
| hypercholesterolemia | 86 (51.2%) | 50 (63.3%) | 36 (40.4%) | 0.005 |
| diabetes mellitus | 40 (23.8%) | 19 (24.1%) | 21 (23.6%) | 0.911 |
| atrial fibrillation | 68 (40.5%) | 18 (22.8%) | 50 (56.2%) | <0.001 |
| coronary heart disease | 30 (17.9%) | 11 (13.9%) | 19 (21.3%) | 0.293 |
| angiotensin convertingenzyme inhibitors | 42 (25.0%) | 16 (20.3%) | 26 (29.2%) | 0.246 |
| angiotensinreceptor antagonists | 21 (12.5%) | 8 (10.1%) | 13 (14.6%) | 0.520 |
| TACS | 33 (19.6%) | 5 (6.3%) | 28 (31.5%) | <0.001 |
| PACS | 101 (60.2%) | 55 (69.6%) | 46 (51.7%) | 0.044 |
| POCS | 22 (13.1%) | 10 (12.7%) | 12 (13.5%) | 0.943 |
| LACS | 12 (7.1%) | 8 (10.1%) | 4 (4.5%) | 0.265 |
| supra–aortic atherosclerosis | 57 (33.9%) | 24 (30.4%) | 33 (37.1%) | 0.452 |
| cardio-aortic embolism | 73 (43.5%) | 26 (32.9%) | 47 (52.8%) | 0.025 |
| small artery occlusion | 12 (7.1%) | 9 (11.4%) | 3 (3.4%) | 0.086 |
| uncommon/undetermined causes of stroke | 26 (15.5%) | 17 (21.5%) | 9 (10.1%) | 0.068 |
Patients were dichotomized into favourable (mRS 0–2) and unfavourable (mRS 3–6) outcomes at day 90 after stroke. P-values for median age and median NIHSS on admission were obtained by Mann-Whitney's U-test. Other p-values were obtained by the Chi-square test or Fisher's exact test.
Figure 1. Plasma midregional pro-adrenomedullin (MR-proADM) levels in patients on admission.
Patients were dichotomized into favourable (mRS 0–2) and unfavourable (mRS 3–6) outcomes at day 90 after stroke. Plots display the median, interquartile range (box), 10th and 90th percentiles (whiskers). Abbreviation: mRS = modified Rankin Scale; MR-proADM = midregional pro-adrenomedullin.
Table 2. Predictive models for an unfavourable functional outcome (modified Rankin Scale 3–6) at day 90 following stroke.
| variables | OR (95% CI) | p | |
| model 1 a | age | 1.097 (1.057–1.139) | <0.001 |
| NIHSS | 1.193 (1.108–1.284) | <0.001 | |
| recanalization therapy | 0.587 (0.277–1.245) | 0.160 | |
| model 2 a | age | 1.090 (1.049–1.132) | <0.001 |
| NIHSS | 1.187 (1.100–1.280) | <0.001 | |
| recanalization therapy | 0.732 (0.332–1.615) | 0.439 | |
| plasma MR-proADM | 4.062 (1.109–14.87) | 0.028 |
Abbreviations: NIHSS = National Institutes of Health Stroke Scale; OR = Odd's ratio; CI = confidence interval.
Areas under receiver operating characteristics (ROC) curves (AUC) 0.803 and 0.819 for models 1 and 2, respectively (p = 0.204); category-free net reclassification improvement (NRI) 0.577 (p<0.001).
Abbreviations: mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale; TACS = total anterior circulation syndrome; PACS = partial anterior circulation syndrome; POCS = posterior circulation syndrome; LACS – lacunar syndrome.
With plasma MR-proADM levels in the upper three quintiles, 100%, 83.3% and 75.5% of patients had an unfavourable functional outcome, respectively, as compared to 36.4% and 47.4% of patients in the lower two quintiles. When using the SPAN-100 for outcome prediction (table 3), in 36 patients with unfavourable outcome reclassification improved by MR-proADM, and in 8 patients it became worse, with a net gain in reclassification proportion of 0.31 (p<0.001). Eleven individuals with favourable outcome were falsely reclassified by MR-proADM quintiles (p<0.01). Overall NRI was 0.175 (p = 0.04) indicating an improvement in reclassification of patients by adding plasma MR-proADM to their SPAN-100 status.
Table 3. Reclassification table for prediction of functional outcome at day 90 following stroke.
| SPAN-100+ MR-proADM, predicted outcome | ||||
| mRS 0–2 | mRS 3–6 | total | ||
| SPAN-100, predicted outcome | events (observed outcome mRS 3–6) | |||
| mRS 0–2 | 33 | 36 | 69 | |
| mRS 3–6 | 8 | 12 | 20 | |
| total | 41 | 48 | 89 | |
| non-events (observed outcome mRS 0–2) | ||||
| mRS 0–2 | 66 | 11 | 77 | |
| mRS 3–6 | 0 | 2 | 2 | |
| total | 66 | 13 | 79 | |
Reclassification was performed using the SPAN-100 alone or in combination with MR-proADM quintiles.
Abbreviations: SPAN = Stroke Prognostication using Age and NIHSS; MR-proADM = midregional pro-adrenomedullin; mRS = modified Rankin Scale.
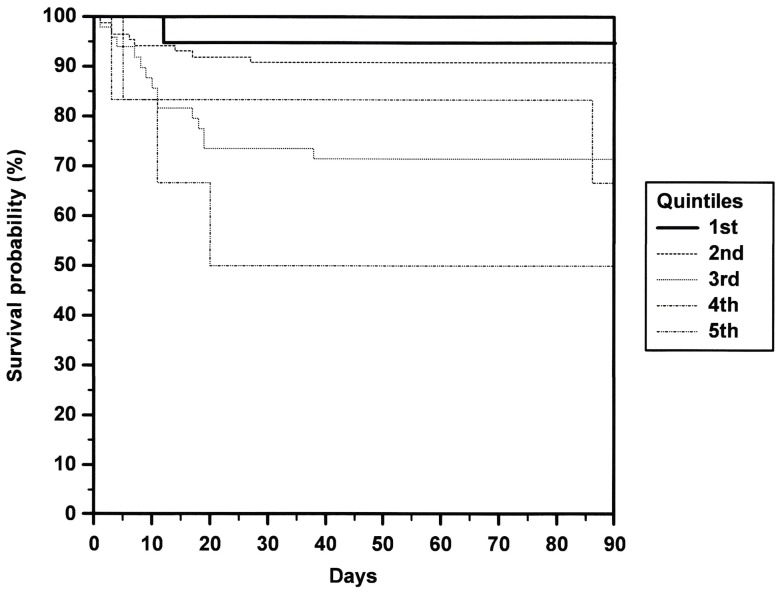
Patients who died within 90 days following stroke (n = 30) had a higher median NIHSS (15 vs. 7; p<0.001) and higher MR-proADM levels (median 0.92 nmol/l vs. 0.73 nmol/l; p<0.001). The difference in age compared to patients who survived did not reach statistical significance (median 79.0 vs. 73.0 years; p = 0.058). In models to predict the patients' death within 90 days following stroke, the AUC of generated ROC curves did not increase significantly when plasma MR-proADM levels were added to the patients' age, NIHSS and the use of recanalisation therapy (data not shown). Category-free NRI of 0.127 (p = 0.523) showed no improvement in reclassification of patients by adding MR-proADM levels to predict patients' death. Kaplan-Meier survival analysis showed a rising risk of death with increasing MR-proADM quintiles (p = 0.011; figure 2).
Figure 2. Kaplan-Meier survival curves.
Time to death related to plasma MR-proADM quintiles (1st: 0.04–0.45 nmol/l; 2nd: 0.46–0.86 nmol/l; 3rd: 0.87–1.27 nmol/l; 4th: 1.28–1.68 nmol/l; 5th: 1.69–2.10 nmol/l).
Discussion
Plasma MR-proADM improves prediction of functional outcome in ischemic stroke when added to the patients' age, NIHSS on admission, and the use of recanalization therapy. Levels of MR-proADM in peripheral blood improve reclassification of patients when the SPAN-100 is used in the prediction of functional outcome. Currently, there are no commonly accepted models to predict functional outcome in ischemic stroke. The SPAN-100 has a great advantage in its ease of use in clinical routine and emergency settings. [29] The mRS at three months is the most prevalent outcome assessment and the preferred outcome measure for treatment trials in acute stroke. [30], [31] As opposed to other stroke biomarker studies, [4]–[6], [32], [33] we have excluded patients with minor stroke or transitory ischemic attack which results in a higher median NIHSS in our study. This contributes to the higher percentage of patients in this study who underwent recanalization therapy as compared to average rates in Austrian stroke units in recent years. [34] We could not approach all eligible patients in the given timeframe for participation in the study. However, we included patients consecutively according to the aforementioned criteria irrespective of any clinical prediction of their prognosis and did not include patients with preexisting disability.
Adrenomedullin has been identified as a tumor survival factor [35] and exerts antimicrobial properties. [36] We have excluded patients with a known malignancy or with signs of infection. In patients with myocardial infarction or CHF, plasma MR-proADM is an independent predictor of death. [8], [9] In our study, Kaplan-Meier survival analysis showed a rising risk of death with increasing plasma level quintiles. MR-proADM levels have previously shown to increase with higher NYHA classes. [9] In that study, MR-proADM appeared to decrease with the intake of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor antagonists. [9] In our cohort, we have excluded patients with NYHA classes III and IV. The proportions of patients who were on ACE inhibitor or angiotensin receptor antagonist therapy in our study did not differ in patients with favourable (mRS 0–2) and unfavourable (mRS 3–6) outcomes (table 1). Patients who took either an ACE inhibitor or an angiotensin receptor antagonist on admission had a higher median mRS at day 90 (4 vs. 2; p = 0.015) and higher median MR-proADM levels (0.82 vs. 0.70 nmol/l; p<0.001).
Adrenomedullin is supposed to counter vasoconstricting and sodium-retaining hormones in patients with CHF. [37] The counter-regulation of vasoconstriction as part of a systemic stress response may also apply to patients with acute ischemic stroke. Data from animal models hint to a role of adrenomedullin in neuroprotection, [38]–[40] an issue to be addressed in future clinical trials. The findings from our exploratory study show that the determination of MR-proADM levels in peripheral blood improves prediction of functional outcome in ischemic stroke patients. This should be reassessed in a larger trial to evaluate its applicability in routine clinical procedures.
Acknowledgments
The excellent technical assistance of Lusik Balayan and Christina Haas is gratefully acknowledged.
Funding Statement
This study was supported by an in-house grant of the Medical University of Graz. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC (2004) Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 35: 158–162. [DOI] [PubMed] [Google Scholar]
- 2. Frendl A, Csiba L (2011) Pharmacological and non-pharmacological recanalization strategies in acute ischemic stroke. Front Neurol 2: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whiteley W, Chong WL, Sengupta A, Sandercock P (2009) Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke 40: e380–389. [DOI] [PubMed] [Google Scholar]
- 4. De Marchis GM, Katan M, Weck A, Fluri F, Foerch C, et al. (2013) Copeptin adds prognostic information after ischemic stroke: Results from the CoRisk study. Neurology 80: 1278–1286. [DOI] [PubMed] [Google Scholar]
- 5. Katan M, Fluri F, Morgenthaler NG, Schuetz P, Zweifel C, et al. (2009) Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol 66: 799–808. [DOI] [PubMed] [Google Scholar]
- 6. Katan M, Fluri F, Schuetz P, Morgenthaler NG, Zweifel C, et al. (2010) Midregional pro-atrial natriuretic peptide and outcome in patients with acute ischemic stroke. J Am Coll Cardiol 56: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 7. Doehner W, von Haehling S, Suhr J, Ebner N, Schuster A, et al. (2012) Elevated plasma levels of neuropeptide proenkephalin a predict mortality and functional outcome in ischemic stroke. J Am Coll Cardiol 60: 346–354. [DOI] [PubMed] [Google Scholar]
- 8. Khan SQ, O'Brien RJ, Struck J, Quinn P, Morgenthaler N, et al. (2007) Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol 49: 1525–1532. [DOI] [PubMed] [Google Scholar]
- 9. von Haehling S, Filippatos GS, Papassotiriou J, Cicoira M, Jankowska EA, et al. (2010) Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur J Heart Fail 12: 484–491. [DOI] [PubMed] [Google Scholar]
- 10. Gumusel B, Chang JK, Hyman A, Lippton H (1995) Adrenotensin: an ADM gene product with the opposite effects of ADM. Life Sci 57: PL87–90. [DOI] [PubMed] [Google Scholar]
- 11. Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, et al. (1994) Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem Biophys Res Commun 203: 719–726. [DOI] [PubMed] [Google Scholar]
- 12. Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, et al. (1994) Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun 201: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 13. Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, et al. (1993) Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 192: 553–560. [DOI] [PubMed] [Google Scholar]
- 14. Kato J, Tsuruda T, Kita T, Kitamura K, Eto T (2005) Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol 25: 2480–2487. [DOI] [PubMed] [Google Scholar]
- 15. Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, et al. (2001) Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem 276: 12292–12300. [DOI] [PubMed] [Google Scholar]
- 16. Meeran K, O'Shea D, Upton PD, Small CJ, Ghatei MA, et al. (1997) Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: a pharmacokinetic study. J Clin Endocrinol Metab 82: 95–100. [DOI] [PubMed] [Google Scholar]
- 17. Struck J, Tao C, Morgenthaler NG, Bergmann A (2004) Identification of an Adrenomedullin precursor fragment in plasma of sepsis patients. Peptides 25: 1369–1372. [DOI] [PubMed] [Google Scholar]
- 18. Morgenthaler NG, Struck J, Alonso C, Bergmann A (2005) Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem 51: 1823–1829. [DOI] [PubMed] [Google Scholar]
- 19. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, et al. (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 20. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 21. Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJ, Algra A, et al. (2010) Comparison of telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc Dis 29: 137–139. [DOI] [PubMed] [Google Scholar]
- 22. Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC (2013) Stroke Prognostication using Age and NIH Stroke Scale: SPAN-100. Neurology 80: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337: 1521–1526. [DOI] [PubMed] [Google Scholar]
- 24. Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, et al. (2007) A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke 38: 2979–2984. [DOI] [PubMed] [Google Scholar]
- 25. DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44: 837–845. [PubMed] [Google Scholar]
- 26. Pencina MJ, D'Agostino RB Sr, Steyerberg EW (2011) Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172; discussion 207–112. [DOI] [PubMed]
- 28. Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 29. Rabinstein A, Rundek T (2013) Prediction of outcome after ischemic stroke: The value of clinical scores. Neurology 80: 15–16. [DOI] [PubMed] [Google Scholar]
- 30. Quinn TJ, Dawson J, Walters MR, Lees KR (2009) Functional outcome measures in contemporary stroke trials. Int J Stroke 4: 200–205. [DOI] [PubMed] [Google Scholar]
- 31. Lees KR, Bath PM, Schellinger PD, Kerr DM, Fulton R, et al. (2012) Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke 43: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 32. Worthmann H, Tryc AB, Goldbecker A, Ma YT, Tountopoulou A, et al. (2010) The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc Dis 30: 85–92. [DOI] [PubMed] [Google Scholar]
- 33. Worthmann H, Kempf T, Widera C, Tryc AB, Goldbecker A, et al. (2011) Growth differentiation factor 15 plasma levels and outcome after ischemic stroke. Cerebrovasc Dis 32: 72–78. [DOI] [PubMed] [Google Scholar]
- 34. Ferrari J, Knoflach M, Kiechl S, Willeit J, Matosevic B, et al. (2010) Stroke thrombolysis: having more time translates into delayed therapy: data from the Austrian Stroke Unit Registry. Stroke 41: 2001–2004. [DOI] [PubMed] [Google Scholar]
- 35. Cuttitta F, Pio R, Garayoa M, Zudaire E, Julian M, et al. (2002) Adrenomedullin functions as an important tumor survival factor in human carcinogenesis. Microsc Res Tech 57: 110–119. [DOI] [PubMed] [Google Scholar]
- 36. Wiesner J, Vilcinskas A (2010) Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1: 440–464. [DOI] [PubMed] [Google Scholar]
- 37. Nicholls MG, Charles CJ, Lainchbury JG, Lewis LK, Rademaker MT, et al. (2003) Adrenomedullin in heart failure. Hypertens Res 26 Suppl: S135–140 [DOI] [PubMed] [Google Scholar]
- 38. Maki T, Ihara M, Fujita Y, Nambu T, Miyashita K, et al. (2011) Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke 42: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 39. Encinas JM, Serrano J, Alonso D, Fernandez AP, Rodrigo J (2002) Adrenomedullin over-expression in the caudate-putamen of the adult rat brain after ischaemia-reperfusion injury. Neurosci Lett 329: 197–200. [DOI] [PubMed] [Google Scholar]
- 40. Serrano J, Alonso D, Encinas JM, Lopez JC, Fernandez AP, et al. (2002) Adrenomedullin expression is up-regulated by ischemia-reperfusion in the cerebral cortex of the adult rat. Neuroscience 109: 717–731. [DOI] [PubMed] [Google Scholar]