Abstract
Strategies to enhance the already established doublet chemotherapy regimen for lung cancer have been investigated for more than 20 years. Initially, the concept was to administer chemotherapy drugs locally to the tumor site for efficient diffusion through passive transport within the tumor. Recent advances have enhanced the diffusion of pharmaceuticals through active transport by using pharmaceuticals designed to target the genome of tumors. In the present study, five patients with non-small cell lung cancer epidermal growth factor receptor (EGFR) negative stage IIIa–IV International Union Against Cancer 7 (UICC-7), and with Eastern Cooperative Oncology Group (ECOG) 2 scores were administered platinum-based doublet chemotherapy using combined intratumoral-regional and intravenous route of administration. Cisplatin analogues were injected at 0.5%–1% concentration within the tumor lesion and proven malignant lymph nodes according to pretreatment histological/cytological results and the concentration of systemic infusion was decreased to 70% of a standard protocol. This combined intravenous plus intratumoral-regional chemotherapy is used as a first line therapy on this short series of patients. To the best of our knowledge this is the first report of direct treatment of involved lymph nodes with cisplatin by endobronchial ultrasound drug delivery with a needle without any adverse effects. The initial overall survival and local response are suggestive of a better efficacy compared to established doublet cisplatin–based systemic chemotherapy in (higher) standard concentrations alone according to the UICC 7 database expected survival. An extensive search of the literature was performed to gather information of previously published literature of intratumoral chemo-drug administration and formulation for this treatment modality. Our study shows a favorable local response, more than a 50% reduction, for a massive tumor mass after administration of five sessions of intratumoral chemotherapy plus two cycles of low-dose intravenous chemotherapy according to our protocol. These encouraging results (even in very sick ECOG 2 patients with central obstructive non-small cell lung cancer having a worse prognosis and quality of life than a non-small cell lung cancer in ECOG 0 of the same tumor node metastasis [TNM]-stage without central obstruction) for a chemotherapy-only protocol that differs from conventional cisplatin-based doublet chemotherapy by the route, target site, and dose paves the way for broader applications of this technique. Finally, future perspectives of this treatment and pharmaceutical design for intratumoral administration are presented.
Keywords: cisplatin, lymph nodes, chemotherapy, intratumoral, lung cancer
Introduction
Lung cancer treatment still remains a challenge. In the last decade an effort has been made to identify the mechanisms and pathways of lung cancer.1,2 Pharmacoethnicity in association with pharmacogenomics has also been investigated as the genetic mutations of lung cancer differ among populations and therefore several stargeted therapies present a higher rate of efficiency, as is the case with epidermal growth factor receptor mutation (EGFR).3–5 Pharmacogenetics have also been investigated for conventional chemotherapy in lung cancer in an effort to identify different pathways and mechanisms of acquired resistance to conventional chemotherapy.6,7 Targeted therapies have been approved as first line treatment for specific genetic mutations and many pathways are still under investigation.8 However, doublet chemotherapy still remains the cornerstone of treatment for many patients.9
Chemotherapy drugs present non-specific cytotoxic activity, which in many cases induce adverse effects from systemic administration. Patients have to stop their treatment and the national health systems are affected by the additional hospitalization days and drugs administered.10 In an effort to enhance the efficiency of conventional chemotherapy, several studies have investigated the addition of immunomodulatory agents with conventional chemotherapy and/or anti-vascular endothelial growth factors with targeted chemotherapy.11–14 However, systemic side effect prevalence still remains the same.
Another conceptual approach, ie, the locoregional administration of chemotherapeutics has been promoted by many groups either in the form of intratumoral chemotherapy (ITC),15–17 brachytherapy,18 photodynamic therapy,19 mechanic debulking,20,21 and aerosol chemotherapy.22–24 Locoregional therapy uses chemicals, genes, biologics, free or formulated drugs, and novel drug carrier systems, as a single therapy or with radiation therapy,25–29 thermal,22,74 or non-thermal local ablative methods. These studies were mostly intended to establish the safety and efficacy of locoregional therapies and investigate whether higher drug concentration could increase efficiency and decrease or prevent systematic adverse effects. Intratumoral drug administration was also investigated as a method to sensitize the tumor to radiotherapy and systematic chemotherapy.30 Nearly 30% of newly diagnosed patients with lung cancer will develop respiratory distress, bleeding, atelectasis, and post-obstructive pneumonia due to partial or complete airway obstruction.31 The tumor core consists of chemotherapy resistant tumor cells and intratumoral chemotherapy has been observed to sensitize these cells by delivering selected drugs into different parts of the core with radioactive coils and other agents for brachytherapy.30,32–34 In particular, it was observed that nanocarriers for chemotherapy drugs lead to improved drug diffusion and sustain release. In addition, brachytherapy studies presented data were the radiation emitted from the coils was only cytotoxic to the site of inplantation.30,35 It has also been observed that local tumor response is accomplished more quickly with local intratumoral drug administration.16,36
Moreover, there are obstacles that have to be bypassed with these treatment techniques, such as: (a) increased interstitial fluid pressure, (b) local hypoxia, (c) heterogeneous distribution of drug formulations due to abnormal vascular architecture, (d) extracellular matrix with collagen, elastin, fibroblasts, and (e) structural abnormalities within the center of the tumor. There are two principal methods of drug permeation of the tumor as a sum effect of diffusion and distribution within the tumor: active and passive transportation, which can be combined with a physical (pre)treatment, like heating the device to enhance uptake or diffusion,37–39 or with a cooling device.40 Several formulations have been investigated either with passive or active transportation in order to investigate their efficiency. In addition, carriers have been added to several pharmaceuticals to prolong their local release and diffusion rates.41,42 Nanocarriers have provided an effective method for drug accumulation within the tumor due to the enhanced permeability and retention effect (EPR) effect.43 The EPR effect has been also observed to be enhanced with the addition of polyethylene glycol (PEG) stealth molecules,44 and controlled by heat shock protein 32 and carbon monoxide.45 The present study illustrates our initial experience with intratumoral chemotherapy compared to previous published studies and we propose future avenues for applications of this methodology.
Patients and methods
Patients and study design
Five patients stage IIIa–IV, performance status 2 (PS2) unfit for surgery, radiation, and chemotherapy were included in the trial. The trial was initiated in May 2009 and was approved by our investigational review board (IRB; Table 1). One additional patient was excluded from the trial when she was diagnosed with mammarian carcinoma stage IV with a stenotic tracheal metastasis and hypercapnia ≥60 mmHg unfit for treatment with laser or argon-plasma-coagulation or radiation; however, she was treated along the same line of this protocol only with slightly different ITC mixture, adding 2 mg mitomycin to 10 mg cisplatin, and observed upon follow up. The staging was decided according to the sixth edition of tumor node metastasis (TNM) classification of non-small cell lung cancer (NSCLC) as the trial took place in 2009 and 2010. All patients had relatively adequate renal function allowing for the administration of at least 70% of the dose defined intravenous standard platinum doublet protocol (or alternatively area under the curve [AUC] 4 in case of carboplatin; defined by a level of serum creatinine of Value1.5 mg/mL), hepatic enzymes (bilirubin of Value1.5 mg/dL), and hematologic function (absolute neutrophil count of 1.5 × 103 mm3 and platelet count of 1.0 × 105 mm3). Patients included had symptomatic obstruction of the trachea or of a major bronchus secondary to inoperable histologically confirmed NSCLC. The patient population in this study included only patients with disease confined to one hemithorax and with the major tumor involvement in the thorax. Two cases with Stage IV NSCLC showed single bone metastasis in upper arm respectively in thigh. For such cases, the therapeutic strategy was to relieve symptoms from the bronchial tumor and to address metastatic bone deposit with radiation therapy and bisphosphonates according to standard oncologic care. Nearly complete airway obstruction, superior to 50% occlusion of at least one major airway was required for inclusion into the study. Other criteria were: isolated lobar obstruction was included only if it was believed to contribute to respiratory insufficiency, chronic (or continuous) bleeding worsening the respiratory situation or post-obstructive pneumonia, or all these conditions together. Patients with small cell carcinoma were excluded, based on anticipated rapid response to systemic chemotherapy or irradiation or both that these patients often experience.46
Table 1.
Patient characteristics
| Patient stage | ECOG | Local 4-week response | Survival (days) | Histology |
|---|---|---|---|---|
| 1. IIIb (cT4N2Mx) | 2 | PR− | 311 | Squamous |
| 2. IV (cT3-4NxM1b) | 2 | PR+ | 429 | Squamous |
| 3. IIIb (cT4N2M0) | 2 | PR+ | 496* | Squamous |
| 4. IV (cT3-4N2M1b) | 2 | PR− | 365 | Adeno |
| 5. IIIa (cT4N1M0) | 2 | NC | 552* | Adeno |
| 6. Mammarian CA IV | 2 | Local CR | 319 | Adeno |
Notes:
Alive upon end of follow-up, other patients died due to cancer-related death. Average PR+ overall survival: 463 days; average PR− overall survival: 338 days. PR+: reduction of >% of initial volume; PR−: reduction of 25%–50% of initial volume.
Expected median survival according to UICC 7 in ECOG 0–1 patients: IIIa 1/5, IIIb 2/5, IV 2/5 = ((1*14 m) + (2*10 m) + (2*6 m))/5 = 9.2 months = 276 days.
Achieved median survival in this ECOG 2 group along our protocol = 13.2 m = 397 days: This means a surplus of 43.8% median survival in comparison to expected survival.
Abbreviations: PR, partial response; ECOG, Eastern Cooperative Oncology Group; NC, no change; CR, complete response; Adeno, adenocarcinoma; CA, carcinoma.
All patient treatment sessions were recorded in order to evaluate the therapeutic protocol (Figures 1–6). Criteria for exclusion were: pregnancy or child-bearing potential without using contraceptive methods, concurrent highly septic situations due to poststenotic pneumonia infections (ie, requiring intravenous antibiotic therapy was allowed), an unstable or serious concurrent medical condition (eg, recent myocardial infarction and superior vena cava syndrome), recent major surgery, or large-field radiation therapy or chemotherapy in the month before entry. Patients were not allowed to receive any concurrent radiotherapy to the lungs, immunotherapy or investigational agents while on the study. These criteria were chosen based on previous publications (Table 2).16,47 Written informed consent was obtained from each patient before study enrollment. Patient characteristics upon admission are presented in Table 1.
Figure 1.
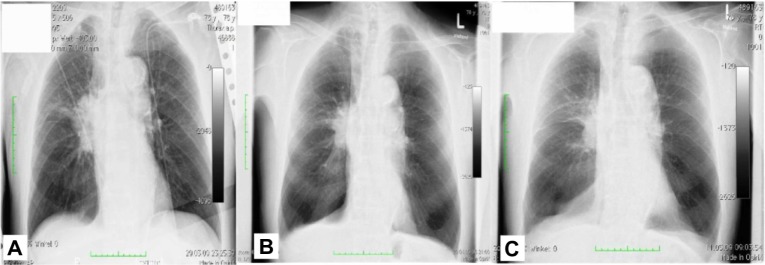
X-ray from patient 1.
Notes: (A) X-ray upon diagnosis. (B) after one session (1 month). (C) and after two sessions (6 weeks after diagnosis).
Figure 6.
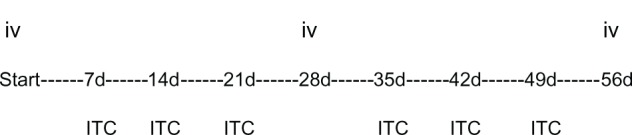
Scheme of proposed intratumoral protocol. Timing of intravenous 4-week-cycle with weekly intratumoral chemotherapy.
Abbreviations: d, day; ITC, intratumoral; iv, intravenous.
Table 2.
Intratumoral therapy experience
| Author | Methodology | Subjects | Cancer cells/tissue | Response | Nanoparticles | Carriers | Reference |
|---|---|---|---|---|---|---|---|
| Jia et al | Intratumoral plus doxorubicin magnetic field | In vitro/in vivo | Lewis lung cancer | √ | Magnetic Fe3O4 | PLGA | 27 |
| Akeda et al | OK-432 | In vivo | Squamous lung carcinoma | √ | – | – | 80 |
| Li et al | Multifunctional theranostic liposome drug delivery system plus doxorubicin | In vitro/in vivo | Squamous cell carcinoma-4 tumor cells | √ | Magnetic | Liposomes | 62 |
| Goldberg et al | Review | Review | Review | Review | Review | Review | 51 |
| Brincker et al | Review | Review | Review | Review | Review | Review | 61 |
| Celikoglu et al | 5-fluorouracil, mitomycin, methotrexate, bleomycin, mitoxantrone, cisplatin | Patients | Lung cancer | √ | – | – | 16 36 46 47 48 56 |
| Fujiwara et al | Intratumoral-P53 | Patients | Lung cancer | √ | – | – | 25 |
Abbreviations: OK, lyophilized incubation mixture of group A Streptococcus pyogenes of human origin; PLGA, poly(lactic-co-glycolic acid).
Dose schedule and technical aspects
The protocol was based on previous publications in the field of intratumoral chemotherapy.16,36,46–48 Previous groups treated their patients with cisplatin 0.5%–4% ITC. In Germany, 0.5% cisplatin is the concentration for intravenous administration; although uncommon, other cisplatin concentrations are available through the hospital’s dispensary when buying cisplatin as a salt. It is believed that upon intratumoral bolus injection, cisplatin will diffuse systemically via lymphatic draining vessels and lymph nodes; such drainage may reach sentinel lymph nodes that would be exposed to the drug as a consequence of ITC and at a higher drug concentration than possible with intravenous administration of the same cisplatin amount (Figure 5). As lymph node involvement in lung cancer is a major concern for local recurrence, we added the direct treatment of involved lymph nodes by drug delivery through a transbronchial needle aspiration (TBNA)-needle of an endobronchial ultrasound (EBUS)-probe to enhance the efficacy over ITC only. This drug delivery by an EBUS-needle (EBUS-transbronchial needle dosing [TBND]) is aiming at six passes per lymph node to optimize the permeation of the drug. We have chosen this number as six passes are the optimal number for EBUS-TBNA to get representative results of the whole lymph node volume.
Figure 5.
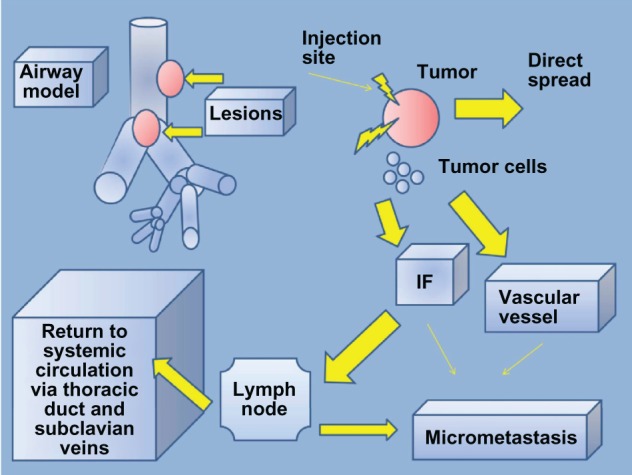
Intratumoral chemotherapy model.
Notes: After the local administration, tumor necrosis is observed and micrometastasis occurs through the local abnormal vascular architecture and diffusion through the interstitial fluid.
Abbreviation: IF, interstitial fluid.
A huge point of contention for the use of ITC is the under or over-dosing due to the leakage or backflow of drug. Such “loss” if not quantified makes it very difficult to relate the dose to the effect over a tumor or target volume unit. We think that even in the case that intratumoral-volume leaks out of the tumor very easily as a so called “downstream effect”, this intratumoral-loss cisplatin dose will be reabsorbed by the alveoli and mucosa in other lung areas and will act as a systemic administration. In other words, ITC that uses aqueous platinum analog offers the advantage of very high concentrations (eg, up to 70-fold higher compared to the same amount of carboplatin given intravenously in ITC with polyethylene glycol (PEG)-carboplatin)49 with less systemic cytotoxicity but in the end it resembles an established intravenous split schedule: the timely separation of intratumoral and intravenous administration leads to the well-known efficient intravenous split cisplatin protocols with two dosages typically 6 to 7 days apart and therefore this dosing regimen makes sense.50
ITC has been demonstrated in animal models with lung cancer to induce a positive antitumor immunoresponse;51 meaning a significant reduction in tumor mass different than the main site directly treated by ITC. The same has been proven in human beings using other local treatment modalities in different cancers.52 This effect is believed or has been partly proven to be caused by demonstrating vast amounts of tumor cell debris toward immunocompetent cells, which are stimulating the immune system to use these “specific” antigens to induce a “specific” antibody reaction; eg, auto-vaccination triggered by local treatment modalities. The cornerstone of these mechanisms is an intact competent immune system which could be negatively influenced by acute administration of intravenous chemotherapy. This is the second reason to separate intravenous from intratumoral administration timing for the first 5–10 days of treatment.
A major factor affecting ITC-efficacy is the distribution of the drug intratumorally. We treated the mass from all possible directions: from endoluminal or from transthoracical direction if feasible under ultrasound or C-arm fluoroscopy guidance due to local anatomy. We used direct central needle insertion in lobar stenosis (with atelectasis, chronic bleeding, and/or post-stenotic pneumonia) and in central airway stenosis >50%. Especially in lobar stenosis, the maneuverability of the needle in the peripheral site is highly reduced; therefore, the needle tip was placed in the most distant part of the tumor and then retracted 0.5 cm delivering at each point a maximum of 1 mL ITC-drug. This approach was only allowed if the needle path was clear inside the inner two thirds of a post-stenotic atelectasis which could represent a tumor portion. In the exophytic parts of mainly central tumors, we additionally directly injected intratumorally and, furthermore, surrounded the exophytic parts on the mucosal layer with small injections (1 mL) intramucosally/superficially around the tumor neck aiming at closing a circle around the tumor neck at this layer. In case of mixed intra-extrabronchial stenosis with a transmucosal part of bulk and/or involved lymph nodes we treated this part of the tumor by EBUS-TBND. In lymph nodes, we aimed at six passes with different angulations for EBUS-TBND. For masses that abutted the thoracic wall, we used ultrasound guided percutaneous transthoracic drug injection (transthoracic ultrasound [tts] ITC) through only one transthoracic puncture receiving the needle tip to the most distal part of the ultrasound picture of the mass and injecting 1 mL ITC-drug after each 0.5 cm pulling step back toward the puncture point. This procedure was repeatedly performed in different angulations with 30 degrees difference in a three-dimensional manner and under bronchoscopy for controlling and calculating the “loss” amount of ITC leaking out of tumor mass and dispersing rapidly down toward the central airways – the so called downstream effect of ITC. The downstream effect may result from the typically inhomogeneous spongy tumor structure or separated by septa, layers, or from puncturing of bronchioli lumen still patent within the tumor. In order to better visualize the downstream effect, we colored the cisplatin-ITC with indigo carmine dye (0.5 mL/10 mL drug volume). If a downstream effect occurred, the injection was stopped, the needle position was changed by retracting 1 cm, followed by the next injection of 1 mL until the subpleural area adjacent to the insertion point was reached. Then a new needle pathway with different angulations was started at the most distal point of the ultrasound picture. In case visualization by ultrasound was not possible we used different angulations under c-arm fluoroscopy control. All this was to achieve a best individual distribution of the ITC-drug to cover up the major part of the tumor volume and involved lymph nodes.
Furthermore, the calculation of the ITC-drug volume was done along the lines of the formula by Monga53 using the help of the computed tomography (CT)-scans (in mL ITC-drug):
| (1) |
covering approximately 1 mL of tumor with 1 mL of ITC-drug. In this study, we were adding the volume for involved lymph nodes which were measured by EBUS during the diagnostic TBNA in addition to the CT-scans prior to therapy. In case a downstream effect was observed, we summed up each observed 1 mL (meaning suddenly rapid unmeasured droplets) of “lost” ITC-drug after planned injection of 1 mL ITC and added this amount afterwards to another “additional” path with a different angulation until the pre-therapeutic calculated ITC-volume was reached. The consequence of this approach was that in the patients with a downstream effect more ITC-volume than previously calculated was used. In reality, this was observed in two patients with no more than 10% additional volume only in the transthoracical approach. We restricted ourselves to a maximum of total ITC volume 2-fold of the pre-therapeutic calculated ITC-volume. A CT-scan for reevaluation and recalculation of the ITC-volumes was repeated after two complete intravenous cycles encompassed by four to six administrations of ITC. The minimal goal in these very sick patients was to administer two cycles intravenous platinum doublet every 3–4 weeks encompassed with at least four sessions ITC. We hoped to achieve four cycles intravenous doublet encompassed by 8–12 ITC-administrations timely separated as mentioned above if the patient was willing to do so and if the local (meanwhile regressed) anatomy was feasible to inject. One potential limitation of this protocol was the fact that these sick patients had to be treated weekly for the ITC-administration with aqueous cisplatin. In palliative patients, too much time inside the hospital should be avoided.
Previous experience of intratumoral administration was 30–40 mg aqueous cisplatin demonstrating good tumor reduction and no adverse effects.16,36,46–48 Therefore we used cisplatin 1% for smaller masses and 0.5% cisplatin concentration for larger masses to realize a compromise between injected volume and drug amount per tumor volume unit for optimization of drug distribution and tumor coverage with ITC. It has to be mentioned that it was allowable to use more than 40 mg cisplatin per ITC-session, but not more than the (theoretical) 70% standard cisplatin intravenous amount in these very sick patients. We restricted ourselves therefore to the maximal dose of 100 mg cisplatin per ITC-session. In reality, this was only used in one ITC-session, all other ITC-sessions were done with 10–50 mg cisplatin in total (average 24 mg cisplatin).
The drug injection rate for ITC was between 1 and 5 mL/minute: if the downstream effect occurred we stopped injection at the preceding injection point and reduced our velocity to 1 mL/minute at the next injection point after retraction of the needle. If there was no downstream effect with 1 mL/minute in the preceding injection point we elevated our injection velocity to 5 mL/minute in the following injection point after retraction. All this was meant to optimize the coverage of ITC-volume in the mass and the time per treatment session. The treatment times for an ITC-session covering tumor mass and node(s) (average mass dimensions: 7 cm; average number of nodes: 2) was between 25 and 60 minutes (average 38 minutes).
Pharmaceuticals
The following pharmaceuticals were used for weekly intratumoral chemotherapy 5–10 days apart from the intravenous administration: cisplatin/hospira solution for infusion 100 mg/100 mL vial BT × 1 vial × 100 mL (Hospira UK Ltd, Queensway, Royal Leamington Spa, Warwickshire, UK) in concentrations between 0.5% and 1%.
For intravenous chemotherapy, 70% of a standard platinum analog containing doublet scheme repeating every 3–4 weeks with the following drugs was used: cisplatin or carboplatin and one of the following drugs GEMZAR® (Lilly USA LLC, Indianapolis, IN, USA; 200 mg/1 g vial), etoposide, and vincristine.
The intravenous administration was separated from ITC by 5–10 days. The protocol aimed to treat these very sick patients was with at least two cycles of intravenous administration encompassed by 4–6 intratumoral administrations, if feasible due to local anatomy. The intravenous combination scheme was chosen based on the performance status of the patients. Putting intravenous and intratumoral administrations together means that between two intravenous administrations it was possible to give an additional two or three ITC-administrations (Figure 6).
Specific treatment effects and complications
In summary, there was no severe adverse event in a total of 22 ITC-sessions even in an ITC with 100 mg cisplatin showing only a one-time moderate hematotoxicity with 1800 leukocytes/mL. This is striking as a proof of the concept that ITC either passed through the lymphatic vessels or was down-streamed or reabsorbed by the bronchial mucosa and alveoli to become systemically active. It is worth mentioning that for all directly visible tumor masses the first injection with ITC, regardless of the approach (endoluminal or transthoracic), changed the tumor color to pale or white almost immediately and sometimes after the injection of only 1 mL. This appears to be an acute interruption of the perfusion or a “shock reaction” of the tumor. Another interesting observation was the fact that in patients with chronic bleeding from the tumor or from peritumoral vessels, the bleeding stopped after only one or two ITC-sessions. No acute local toxic effect in the mucosa or in the healthy central airways could be identified; no late stenotic process after ITC-sessions occurred either. Similar findings have been reported in previous studies (Table 1).16,36,46–48
Discussion
Intratumoral injection of chemotherapeutics is an efficient and safe local therapeutic method. ITC as adjuvant therapy using the protocol described herein showed, as in other ITC-studies, an expected relief concerning the acute local problems caused by stenosis, bleeding, or atelectasis in four of five NSCLC patients in the protocol and in one patient (out of protocol) with mammarian cancer and tracheal metastasis.32,42,44,54–60 Moreover, this trial demonstrated for the first time that direct treatment of involved lymph nodes with ITC by aqueous cisplatin simultaneously to the tumor mass is possible and safe. As the results concerning debulking are similar to those of other studies, loss of ITC-volume as a downstream effect seems to be negligible.32,42,44,54–60 Such a downstream effect was only seen with small “loss” volumes in two patients only when applying transthoracical ITC. It is worth mentioning that to the best of our knowledge we applied for the first time this protocol using a dye and simultaneous bronchoscopy during transthoracical approach of ITC to control this unintended effect of downstreaming and thereby optimized the application of aqueous cisplatin for ITC in a transthoracical approach. A big difference in this study compared to other studies was that we took this approach in very sick ECOG 2 patients who were not eligible for standard oncologic care procedures. Under general conditions these patients would have only received best supportive care with a survival prognosis around 3 months. Even comparing these very sick patients with the actual database UICC 7, which is referred to as ECOG 0–1 patients treated in general with full standard dose of intravenous platinum doublet schedules, it appears as if this simple and less toxic protocol is superior to standard intravenous regimens with respect to not only quality of life but as well to overall survival measured as cancer related death. For now, the technique described herein using free drug is applicable everywhere and induces relatively low costs compared to the so called personalized tumor treatment with modern drugs like tyrosine-kinase-inhibitors.
However, there is much room for optimization and further investigation into the formulations of the drugs administered in this study is warranted.36,48,51,61 First, passive or active targeting should be explored. Active targeting has the advantage that the formulation will bind locally to tumor cells and will not leak through the abnormal vascular structures. Toward this end, several efforts have been made to identify molecules that can target actively with or without an additional chemotherapy agent the tumor mutations.62 Several molecules/pathways have been investigated in lung cancer patients, such as Galectin-3 and Cyclin D1, however there are still no candidates for active targeting. This is an example where molecules and pathways, although similar in different tumors, play different roles.63 In an effort to identify new methods of sustaining drug release, co-encapsulation of magnetic Fe3O4 with chemotherapeutic agents have been investigated.27,64 However, although this method of local drug entrapment is effective in small animals, it can be lethal in larger (>dogs) as a larger magnetic field is required. Also, extravasation has been observed when the drug formulation is injected superficially. Another method of drug release which is under investigation by Patrick Le Pivert (Interventional Drug Delivery Systems and Strategies [ID2S2], Medical Cryogenics, Jupiter, FL, USA) is intratumoral drug injection while simultaneously freezing tissue locally.40 Another method for temporary local entrapment based on passive transportation is the addition of epinephrine with the cisplatin drug formulation.65 The pH release system has also been investigated with chemotherapy, the principal theory being that at a low pH (<6.5; acidic environment) the formulated complex releases the encapsulated drug.27 In the study by Callahan et al,66 for the first time a pH responsive genetically encoded drug release nanoparticle system was engineered. This pH delivery system is designed to release drug formulations in the mildly acidic environment that prevails in the extracellular matrix (ECM) of many solid tumors. Temperature-sensitive gels as an intratumoral sustain release system were also investigated (β-Lapachone). This formulation can be combined in a drug formulation complex and act as the trigger for the drug release.28
Furthermore, a new methodology for evaluating drug formulations for intratumoral delivery has to be pursued. Specifically, as previously presented in gene therapy studies, prediction models for drug diffusion within the tumor have to be established before initiation of drug administration.67 The ITASSER ([http://zhanglab.ccmb.med.umich.edu], Ann Arbor, MI, USA) software is an established evaluation method with many applications that has been used in previous studies.67,68
Moreover, several other studies were performed using the ITASSER methodology; however, they were all implemented in other organs, but they did demonstrate both efficiency and safety.30 Radioactive wires have been applied with or without the combination of a chemotherapy agent. These studies used nanoparticles for better tumor penetration and diffusion (Table 4).42,44,64,69,70 In these studies, it was observed that healthy tissue was not affected by this therapy. In a study by Watson et al70 an ultrasound methodology was investigated to efficiently deliver nanoparticles in epithelial and epithelial-mesenchymal transition tumors. Nanoparticles have also been used in association with thermal ablation as a method for enhancing drug delivery/diffusion within the tumor and to enhance local cytotoxicity.29 Intratumoral gene therapy was also investigated with or without the addition of a chemotherapy agent and or radiotherapy.44,67,71–73 In a gene therapy study by Hanna et al72 the methodology of intratumoral injection was presented making this study an example for others to follow. In these studies, it was observed that larger particles have a higher probability of being absorbed by macrophages. New photo-absorbent agents have also been tested as intratumoral therapy. Indocyanine green was conjugated with phospholipid-polyethylene glycol-monoclonal antibody (PL)-PEG-mAb in order to create a formulation with slow clearance times. This drug formulation has numerous applications for several cancer types.74 Moreover, fibers bearing chemotherapy agents were constructed for intratumoral therapy and were evaluated for their pharmacokinetic profile and efficiency in vitro and in vivo.75,76 The fibers presented efficient tumor control and a correlation with radiolabelled coils was established.
Table 4.
Intratumoral studies using different approaches
| Author | Methodology | Subjects | Cancer cells/tissue | Response | Nanoparticles | Carriers | Reference |
|---|---|---|---|---|---|---|---|
| Horev-Drori et al | 224Ra-loaded wires plus gemcitabine/5-FU | In vitro/in vivo | Pancreas | √ | – | – | 30 |
| Xie et al | 64Cu-nanoshells | Nude rats | Head-neck | √ | √ | Nanoshells | 42 |
| Hecht et al | TNFerade (AdGVEGR.TNF.11D) | Patients | Pancreas | √ | – | – | 71 |
| Govindarajan et al | TMAF | In vitro/in vivo | Breast-ovarian | √ | – | – | 67 |
| Lin et al | Review | Review | Review | Review | Lipid nanoparticles | Review | 41 |
| Cunha-Filho et al | β-Lapachone | In vitro | Intratumoral implant | √ | – | – | 28 |
| Zheng et al | ICG-PL-PEG-mAb | In vitro/in vivo | U87-MG human glioblastoma cancer cells | √ | ICG-PL-PEG-mAb | PL-PEG | 74 |
| Luo et al | Core-loaded fibers with hydroxycam ptothecin | In vitro/in vivo | H22 hepatoma cells | √ | – | Fibers | 75 |
| Tsuda et al | Propionibacterium acnes | In vitro/in vivo | BI6 melanoma cell line | √ | – | – | 77 |
| Oh et al | SLC-Fc, CpG-ODN | In vitro/in vivo | B16F10 murine melanoma model | √ | – | – | 78 |
| Yang et al | Hul4.18-IL-2 | In vitro/in vivo | NXS2 neuroblastoma Cell line | √ | – | – | 79 |
| Peiris et al | Three nanoparticle magnetic chain with doxorubicin | In vitro/in vivo | MAT Bill tumor-bearing animals | √ | Nano chain magnetic particles | – | 64 |
| Hanna et al | BC-819 | In vitro/in vivo | Pancreas | √ | – | – | 72 |
| Liu et al | mPEG-PCL-Docetaxel | In vitro/in vivo | H22 hepatoma cells | √ | mPEG-PCL | Poly (caprolactone) | 69 |
| Luo et al | PELA fibers plus hydroxycam ptothecin | In vitro/in vivo | H22 hepatoma cells | √ | PELA | Poly(D,L-lactide) | 76 |
| Geletneky et al | Parvovirus H-1 | In vivo | Glioblastoma multiforme | √ | – | – | 73 |
| Zhao et al | NLP-PEG, CLP-PEG plus DOX | In vitro/in vivo | H22 hepatoma cells | √ | DOX-NLPs, DOX-CLPs, DOX-NLP-PEG, DOX-CLP-PEG | Cationic liposomes, nanolipid particles | 44 |
| Ahmed et al | Nanoparticles and thermal ablation | Review | Review | Review | Review | Review | 29 |
| Betting et al | CpG plus rituximab/cyclophosphamide | In vitro/in vivo | B-cell lymphoma | √ | – | – | 81 |
| Son et al | Dendritic cells plus cyclophosphamide/irradiation | In vitro/in vivo | CT-26 colon carcinoma cell line | √ | – | – | 83 |
| Galili | Anti-gal human antibody | Review | Review | Review | Review | Review | 84 |
| Hamalukic et al | HMG-CoA reductase inhibitor lovastatin | In vitro/in vivo | HT29 human colon carcinoma cells | √ | – | – | 85 |
| Raut et al | Sorafenib | Patients | Refractory sarcomas | √ | – | – | 86 |
| Werner et al | Cisplatin/epinephrine | Patients | Head neck | √ | – | – | 65 |
Abbreviations: FU, fluorouracil; PEG, polyethylene glycol;U-87-MG, human glioblastoma-astrocytoma, epithelial-like cell line; DOX, doxorubicin; B 16, melanoma cell line; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; PL, polylactic; H22, hepatoma cells; CT-26, colon carcinoma cell line; ODN, oligodeoxynucleotide; HT29, human colon carcinoma cell lines; B16F10, murine metastatic melanoma in the tails of C57BL/6 mice; TNFerade, a replication-deficient adenoviral vector that expresses tumor necrosis factor-α (TUMOR NECROSIS FACTOR-α); BC-819, a plasmid comprised of the H 19 gene regulatory sequences; mPEG-PCL, poly(caprolactone); PELA, poly(D,L-lactide); SLC-Fc, secondary lymphoid tissue chemokine-Fc; CLP, cationic liposomes; NLP, neutral liposomes; ICG-PL-PEG-mAb, Indocyanine green-polylactic-polyethylene glycol-integrin α(v)β(3) monoclonal antibody; AdGVEGRTNF. 11D, a replication-deficient adenoviral vector that expresses tumor necrosis factor-a (TNF-α); Hu14.18-IL-2, an immunocytokine consisting of human IL-2 linked to hu14.18 mAb, which recognizes the GD2 disialoganglioside; NXS2, neuroblastoma cell line; MAT B, animals (inoculated with Mat B-lll-uPAR cells).
Immunotherapy as intratumoral therapy has also been investigated: (a) Propionibacterium acnes induces immunestimulation by increasing interleukin-12, tumor necrosis factor-α, and interferon-γ,77 (b) secondary lymphoid chemokine and unmethylated cytosine-phosphorothioate-guanine-oligodeoxynucleotide were used to mobilize lymphocytes and dendritic cells and increased the infiltration of CD4+ T-cells and CD11c+ cells in the tumor mass with observed reduction in tumor mass,78 (c) hu14.18-interleukin-2 administration resulted in increased natural killer (NK) group 2, member D receptors on intratumoral NKG2A/C/E+NKp46+ NK cells,79 (d) OK-432 efficiently suppressed metastatic squamous cell carcinoma lesion by inducing interferon-γ and tumor necrosis factor-α,80 (e) pre-treatment with cyclophosphamide and oligodeoxynucleotides plus rituximab enhanced immune activation against tumor cells and reduced tumor evolution,81 (f) dendritic cells and dendritic cells plus cyclophosphamide or paclitaxel at low doses enhanced immune system activation,82,83 (g) anti-gal antibody injection.84 Furthermore, several biomarkers were used specifically in intratumoral therapies as independent predictive factors, such as T cells and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, an inhibitor of lovastatin, as a formulation that blocks local metastasis after irradiation.85
Recently, sorafenib, a multi-targeted tyrosine kinase inhibitor, was used in an effort to modify interstitial fluid pressure (IFP) and vascular density86 It was observed that sorafenib and imatinib both decreased IFP, increased vascular endothelial growth factor, human placental growth factor, stromal cell-derived factor α, and decreased soluble vascular endothelial growth factor receptor-2, and consequently, disease control was observed.
Finally, lymphatic vascular circulation biology plays a key role in micrometastasis formation. Tumor cells are mobilized from the tumor site to the lymphatics before returning to the systemic vascular circulation.87–90 Lymphatics have thin walls and low pressure and in addition, they collect fluid of various substances from the interstitium and return it to the vascular circulation.53,90 The lymphatics also act as a filter for tumor cells and although they redeposit cancer cells they additionally destroy cancer cells using their immunomodulatory activity87 Sentinel lymph node mapping has been well described with tracer substances. Specifically, it was observed that when a tracer was injected through the bronchial wall the tracer was transported to the regional lymph nodes by local lymphatic drainage within 20–60 minutes depending on the site of injections and available lymph node vasculature.5,56,91,92 The same principle is in effect with regard to cytotoxic drug tissue injection.
At this point, the authors would like to state that the major limitation of this study is the small number of patients; however, statistically significant overall survival was observed P = 0.048 (Table 1). Thus, our data provide us with the necessary support to evaluate this modality in a larger prospective study.
Based on our data and published literature we are confident that intratumoral chemotherapy should be considered for the following uses:16
As a debulking tool in central NSCLC with a high efficacy rate of >75% after four weekly sessions of ITC, demonstrated efficacy in 378 published patients including this trial over two decades in different countries;32,42,44,54,55–60
As an adjunct to standard therapies even now in healing trials in PS2-patients with central obstructive palliative NSCLC;
Five studies, including this trial using ITC as an adjunct to different standard therapies, are observational studies using different modalities (to the best knowledge Phase IIb), but they have shown in 68 patients with NSCLC IIIa–IV unexpected median survival with an improvement of 21%–78% compared to UICC 7 data (4–6 months in total);42,57,59,60
Direct treatment with ITC (cisplatin 0.5%–1%) of central tumor mass and involved lymph nodes by EBUS-TBND is possible without severe adverse effects;
Direct treatment with ITC (para-toluenesulfonamide) of peripheral nodules in combination with standard carboplatin doublet chemotherapy shows very promising results;60 and
ITC as an adjuvant local procedure in combination with standard, timely separated intravenous protocols should be tested against adjuvant radiotherapy especially in regards to (reduced) toxicity and survival.
Figure 2.
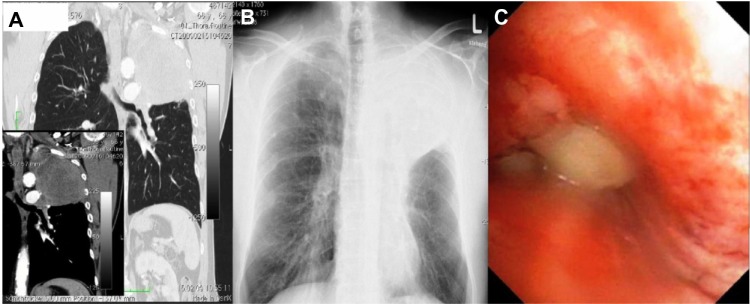
Patient 2 upon diagnosis.
Notes: (A) Vertical computed tomography image upon diagnosis with ans without contrast. (B) X-ray upon diagnosis. (C) bronchoscopy image upon diagnosis.
Figure 3.
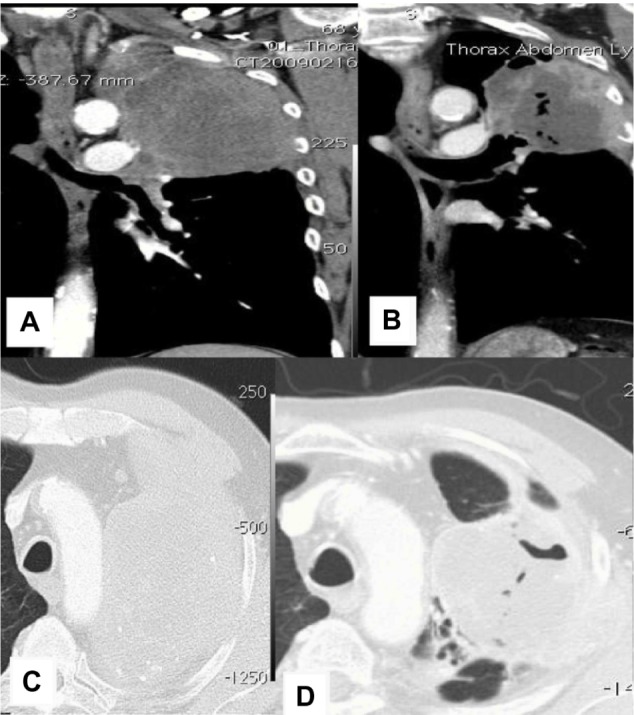
CT images from patient 2.
Notes: (A and C) CT upon diagnosis. (B and D) CT after 10 weeks.
Abbreviation: CT, computed tomography.
Figure 4.
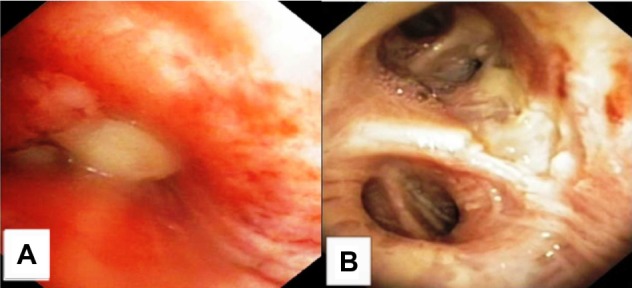
Patient 2 bronchoscopy findings upon diagnosis and after 10 weeks of therapy including four ITC-sessions and three reduced systemic carboplatin doublet applications.
Notes: (A) Bronchoscopy image upon diagnosis. (B) Bronchoscopy image after local intratumoral treatment.
Abbreviation: ITC, intratumoral chemotherapy.
Table 3.
Effects and safety features
| Patient | Adverse effect | Positive effect | Comment |
|---|---|---|---|
| 1 | Enforced bleeding after 1 ITC (infiltration of the LLL-vein) | Stopped moderate chronical bleeding after second ITC, no recurrence of bleeding. | PR– (main symptom was bleeding). |
| 2 | Vomiting, nausea, and hematotoxicity. (1800 Leuc./uL) after first ITC with 100 mg cisplatin. | Could swallow again after second ITC stopping muscle waste, walks alone. | PR+; possible infiltration of the esophagus in reference to thorax-CT. Hematotoxicity as a proof of systemic effect. |
| 3 | None | Relief of dyspnoea and mucous retention, stopped chronical bleeding after first ITC. | PR+ |
| 4 | None | Relief of retention, reduced pCO2. | PR−; heavy smoker until death. |
| 5 | Acute cytotoxicity in the complete ROL as COP (including S3) radiological without clinical relevance and spontaneous regression without any lasting damage; needle was steered from inside at the inner 2/3 margin of the lobar atelectasis which occluded orifice S1+2 in ROL. | None (slightly less back pain). | NC |
| 6 | Fever for 3 days after ITC. | Local CR, no longer bleeding or retention. | Local CR; acute short mediastinitis? Breast cancer stage IV with tracheal metastasis (out of protocol). |
Abbreviations: CT, computed tomography; ITC, intratumoral chemotherapy; CR, complete response; COP, cryptogenic organising pneumonia; Leuc, leucocytes; ROL, right upper lobe (rul); PR, partial response; pCO2, partial carbondioxide measurement; LLL, left lower lobe; S, subsegment; NC, no change.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hinoda Y. [Molecular targeted cancer therapy and genetic tests – chairman’s introductory remarks.] Rinsho Byori. 2012;60(10):967–968. Japanese. [PubMed] [Google Scholar]
- 2.Domvri K, Darwiche K, Zarogoulidis P, Zarogoulidis K. Following the crumbs: from tissue samples, to pharmacogenomics, to NSCLC therapy. Transl Lung Cancer Res. 2012 doi: 10.3978/j.issn.2218-6751.2012.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saijo N. The role of pharmacoethnicity in the development of cytotoxic and molecular targeted drugs in oncology. Yonsei Med J. 2013;54(1):1–14. doi: 10.3349/ymj.2013.54.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savonarola A, Palmirotta R, Guadagni F, Silvestris F. Pharmacogenetics and pharmacogenomics: role of mutational analysis in anti-cancer targeted therapy. Pharmacogenomics J. 2012;12(4):277–286. doi: 10.1038/tpj.2012.28. [DOI] [PubMed] [Google Scholar]
- 5.Mamot C, Drummond DC, Noble CO, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 6.Giovannetti E, Toffalorio F, De Pas T, Peters GJ. Pharmacogenetics of conventional chemotherapy in non-small-cell lung cancer: a changing landscape? Pharmacogenomics. 2012;13(9):1073–1086. doi: 10.2217/pgs.12.91. [DOI] [PubMed] [Google Scholar]
- 7.Yin JY, Huang Q, Zhao YC, Zhou HH, Liu ZQ. Meta-analysis on pharmacogenetics of platinum-based chemotherapy in non small cell lung cancer (NSCLC) patients. PLoS One. 2012;7(6):e38150. doi: 10.1371/journal.pone.0038150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayo C, Bertran-Alamillo J, Molina-Vila MA, Gimenez-Capitan A, Costa C, Rosell R. Pharmacogenetics of EGFR in lung cancer: perspectives and clinical applications. Pharmacogenomics. 2012;13(7):789–802. doi: 10.2217/pgs.12.54. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Peng L, Li J, et al. A trial-based cost-effectiveness analysis of erlotinib alone versus platinum-based doublet chemotherapy as first-line therapy for eastern asian nonsquamous non-small-cell lung cancer. PLoS One. 2013;8(3):e55917. doi: 10.1371/journal.pone.0055917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ihbe-Heffinger A, Paessens B, Berger K, et al. The impact of chemotherapy-induced side effects on medical care usage and cost in German hospital care – an observational analysis on non-small-cell lung cancer patients. Support Care Cancer. 2013;21(6):1665–1675. doi: 10.1007/s00520-012-1711-5. [DOI] [PubMed] [Google Scholar]
- 11.Mylonaki E, Manika K, Zarogoulidis P, et al. In vivo synergistic cytogenetic effects of aminophylline on lymphocyte cultures from patients with lung cancer undergoing chemotherapy. Mutat Res. 2012;740(1–2):1–5. doi: 10.1016/j.mrfmmm.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Boutsikou E, Kontakiotis T, Zarogoulidis P, et al. Docetaxel-carboplatin in combination with erlotinib and/or bevacizumab in patients with non-small cell lung cancer. Onco Targets Ther. 2013;6:125–134. doi: 10.2147/OTT.S42245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarogoulidis K, Eleftheriadou E, Kontakiotis T, et al. Long acting somatostatin analogues in combination to antineoplastic agents in the treatment of small cell lung cancer patients. Lung Cancer. 2012;76(1):84–88. doi: 10.1016/j.lungcan.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Zarogoulidis K, Ziogas E, Papagiannis A, et al. Interferon alpha-2a and combined chemotherapy as first line treatment in SCLC patients: a randomized trial. Lung Cancer. 1996;15(2):197–205. doi: 10.1016/0169-5002(95)00583-8. [DOI] [PubMed] [Google Scholar]
- 15.Hohenforst-Schmidt W. Intratumoral Chemotherapy (ITC) – a forgotten option at least in functionally or oncologically driven palliation!; Paper presented at: 16th WCBE 20102010. [Google Scholar]
- 16.Celikoglu F, Celikoglu SI, Goldberg EP. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer. 2008;61(1):1–12. doi: 10.1016/j.lungcan.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Kar UK, Srivastava MK, Andersson A, et al. Novel CCL21-vault nanocapsule intratumoral delivery inhibits lung cancer growth. PLoS One. 2011;6(5):e18758. doi: 10.1371/journal.pone.0018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macha HN, Freitag L. The role of brachytherapy in the treatment and control of central bronchial carcinoma. Monaldi Arch Chest Dis. 1996;51(4):325–328. [PubMed] [Google Scholar]
- 19.Freitag L, Ernst A, Thomas M, Prenzel R, Wahlers B, Macha HN. Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax. 2004;59(9):790–793. doi: 10.1136/thx.2003.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porpodis K, Karanikas M, Zarogoulidis P, et al. A case of typical pulmonary carcinoid tumor treated with bronchoscopic therapy followed by lobectomy. J Multidiscip Healthc. 2012;5:47–51. doi: 10.2147/JMDH.S29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freitag L, Macha H-N, Loddenkemper R. Interventional bronchoscopic procedures. Eur Resp mon. 2001;17:272–304. [Google Scholar]
- 22.Zarogoulidis P, Chatzaki E, Porpodis K, et al. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. Int J Nanomedicine. 2012;7:1551–1572. doi: 10.2147/IJN.S29997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarogoulidis P, Eleftheriadou E, Sapardanis I, et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Invest New Drugs. 2012;30(4):1628–1640. doi: 10.1007/s10637-011-9714-5. [DOI] [PubMed] [Google Scholar]
- 24.Darwiche K, Zarogoulidis P, Karamanos NK, et al. Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: a future dilemma for micro-oncology. Future Oncol. 2013;9(4):505–525. doi: 10.2217/fon.12.205. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara T, Tanaka N, Kanazawa S, et al. Multicenter phase I study of repeated intratumoral delivery of adenoviral p53 in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2006;24(11):1689–1699. doi: 10.1200/JCO.2005.03.4116. [DOI] [PubMed] [Google Scholar]
- 26.Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther. 2012;19(9):593–600. doi: 10.1038/cgt.2012.36. [DOI] [PubMed] [Google Scholar]
- 27.Jia Y, Yuan M, Yuan H, et al. Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biodegradable PLGA nanocarriers for intratumoral drug delivery. Int J Nanomedicine. 2012;7:1697–1708. doi: 10.2147/IJN.S28629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunha-Filho MS, Alvarez-Lorenzo C, Martinez-Pacheco R, Landin M. Temperature-sensitive gels for intratumoral delivery of beta- lapachone: effect of cyclodextrins and ethanol. ScientificWorldJournal. 2012;2012:126723. doi: 10.1100/2012/126723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed M, Moussa M, Goldberg SN. Synergy in cancer treatment between liposomal chemotherapeutics and thermal ablation. Chem Phys Lipids. 2012;165(4):424–437. doi: 10.1016/j.chemphyslip.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horev-Drori G, Cooks T, Bittan H, et al. Local control of experimental malignant pancreatic tumors by treatment with a combination of chemotherapy and intratumoral 224 radium-loaded wires releasing alpha-emitting atoms. Transl Res. 2012;159(1):32–41. doi: 10.1016/j.trsl.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg RJ. Resection of non-small cell lung cancer: how much and by what route. Chest. 1997;112(Suppl 4):203S–205S. doi: 10.1378/chest.112.4_supplement.203s. [DOI] [PubMed] [Google Scholar]
- 32.McGinn CJ, Shewach DS, Lawrence TS. Radiosensitizing nucleosides. J Natl Cancer Inst. 1996;88(17):1193–1203. doi: 10.1093/jnci/88.17.1193. [DOI] [PubMed] [Google Scholar]
- 33.Pauwels B, Korst AE, Lardon F, Vermorken JB. Combined modality therapy of gemcitabine and radiation. Oncologist. 2005;10(1):34–51. doi: 10.1634/theoncologist.10-1-34. [DOI] [PubMed] [Google Scholar]
- 34.Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol. 2007;25(26):4043–4050. doi: 10.1200/JCO.2007.11.5287. [DOI] [PubMed] [Google Scholar]
- 35.Arazi L, Cooks T, Schmidt M, Keisari Y, Kelson I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys Med Biol. 2007;52(16):5025–5042. doi: 10.1088/0031-9155/52/16/021. [DOI] [PubMed] [Google Scholar]
- 36.Celikoglu F, Celikoglu SI. Intratumoural chemotherapy with 5-fluorouracil for palliation of bronchial cancer in patients with severe airway obstruction. J Pharm Pharmacol. 2003;55(10):1441–1448. doi: 10.1211/0022357021936. [DOI] [PubMed] [Google Scholar]
- 37.Hohenforst-Schmidt W. Intratumoral chemotherapy (ITC) as adjunct therapy in NSCLC iiia–iv prolongs life; Poster presented at: International Conference and Exhibition on Cancer Science and Therapy; August 15–17, 2011; Las Vegas, NV. [Google Scholar]
- 38.Bae YH. Interview with Dr You Han Bae: ligand-mediated versus ‘passive’ targeting approaches in nanoparticle oncology research. Ther Deliv. 2012;3(8):933–936. doi: 10.4155/tde.12.62. [DOI] [PubMed] [Google Scholar]
- 39.Yang W, Ahmed M, Elian M, et al. Do liposomal apoptotic enhancers increase tumor coagulation and end-point survival in percutaneous radiofrequency ablation of tumors in a rat tumor model? Radiology. 2010;257(3):685–696. doi: 10.1148/radiol.10100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Pivert PJ, Morrison DR, Haddad RS, et al. Percutaneous tumor ablation: microencapsulated echo-guided interstitial chemotherapy combined with cryosurgery increases necrosis in prostate cancer. Technol Cancer Res Treat. 2009;8(3):207–216. doi: 10.1177/153303460900800305. [DOI] [PubMed] [Google Scholar]
- 41.Lin X, Gao R, Zhang Y, et al. Lipid nanoparticles for chemotherapeutic applications: strategies to improve anticancer efficacy. Expert Opin Drug Deliv. 2012;9(7):767–781. doi: 10.1517/17425247.2012.685933. [DOI] [PubMed] [Google Scholar]
- 42.Xie H, Goins B, Bao A, Wang ZJ, Phillips WT. Effect of intratumoral administration on biodistribution of 64Cu-labeled nanoshells. Int J Nanomedicine. 2012;7:2227–2238. doi: 10.2147/IJN.S30699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sim H, Bibee K, Wickline S, Sept D. Pharmacokinetic modeling of tumor bioluminescence implicates efflux, and not influx, as the bigger hurdle in cancer drug therapy. Cancer Res. 2011;71(3):686–692. doi: 10.1158/0008-5472.CAN-10-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao W, Zhuang S, Qi XR. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int J Nanomedicine. 2011;6:3087–3098. doi: 10.2147/IJN.S25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, Qin H, Nakamura H, Tsukigawa K, Shin T, Maeda H. Carbon monoxide, generated by heme oxygenase-1, mediates the enhanced permeability and retention effect in solid tumors. Cancer Sci. 2012;103(3):535–541. doi: 10.1111/j.1349-7006.2011.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celikoglu F, Celikoglu SI, York AM, Goldberg EP. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer. 2006;51(2):225–236. doi: 10.1016/j.lungcan.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Celikoglu F, Celikoglu SI, Goldberg EP. Intratumoural chemotherapy of lung cancer for diagnosis and treatment of draining lymph node metastasis. J Pharm Pharmacol. 2010;62(3):287–295. doi: 10.1211/jpp.62.03.0001. [DOI] [PubMed] [Google Scholar]
- 48.Celikoglu SI, Karayel T, Demirci S, Celikoglu F, Cagatay T. Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction. Postgrad Med J. 1997;73(857):159–162. doi: 10.1136/pgmj.73.857.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horng G, Askari S, Choi Y, Grundfest W. Sustained Localized Drug Delivery In Mammalian Lung By Bronchoscopy; Paper presented at: American Thoracic Society 2012 International Conference; May 18–23, 2012; San Francisco, CA. [Google Scholar]
- 50.Yeh CT, Chen HC, Sung CM, et al. Retrospective comparison between a regular and a split-dose protocol of 5-fluorouracil, cisplatin, and mitoxantrone for the treatment of far advanced hepatocellular carcinoma. BMC Cancer. 2011;11:117. doi: 10.1186/1471-2407-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldberg EP, Hadba AR, Almond BA, Marotta JS. Intratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug delivery. J Pharm Pharmacol. 2002;54(2):159–180. doi: 10.1211/0022357021778268. [DOI] [PubMed] [Google Scholar]
- 52.Vogl TJ, Wissniowski TT, Naguib NN, et al. Activation of tumor-specific T lymphocytes after laser-induced thermotherapy in patients with colorectal liver metastases. Cancer Immunol Immunother. 2009;58(10):1557–1563. doi: 10.1007/s00262-009-0663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monga SP, Wadleigh R, Sharma A, et al. Intratumoral therapy of cisplatin/epinephrine injectable gel for palliation in patients with obstructive esophageal cancer. Am J Clin Oncol. 2000;23(4):386–392. doi: 10.1097/00000421-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Wagai F, Kinoshita M, Shiraki R, Watanabe H, Kitamura S. [The direct injection of several anti-cancer drugs into the primary lung cancer lesion through a fiberoptic bronchoscope (author’s transl).] Nihon Kyobu Shikkan Gakkai Zasshi. 1982;20(2):170–175. Japanese. [PubMed] [Google Scholar]
- 55.Liu M, Ma P, Lu Z. [Local chemotherapy by fibrobronchoscopy for advanced bronchogenic carcinoma.] Zhonghua Jie He He Hu Xi Za Zhi. 2000;23(9):550–551. Chinese. [PubMed] [Google Scholar]
- 56.Celikoglu SI, Celikoglu F, Goldberg EP. Endobronchial intratumoral chemotherapy (EITC) followed by surgery in early non-small cell lung cancer with polypoid growth causing erroneous impression of advanced disease. Lung Cancer. 2006;54(3):339–346. doi: 10.1016/j.lungcan.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Nader AD. Intratumoral chemotherapy as an adjuvant to endobronchial brachytherapy. Chest. 2007;132(Suppl):459. [Google Scholar]
- 58.Jabbardarjani H, Safara H, Kharabian S, Reza M. Endobronchial chemotherapy in malignant airway lesions of the lung: Report of 3 years experience. J Bronchology. 2007;14:242–245. [Google Scholar]
- 59.Ramos HC, Ruivo I, deBrito U. Use of intratumoral cisplatinium in lung cancer. Experimental Pathology and Healt Science. 2008;2(2):45–46. [Google Scholar]
- 60.He J, Ying W, Yang H, et al. Gemcitabine plus cisplatin chemotherapy with concurrent para-toluenesulfonamide local injection therapy for peripherally advanced nonsmall cell lung cancer larger than 3 cm in the greatest dimension. Anticancer Drugs. 2009;20(9):838–844. doi: 10.1097/CAD.0b013e32832fe48f. [DOI] [PubMed] [Google Scholar]
- 61.Brincker H. Direct intratumoral chemotherapy. Crit Rev Oncol Hematol. 1993;15(2):91–98. doi: 10.1016/1040-8428(93)90049-a. [DOI] [PubMed] [Google Scholar]
- 62.Li C, Wang Y, Zhang X, Deng L, Zhang Y, Chen Z. Tumor-targeted liposomal drug delivery mediated by a diseleno bond-stabilized cyclic peptide. Int J Nanomedicine. 2013;8:1051–1062. doi: 10.2147/IJN.S40498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kosacka M, Piesiak P, Kowal A, Golecki M, Jankowska R. Galectin-3 and cyclin D1 expression in non-small cell lung cancer. J Exp Clin Cancer Res. 2011;30:101. doi: 10.1186/1756-9966-30-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peiris PM, Bauer L, Toy R, et al. Enhanced delivery of chemotherapy to tumors using a multicomponent nanochain with radio-frequency-tunable drug release. ACS Nano. 2012;6(5):4157–4168. doi: 10.1021/nn300652p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werner JA, Kehrl W, Pluzanska A, et al. A phase III placebo-controlled study in advanced head and neck cancer using intratumoural cisplatin/epinephrine gel. Br J Cancer. 2002;87(9):938–944. doi: 10.1038/sj.bjc.6600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Callahan DJ, Liu W, Li X, et al. Triple stimulus-responsive polypeptide nanoparticles that enhance intratumoral spatial distribution. Nano Lett. 2012;12(4):2165–2170. doi: 10.1021/nl300630c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Govindarajan S, Sivakumar J, Garimidi P, et al. Targeting human epidermal growth factor receptor 2 by a cell-penetrating peptide-affibody bioconjugate. Biomaterials. 2012;33(8):2570–2582. doi: 10.1016/j.biomaterials.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Gopal V, Guruprasad K. Structure prediction and validation of an affibody engineered for cell-specific nucleic acid targeting. Syst Synth Biol. 2010;4(4):293–297. doi: 10.1007/s11693-011-9074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Q, Li R, Zhu Z, et al. Enhanced antitumor efficacy, biodistribution and penetration of docetaxel-loaded biodegradable nanoparticles. Int J Pharm. 2012;430(1–2):350–358. doi: 10.1016/j.ijpharm.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Watson KD, Lai CY, Qin S, et al. Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res. 2012;72(6):1485–1493. doi: 10.1158/0008-5472.CAN-11-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hecht JR, Farrell JJ, Senzer N, et al. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. Gastrointest Endosc. 2012;75(2):332–338. doi: 10.1016/j.gie.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanna N, Ohana P, Konikoff FM, et al. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012;19(6):374–381. doi: 10.1038/cgt.2012.10. [DOI] [PubMed] [Google Scholar]
- 73.Geletneky K, Huesing J, Rommelaere J, et al. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99. doi: 10.1186/1471-2407-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng X, Zhou F, Wu B, Chen WR, Xing D. Enhanced tumor treatment using biofunctional indocyanine green-containing nanostructure by intratumoral or intravenous injection. Mol Pharm. 2012;9(3):514–522. doi: 10.1021/mp200526m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo X, Xie C, Wang H, Liu C, Yan S, Li X. Antitumor activities of emulsion electrospun fibers with core loading of hydroxycamptothecin via intratumoral implantation. Int J Pharm. 2012;425(1–2):19–28. doi: 10.1016/j.ijpharm.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Luo X, Xu G, Song H, et al. Promoted antitumor activities of acid-labile electrospun fibers loaded with hydroxycamptothecin via intratumoral implantation. Eur J Pharm Biopharm. 2012;82(3):545–553. doi: 10.1016/j.ejpb.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 77.Tsuda K, Yamanaka K, Linan W, et al. Intratumoral injection of Propionibacterium acnes suppresses malignant melanoma by enhancing Th1 immune responses. PLoS One. 2011;6(12):e29020. doi: 10.1371/journal.pone.0029020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oh SM, Oh K, Lee DS. Intratumoral administration of secondary lymphoid chemokine and unmethylated cytosine-phosphorothioate-guanine oligodeoxynucleotide synergistically inhibits tumor growth in vivo. J Korean Med Sci. 2011;26(10):1270–1276. doi: 10.3346/jkms.2011.26.10.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, et al. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK cells as well as enhanced IC retention. J Immunol. 2012;189(5):2656–2664. doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akeda T, Yamanaka K, Kitagawa H, et al. Intratumoral injection of OK-432 suppresses metastatic squamous cell carcinoma lesion inducing interferon- γ and tumor necrosis factor-α. Clin Exp Dermatol. 2012;37(2):193–194. doi: 10.1111/j.1365-2230.2011.04151.x. [DOI] [PubMed] [Google Scholar]
- 81.Betting DJ, Hurvitz SA, Steward KK, et al. Combination of cyclophosphamide, rituximab, and intratumoral CpG oligodeoxynucleotide successfully eradicates established B cell lymphoma. J Immunother. 2012;35(7):534–543. doi: 10.1097/CJI.0b013e318261e679. [DOI] [PubMed] [Google Scholar]
- 82.Zhong H, Han B, Tourkova IL, et al. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13(18 Pt 1):5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Son CH, Shin DY, Kim SD, et al. Improvement of antitumor effect of intratumoral injection of immature dendritic cells into irradiated tumor by cyclophosphamide in mouse colon cancer model. J Immunother. 2012;35(8):607–614. doi: 10.1097/CJI.0b013e31826f79a6. [DOI] [PubMed] [Google Scholar]
- 84.Galili U. Conversion of tumors into autologous vaccines by intratumoral injection of alpha-Gal glycolipids that induce anti-Gal/alpha-Gal epitope interaction. Clin Dev Immunol. 2011;2011:134020. doi: 10.1155/2011/134020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamalukic M, Huelsenbeck J, Schad A, Wirtz S, Kaina B, Fritz G. Rac1-regulated endothelial radiation response stimulates extravasation and metastasis that can be blocked by HMG-CoA reductase inhibitors. PLoS One. 2011;6(10):e26413. doi: 10.1371/journal.pone.0026413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raut CP, Boucher Y, Duda DG, et al. Effects of sorafenib on intra-tumoral interstitial fluid pressure and circulating biomarkers in patients with refractory sarcomas (NCI protocol 6948) PLoS One. 2012;7(2):e26331. doi: 10.1371/journal.pone.0026331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438(7070):946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 88.Fan Y, Du W, He B, et al. The reduction of tumor interstitial fluid pressure by liposomal imatinib and its effect on combination therapy with liposomal doxorubicin. Biomaterials. 2013;34(9):2277–2288. doi: 10.1016/j.biomaterials.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Shayan R, Achen MG, Stacker SA. Lymphatic vessels in cancer metastasis: bridging the gaps. Carcinogenesis. 2006;27(9):1729–1738. doi: 10.1093/carcin/bgl031. [DOI] [PubMed] [Google Scholar]
- 90.Oliver G, Harvey N. A stepwise model of the development of lymphatic vasculature. Ann N Y Acad Sci. 2002;979:159–165. doi: 10.1111/j.1749-6632.2002.tb04876.x. discussion 188–196. [DOI] [PubMed] [Google Scholar]
- 91.Lardinois D, Brack T, Gaspert A, et al. Bronchoscopic radioisotope injection for sentinel lymph-node mapping in potentially resectable non-small-cell lung cancer. Eur J Cardiothorac Surg. 2003;23(5):824–827. doi: 10.1016/s1010-7940(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 92.Chen X, Wang X, Wang Y, et al. Improved tumor-targeting drug delivery and therapeutic efficacy by cationic liposome modified with truncated bFGF peptide. J Control Release. 2010;145(1):17–25. doi: 10.1016/j.jconrel.2010.03.007. [DOI] [PubMed] [Google Scholar]


