Abstract
BACKGROUND
Assessment of the vasculature is critical for overall success in cranial vascular neurological surgery procedures. Although several methods of monitoring cortical perfusion intraoperatively are available, not all are appropriate or convenient in a surgical environment. Recently, 2 optical methods of care have emerged that are able to obtain high spatial resolution images with easily implemented instrumentation: indocyanine green (ICG) angiography and laser speckle contrast imaging (LSCI).
OBJECTIVE
To evaluate the usefulness of ICG and LSCI in measuring vessel perfusion.
METHODS
An experimental setup was developed that simultaneously collects measurements of ICG fluorescence and LSCI in a rodent model. A 785-nm laser diode was used for both excitation of the ICG dye and the LSCI illumination. A photothrombotic clot model was used to occlude specific vessels within the field of view to enable comparison of the 2 methods for monitoring vessel perfusion.
RESULTS
The induced blood flow change demonstrated that ICG is an excellent method for visualizing the volume and type of vessel at a single point in time; however, it is not always an accurate representation of blood flow. In contrast, LSCI provides a continuous and accurate measurement of blood flow changes without the need of an external contrast agent.
CONCLUSION
These 2 methods should be used together to obtain a complete understanding of tissue perfusion.
Keywords: Cerebral blood flow, Indocyanine green, Laser speckle contrast imaging, Neurosurgery, Photothrombosis, Rat
Assessment of vasculature is critical for overall success in cranial vascular neurological surgery procedures such as extracranial-intracranial bypass, arteriovenous malformation, and cerebral aneurysm.1 Several methods currently exist for intraoperative monitoring of cortical perfusion, including digital subtraction angiography, indocyanine green angiography, laser Doppler, etc; however, these techniques can be challenging to implement intraoperatively and have several limitations in spatial and/or temporal resolution.2 In this study, the feasibility of using laser speckle contrast imaging (LSCI) to monitor cerebral vessel perfusion was assessed in rat models by comparing LSCI with one of the current clinical methods of care, indocyanine green (ICG) angiography.
Indocyanine green was chosen as the comparison modality for this study because it is a well-established method of measuring blood vessel perfusion.3 Recently, ICG has been gaining popularity in the neurosurgery field, because ICG monitoring hardware can be easily configured with neurosurgical microscopes.4 Therefore, the dye can be easily used for monitoring the direction of vessel perfusion as well as obtaining a relative measure of cerebral blood flow via the “blood flow index.” LSCI has been used for a number of years for research studies; however, its clinical applications have only recently been investigated.5,6
As scientists and clinicians alike begin to explore the uses of LSCI for measuring the relative speed of blood flow in the human cortex, it is important to understand what advantages (or disadvantages) this method can provide over those currently available. Therefore, we performed a direct comparison of ICG angiography and LSCI for visualization and quantification of vessel perfusion to better evaluate the usefulness of these 2 imaging modalities. A rodent model was used to allow controlled occlusion of cortical vessels, and the same light source was used for both ICG excitation and LSCI.
MATERIALS AND METHODS
Indocyanine Green
Indocyanine green is a tricarbocyanine dye that was originally approved by the US Food and Drug Administration in 1956 for monitoring cardiocirculatory and liverfunction.3 Since its introduction, the usefulness of the dye for vascular imaging has spread to other fields such as ophthalmology and neurosurgery. In neurosurgery, ICG has become an important method for microsurgical clipping and revascularization.4 The dye excitation wavelength varies between 780 nm and 805 nm depending on concentration and the formation of aggregates.7 The emission spectra of the dye also vary between 820 nm and 835 nm depending on specific interactions with phospholipids.7,8 The plasma half-life of ICG is 3 to 4 minutes, with almost complete clearing noticeable after 10 minutes.3 Because ICG is not metabolized and is eliminated exclusively by the liver, it has become a safe and repeatable measure of vessel perfusion. ICG angiography instrumentation consists of a near-infrared light source that is capable of exciting the dye and a high-sensitivity camera capable of detecting the fluorescence. These components are easily adaptable to modern surgical microscopes, and thus this technique is steadily growing in use. ICG angiography has also been demonstrated as a useful tool for visualizing flow in vasculature by using a relative measure of cerebral blood flow, known as the blood flow index (BFI), which is based on the time the dye takes to appear within a vessel, rise time, and the increase in signal within that time.9,10 The rise time of the signal can also be used to determine the type of vessel within the region, assuming arteries fill faster than the veins. Despite these advantages, ICG has several drawbacks because quantitative information is only obtained during the initial wash-in of the dye (5-10 seconds), multiple injections are not always feasible, and adverse reactions to the dye have been observed.11
Laser Speckle Contrast Imaging
Laser speckle contrast imaging is well established for imaging blood flow by using simple instrumentation in a laboratory setting.5,6,12,13 The basic technique involves using a laser light source to uniformly illuminate the field of interest, while a camera captures the backscattered light. The raw images are then processed based on the decorrelation of the randomly scattered photons, resulting in an image of relative blood flow. The spatial resolution of this technique depends on the camera being used, and images can be acquired at the frame rate of the camera as well as the processing software. With the use of custom-designed data acquisition software, real-time measures of blood flow are obtainable (70-100 Hz).14 Similar to ICG, it has been demonstrated that the LSCI system can be easily integrated into a surgical microscope such that image acquisition can be performed intraoperatively without hindering the surgeon.15 Because exogenous contrast agents are not required for LSCI, continuous imaging of blood flow is possible.
Instrumentation
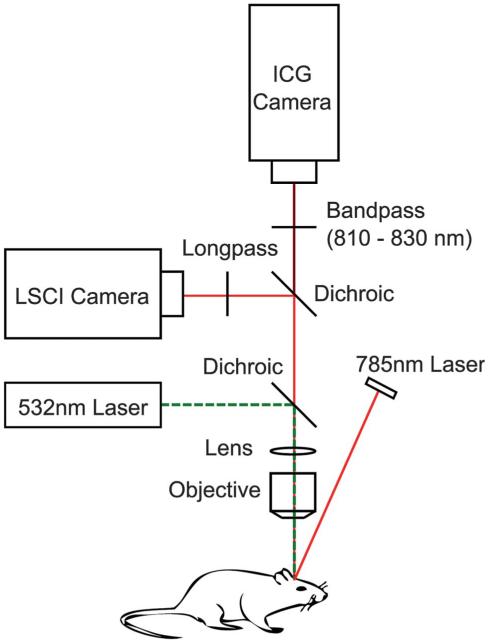
To achieve a fair comparison of the 2 techniques, a 2-camera setup was used so that ICG and LSCI images could be acquired simultaneously. A 785-nm laser diode (Thorlabs DL7140-201S) was used both as the excitation source for the ICG dye and the coherent source for LSCI. A dichroic beam splitter (Semrock ICG-A) was used to direct the backscattered excitation light toward the LSCI camera (Basler 641f) and the ICG fluorescence emission light to the ICG camera (Cascade 512B Photometrics). An additional band-pass filter was added in front of the ICG camera to eliminate any excess excitation light that might have leaked through the dichroic (Figure 1). The 2 cameras were adjusted so that they both formed an image of the same area of cortex through the objective lens.
FIGURE 1.
Multicamera experimental setup for simultaneous LSCI and ICG measurements. LSCI, laser speckle contrast imaging; ICG, indocyanine green. Scale bar = 0.5 mm.
To induce a change in blood flow, localized photothrombosis of a branching vessel within the field of view was performed by using rose-bengal and a 532-nm laser. Collimated green laser light was passed through the objective lens of the imaging system so that the resulting focused beam could be accurately positioned on the vessel of interest within the field of view. Rose-bengal injections of 0.5 mL were used in conjunction with an output laser power of 5 mW to achieve photothrombosis. A long-pass filter was added in front of the LSCI camera to prevent any green laser light from reaching the camera.
Animal Preparation
For these experiments, Sprague-Dawley rats (n = 5, 300-450 g) were anesthetized with 2% isoflurane in O2, ventilated (48 breaths per minute, 2-mL tidal volume) through a tracheotomy and temperature regulated at 37 °C. A 0.5-cm2 craniotomy was performed on the left hemisphere to ensure that adequate branching vessels could be visualized, and the femoral vein was cannulated to facilitate intravenous injections of the photothrombosis agent, rose-bengal (Sigma Aldrich) and ICG dye (Sigma Aldrich CardioGreen). Heart rate and blood oxygenation were continuously monitored throughout the experiment (Mouse-Ox system, STARR Life Sciences Corp). Bolus injections of 0.05 mL of ICG solution (1 mg/mL) were prepared immediately before imaging in a 5% bovine serum albumin-saline solution to minimize clotting.16
Image Analysis
ICG images are represented as raw fluorescence intensity images that were collected by the camera. Temporal results are normalized by using the first minute of collection as a baseline to remove any noise that might be present in the system. The wash-in of the ICG dye was analyzed for each pixel within the image from an initial start point 0.1 seconds before the onset of the injection to when the signal peaked for that injection. The BFI of the ICG images were also calculated using
| (1) |
where ΔICG indicates the change in signal intensity and trise corresponds to the time taken for the dye to reach the peak value.7 To help remove noise, the start and end points in the analysis were calculated to be 10% and 90% of the maximum signal within each injection.
LSCI raw images collected by the camera are processed by calculating the spatial speckle contrast value (K) within a specified moving window (usually 7 × 7 pixels) (Equation 2),
| (2) |
where σs is the standard deviation, and,<I> is the average intensity within the region. Speckle contrast images were then converted into a more quantitative measure of blood flow, correlation time (τc),5,17,18 by looking at the average decay time of the speckle electric field autocorrelation function, Equation 3,
| (3) |
where T is the exposure duration of the camera (5 ms), and β is an instrumentation factor that depends on several variables such as the polarization of light and the number of speckles imaged onto a single camera pixel. For the purposes of these experiments, β was assumed to be equal to 1.
Differences in the size and orientation of the 2 camera sensors made coregistration of the images collected from both techniques necessary in order to easily compare them. This was achieved during postprocessing by using vasculature landmarks chosen within the first frame of each imaging technique, and a transformation matrix was generated and applied to subsequent frames of the LSCI image so that the 2 imaging techniques would be anatomically coregistered.19
RESULTS
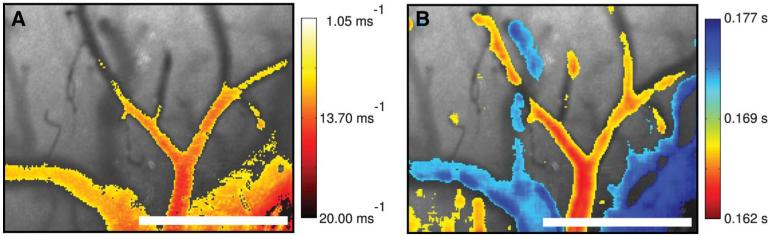
Baseline LSCI images are shown for 1 animal overlaid with the respective changes in blood flow and wash-in time calculated by using the LSCI and ICG techniques, respectively. For each baseline image, darker areas indicate regions of higher flow (vasculature), whereas lighter areas represent slower flow or static areas (surrounding tissue). Figure 2A has been thresholded to show only high-flow areas in which the flow rate was 3 times greater than the surrounding static tissue. On the right, Figure 2B, is overlaid with the wash-in time of the dye. These values were thresholded to separate the slower changes (0.172-0.177 seconds) in blue and faster changes (0.163-0.168 seconds) in red, thus separating the arteries from the veins. These baseline results demonstrate that both methods are capable of imaging the vasculature within the field of view with similar spatial resolution; however, this also begins to demonstrate how each method provides very different measurements of the vasculature. Although the LSCI and ICG images are visually similar, it is important to recognize that the 2 techniques are actually providing very different information. LSCI images are sensitive to the motion of scattering particles. In other words, darker areas represent more dynamic light-scattering events and therefore a higher flow (lighter areas indicate slower flow). Thus, as the vessel is occluded, fewer dynamic scattering events occur and the area becomes lighter. The ICG images, however, are indicative of the volume of plasma within the vessel. This means that larger vessels (which inherently carry more plasma) will result in a stronger signal (brighter) due to the increased amount of fluorescent dye. Once the vessel has been occluded, the dye is no longer able to reach the area, and thus no signal is detected (darker).
FIGURE 2.
Relative blood flow overlay where red indicates faster flow (yellow slower) (A), and B represents the ICG rise time overlay where red indicates a shorter rise time (faster wash-in) and blue signifies longer rise time (slower wash-in). ICG, indocyanine green.
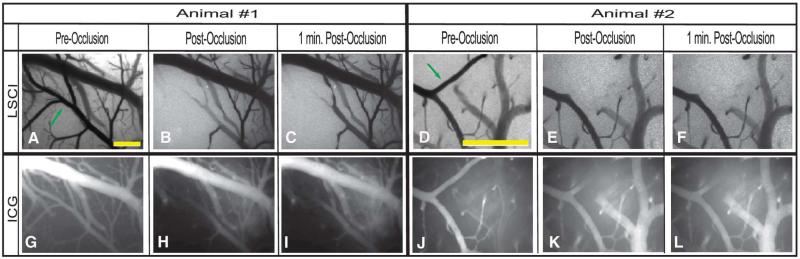
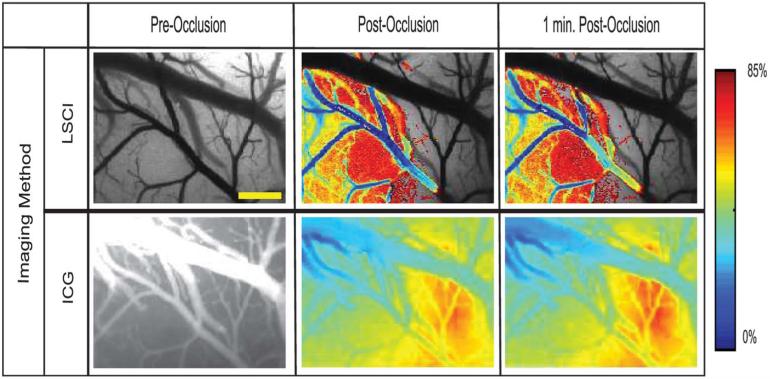
In the preocclusion images (Figure 3A, D, G, J), the complete vasculature is clearly visualized by both techniques. Once the clot is formed, however, the branching vessel (indicated by the arrow) disappears from the field of view. In the LSCI images, the apparent loss in vasculature is a result of a reduction in the flow rate within that branched vessel. In other words, the vessel still exists within the field of view; however, the flow rate has become so low that the vessel blends in with the surrounding static tissue. In the ICG images, the loss of contrast in the branching vessel is a result of the dye being blocked from getting to that region. These results imply that the occlusion was complete and that both methods are capable of assessing the perfusion of a vessel. Relative LSCI images were also generated by calculating the change in the correlation time (or blood flow) with respect to the baseline measure. Similarly, relative ICG images were generated to show the change in signal intensity between the pre- and postocclusion injections. These relative images are shown in Figure 4 where baseline LSCI images were used as a background and each relative image has been thresholded to show any areas with a flow decrease of 15% or more (85% of baseline).
FIGURE 3.
Images captured before and after the occlusion for both methods are shown for 2 different animals in the study. In each case, baseline LSCI images were acquired for 1 minute and ICG images were acquired during a single-bolus intravenous injection of the ICG dye. Once a stable clot had been formed within the selected branching vessel (arrow), a second bolus injection of the ICG dye was again administered intravenously to visualize the postocclusion vasculature. Scale bars = 0.5 mm. LSCI, laser speckle contrast imaging; ICG, indocyanine green.
FIGURE 4.
Images collected from animal 1 by the use of both imaging techniques at multiple time points before and after vessel occlusion that have been overlaid with a color map of changes in correlation time (relative blood flow) and ICG intensity with respect to the baseline image where blue indicates a decrease in overall intensity (red indicates increase). Images have been thresholded to show any areas with a flow decrease of 15% or more (85% of baseline). Scale bar = 0.5 mm. LSCI, laser speckle contrast imaging; ICG, indocyanine green.
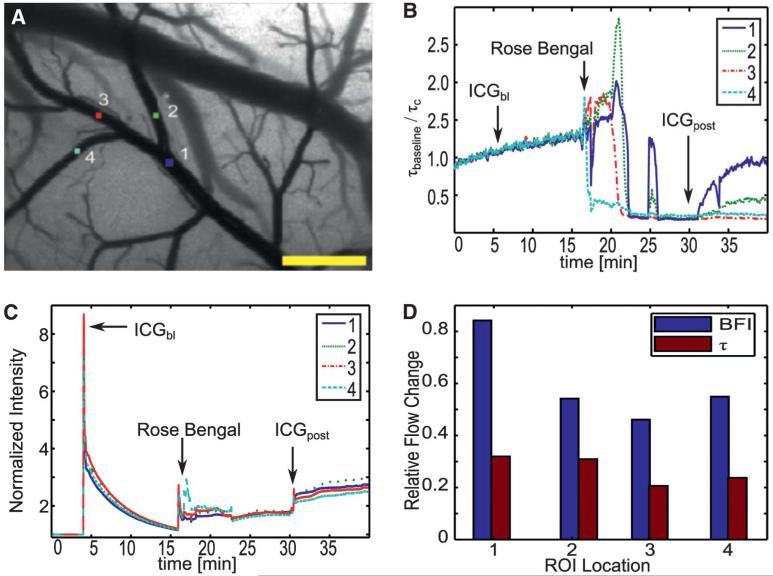
From the LSCI images of Figure 4, the large decreases in blood flow are clearly visualized and restricted to the area in which the occlusion was created, implying that this was a very localized occlusion resulting in a decrease of flow within that vessel. The ICG images, however, indicate a global change throughout the field of view, which is a result of minor inconsistencies between injections and changes in ICG concentration within the vessels after the dye becomes trapped at the occlusion site. The relative ICG images also indicate a significant reduction in signal obtained from the vein; however, this change is not observed in the LSCI images. This is most likely because a significant amount of the ICG dye has been blocked by the clot, thus reducing the amount of dye that reaches the vein and causing a lower signal. Because the LSCI images are not dependent on an exogenous dye, the vein is still visualized in the same way as before. These changes can be further examined by picking several regions of interest within the field of view and plotting the temporal fluctuations as shown in Figure 5.
FIGURE 5.
Measured temporal changes for animal 1 in 4 various regions of interest (ROIs) (A), shown as relative blood flow change for LSCI (B) and relative intensity changes for ICG images (C, D). Scale bar = 0.5 mm. LSCI, laser speckle contrast imaging; ICG, indocyanine green; BFI, blood flow index.
Figure 5 depicts the temporal changes occurring within the vasculature through several manually selected regions of interest. The temporal fluctuations in relative blood flow (Figure 5B) are easily visualized using the LSCI method by calculating the relative correlation time discussed in Materials and Methods. Rapid changes in flow were shown in the second region of interest (Figure 5B). Initially, the relative correlation time remained constant until the rose-bengal injection was administered (t = 16 min). As the photothrombosis was formed within the blocked vessel, LSCI was able to detect an increase in the flow as the vessel narrowed but blood volume remained constant (16-23 minutes). Once the clot was formed, flow within the vessels was stopped completely, and the relative correlation time decreased dramatically. Occasionally, pressure buildup caused portions of the clot to break off within the vessel, resulting in a sharp increase to the flow (t = 25 min). However, because of the excess rose-bengal in the system, the clot was able to re-form quickly and flow was again stopped within that region. A movie demonstrating the continuous acquisition of these changes observed from the LSCI measurements has been provided in the supplemental material (see Video 1, Supplemental Digital Content 1, http://links.lww.com/NEU/A478, which demonstrates the feasibility of LSCI for measuring vessel perfusion).
The ICG time course, however, is more difficult to interpret, because the only truly useful information is obtained at the points of the injection(t = 5 min and t = 30 min), as shown in Figure 5C. Four points of interest are shown, which correspond with the baseline ICG injection at 5 minutes, rose-bengal injection at 16 minutes, residual dye trapped within the formed clot at 17 minutes, and the postocclusion injection at 30 minutes (Figure 5C). Although the baseline and postocclusion injections were intended ICG injections, some residual dye remained in the femoral line during the rose-bengal injection, resulting in the fluorescence at that time (t = 16 min). A gross comparison of ICG intensities made between the baseline injection and the postocclusion injection might indicate that there was a significant change in blood flow due to the significant decrease in overall signal given the same amount of dye being injected; however, no absolute conclusion can be drawn from these 2 peaks.
In comparison with the baseline injection (t = 5 min), the decrease in ICG intensity at the rose-bengal injection point (t = 16 min) might lead the observer to believe that a change has occurred in the physiology, as shown in Figure 5C. However, the LSCI data taken at the same time point indicate that flow is still present in the vessel, as shown in Figure 5B. Therefore, the intensity difference was a result of a lowered dye concentration rather than a change in physiology. This demonstrates that changes in the administration of the dye might lead to erroneous information to the surgeon when only the change in ICG signal is investigated.
As previously mentioned, the ICG data are not capable of visualizing the changes in blood flow over a continuous time course. Therefore, it is also important to compare the LSCI correlation time with the discrete relative changes in ICG as indicated by the BFI. The bar chart in Figure 5D demonstrates the changes in both correlation time, t, and BFI relative to their values before the occlusion. It is observed visually in Figure 3B, the occluded vessel was completely affected by the photothrombosis. The relative BFI values, however, suggest that the regions were minimally affected (20%–50% reduction from baseline). In contrast, LSCI depicted a 70% to 80% decrease in flow across all regions. It is suggested that, despite the lack of flow in the region, traces of the contrast agent were able to leak into the area, resulting in misleading information.
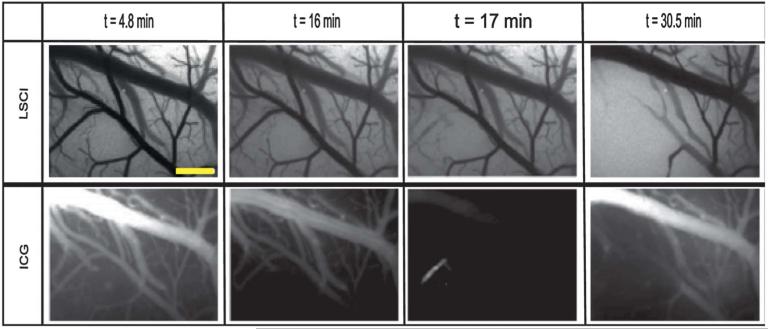
The actual ICG image acquired at the point of the rose-bengal injection at 16 minutes, however, is in agreement with the LSCI image in that flow is still being observed in the vessel, as shown in Figure 6. During the formation of the clot, however, the residual ICG becomes trapped (t = 17 min), resulting in a visualization of the occluded portion of the vessel despite a lack of flow to that area, thus demonstrating the idea that ICG is a method of visualizing blood volume rather than blood flow. This also suggests misleading information might be provided to the surgeon if any residual dye were to become trapped within a vessel during the procedure.
FIGURE 6.
LSCI and ICG images acquired at each observable ICG intensity peak for animal 1. Scale bar = 0.5 mm. LSCI, laser speckle contrast imaging; ICG, indocyanine green.
DISCUSSION
Both methods are capable of being used in a surgical environment to image vasculature, and they have both proven adequate for assessing cortical tissue perfusion. However, each technique has been shown to provide slightly different physiological information about the vasculature; therefore, careful consideration must be used when deciding which method is appropriate for a given procedure.
ICG was able to provide accurate and reliable identification of feeder and drainer vessels within the field of view by observing the wash-in time of the dye. This information has been of great use to the clinician during vessel bypass and aneurysm procedures20-22 and is currently not available by using only the LSCI technique. Recently, it has been shown that the direction of flow in certain vessels can be determined without exogenous contrast agents (and thus the ability to identify feeder vs drainer vessels) by high-speed reflectance imaging of the movement of individual red blood cells within a vessel. The use of this technology in conjunction with LSCI might be able to overcome some of the limitations of ICG angiography while still being able to obtain all of the same information about vasculature perfusion.
One of the important distinctions of LSCI vs ICG is that LSCI is a true measure of the flow of a particular area, whereas ICG is innately measuring the blood volume. This is demonstrated in the baseline images in Figure 3. For animal 1, the ICG image shows a much stronger signal in the larger vein at the top of the image, whereas less signal is observed in the artery below because a higher volume of dye is found within the larger vessel. When the vessels are more uniform in size throughout the image, as in animal 2, a more uniform intensity is observed in the ICG image. In contrast, LSCI images are able to distinguish different flow rates independently of size. In animal 1, LSCI images show a higher flow exists in the artery (darker) while a slower flow exists in the draining vein (lighter). This distinction again demonstrates that, because the LSCI images are a measure of flow, they are capable of obtaining relative flow measures within a field of view despite variations in vessel size. Because ICG images are only a measure of the volume of a vessel, the relative intensities within the field of view are strongly influenced by the size of the vessels that are present in the area.
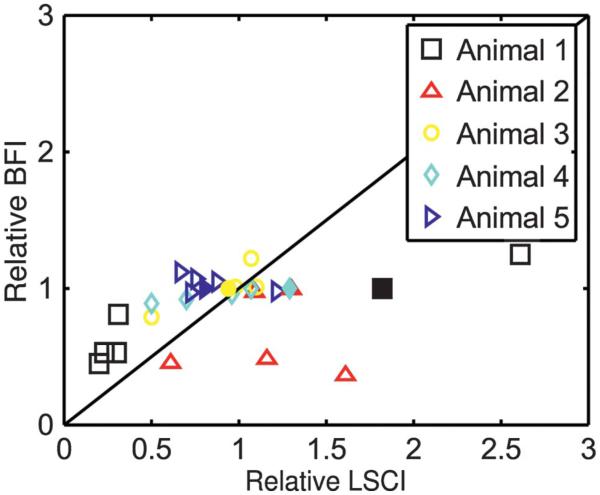
As previously described in Materials and Methods, it is possible to calculate a relative measure of flow based on the rise time of the ICG signal in a measurement known as the BFI. For each animal, BFI values were calculated at each injection point, and normalized to an area in the parenchyma to remove any error that resulted from inconsistencies within the injections. These normalized BFI values were then compared at the injection time points to determine the relative changes in flow as measured by BFI. These values were then compared with the relative change measured by LSCI at the same time point. Figure 7 shows a scatter plot of these data across all of the animals. For each animal, these calculations were performed for the following regions of interest: surrounding tissue located far away from the occlusion site, branching vessel before the vessel diverges, each branch of the vessel (occluded and unoccluded), and a larger vessel outside the occlusion.
FIGURE 7.
Relative change in blood flow index (BFI) in comparison with the measured relative change in flow as measured by LSCI. Filled markers indicate vessels that were not within the occlusion area, and open markers reflect changes in the various occluded branches. LSCI, laser speckle contrast imaging.
From Figure 7, it is observed that both methods are able to predict relatively constant flow changes. However, when the flow within a vessel significantly decreased, the BFI measurements overpredicted the flow in comparison with LSCI. When flow was increased, the BFI was unable to predict these changes entirely. LSCI was able to predict these changes more accurately because it is a direct measurement of the flow. Despite normalizing the data, the ICG measurements were still subject to small variations in the injection rate as well as changes in the physiology that occur from multiple injections.
Although it has been demonstrated that ICG is capable of quantifying changes in flow, these changes are very discrete in that they are limited to only 1 measurement obtained at the time of the injection. LSCI, however, is able to obtain the measurements without the use of a contrast agent and thus provides a continuous monitor of the dynamics within the field of view.
Clinically, the use of ICG has significant advantages, because the method has already proven to be a safe and effective method of measuring cerebral perfusion during neurosurgery. As far as safety is concerned, LSCI poses no significant risks to the patient outside standard hospital procedures. The typical power required for LSCI measurement is less than 50 mW. Because power is distributed over an area that is typically greater than 1 cm in diameter, the maximum optical fluence at the cortex is 0.015 W/cm2. This value is significantly lower than the maximum permissible exposure for skin (0.2 W/cm2 for visible wavelengths between 400 and 700 nm), as indicated by the American National Standards Institute.23 Although cortical tissue is different from skin tissue, no standards exist for direct exposure of laser light to the cortex. However, it is expected that the maximum permissible exposure of cortical tissue would be much higher than that of skin, because the cortical tissue has greater perfusion than skin, and therefore, a greater capacity to dissipate heat.
It is also important to recognize that vessels in a clinical setting will be much larger than those in a rodent model, and therefore they are likely to contain a very low velocity backflow. This backflow has been observed by using ICG; however, because of the limited number of clinical trials, it has yet to be observed by using LSCI. It is hypothesized that LSCI would be able to resolve this low-velocity backflow given the wide range of flow speed that LSCI is sensitive to. Future clinical studies using LSCI should investigate this parameter to ensure that there is no loss of information when using LSCI in comparison with ICG video angiography.
CONCLUSION
Laser speckle contrast imaging and ICG fluorescence angiography provide complementary information about vessel perfusion. The primary advantage of ICG angiography is its ability to identify feeding and draining vessels. Because LSCI does not require any contrast agents, it can provide continuous assessment of vessel perfusion during surgical procedures. Furthermore, LSCI provides a more direct measure of flow than ICG angiography. Both techniques can be performed simultaneously with a single light source, provided a laser is used.
Supplementary Material
Acknowledgments
The authors acknowledge the NeuroTexas Institute at St. David’s Hospital in Austin, Texas, for all their help in understanding the clinical applications of ICG during neurosurgeries.
ABBREVIATIONS
- BFI
blood flow index
- ICG
indocyanine green
- LSCI
laser speckle contrast imaging
Footnotes
Disclosures
This project was funded by the Coulter Foundation, National Institutes of Health (EB008715), The American Heart Association (0735136N), National Science Foundation (CBET-0644638), and the Consortium Research Fellows Program. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.neurosurgery-online.com).
REFERENCES
- 1.Takagi Y, Sawamura K, Hashimoto N. Intraoperative near-infrared indocyanine green videoangiography performed with a surgical microscope—applications in cerebrovascular surgery. Eur Neurol Rev. 2008;3(1):66–68. [Google Scholar]
- 2.Pouratian N. Shedding light on brain mapping: advances in human optical imaging. Trends Neurosci. 2003;26(5):277–282. doi: 10.1016/S0166-2236(03)00070-5. [DOI] [PubMed] [Google Scholar]
- 3.Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52(1):132–139. doi: 10.1097/00006123-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Ma CY, Shi JX, Wang HD, Hang CH, Cheng HL, Wu W. Intraoperative indocyanine green angiography in intracranial aneurysm surgery: microsurgical clipping and revascularization. Clin Neurol Neurosurg. 2009;111(10):840–846. doi: 10.1016/j.clineuro.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21(3):195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage. 2005;27(2):279–290. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Desmettre T, Devoisselle JM, Mordon S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv Ophthalmol. 2000;45(1):15–27. doi: 10.1016/s0039-6257(00)00123-5. [DOI] [PubMed] [Google Scholar]
- 8.Landsman ML, Kwant G, Mook GA, Zijlstra WG. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol. 1976;40(4):575–583. doi: 10.1152/jappl.1976.40.4.575. [DOI] [PubMed] [Google Scholar]
- 9.Kuebler WM, Sckell A, Habler O, et al. Noninvasive measurement of regional cerebral blood flow by near-infrared spectroscopy and indocyanine green. J Cereb Blood Flow Metab. 1998;18(4):445–456. doi: 10.1097/00004647-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Gora F, Shinde S, Elwell CE, et al. Noninvasive measurement of cerebral blood flow in adults using near-infrared spectroscopy and indocyanine green: a pilot study. J Neurosurg Anesthesiol. 2002;14(3):218–222. doi: 10.1097/00008506-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Owens SL. Indocyanine green angiography. Br J Ophthalmol. 1996;80(3):263–266. doi: 10.1136/bjo.80.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn AK. Laser speckle contrast imaging of cerebral blood flow. Ann Biomed Eng. 2012;40(2):367–377. doi: 10.1007/s10439-011-0469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boas DA, Dunn AK. Laser speckle contrast imaging in biomedical optics. J Biomed Opt. 2010;15(1):011109. doi: 10.1117/1.3285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tom WJ, Ponticorvo A, Dunn AK. Efficient processing of laser speckle contrast images. IEEE Trans Med Imaging. 2008;27(12):1728–1738. doi: 10.1109/TMI.2008.925081. [DOI] [PubMed] [Google Scholar]
- 15.Parthasarathy AB, Weber EL, Richards LM, Fox DJ, Dunn AK. Laser speckle contrast imaging of cerebral blood flow in humans during neurosurgery: a pilot clinical study. J Biomed Opt. 2010;15(6):066030. doi: 10.1117/1.3526368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Visscher G, van Rossem K, Van Reempts J, Borgers M, Flameng W, Reneman RS. Cerebral blood flow assessment with indocyanine green bolus transit detection by near-infrared spectroscopy in the rat. Comp Biochem Physiol A Mol Integr Physiol. 2002;132(1):87–95. doi: 10.1016/s1095-6433(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 17.Ayata C, Dunn AK, Gursoy-OZdemir Y, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24(7):744–755. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- 18.Strong AJ, Bezzina EL, Anderson PJ, Boutelle MG, Hopwood SE, Dunn AK. Evaluation of laser speckle flowmetry for imaging cortical perfusion in experimental stroke studies: quantitation of perfusion and detection of peri-infarct depolarisations. J Cereb Blood Flow Metab. 2006;26(5):645–653. doi: 10.1038/sj.jcbfm.9600240. [DOI] [PubMed] [Google Scholar]
- 19.Jones PB, Shin HK, Boas DA, et al. Simultaneous multispectral reflectance imaging and laser speckle flowmetry of cerebral blood flow and oxygen metabolism in focal cerebral ischemia. J Biomed Opt. 2008;13(4):04407. doi: 10.1117/1.2950312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woitzik J, Horn P, Vajkoczy P, Schmiedek P. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005;102(4):692–698. doi: 10.3171/jns.2005.102.4.0692. [DOI] [PubMed] [Google Scholar]
- 21.Wong G, Poon W. Near-infrared indocyanine green videoangiography-guided intracranial aneurysm surgery. Surg Pract. 2011;15(3):109–110. [Google Scholar]
- 22.de Oliverira JG, Beck J, Seifert V, Teixeira MJ, Raabe A. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery. 2007;61(3 suppl):63–72. doi: 10.1227/01.neu.0000289715.18297.08. [DOI] [PubMed] [Google Scholar]
- 23.ANSI (American National Standards Institute) American national standard for safe use of lasers, Z136.1. Laser Institute of America; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.