Synopsis
Inositol trisphosphate (InsP3)-mediated puffs are fundamental building blocks of cellular Ca2+ signalling, and arise through the concerted opening of clustered InsP3 receptors (InsP3Rs) coordinated via Ca2+-induced Ca2+ release. Although the Ca2+ dependency of InsP3Rs has been extensively studied at the single channel level, little is known as to how changes in basal cytosolic [Ca2+] would alter dynamics of InsP3-evoked Ca2+ signals in intact cells. To explore this question, we expressed Ca2+-permeable channels (nicotinic acetylcholine receptors) in the plasma membrane of voltage-clamped Xenopus oocytes to regulate cytosolic [Ca2+] by changing the electrochemical gradient for extracellular Ca2+ entry, and imaged Ca2+ liberation evoked by photolysis of caged InsP3. Elevation of basal cytosolic [Ca2+] strongly increased the amplitude and shortened the latency of global Ca2+ waves. In oocytes loaded with EGTA to localize Ca2+ signals, the number of sites at which puffs were observed, and the frequency and latency of puffs were strongly dependent on cytosolic [Ca2+], whereas puff amplitudes were only weakly affected. Our result indicates that basal cytosolic [Ca2+] strongly affects the triggering of puffs, but has less effect on puffs once they have been initiated.
Keywords: Ca2+ puffs, InsP3, cytosolic Ca2+, InsP3 receptor
INTRODUCTION
The inositol 1, 4, 5 trisphosphate receptor (InsP3R) is a Ca2+-permeable channel expressed in the endoplasmic reticulum (ER) which is gated by the binding of the second messenger InsP3 and by cytosolic Ca2+ itself [1–7]. Ca2+ liberation occurs at discrete functional release sites, formed by clusters of InsP3R on the endoplasmic reticulum (ER) membrane. These participate in generating a hierarchy of cellular Ca2+ signals involving opening of single InsP3R channels [8, 9], concerted release from several channels within a cluster [10], and global Ca2+ waves that propagate from cluster to cluster [11, 12]. The positive feedback mechanism of Ca2+-induced Ca2+ release (CICR) by which Ca2+ released from one InsP3R channel promotes opening of neighbouring channels underlies these processes, and factors including cytosolic Ca2+ buffering, [InsP3], and basal cytosolic [Ca2+] determine the transition between local and global signalling patterns.
The role of cytosolic Ca2+ in modulating InsP3R channel gating has been extensively studied by single-channel recordings from InsP3R in excised nuclei and after reconstitution in lipid bilayers, revealing the well-known 'bell-shaped' curve of Ca2+ facilitation and inhibition [1, 2, 13, 14]. However, less is known of how cytosolic Ca2+ modulates local InsP3-mediated signals in the intact cell, although imaging studies in Xenopus oocytes demonstrate a profound potentiation of global Ca2+ waves [13, 15–17].
Here, we expressed Ca2+-permeable nicotinic acetylcholine receptor/channels (nAChRs) in the plasma membrane of Xenopus oocytes so as to experimentally regulate basal cytosolic [Ca2+] concentration [18], and examined how elevations of cytosolic [Ca2+] affected the dynamics of local and global Ca2+ signals evoked by photoreleased InsP3. We show that an increased probability of triggering local Ca2+ release at puff sites underlies the strong augmentation of global InsP3-mediated Ca2+ waves, whereas puff amplitudes and durations were unaffected.
EXPERIMENTAL
Oocyte preparation and expression of nAChRs
Xenopus laevis were purchased from Nasco International (Fort Atkinson, WI, USA), and oocytes were surgically removed [19] following protocols approved by the UC Irvine Institutional Animal Care and Use committee. Stage V–VI oocytes were isolated and treated with collagenase (1 mg/ml of collagenase type A1 for 30 min) to remove follicular cell layers. One day after isolation oocytes were injected with a cRNA mixtures for nAChR expression (α,β,γ,δ subunits at a ratio of 2: 1: 1: 1; 50 nl at final concentration of 0.1–1 mg/ml) and were then maintained in modified Barth’s solution (mM: NaCl, 88; KCl, 1; NaHCO3, 2.4; MgSO4, 0.82; Ca(NO3)2, 0.33; CaCl2, 0.41; HEPES, 5; gentamicin, 1mg/ml ; pH 7.4) for 1 – 3 days at 16 °C before use. Expression of nAChR was evaluated using a voltage clamp to measure currents evoked by 500 nM ACh: oocytes showing currents >1 µA at −80 mV were selected for experiments.
Microinjection of oocytes
Intracellular microinjections were performed using a Drummond microinjector. About 1 h before Ca2+ imaging experiments, oocytes in Ca2+ -free Barth’s solution were injected with Fluo–4 dextran (high affinity; Kd = 800 nM) to a final concentration of 40 µM, assuming equal distribution throughout a cytosolic volume of 1 µl, and with caged Ins (1,4,5) P3 (D-myo-inositol 1,4,5-trisphosphate P4(5)-[1-(2-nitrophenyl)ethyl]ester (final concentration 8 µM). EGTA (final concentration 300 µM) was further injected for puff studies.
Ca2+ imaging and flash photolysis
Oocytes were voltage-clamped using a conventional two-microelectrode technique. The membrane potential was held at 0 mV during superfusion with a non-desensitizing concentration of ACh (100 – 500 nM) in Ringer's solution and was briefly stepped to −120 mV to strongly increase the electrical driving force for Ca2+ influx [20]. Global Ca2+ signals were imaged at room temperature by a custom-build confocal line scanner [21] interfaced to an Olympus inverted microscope IX 70, and fluorescence excitation was provided by the 488 nm line of an argon ion laser, with the laser spot focused by a 40 × oil immersion objective (NA 1.35) and scanned along at a rate of 10 ms /50 µm line. To image puffs, we applied a wide-field fluorescence microscopy using an Olympus IX 71 inverted microscope equipped with a 40 × oil-immersion objective, a 488 nm argon ion laser for fluorescence excitation and an electron-multiplied charge-coupled device (ccd) camera (Cascade 128+: Roper Scientific) for imaging fluorescence emission (510–600 nm) at frame rates of 500 s−1. Fluorescence was imaged from a 40 × 40 µm (128 × 128 pixel) region within the animal hemisphere of the oocyte. Fluorescence measurements made by line-scan and camera imaging are expressed as a ratio (ΔF/Fo) of the mean change in fluorescence (ΔF) at a pixel relative to the resting fluorescence at that pixel before stimulation (Fo). Mean values of Fo were obtained by averaging over several scans/frames before stimulation. To calibrate changes in ΔF/Fo values in terms of nM increases of free [Ca2+] we determined maximal (Fmax) and minimal (Fmin) fluorescence values by injecting Fluo-4 dextran-loaded oocytes (n = 5) with, respectively, 30 nl of 100 mM CaCl2 or 100 mM EGTA from a micropipette located close to the imaging site. After correcting for oocyte autofluorescence, mean values were Fmax = 8.52 ± 1.16, Fmin = 0.857 ± 0.024 relative to a resting fluorescence F0 before injection. We assumed a Kd for fluo-4 dextran of 2400nM, based on measurements of ~800 nM in free solution [22] and a roughly three-fold reduction in affinity in cytoplasmic environment [22]. A fluorescence increase of ΔF/Fo = 1 above baseline would then correspond to an increase of [Ca2+]cyt of about 360 nM. Photolysis of caged IP3 was evoked by flashes of UV light (350–400 nm) from a mercury arc lamp, delivered through the microscope objective and adjusted to uniformly irradiate a circular region slightly larger than the imaging frame or scan line. Flash durations were set using a Uniblitz shutter and digital controller.
Reagents
Fluo-4 dextran, high affinity (Kd: ~800nM), and caged InsP3 were purchased from Invitrogen (Carlsbad, CA, U.S.A.). All other reagents were from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Data analysis
Custom routines written in the IDL programming environment (Research Systems, Boulder, CO, USA) were used for line scan image processing and measurements. MetaMorph (Molecular Devices) was used to process and measure data obtained from wide-field camera-based imaging. Further analysis and graphing was accomplished using Microcal Origin version 6.0 (OriginLab, Northamptom, MA, USA). Data are expressed as mean ± 1 SEM, and significance was assessed by t-tests.
RESULTS
Elevated basal cytosolic [Ca2+] enhances InsP3-evoked Ca2+ waves
In order to evoke cytosolic [Ca2+] elevations, Ca2+ influx was induced through nAChRs expressed in the oocyte plasma membrane. Oocytes were continuously superfused with Ringer's solution containing 1.8 mM Ca2+ together with a low, non-desensitizing concentration (100 – 500 nM) of acetylcholine, and were voltage-clamped to control the electrochemical gradient for Ca2+ entry. The membrane potential was held at 0 mV to minimize Ca2+ influx, and was then stepped to more negative values to promote Ca2+ influx, beginning 2.5 s before delivery of a UV flash to photorelease InsP3 from a caged precursor loaded into the oocyte (Fig. 1A). The resulting changes in Fluo-4 fluorescence were imaged to compare InsP3-evoked Ca2+ responses evoked by identical UV flashes during cytosolic [Ca2+] elevation with control records when the voltage pulse was not applied.
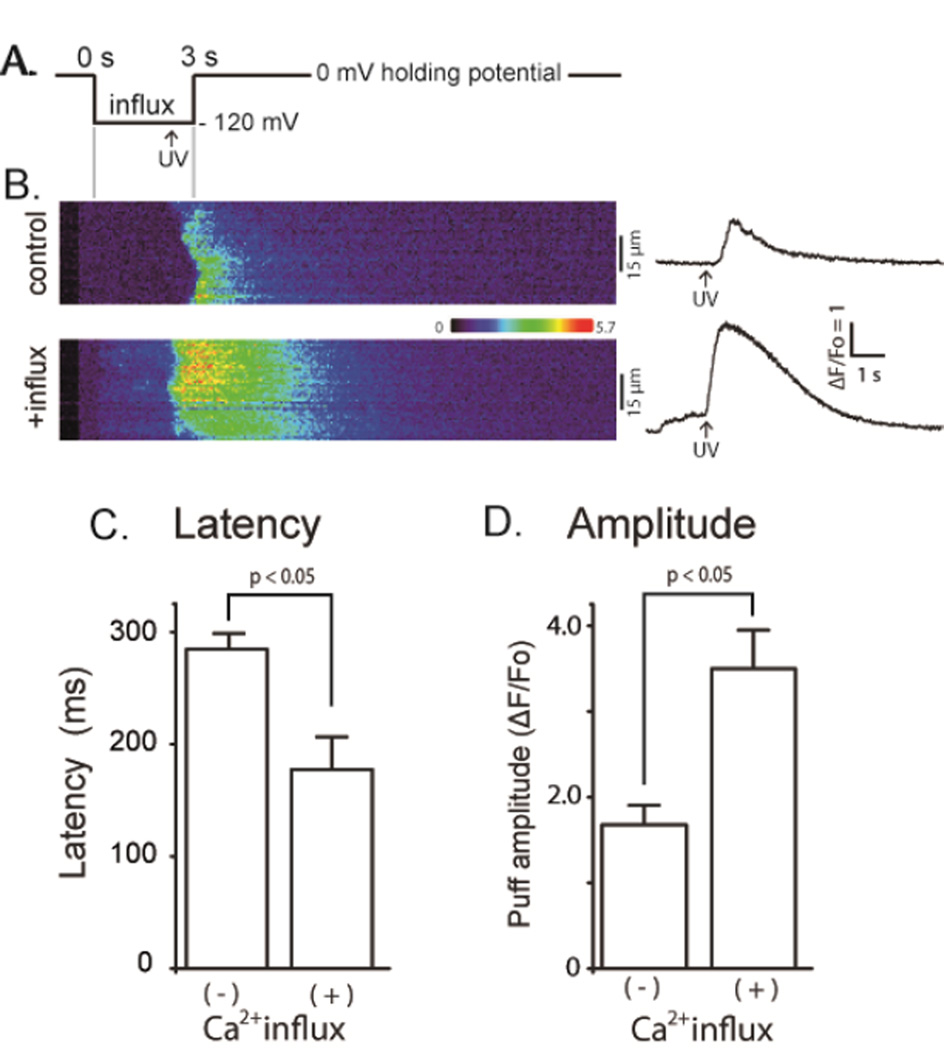
FIGURE 1. Elevated basal cytosolic [Ca2+] enhances InsP3-induced Ca2+ waves.
(A) Schematic of the experimental protocol. (B) Representative confocal linescan images illustrating fluo-4 dextran fluorescence signals evoked by photoreleased InsP3 under control conditions without elevation of cytosolic [Ca2+] (upper) and with cytosolic [Ca2+] elevation (lower). Increasing fluorescence (ΔF/Fo: Ca2+ level) is depicted on a pseudocolor scale as indicated by the colour bar. Traces on the right show corresponding fluorescence profiles averaged across 15 µm widths of the linescans (indicated by bars). (C) Mean values of latency between the photolysis flash and initial rise in fluorescence derived from traces like those in (B). Latency without Ca2+ influx 284 ± 14 ms; during Ca2+ influx 177 ± 30 ms, p < 0.05. (D) Mean peak amplitudes of Ca2+ waves derived from traces like those in (B). ΔF/Fo without Ca2+ influx 1.68 ± 0.23; during Ca2+ influx 3.50 ± 0.45, p < 0.05: n = 6 and 4 oocytes, respectively).
We first examined global Ca2+ signals evoked in oocytes that were not loaded with EGTA. The panels on the left of Figure 1B show representative linescan images of fluorescence changes evoked by photoreleased InsP3 without (top) and with (bottom) Ca2+ influx; corresponding florescence profiles are presented on the right. We compared the latencies and peak amplitude of InsP3-evoked Ca2+ signals under resting cytosolic [Ca2+], and during Ca2+ influx that increased the basal fluorescence signal by a mean of 0.60 ± 0.06 ΔF/Fo (6 oocytes from 3 different frogs). Latencies (time from the UV flash to the initial rise of fluorescence) were significantly shorter during cytosolic [Ca2+] elevation (Fig. 1C: control, 284 ± 14 ms: during Ca2+ influx, 177 ± 30 ms, p < 0.05). The mean peak amplitude of Ca2+ waves was profoundly augmented by cytosolic Ca2+ elevation (Fig. 1D: control, ΔF/Fo = 1.68 ± 0.23; during Ca2+ influx; ΔF/Fo = 3.50 0.45, p < 0.05, n = 6). These results are consistent with previous observations showing facilitation of InsP3-evoked Ca2+ signals by cytosolic [Ca2+] [1–6] [7].
Elevated basal cytosolic [Ca2+] promotes InsP3-evoked Ca2+ puffs
We next examined the effects of basal cytosolic [Ca2+] elevations on local Ca2+ puffs. For this purpose oocytes were loaded with EGTA (final intracellular concentration 300 µM) to suppress generation of Ca2+ waves by inhibiting inter-cluster diffusion of Ca2+ ions [23]. We further employed wide-field fluorescence microscopy to image a 40 × 40 µm field of view with a fast (500 fps) electron-multiplied c.c.d. camera, so as to sample many more puff sites than possible by one-dimensional linescan imaging. Figure 2 shows the experimental protocol (Fig. 2Aa), and representative fluorescence traces monitored from small regions of interest centred on puff sites illustrating responses evoked by photoreleased InsP3 at resting cytosolic Ca2+ (Fig. 2Ab) and when Ca2+ was elevated by Ca2+ influx (Fig. 2Ac,d). The photolysis flash was delivered 4 s after the onset of the hyperpolarizing pulse so as to allow cytosolic [Ca2+] to equilibrate, and puffs were then recorded for 6 s while the hyperpolarization was maintained. We varied the duration of the photolysis flash to evoke differing numbers of puffs at resting cytosolic [Ca2+]; 'weak' flashes (25 – 50 ms) were chosen to evoke on average about a single puff in the entire imaging field 40 × 40 µm, and 'strong' flashes (50 – 100 ms) to evoke up to 4 puffs. Even the 'strong' stimulus was chosen to evoke responses well below the maximal, so as to avoid possible saturation effects when responses were further potentiated by Ca2+ influx.
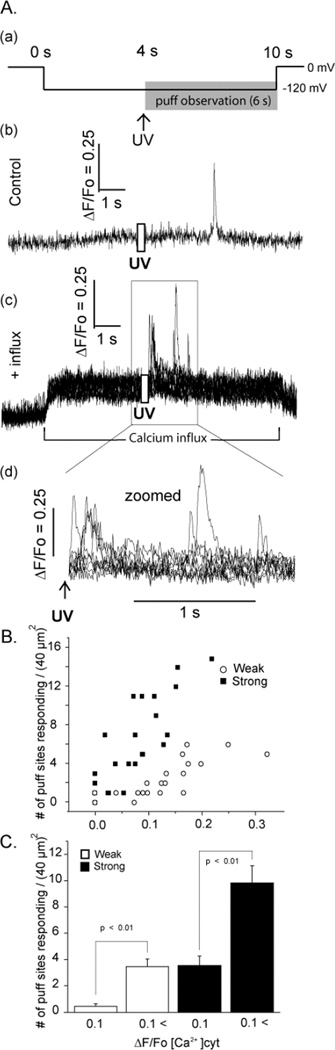
FIGURE 2. Cytosolic [Ca2+]-dependent potentiation of InsP3-evoked Ca2+ puffs.
(A) (a) schematic of the experimental protocol. (b,c) Representative fluorescence profiles of puffs evoked, respectively, without (control) and with (+ influx) basal cytosolic [Ca2+] elevation, obtained from the same oocyte. The record in (b) was obtained from the single responding site within the image field. That in (c) shows superimposed traces from 7 responding sites. (d) Zoomed version of (c) on an expanded timescale to illustrate more clearly the variation in puff latencies following photorelease of InsP3. Traces in (b–d) are blanked out during the photolysis flash. (B) Scatter plot showing the numbers of sites within the imaging field that showed puffs following weak (open symbols; 25–50 ms flash duration) or strong (filled symbols; 50–100 ms) photorelease of InsP3 as a function of cytosolic Ca2+ elevation during influx (ΔF/Fo[Ca2+]cyt). (C) Mean numbers of responding puff sites within imaging field, grouped by photolysis strength (weak, open bars; strong, filed bars) and by elevation of basal cytosolic [Ca2+] (ΔF/Fo < 0.1 or > 0.1).
Figure 2B shows a scatter plot of the relationship between the numbers of individual sites in the imaging field where puffs were observed during 6 s following photorelease of InsP3 as a function of the elevation of cytosolic [Ca2+] evoked by hyperpolarizing pulses. We express the [Ca2+] elevation in terms of fluorescence ratio change, without correction for oocyte autofluorescence (about 50% of resting Fluo-4 fluorescence). Based on the calibration described in Methods, an increase of ΔF/Fo of 0.1 corresponds to a rise in [Ca2+] of about 36 nM. With both weak and strong photolysis flashes the numbers of responding puff sites increased steeply with increasing basal cytosolic [Ca2+], with strong flashes giving greater numbers at any given basal [Ca2+]. Fig. 2C shows mean data, grouped according to flash duration and whether basal cytosolic levels just before the photolysis flash were at or close to the resting level (ΔF/Fo 0 – 0.1) or were appreciably elevated (ΔF/Fo > 0.1).
Elevated cytosolic [Ca2+] shortens puff latency
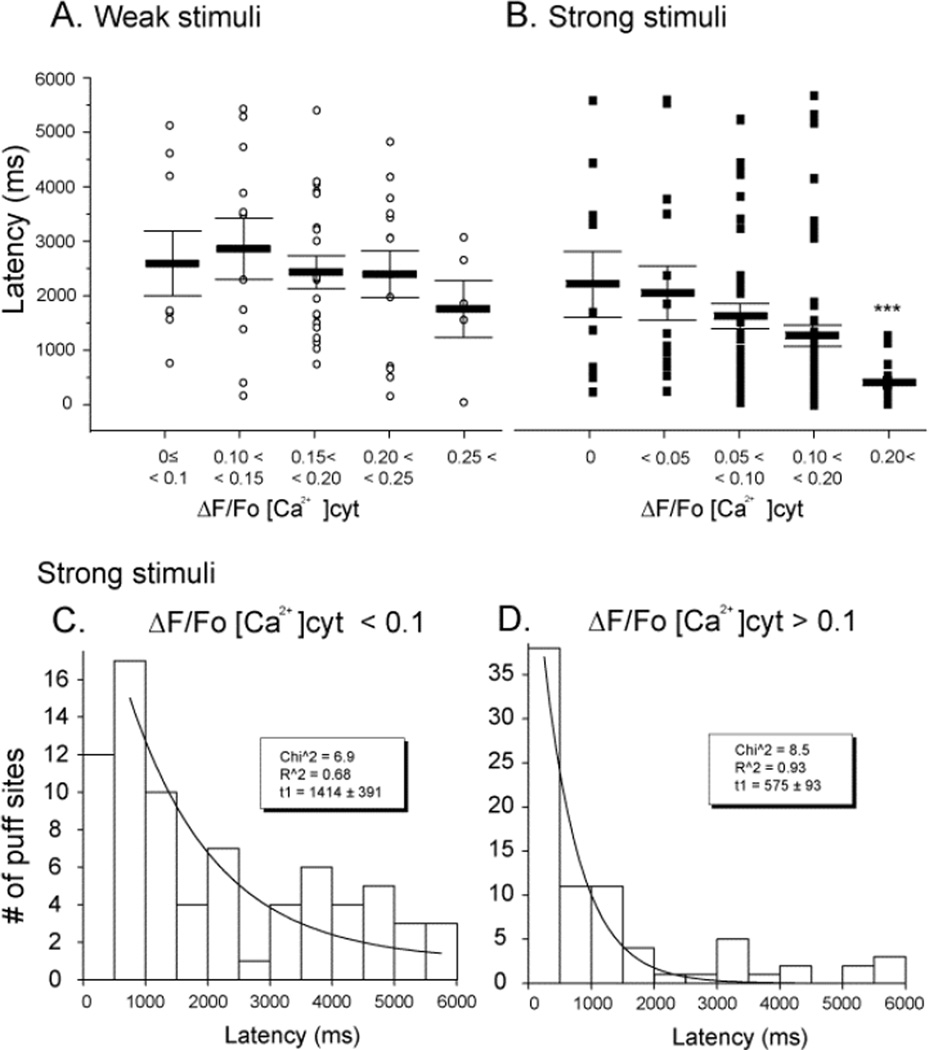
Figures 3A and B show scatter plots of individual and mean latencies of puffs, grouped according to cytosolic [Ca2+] elevation at the time of the photolysis flash. Puffs evoked by weak stimuli arose with relatively long (2–3s) latencies, which tended to shorten with increasing cytosolic [Ca2+] but did not show a statistically significant correlation (Fig. 3A). On the other hand, mean puff latencies were shorter with stronger photorelease of InsP3 (Fig. 3B), and showed a marked dependence on cytosolic [Ca2+], reducing from 2133 ± 200 ms at near resting level (ΔF/F <0.1) to 1240 ± 174 ms when the fluorescence was elevated to >0.1 ΔF/Fo during Ca2+ influx (p < 0.01). Puff latencies followed roughly exponential distributions at both relatively low and high cytosolic [Ca2+] (Figs. 3C and D, respectively), with a markedly shorter time constant at higher [Ca2+] (Fig. 3C and D).
FIGURE 3. Puff latencies shorten with increasing cytosolic [Ca2+].
Latencies were measured as the time from end of the photolysis flash to the observation of the first puff at a given site. (A, B) Mean latencies of puffs evoked, respectively, by weak and strong photorelease of InsP3. Open circles in (A) and filled squares in (B) indicate data from individual puffs; bars indicate mean ± SEM. (C, D) Histograms showing distributions of latencies of puffs evoked by strong stimuli during cytosolic Ca2+ elevations < 0.1 ΔF/F0 (C) and > 0.1 (D) Curves are single exponential fits to the data with respective time constants of 1414 ± 391 ms and 575 ± 93 ms.
Puff amplitude are only weakly dependent on cytosolic [Ca2+]
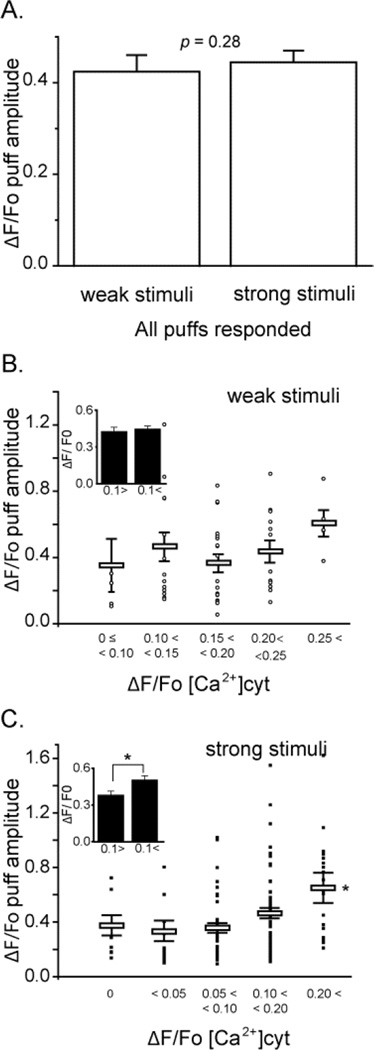
Next, we analyzed the effects of changes in cytosolic [Ca2+] on puff amplitudes. After pooling data across all different basal cytosolic [Ca2+] levels we found no significant difference in mean puff amplitudes evoked by weak or strong photorelease of InsP3 (Fig. 4A, weak flash mean puff amplitude ΔF/Fo = 0.43 ± 0.04, n = 56; strong flash, ΔF/Fo = 0.42 ± 0.03, n = 155, p > 0.05). Looking then at the effect of elevating cytosolic [Ca2+] levels, we observed little or no effect on the amplitudes of puffs evoked by weak photorelease (Fig. 4B). On the other hand, puffs evoked by strong photorelease of InsP3 showed a significant increase in puff amplitude with higher elevations of cytosolic [Ca2+] (ΔF/Fo >0.2) (Fig. 4C).
FIGURE 4. The amplitude of InsP3-evoked puffs is only weakly dependent on the basal cytosolic [Ca2+].
(A) Mean puff amplitudes evoked by weak photorelease of InsP3 (ΔF/Fo = 0.42 ± 0.04, n = 56) and strong photorelease (ΔF/Fo = 0.44 ± 0.23, n = 146. p > 0.05), after pooling data across all basal cytosolic [Ca2+] levels. (B) Main panel shows a scatter plot of amplitudes (ΔF/F0) of puffs evoked by weak photorelease of InsP3 as a function of increase in basal fluorescence during Ca2+ influx. Open circles mark data from individual puffs and bars show mean ± SEM. (C) Corresponding measurements of puff amplitudes following strong photorelease of InsP3. Inset graphs in (B) and (C) represent mean values of puff amplitudes evoked, respectively, by weak and strong photolysis flashes grouped for cytosolic [Ca2+] elevations < 0.1 and > 0.1 ΔF/Fo.
Puff durations are independent of basal cytosolic [Ca2+]
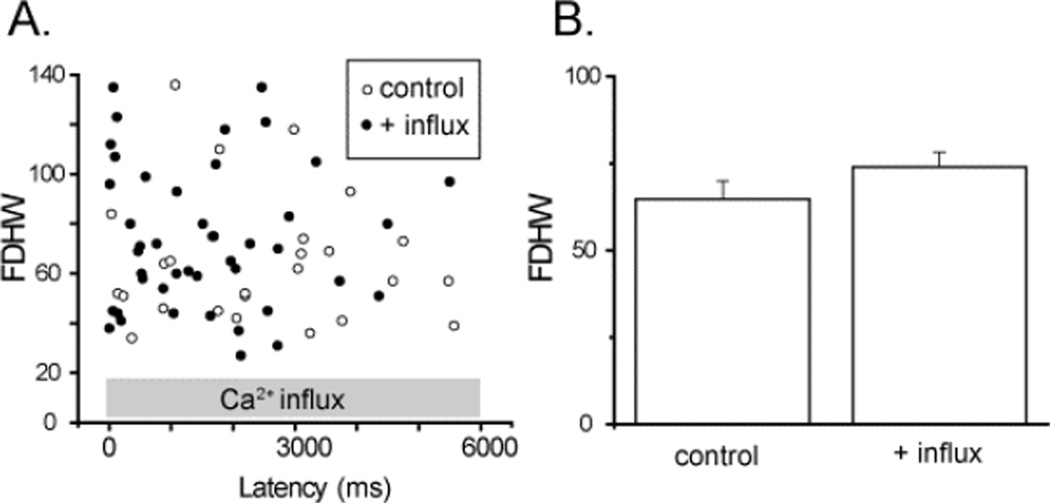
We had previously observed a prolongation of puff duration when puffs were evoked after loading ER Ca2+ stores by inducing a prior Ca2+ influx in oocytes transfected to over-express SERCA, but not in control (non-expressing) oocytes. We now examined the effect of elevated [Ca2+]cyt on puff durations. Puffs evoked by strong photorelease of InsP3 were compared in the same imaging field at basal [Ca2+]cyt and during induction of Ca2+ influx. Figure 5A shows a scatter plot of durations of puffs (measured as full duration at half-maximal amplitude: FDHM) against the latency of the puffs following the UV flash. No differences were apparent in puff durations between control and Ca2+ influx records, and puff durations did not show any obvious systematic dependence on latency following the UV flash. Figure 5B further plots mean values of FDHM of control puffs and puffs during Ca2+ influx, showing no significant difference. [p = 0.64].
FIGURE 5. Duration of InsP3-evoked puffs is independent on the basal cytosolic [Ca2+].
(A) Scatter plot showing full-duration at half-maximal amplitude (FDHM) of all puffs observed within the imaging field as a function of their latencies. Open circles are control puffs evoked by the strong photolysis flash, and filled circles represents FDHM of puffs observed during Ca2+ influx (mean ΔF/Fo[Ca2+]cyt = 0.23 ± 0.03, 4 trials). (B) Mean FDHM of puffs. (control FDHM; 64.8 ± 5.2, n = 25, with influx; FDHM 64.8 ± 5.2, n = 44, 4 oocytes).
DISCUSSION
The aim of the present study was to investigate how elevated basal cytosolic [Ca2+] would affect InsP3-evoked Ca2+ signals. We utilized the expression of Ca2+-permeable nicotinic receptor/channels in the plasma membrane as a means to evoke controlled entry of extracellular Ca2+ into the cell during hyperpolarizing voltage-clamped pulses. Consistent with previous observations [4, 5], we confirmed that cytosolic [Ca2+] elevations powerfully facilitated InsP3-mediated Ca2+ waves in terms of increased peak amplitude and shortened latency (Figures 1A and B). We further investigated the effect of elevated basal [Ca2+]cyt on the local InsP3-mediated Ca2+ puffs that are the triggers and fundamental building blocks of Ca2+ waves, as well as serving signalling functions in their own right. Our results show that the numbers of puff sites that respond at a given [InsP3] are strongly potentiated in a graded manner with increasing [Ca2+]cyt, and that the mean latency of puffs markedly shortens. In contrast, puff amplitudes were little affected except at high [Ca2+]cyt, and we observed no significant effects of [Ca2+]cyt on puff durations.
The effects we describe on InsP3-evoked Ca2+ liberation from the ER can be directly attributed to changes in basal [Ca2+]cyt, and not to any increase in Ca2+ store filling within the ER. We had previously utilized Ca2+ influx through nicotinic receptors as a means to increase ER Ca2+ loading, by applying a transient hyperpolarizing pulse and then allowing [Ca2+]cyt to subside to the resting level before examining responses to photoreleased InsP3. However, changes in puff properties were observed only when SERCA activity was accelerated by cADP ribose [20] [24] or when SERCA 2b was overexpressed [18]. With basal SERCA activity, no significant changes in puff triggering, kinetics or amplitude were apparent following even strong Ca2+ influx.
We have proposed that the puff is itself triggered by the stochastic opening of a single InsP3R channel within the cluster [25, 26]. Factors that determine the occurrence of puffs thus include the number of channels present in the cluster and the open probability of each channel. The latter, in turn, is a function of the concentrations of InsP3 and Ca2+, acting as co-agonists to open the channel [3, 7, 27]. Concordant with this mechanism, increasing [InsP3] results in an increased frequency of puffs and a shortening of the latency to the first puff evoked at a site following photorelease of InsP3 [26, 28]). Similarly, modest elevations of [Ca2+]cyt will increase the open channel probability at a given [InsP3], and hence increase the probability of puff triggering, leading to a greater number of sites that generate puffs following photorelease of InsP3 and a shortening in mean latency of these puffs. Although gating of the InsP3R channel is biphasically regulated by [Ca2+], inhibition of the native Xenopus InsP3R arises only when [Ca2+] exceeds several hundred µM [27] and thus would not be expected to be apparent in our experiments, where we estimate that the maximal Ca2+ influx (ΔF/Fo ~ 0.3) corresponded to an increase of [Ca2+]cyt of < 100 nM.
Because the resting [Ca2+]cyt is very low, small elevations above this level will strongly potentiate puff triggering. On the other hand, once an initial 'trigger' channel opens, the Ca2+ flux passing through it will elevate the local free [Ca2+] at the puff site to much higher levels, predicted to reach a few hundred µM at the mouth of the open channel and at least several µM at neighboring InsP3R within the cluster [29]. This will effectively 'swamp' the effect of any smaller elevation of basal [Ca2+]. Once triggered, the puff thus becomes a self-regenerative process and its subsequent evolution is expected to be substantially independent of the preceding conditions; likely explaining why we found little dependence of puff amplitudes and kinetics on basal [Ca2+]cyt.
The sensitization of global Ca2+ waves by elevated basal [Ca2+]cyt may similarly be explained by enhanced coupling between neighbouring release sites. Ca2+ waves propagate because Ca2+ released from one site diffuses to evoke CICR from adjacent sites [11, 12], and this triggering will be facilitated if [Ca2+]cyt is already elevated. The results in Fig. 1 were obtained using relatively weak photorelease of InsP3 that evoked only abortive Ca2+ waves, and basal [Ca2+] elevation promoted a more robust propagation by CICR resulting in strong potentiation of the spatially-averaged Ca2+ signal. With stronger stimulation by InsP3 the amplitude of repetitive Ca2+ waves is not potentiated by Ca2+ influx [17], presumably because the more substantial Ca2+ release through InsP3R swamps any effect of elevated basal [Ca2+], but wave velocities and frequency of repetitive spikes are increased [17].
InsP3-mediated Ca2+ signalling can function as a coincidence detector, whereby release of Ca2+ from intracellular stores is potentiated by extracellular Ca2+ entering through plasmalemmal ligand- or voltage-operated channels. This interaction may arise through two different mechanisms, operating on different timescales. Most directly, as we describe here, elevation of basal [Ca2+]cyt enhances the probability of triggering of local and global [Ca2+] signals by binding to activating sites on the cytosolic face of the InsP3R. In addition, we have described a more circuitous mechanism, whereby extracellular [Ca2+] entering the cytosol is taken up by the action of SERCA pumps, leading to enhanced filling of ER Ca2+ stores [18]. That, in turn, promotes Ca2+ puffs and waves, likely because increased Ca2+ flux through the InsP3R channel enhances CICR via the cytosolic activating sites on the InsP3R, and possibly also through luminal regulation of InsP3R function [30–32]. The direct action of Ca2+ influx on InsP3R is immediate and short lasting, depending on clearance rate from the cytosol. In contrast, potentiation via ER store filling is slower to develop, more persistent, and subject to potential modulation by other messenger pathways, such as cADPR, that affect SERCA activity either directly or indirectly [20, 33, 34]. Interactions between these different modulatory mechanisms are likely to be of particular importance for Ca2+ signalling in neurons in regard to activity-dependent synaptic plasticity as well as gene expression and protein synthesis [35–37].
Acknowledgments
FUNDING
This work was supported by a grant (GM048071) from the National Institutes of Health.
Abbreviations used
- InsP3
Inositol trisphosphate
- caged InsP3
Ins (1,4,5) P3 (D-myo-inositol 1,4,5-trisphosphate P4(5)-[1-(2-nitrophenyl)ethyl]ester
- CICR
Ca2+-induced Ca2+ release
- nAChRs
nicotinic acetylcholine receptor/channels
- ER
the endoplasmic reticulum
- SERCA
the sarcoplasmic/endoplasmic reticulum calcium ATPase
REFERENCES
- 1.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J. Gen. Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 3.Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 4.Yao Y, Parker I. Potentiation of inositol trisphosphate-induced Ca2+ mobilization in Xenopus oocytes by cytosolic Ca2+ J. Physiol. 1992;458:319–338. doi: 10.1113/jphysiol.1992.sp019420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLisle S, Welsh MJ. Inositol trisphosphate is required for the propagation of calcium waves in Xenopus oocytes. J. Biol. Chem. 1992;267:7963–7966. [PubMed] [Google Scholar]
- 6.Marshall IC, Taylor CW. Biphasic effects of cytosolic Ca2+ on Ins(1,4,5)P3-stimulated Ca2+ mobilization in hepatocytes. J. Biol. Chem. 1993;268:13214–13220. [PubMed] [Google Scholar]
- 7.Marchant JS, Taylor CW. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr. Biol. 1997;7:510–518. doi: 10.1016/s0960-9822(06)00222-3. [DOI] [PubMed] [Google Scholar]
- 8.Parker I, Choi J, Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell calcium. 1996;20:105–121. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- 9.Parker I, Yao Y. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. J. Physiol. 1996;491(Pt 3):663–668. doi: 10.1113/jphysiol.1996.sp021247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker I, Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc. Biol. Sci. 1991;246:269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- 11.Marchant JS, Parker I. Role of elementary Ca(2+) puffs in generating repetitive Ca(2+) oscillations. EMBO J. 2001;20:65–76. doi: 10.1093/emboj/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson SP, Keizer J, Pearson JE. Fire-diffuse-fire model of dynamics of intracellular calcium waves. Proc. Natl. Acad. Sci. U S A. 1999;96:6060–6063. doi: 10.1073/pnas.96.11.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker I, Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+ Proc. Natl. Acad. Sci. U S A. 1990;87:260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mak DO, Pearson JE, Loong KP, Datta S, Fernandez-Mongil M, Foskett JK. Rapid ligand-regulated gating kinetics of single inositol 1,4,5-trisphosphate receptor Ca2+ release channels. EMBO Rep. 2007;8:1044–1051. doi: 10.1038/sj.embor.7401087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lechleiter JD, Clapham DE. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992;69:283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- 16.Meyer T. Cell signaling by second messenger waves. Cell. 1991;64:675–678. doi: 10.1016/0092-8674(91)90496-l. [DOI] [PubMed] [Google Scholar]
- 17.Yao Y, Parker I. Ca2+ influx modulation of temporal and spatial patterns of inositol trisphosphate-mediated Ca2+ liberation in Xenopus oocytes. J. Physiol. 1994;476:17–28. [PMC free article] [PubMed] [Google Scholar]
- 18.Yamasaki-Mann M, Parker I. Enhanced ER Ca2+ store filling by overexpression of SERCA2b promotes IP3-evoked puffs. Cell calcium. 2011;50:36–41. doi: 10.1016/j.ceca.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demuro A, Parker I. "Optical patch-clamping": single-channel recording by imaging Ca2+ flux through individual muscle acetylcholine receptor channels. The Journal of general physiology. 2005;126:179–192. doi: 10.1085/jgp.200509331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamasaki-Mann M, Demuro A, Parker I. cADPR stimulates SERCA activity in Xenopus oocytes. Cell calcium. 2009;45:293–299. doi: 10.1016/j.ceca.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker I, Callamaras N, Wier WG. A high-resolution, confocal laser-scanning microscope and flash photolysis system for physiological studies. Cell calcium. 1997;21:441–452. doi: 10.1016/s0143-4160(97)90055-5. [DOI] [PubMed] [Google Scholar]
- 22.Martin VV, Beierlein M, Morgan JL, Rothe A, Gee KR. Novel fluo-4 analogs for fluorescent calcium measurements. Cell calcium. 2004;36:509–514. doi: 10.1016/j.ceca.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Dargan SL, Parker I. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J. Physiol. 2003;553:775–788. doi: 10.1113/jphysiol.2003.054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki-Mann M, Demuro A, Parker I. Modulation of Endoplasmic Reticulum Ca(2+) Store Filling by Cyclic ADP-ribose Promotes Inositol Trisphosphate (IP(3))-evoked Ca(2+) Signals. J. Biol. Chem. 2010;285:25053–25061. doi: 10.1074/jbc.M109.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose HJ, Dargan S, Shuai J, Parker I. 'Trigger' events precede calcium puffs in Xenopus oocytes. Biophys J. 2006;91:4024–4032. doi: 10.1529/biophysj.106.088872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickinson GD, Swaminathan D, Parker I. The probability of triggering calcium puffs is linearly related to the number of inositol trisphosphate receptors in a cluster. Biophysical journal. 2012;102:1826–1836. doi: 10.1016/j.bpj.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuai J, Pearson JE, Foskett JK, Mak DO, Parker I. A kinetic model of single and clustered IP3 receptors in the absence of Ca2+ feedback. Biophysical journal. 2007;93:1151–1162. doi: 10.1529/biophysj.107.108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ullah G, Parker I, Mak DO, Pearson JE. Multi-scale data-driven modeling and observation of calcium puffs. Cell calcium. 2012 doi: 10.1016/j.ceca.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horne JH, Meyer T. Luminal calcium regulates the inositol trisphosphate receptor of rat basophilic leukemia cells at a cytosolic site. Biochemistry. 1995;34:12738–12746. doi: 10.1021/bi00039a033. [DOI] [PubMed] [Google Scholar]
- 31.Sienaert I, De Smedt H, Parys JB, Missiaen L, Vanlingen S, Sipma H, Casteels R. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1996;271:27005–27012. doi: 10.1074/jbc.271.43.27005. [DOI] [PubMed] [Google Scholar]
- 32.Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 33.Lukyanenko V, Gyorke I, Wiesner TF, Gyorke S. Potentiation of Ca(2+) release by cADP-ribose in the heart is mediated by enhanced SR Ca(2+) uptake into the sarcoplasmic reticulum. Circ. Res. 2001;89:614–622. doi: 10.1161/hh1901.098066. [DOI] [PubMed] [Google Scholar]
- 34.Macgregor AT, Rakovic S, Galione A, Terrar DA. Dual effects of cyclic ADP-ribose on sarcoplasmic reticulum Ca2+ release and storage in cardiac myocytes isolated from guinea-pig and rat ventricle. Cell calcium. 2007;41:537–546. doi: 10.1016/j.ceca.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Rose CR, Konnerth A. Stores not just for storage. intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- 36.Barbara JG. IP3-dependent calcium-induced calcium release mediates bidirectional calcium waves in neurones: functional implications for synaptic plasticity. Biochimica et biophysica acta. 2002;1600:12–18. doi: 10.1016/s1570-9639(02)00439-9. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe S, Hong M, Lasser-Ross N, Ross WN. Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons. The Journal of physiology. 2006;575:455–468. doi: 10.1113/jphysiol.2006.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]