Abstract
DNA double-strand breaks (DSBs) occur frequently during replication in sister chromatids, and are dramatically increased when cells are exposed to chemotherapeutic agents including camptothecin. Such DSBs are efficiently repaired specifically by homologous recombination (HR) with the intact sister chromatid. HR hence plays pivotal roles in cellular proliferation and cellular tolerance to camptothecin. Mammalian cells carry several structure-specific endonucleases, such as Xpf-Ercc1 and Mus81-Eme1, in which Xpf and Mus81 are the essential subunits for enzymatic activity. Here we show the functional overlap between Xpf and Mus81 by conditionally inactivating Xpf in the chicken DT40 cell line, which has no Mus81 ortholog. Although mammalian cells deficient in either Xpf or Mus81 are viable, Xpf inactivation in DT40 cells was lethal, resulting in a marked increase in the number of spontaneous chromosome breaks. Similarly, inactivation of both Xpf and Mus81 in human HeLa cells and murine embryonic stem cells caused numerous spontaneous chromosome breaks. Furthermore, the phenotype of Xpf-deficient DT40 cells was reversed by ectopic expression of human Mus81-Eme1 or human Xpf-Ercc1 heterodimers. These observations indicate the functional overlap of Xpf-Ercc1 and Mus81-Eme1 in the maintenance of genomic DNA. Both Mus81-Eme1 and Xpf-Ercc1 contribute to the completion of HR as evidenced by the following data that the expression of Mus81-Eme1 or Xpf-Ercc1 diminished the number of camptothecin-induced chromosome breaks in Xpf-deficient DT40 cells, and preventing early steps in HR by deleting XRCC3 suppressed the inviability of Xpf-deficient DT40 cells. In summary, Xpf and Mus81 have a substantially overlapping function in completion of HR.
Keywords: homologous recombination, Xpf, Mus81, nuclease, chemotherapeutic agents
Introduction
Homologous recombination (HR) mediated double-strand break (DSB) repair is initiated by resection of DSBs and formation of 3′ single-strand overhangs, followed by polymerization of Rad51 (1, 2). The resulting nucleoprotein filaments, consisting of the 3′ single-strand tail and the polymerized Rad51, undergo homology search and pairing with the intact duplex DNA donor to form a displacement (D)-loop structure. Extensive strand exchange of the D-loop leads to the generation of HR intermediates. HR intermediates are processed into either crossover products or non-crossover products (3). HR intermediates including Holliday junction (HJ) are hereafter called joint molecules (JMs).
The processing of JMs is carried out by dissolution and resolution pathways. In the dissolution pathway, Sgs1 (Blm, an ortholog of Sgs1 in human), topoisomerase III, and RMI1/2 collaboratively catalyze the decatenation of HJs and generate non-crossover products (4-6). In the resolution pathway, structure-specific endonucleases cleave the HJs and produce crossover and non-crossover products, depending on the choice of cleaved strands at the four-way junction (7). In E. coli, the RusA nuclease and the RuvABC complex cleave HJs symmetrically and play a key role in the resolution pathway (8-10). Recent work with eukaryotes has revealed two resolvases, Gen1 (11) and the Slx1-Slx4 complex (12-15), both of which can symmetrically cleave the four-way junction. A third enzyme called Mus81 together with its partner Eme1/Mms4 has also been implicated in resolving HJs and the formation of crossover recombinants in both mitotic and meiotic recombination in yeast and mammals (16, 17). In vitro data show an ability of Mus81 to incise HJs asymmetrically, however a greater predilection for cleaving D-loops and nicked HJs suggests that unlike “classical” HJ resolvases Mus81 may process JMs before they mature into fully ligated HJs (16).
Mus81 is a member of the Xpf family of structure-specific endonucleases. Human Xpf is best known for its role in nucleotide excision repair (NER), together with its partner Ercc1. Moreover, Xpf, but not the other NER factors, is involved in DSB repair including single-strand annealing (18, 19), incision at interstrand crosslinks (20), and gene targeting (21). However, no studies have reported a functional overlap between Mus81 and Xpf in any DNA repair or recombination reactions, or a role of Xpf in HR after the formation of JMs. Intriguingly the Xpf orthologs in both Drosophila and C. elegans have been implicated in JM processing and the formation of crossovers during meiosis. Whether Xpf is similarly involved in JM processing in vertebrates alongside enzymes like Mus81 is currently unknown.
To investigate the role of Xpf in HR, we conditionally disrupted the XPF gene in the chicken B lymphocyte line DT40 (22). Because this line does not possess the MUS81 gene, we propose that the loss of Xpf in DT40 cells is equivalent to mammalian cells deficient in both Xpf and Mus81. Deletion of XPF caused extensive chromosomal aberrations and cell death. However, this lethality was substantially reversed by ectopic expression of human Mus81 together with Eme1 (HsMus81-Eme1), indicating a compensatory relationship between Xpf and Mus81 in the maintenance of chromosomal DNA. The phenotypic analysis of Xpf-depleted DT40 cells indicated that a marked genomic instability may result from the defective processing of JMs. Our data uncovered that the Xpf and Mus81 endonucleases play overlapping and essential roles in completion of HR.
Materials and Methods
Cell culture, plasmid constructs, and siRNAs
Chicken DT40, mouse embryonic stem (ES) (wild-type (IB10) and MUS81−/−), and HeLa cells were cultured as described previously (23-25). DT40 or ES cells have been maintained by S. Takeda or J. Essers since 1991 or 2004, respectively (22, 24). HeLa cells were obtained from K. Myung (NHGRI) in 2007 (25). All of cell lines were tested routinely for various criteria such as morphology, growth rate, and karyotype. Details for plasmid constructs, and siRNAs are provided in SI materials and methods.
Cell cycle analysis
After BrdU pulse-labeling in the cells exposed to tamoxifen (TAM), cell-cycle distribution was measured as described previously (26).
Measurement of chromosomal aberrations
Chromosomal aberrations were measured as described previously (23). Briefly, the cells were exposed to TAM for 1, 2, or 3 days, with 0.1 μg/ml of colcemid added for the last 3 h of incubation before harvest. To measure IR-induced chromosomal aberrations, the cells were exposed to TAM for 24 h, and colcemid was added immediately after cells were irradiated with 0.3 Gy γ-rays. To test the response to CPT, cells were continuously exposed to TAM for 33 h, with the cells treated with 100 nM CPT for the last 9 h, and also treated with colcemid for the last 3 h before harvest of mitotic cells.
Measurement of Rad51 subnuclear foci
Before measurement of Rad51 subnuclear foci, cells were exposed to TAM for 2 days. Rad51 foci were visualized with anti-Rad51 antibody in untreated cells and in cells γ-irradiated with 4 Gy, 3 h and 6 h after treatment as previously described (26).
Measurement of SCE levels
SCE levels were measured as described previously (27). Before BrdU labeling, cells were exposed to TAM for 30 h. Cells were cultured in the presence of 10 μM BrdU for 21 h and treated with 0.1 μg/ml of colcemid for the last 3 h. CPT (5 nM or 100 nM) was added 9 h before harvest.
Measurement of chromosome aberrations in HeLa and mouse ES cells
Chromosomal aberrations were measured as described previously (23). Briefly, transfection of siRNA into HeLa or mouse ES cells using lipofectamine RNAiMAX or lipofectamine 2000 was performed according to the manufacturer’s instructions, respectively. 45 h after the transfection, HeLa cells were incubated for 1 h with 0.2 μg/ml colcemid after which metaphase cells were collected by mitotic shake-off. Alternatively, 72 h after the transfection, mouse ES cells were incubated for 2 h with 0.1 μg/ml colcemid after which metaphase cells were collected by mitotic shake-off.
Statistics
We did three independent experiments for all of data sets. The results were expressed as mean ± SD (growth curves and sensitivity to the genotoxic agents) or mean ± SEM (chromosomal aberrations and SCEs). Differences between the data were tested for statistical significance using t-test.
Additional details are provided in SI materials and methods.
Results
Mus81 protein is absent in chicken DT40 cells
Chicken XPF cDNA encodes a putative 903 amino-acid proteins, compared to the 905 amino-acids of human Xpf (Supplementary Fig. S1). The sequence identity between the two proteins is 76.8%. As expected, immunoprecipitants of tagged Xpf included Ercc1 (Supplementary Fig. S2A and B), indicating that Xpf associates with Ercc1 in DT40 cells. Since there is an ortholog of Eme1 but not Mus81 registered in the chicken genome database (Supplementary Fig. S3A and B) (28), we analyzed proteins that interact with Eme1, which forms a heterodimer with Mus81 in mammalian cells. Immunoprecipitants of tagged Eme1 included Xpf, but not Mus81 (Supplementary Fig. S4A), indicating the absence of functional Mus81-Eme1 complex in DT40 cells. To verify the absence of Mus81-Eme1, we disrupted the EME1 gene (Supplementary Fig. S5A). EME1−/− DT40 cells were able to proliferate and showed moderate sensitivity only to cisplatin (Supplementary Fig. S5B and C), which phenotype is in marked contrast with the hypersensitivity of mouse EME1−/− and MUS81−/− ES cells to a wide variety of DNA damaging agents (29, 30). We therefore concluded no Mus81 ortholog in DT40 cells.
The data indicate that the relationship among Xpf, Mus81, Eme1 and Ercc1 differs between DT40 and mammalian cells. The moderate sensitivity of EME1−/− DT40 cells to cisplatin (Supplementary Fig. S5C) indicates that Xpf might form a functional complex with Eme1, whereas mammalian Mus81 and Xpf form a heterodimer with Eme1 and Ercc1, respectively. This idea is supported by the data that, consistent with a previous report (31), interaction of Xpf with Eme1 was confirmed by co-immunoprecipitation of recombinant proteins (Supplementary Fig. S4B and C). In addition to the presence of Xpf-Eme1, it should be noted that we could not formally exclude the possibility that a functional homolog of Mus81 is present in chicken cells. In conclusion, Xpf-Ercc1, but not Xpf-Eme1, plays the major role in DNA damage response in chicken cells.
Deletion of XPF results in an accumulation of chromosomal aberrations and subsequent cell death
We generated XPF gene-disruption constructs, which deleted amino-acid coding sequences 1 to 148 together with the transcriptional promoter sequences (Fig. 1A and supplementary Fig. S6A). Because we failed to establish XPF−/− cells from XPF+/− cells, we generated conditional Xpf-deficient cells using a chicken XPF transgene flanked by loxP-signal sequences (GdXPF-loxP), which is excised by the chimeric Cre recombinase carrying the TAM-binding domain (Supplementary Fig. S6B). Since the GdXPF-loxP transgene also carries a marker gene encoding the Green Fluorescent Protein (GFP), TAM-mediated excision of the GdXPF-loxP transgene can be evaluated by monitoring the loss of the GFP signal (Supplementary Fig. S6C and D). We also confirmed depletion level of XPF mRNA by RT-PCR (Supplementary Fig. 6E). We generated XPF+/−GdXPF-loxP clones, and subsequently generated two XPF−/−GdXPF-loxP clones, which displayed indistinguishable phenotypes.
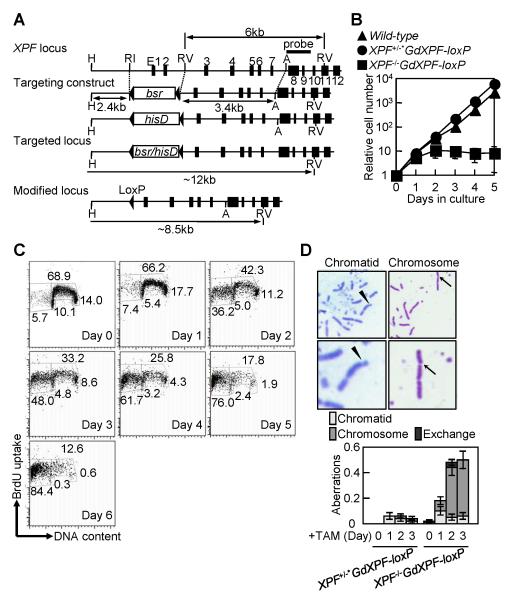
Figure 1. Xpf is essential for the maintenance of genome stability.
(A) Targeted disruption of the chicken XPF gene. The chicken XPF locus, the two targeting constructs and the resulting targeted locus are shown. The black boxes represent the exons of the XPF gene. The triangles flanking the blastidine-resistance (bsrR) and histidinol-resistance (hisDR) genes represent the loxP sequences, the recognition site of the Cre recombinase. (B) Growth curve after addition of tamoxifen (TAM) to XPF−/−GdXPF-loxP cells at time zero for the excision of the GdXPF-loxP transgene. (C) Flow-cytometric analysis of cell-cycle distribution after BrdU pulse-labeling in XPF−/−GdXPF-loxP cells. (D) Spontaneous chromosomal aberrations in XPF−/−GdXPF-loxP cells. Top panels, a representative chromatid-type break (shown by arrowhead) and a chromosome-type break (shown by arrow). Breaks are magnified in the lower panels. Bottom panels, measurement of spontaneous chromosomal aberrations. A chromatid-type break indicates a discontinuity in one of the two sister chromatids, and a chromosome-type break indicates discontinuities at the same site of both sisters. Exchange indicates chromosomal translocation. The vertical axis shows the number of aberrations per cell.
We analyzed the proliferation kinetics of the cells with and without treatment with TAM (Fig. 1B and C). The XPF+/+, XPF+/−GdXPF-loxP and XPF−/−GdXPF-loxP cells without the TAM treatment divided every 8 h (Supplementary Fig. S6F). By contrast, at ~2 day after treatment with TAM, the XPF−/− cells ceased to proliferate (Fig. 1B and C). To explore the cause of this mortality, we examined spontaneously arising chromosomal aberrations in mitotic cells. It should be noted that chromosomal breaks hereafter represent discontinuities, which appear on chromosomes in metaphase spreads as regions unstained by Giemsa, and do not always result from DSBs. We found a dramatic increase in the number of chromosomal aberrations prior to cell death in the Xpf-deficient cells (Fig. 1D), an occurrence that is consistently observed in cells that have a severe defect in HR (26, 32-34), but is not observed in DT40 cells deficient in the XPA or XPG genes involved in NER (35, 36). To test whether the nuclease activity of Xpf is required for cellular viability, we created two cDNAs mutated at the catalytic center of Xpf, GdXPF(D674A) and GdXPF(D702A) (Supplementary Figs. S1 and S6G). In contrast to a wild-type Xpf transgene, these mutant transgenes yielded no stable clones (Supplementary Fig. S6G), suggesting that the mutant Xpf proteins may interfere with the endogenous Xpf by competing for the association with Ercc1.
HR-mediated DSB repair is severely compromised in Xpf-deficient cells
To analyze the role of Xpf in HR, we assessed the capability of HR-mediated DSB repair at 24 h after TAM treatment, when the cells were still able to proliferate exponentially. To selectively evaluate HR-mediated DSB repair, we measured the number of chromosomal aberrations in mitotic cells following exposure of cells to camptothecin (CPT), a DNA topoisomerase I poison, and to γ-rays in the G2 phase. CPT induces single-end breaks during replication, and the restart of replication requires HR with the intact sister chromatid (37). Similarly, γ-ray-induced chromosomal aberrations are repaired exclusively by HR in the G2 phase in DT40 cells (38).
Xpf-depleted cells had a greater number of CPT-induced chromosomal aberrations than did Xpf-expressing cells (Fig. 2A). We next exposed an asynchronous population of cells to γ-rays and measured the number of chromosomal aberrations in cells that entered the M phase within 3 h after irradiation. This protocol allows for the evaluation of DSB repair selectively during the G2 phase, where HR plays a dominant role in DSB repair (38). Following irradiation, the total number of chromosomal aberrations in Xpf-deficient cells increased to 0.26 per cell, whereas the total number in XPF+/+ cells increased only to 0.08 (Fig. 2B). Taken together, these results indicate that Xpf plays a key role in HR-mediated DSB repair.
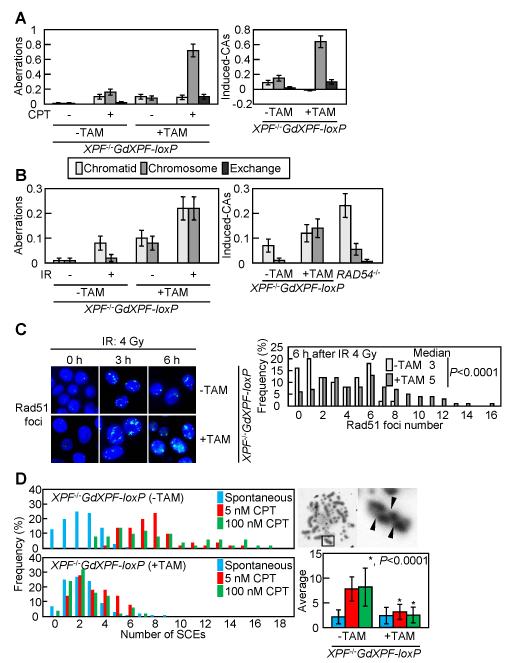
Figure 2. A defect in homologous-recombination-dependent DSB repair in Xpf-depleted cells.
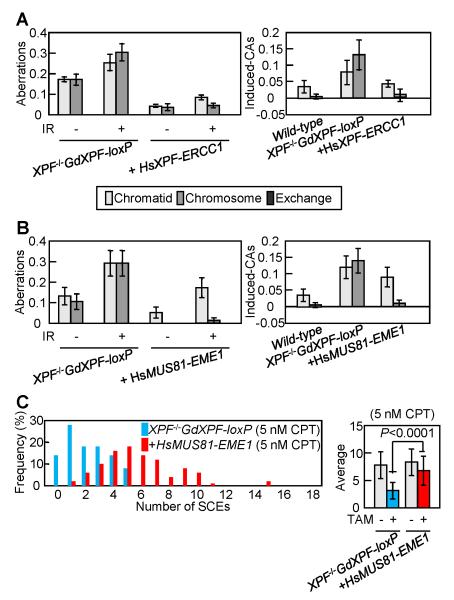
(A) Camptothecin (CPT)-induced chromosomal aberrations in Xpf-depleted cells. XPF−/−GdXPF-loxP cells were continuously exposed to TAM for 33 h, during which the cells were treated with 100 nM CPT for the last 9 h, and with colcemid for the last 3 h before harvest of mitotic cells. Left panels, measurement of chromosomal aberrations after treatment with or without CPT. Right panels, CPT-induced chromosomal aberrations were calculated by subtracting the number of spontaneously occurring chromosomal aberrations from the number of chromosomal aberrations observed in the CPT-treated sample of the same genotype. 100 mitotic cells were examined for each analysis. The vertical axis shows the number of aberrations per cell in A and B. (B) Ionizing radiation (IR)-induced chromosomal aberrations in Xpf-depleted cells. Cells were exposed to TAM for 24 h, exposed to γ-rays, then treated with colcemid for 3 h. Left panels, measurement of chromosomal aberrations after exposure to γ-rays. Right panels, IR-induced chromosomal aberrations were calculated by subtracting the number of spontaneously occurring chromosomal aberrations from the number of chromosomal aberrations observed in the γ-rays-exposed sample of the same genotype. Results of IR-induced chromosomal aberrations in RAD54−/− cells were described in a previous report (26). (C) The formation of γ-induced Rad51 foci in Xpf-expressing and Xpf-depleted cells. Left panels, a fraction of the γ-irradiation-induced Rad51 subnuclear foci persists for extended periods. Cells were exposed to TAM for 2 days, irradiated with 4 Gy γ-ray, fixed at 3 and 6 h post-IR, and subjected to immuno-cytochemistry using anti-Rad51 antibody. Right panels, quantification of Rad51 foci number at 6 h post-IR. 100 cells were examined for each analysis. (D) Xpf depletion reduces sister-chromatid exchange (SCE) events induced by CPT. The distribution of SCE events per cell is shown for the indicated cell samples to the left panels. Blue bars represent no CPT treatment, and red and green bars represent data for 5 nM and 100 nM CPT treatment, respectively. Mean values and photo of a representative SCE (shown by arrowhead) are shown to the right panels.
HR-mediated DSB repair in XPF mutant cells is compromised at a late step
To differentiate between the early and late steps of HR, we analyzed the formation of γ-ray-induced Rad51 foci (Fig. 2C). At 3 h after IR, there was no change in the rate of Rad51 focus formation for Xpf-expressing or Xpf-depleted cells. However, the Rad51 foci continued to be formed by 6 h in Xpf-depleted cells, whereas the foci were decreased in Xpf-expressing cells. This suggests that Xpf is required for the completion of HR after formation of the Rad51 nucleofilament. To assess the role of Xpf in the late steps of HR, i.e., during JM formation and processing, we measured the number of SCEs, which represent crossover-type HR (27). The number of CPT-induced SCEs in Xpf-depleted cells was only 40% of the number in Xpf-expressing cells (Fig. 2D), indicating that Xpf is required for crossover-type HR.
If the mortality of the XPF−/− cells is caused by impaired completion of HR, it might be reversed by blocking the initiation of HR. To test this hypothesis, we disrupted the XRCC3 gene in XPF−/−GdXPF-loxP cells. Xrcc3 facilitates an initial step of HR by promoting the polymerization of Rad51 at DNA lesions (39). To generate XPF−/−/XRCC3−/− cells, we exposed the resulting XPF−/−GdXPF-loxP/XRCC3−/− cells to TAM. This mutant displayed lower levels of cell death (Fig. 3A) and a significant decrease in the number of chromosomal aberrations (Fig. 3B), compared to Xpf-depleted cells, though the XRCC3−/− cells did display moderate genomic instability (23, 39). These observations support the conclusion that Xpf plays a critical role in the completion of HR-mediated repair after the polymerization of Rad51 at DNA lesions.
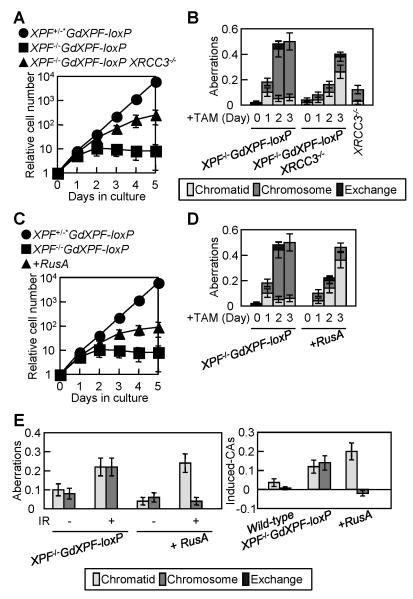
Figure 3. Deletion of the XRCC3 reverses the mutant phenotype of XPF−/− cells.
(A) Growth curve after adding TAM to XPF−/−GdXPF-loxP/XRCC3−/− cells at time zero to inactivate the GdXPF-loxP transgene. (B) Spontaneous chromosomal aberrations in XPF−/−GdXPF-loxP/XRCC3−/− cells were measured as described in Figure 1D. Results of spontaneous chromosomal aberrations for XRCC3−/− cells were described in a previous report (23). (C) Growth curve after adding TAM to the indicated cells at time zero. +RusA represents XPF−/−GdXPF-loxP cells expressing RusA. (D) Spontaneous chromosomal aberrations in the indicated cells were measured as described in Figure 1D. (E) IR-induced chromosomal aberrations in XPF−/−/RusA cells at 1 day after addition of TAM. Chromosomal aberrations induced by γ-rays were measured and calculated as described in Figure 2B.
We next considered that if Xpf contributes to HR after the formation of JMs, the severe phenotype of Xpf-depleted cells might be suppressed by ectopic expression of E. coli RusA resolvase (40). To generate XPF−/− cells stably expressing RusA, XPF−/−GdXPF-loxP cells were transfected with RusA cDNA, and the resulting XPF−/−GdXPF-loxP/RusA cells were exposed to TAM. The RusA expression improved cellular viability (Fig. 3C) and decreased the number of spontaneous chromosomal aberrations in Xpf-depleted cells (Fig. 3D). Intriguingly, the reduction in chromosome-type breaks is associated with an increase in chromatid-type breaks. Similarly RusA expression resulted in a decrease in γ-ray-induced chromosome-type breaks without affecting the number of chromatid-type breaks (Fig. 3E). These data are consistent with the idea that chromosome-type breaks result from a failure to complete HR after the formation of JMs in Xpf-deficient cells.
Human Mus81-Eme1 and Xpf-Ercc1 contribute to HR by processing joint molecules
The absence of both Xpf and Mus81 in XPF−/− DT40 cells provides us with the novel opportunity of investigating the role of human Xpf-Ercc1 and Mus81-Eme1 in HR. To generate XPF−/−/HsXPF-ERCC1 and XPF−/−/HsMUS81-EME1 cells, XPF−/−GdXPF-loxP cells were transfected with HsXpf-Ercc1 or HsMus81-Eme1 cDNA, and XPF−/−GdXPF-loxP cells stably expressing HsXpf-Ercc1 or HsMus81-Eme1 were exposed to TAM. Note that we failed to generate XPF−/−/HsMUS81 cells probably due to instability of HsMus81 as a consequence of poor association with GdEme1. HsXpf-Ercc1 reversed the mortality in XPF−/− DT40 cells (Fig. 4A). Remarkably, HsMus81-Eme1 also significantly restored the cellular proliferation of XPF−/− DT40 cells to a level comparable to the cells complemented with HsXpf-Ercc1 (Fig. 4C), though the amino acid sequence identity between GdXpf and HsMus81 is only 9.7%. Next we analyzed XPF−/−/HsXPF-ERCC1 and XPF−/−/HsMUS81-EME1 cells by counting both spontaneous (Fig. 4B and D) and γ-ray-induced chromosomal aberrations (Fig. 5A and B). In all cases, the ectopic expression of the human enzymes suppressed the chromosomal aberrations caused by Xpf deficiency and particularly chromosome-type breaks. These observations indicate that HsMus81-Eme1 and HsXpf-Ercc1 have very similar functions in HR-mediated DNA repair and genome maintenance.
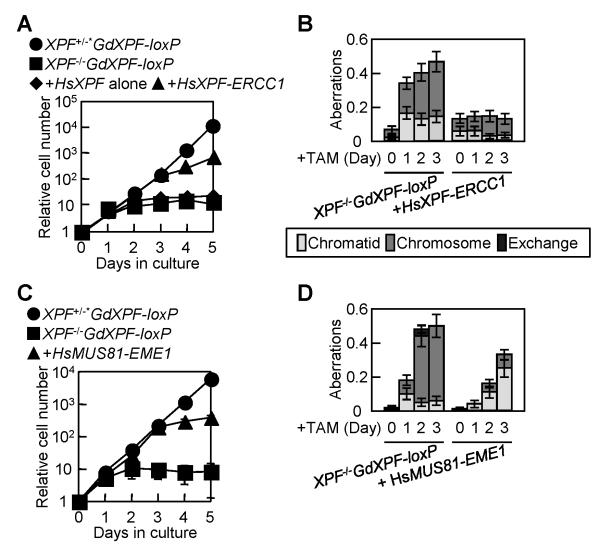
Figure 4. Ectopic expression of HsXpf-Ercc1 or HsMus81-Eme1 suppresses the lethality in XPF−/− cells.
(A) Growth curve after addition of TAM to the indicated cells at time zero. + HsXPF alone and +HsXPF-ERCC1 represent XPF−/−GdXPF-loxP cells expressing HsXpf alone and XPF−/−GdXPF-loxP cells expressing HsXpf-Ercc1, respectively. (B) Spontaneous chromosomal aberrations in the indicated cells were measured as described in Figure 1D. (C) Growth curve after addition of TAM to the indicated cells at time zero. +HsMUS81-EME1 represents XPF−/−GdXPF-loxP cells expressing HsMus81-Eme1. (D) Spontaneous chromosomal aberrations in the indicated cells were measured as described in Figure 1D.
Figure 5. Ectopic expression of H HsXpf-Ercc1 or HsMus81-Eme1 reverses the mutant phenotype of XPF−/− cells.
(A) IR-induced chromosomal aberrations in XPF−/−/HsXPF-ERCC1 cells at 1 day after addition of TAM. Chromosomal aberrations induced by γ-rays were measured and calculated as described in Figure 2B. (B) IR-induced chromosomal aberrations in XPF−/−/HsMUS81-EME1 cells at 1 day after addition of TAM. Chromosomal aberrations induced by γ-rays were measured and calculated as described in Figure 2B. (C) CPT-induced SCE events at 2 days after addition of TAM. The histograms to the left display the distribution of SCEs per cell following treatment with 5 nM CPT. Blue and red bars represent XPF−/− and XPF−/−/HsMUS81-EME1 cells, respectively. Mean values are shown to the right panels.
To determine whether HsMus81-Eme1 promotes crossover-type HR, we counted the number of SCEs in XPF−/−/HsMUS81-EME1 cells. The expression of HsMus81-Eme1 restored the number of CPT-induced SCEs (Fig. 5C) to the number of SCEs in GdXpf-expressing cells (Fig. 2D). We conclude that Mus81 and Xpf have very similar functions in promoting crossover-type HR.
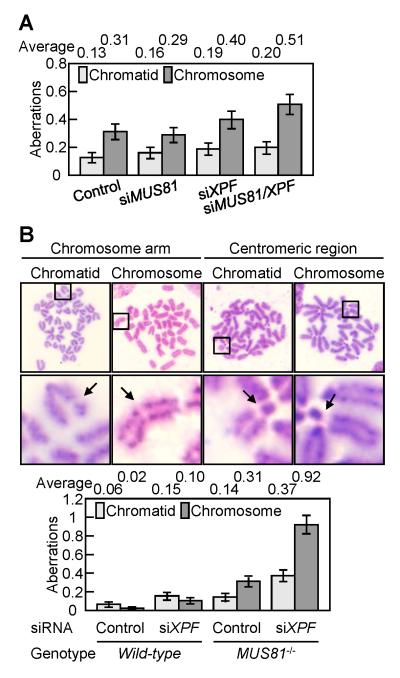
Finally, we tested whether Xpf and Mus81 compensate for each other in the completion of HR in human and mouse cell lines. We depleted Xpf and Mus81 in human HeLa cells or depleted Xpf in mouse MUS81−/− ES cells, using small interfering RNA (siRNA) (Supplementary Fig. S7A and B). In HeLa cells, the depletion of both Xpf and Mus81, but not the depletion of either Xpf or Mus81 alone, increased the number of spontaneous chromosome-type breaks (Fig. 6A). Similarly, depletion of Xpf in mouse MUS81−/− embryonic stem (ES) cells greatly increased the number of spontaneous chromosome-type breaks, compared with Xpf-depleted wild-type and mock-transfected MUS81−/− ES cells, while they showed only a moderate increase in the number of spontaneous chromosome-type breaks (Fig. 6B). These results indicate that the role of Xpf in HR is conserved in mammalian and DT40 cells.
Figure 6. Xpf and Mus81 compensate for each other in the completion of HR in human and mouse cell lines.
(A) After treatment with the indicated siRNAs in human HeLa cells, aberrant chromosomes in metaphase cells (n=200) were analyzed. The vertical axis shows the number of aberrations per cell in A and B.(B) After treatment with the indicated siRNAs in mouse wild-type or MUS81−/− cells, aberrant chromosomes in metaphase cells (n=100) were analyzed. A representative chromatid-type break and a chromosome-type break are magnified in the middle panels and are shown by arrow.
Discussion
We have shown that the Xpf and Mus81 structure-specific endonucleases compensate for each other in the maintenance of chromosomal DNA. This compensatory relationship is verified by the following phenotypic analysis of Xpf-deficient DT40 cells, which lack a MUS81 ortholog. First, the expression of HsMus81-Eme1 as well as HsXpf-Ercc1 reversed the mortality of Xpf-deficient DT40 cells (Figs. 4 and 5). Second, the mortality of Xpf-deficient DT40 cells is in marked contrast to the viability of Xpf-deficient mammalian cells (41, 42) and to the normal development of Mus81-deficient mice (24, 43). Third, although chicken Xpf and Eme1 physically interact with each other (Supplementary Fig. S4B and C), GdXpf-Eme1 has a minor role in genome maintenance (Supplementary Fig. S5), and does not account for mortality of Xpf-deficient DT40 cells. Finally, our findings using DT40 cells are applicable to mammals as evidenced by the fact that inactivation of both Xpf and Mus81 caused increased the number of chromosome-type breaks in human HeLa and mouse ES cells (Fig. 6). These observations demonstrate that the two structure-specific endonucleases have substantially overlapping functions in the maintenance of genomic DNA.
We have provided a few different lines of evidence that collectively indicate that Xpf contributes to HR-dependent DSB repair probably after the formation of JMs made of broken sister chromatids and the other intact ones. First, the inactivation of Xpf resulted in prolonged Rad51 foci formation (Fig. 2C). Second, the expression of Xpf and Mus81 enhanced the formation of SCEs (Fig. 2D and 5C). Third, preventing the initiation of HR by deleting XRCC3 suppressed the mutant phenotype of Xpf-deficient DT40 cells (Fig. 3A and B). These results indicated that Xpf plays a role in HR at a late step. Conceivably, the inactivation of Xpf may cause a defect in the separation of JMs prior to mitosis, which defect is more toxic than the defective formation of JMs due to the absence of XRCC3.
The loss of viability in Xpf-deficient DT40 cells is associated with chromosome aberrations, which appear on metaphase spreads as regions unstained by Giemsa. Throughout we have referred to these as chomatid-type breaks and chromosome-type breaks depending on whether the discontinuity in staining affects one or both sister chromatids, respectively. In the case of chromatid-type breaks, we suspect that the absence of Giemsa staining actually represents a DSB in the sister chromatid as these aberrations are also observed in mutants that are defective for early steps of HR (44). However, we think that the chromosome-type breaks represent regions of uncondensed DNA rather than actual broken chromosomes similar to what was recently reported (45). It is proposed that regions devoid of Giemsa staining occur at sites of sister chromatid entanglement, which inhibits chromosome condensation. Importantly the chromosome-type breaks that we observe in Xpf-deficient cells both spontaneously and following γ-ray exposure are suppressed by ectopic expression of the HJ resolvase RusA (Fig. 3D and E) providing strong evidence that they result from unprocessed JMs. The fact that ectopic expression of HsXpf-Ercc1 and HsMus81-Eme1 also suppress chromosome-type breaks in Xpf-deficient DT40 cells suggests that there is a significant overlap in function between these enzymes that is most likely related to processing JMs such as D-loops and nicked single HJs.
Cells deficient in Xpf-Ercc1 are considerably more sensitive to chemical crosslinking agents than cells deficient in the other NER factors, indicating the critical role for Xpf-Ercc1 in interstrand crosslink (ICL) repair. Although there is compelling evidence that the critical role played by Xpf is carried out by introducing single-strand breaks at cross-links to initiate ICL repair (20, 46), Xpf may function in ICL repair also by facilitating HR. We recently found that Slx4 links the FA-dependent ICL pathway with both Mus81 and Xpf, as Slx4 serves as docking sites for the two nucleases and the ubiquitinated FancD2 protein, which finding agrees with recent reports showing FA patients carrying mutations in the SLX4 gene (47-49). Taking into account the fact that Slx4 also binds to the Slx1 5′-flap endonucleaseand in addition to the Mus81-Eme1 and Xpf-Ercc1 3′-flap endonucleases (Supplementary Fig. S2A and S4D) (12-15), these endonucleases may collaboratively work in ICL repair by promoting the completion of HR. In this scenario, it is not surprising that the two 3′-flap endonucleases are complementary to each other. Future studies will analyze the interdependent and complementary relationships of multiple endonucleases when they carry out a variety of DNA repair reactions.
Supplementary Material
Acknowledgements
We thank R. Ohta and Y. Satoh for their technical assistance. We also thank Dr. M. Lisby for sharing unpublished data.
Grant Support Financial support was provided in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (MEXT) (grant number 20241012 to S.T. and grant number 22700881 to K.K.), a grant from The Kanae Foundation (to K.K.), grants from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement No. HEALTH-F2-2010-259893 and from the Netherlands Genomics Initiative / Netherlands Organization for Scientific Research (to R.K.), and NIH grants GM68418 and CA133093 (to K.Kitagawa).
Footnotes
Conflict of interest: No conflict of interest exists.
References
- 1.Takeda S, Nakamura K, Taniguchi Y, Paull TT. Ctp1/CtIP and the MRN complex collaborate in the initial steps of homologous recombination. Mol. Cell. 2007;28:351–2. doi: 10.1016/j.molcel.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein KA, Rothstein R. At loose ends: resecting a double-strand break. Cell. 2009;137:807–10. doi: 10.1016/j.cell.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bzymek M, Thayer NH, Oh SD, Kleckner N, Hunter N. Double Holliday junctions are intermediates of DNA break repair. Nature. 2010;464:937–41. doi: 10.1038/nature08868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu LaH ID. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–4. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 5.Xu D, Guo R, Sobeck A, Bachrati CZ, Yang J, Enomoto T, Brown GW, Hoatlin ME, Hickson ID, Wang W. RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability. Genes Dev. 2008;22:2843–55. doi: 10.1101/gad.1708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat. Rev. Cancer. 2009;9:644–54. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz E, Heyer WD. Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes. Chromosoma. 2011;120(2):109–27. doi: 10.1007/s00412-010-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macmaster R, Sedelnikova S, Baker PJ, Bolt EL, Lloyd RG, Rafferty JB. RusA Holliday junction resolvase: DNA complex structure—insights into selectivity and specificity. Nucleic Acids Res. 2006;34:5577–84. doi: 10.1093/nar/gkl447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinagawa H, Iwasaki H. Processing the holliday junction in homologous recombination. Trends Biochem. Sci. 1996;21:107–11. [PubMed] [Google Scholar]
- 10.West SC. Processing of recombination intermediates by the RuvABC proteins. Annu. Rev. Genet. . 1997;31:213–44. doi: 10.1146/annurev.genet.31.1.213. [DOI] [PubMed] [Google Scholar]
- 11.Ip SC, Rass U, Blanco MG, Flynn HR, Skehel JM, West SC. Identification of Holliday junction resolvases from humans and yeast. Nature. 2008;456:357–61. doi: 10.1038/nature07470. [DOI] [PubMed] [Google Scholar]
- 12.Andersen SL, Bergstralh DT, Kohl KP, LaRocque JR, Moore CB, Sekelsky J. Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Mol. Cell. 2009;35:128–35. doi: 10.1016/j.molcel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fekairi S, Scaglione S, Chahwan C, Taylor ER, Tissier A, Coulon S, Dong MQ, Ruse C, Yates JR, 3rd, Russell P, Fuchs RP, McGowan CH, Gaillard PH. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell. 2009;138:78–89. doi: 10.1016/j.cell.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz IM, Hain K, Declais AC, Gardiner M, Toh GW, Sanchez-Pulido L, Heuckmann JM, Toth R, Macartney T, Eppink B, Kanaar R, Chris P, Ponting CP, David MJ, Lilley DM, Rouse J. Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol. Cell. 2009;35:116–27. doi: 10.1016/j.molcel.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Svendsen JM, Smogorzewska A, Sowa ME, O’Connell BC, Gygi SP, Elledge SJ, Harper JW. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138:63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osman F, Dixon J, Doe CL, Whitby MC. Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol Cell. 2003;12(3):761–74. doi: 10.1016/s1097-2765(03)00343-5. [DOI] [PubMed] [Google Scholar]
- 17.Holloway JK, Booth J, Edelmann W, McGowan CH, Cohen PE. MUS81 generates a subset of MLH1-MLH3-independent crossovers in mammalian meiosis. PLoS Genet. 2008;4(9):e1000186. doi: 10.1371/journal.pgen.1000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Alex Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo B, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell. Biol. 2004;24:5776–87. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pâques FaH, J.E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knipscheer P, Räschle M, Smogorzewska A, Enoiu M, Ho TV, Schärer OD, Elledge SJ, Walter JC. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science. 2009;326(5960):1698–701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niedernhofer LJ, Essers J, Weeda G, Beverloo B, de Wit J, Muijtjens M, Odijk H, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required for targeted gene replacement in embryonic stem cells. EMBO J. 2001;20:6540–9. doi: 10.1093/emboj/20.22.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buerstedde JMaT, S Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell. 1991;67:179–88. doi: 10.1016/0092-8674(91)90581-i. [DOI] [PubMed] [Google Scholar]
- 23.Fujimori A, Tachiiri S, Sonoda E, Thompson LH, Dhar PK, Hiraoka M, Takeda S, Zhang Y, Reth M, Takata M. Rad52 partially substitutes for the Rad51 paralog XRCC3 in maintaining chromosomal integrity in vertebrate cells. EMBO J. 2001;20:5513–20. doi: 10.1093/emboj/20.19.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPherson JP, Lemmers B, Chahwan R, Pamidi A, Migon E, Matysiak-Zablocki E, Moynahan ME, Essers J, Hanada K, Poonepalli A, Sanchez-Sweatman O, Khokha R, Kanaar R, Jasin M, Hande MP, Hakem R. Involvement of mammalian Mus81 in genome integrity and tumor suppression. Science. 2004;304:1822–6. doi: 10.1126/science.1094557. [DOI] [PubMed] [Google Scholar]
- 25.Motegi A, Sood R, Moinova H, Markowitz SD, Liu PP, Myung K. Human SHPRH suppresses genomic instability through proliferating cell nuclear antigen polyubiquitination. J. Cell Biol. 2006;175(5):703–8. doi: 10.1083/jcb.200606145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi-Iwai Y, Sonoda E, Sasaki MS, Morrison C, Haraguchi T, Hiraoka Y, Yamashita YM, Yagi T, Takata M, Price C, Kakazu N, Takeda S. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 1999;18:6619–29. doi: 10.1093/emboj/18.23.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonoda E, Sasaki MS, Morrison C, Yamaguchi-Iwai Y, Takata M, Takeda S. Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol. Cell. Biol. 1999;19:5166–9. doi: 10.1128/mcb.19.7.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. http://genome.ucsc.edu/cgi-bin/hgBlat.
- 29.Abraham J, Lemmers B, Hande M,P, Moynahan ME, Chahwan C, Ciccia A, Essers J, Hanada K, Chahwan R, Khaw AK, McPherson P, Shehabeldin A, Laister R, Arrowsmith C, Kanaar R, West SC, Jasin M, Hakem R. Eme1 is involved in DNA damage processing and maintenance of genomic stability in mammalian cells. EMBO J. 2003;22:6137–47. doi: 10.1093/emboj/cdg580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanada K, Budzowska M, Modesti M, Maas A, Wyman C, Essers J, Kanaar R. The structure-specific endonuclease Mus81-Eme1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. EMBO J. 2006;25:4921–32. doi: 10.1038/sj.emboj.7601344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciccia A, Ling C, Coulthard R, Yan Z, Xue Y, Meetei AR, et al. Identification of FAAP24, a Fanconi anemia core complex protein that interacts with FANCM. Mol Cell. 2007;25(3):331–43. doi: 10.1016/j.molcel.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Sonoda E, Sasaki MS, Buerstedde JM, Bezzubova O, Shinohara A, Ogawa H, Takata M, Yamaguchi-Iwai Y, Takeda S. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 1998;17:598–608. doi: 10.1093/emboj/17.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakahara M, Sonoda E, Nojima K, Sale JE, Takenaka K, Kikuchi K, Taniguchi Y, Nakamura K, Sumitomo Y, Bree RT, Lowndes NF, Takeda S. Genetic evidence for single-strand lesions initiating Nbs1-dependent homologous recombination in diversification of Ig v in chicken B lymphocytes. PLoS Genet. 2009;5:e1000356. doi: 10.1371/journal.pgen.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K, Kogame T, Oshiumi H, Shinohara A, Sumitomo Y, Agama K, Pommier Y, Tsutsui KM, Tsutsui K, Hartsuiker E, Ogi T, Takeda S, Taniguchi Y. Collaborative action of Brca1 and CtIP in elimination of covalent modifications from double-strand breaks to facilitate subsequent break repair. PLoS Genet. 2010;6:e1000828. doi: 10.1371/journal.pgen.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada T, Sonoda E, Yamashita YM, Koyoshi S, Tateishi S, Yamaizumi M, Takata M, Ogawa O, Takeda S. Involvement of vertebrate polkappa in Rad18-independent postreplication repair of UV damage. J. Biol. Chem. 2002;277:48690–5. doi: 10.1074/jbc.M207957200. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi K, Taniguchi Y, Hatanaka A, Sonoda E, Hochegger H, Adachi N, Matsuzaki Y, Koyama H, van Gent DC, Jasin M, Takeda S. Fen-1 facilitates homologous recombination by removing divergent sequences at DNA break ends. Mol. Cell. Biol. 2005;25:6948–55. doi: 10.1128/MCB.25.16.6948-6955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010;17:421–33. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair. 2006;5:1021–9. doi: 10.1016/j.dnarep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Takata M, Sasaki MS, Tachiiri S, Fukushima T, Sonoda E, Schild D, Thompson LH, Takeda S. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralog. Mol. Cell. Biol. 2001;21:2858–66. doi: 10.1128/MCB.21.8.2858-2866.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blais V, Gao H, Elwell CA, Boddy MN, Gaillard PH, Russell P, McGowan CH. RNA interference inhibition of Mus81 reduces mitotic recombination in human cells. Mol. Biol. Cell. 2004;15(2):552–62. doi: 10.1091/mbc.E03-08-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brookman KW, Lamerdin JE, Thelen MP, Hwang M, Reardon JT, Sancar A, Zhou ZQ, Walter CA, Parris CN, Thompson LH. ERCC4 (XPF) encodes a human nucleotide excision repair protein with eukaryotic recombination homologs. Mol. Cell. Biol. 1996;16:6553–62. doi: 10.1128/mcb.16.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian M, Shinkura R, Shinkura N, Alt FW. Growth retardation, early death, and DNA repair defects in mice deficient for the nucleotide excision repair enzyme XPF. Mol. Cell. Biol. 2004;24:1200–5. doi: 10.1128/MCB.24.3.1200-1205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dendouga N, Gao H, Moechars D, Janicot M, Vialard J, McGowan CH. Disruption of Murine Mus81 Increases Genomic Instability and DNA Damage Sensitivity but Does Not Promote Tumorigenesis. Mol. Cell. Biol. 2005;25:7569–79. doi: 10.1128/MCB.25.17.7569-7579.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonoda E, Okada T, Zhao GY, Tateishi S, Araki K, Yamaizumi M, Yagi T, Verkaik NS, van Gent DC, Takata M, Takeda S. Multiple roles of Rev3, the catalytic subunit of polzeta in maintaining genome stability in vertebrates. EMBO J. 2003;22:3188–97. doi: 10.1093/emboj/cdg308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wechsler T, Newman S, West SC. Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature. 2011;471(7340):642–6. doi: 10.1038/nature09790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang AT, Sengerová B, Cattell E, Inagawa T, Hartley JM, Kiakos K, Burgess-Brown NA, Swift LP, Enzlin JH, Schofield CJ, Gileadi O, Hartley JA, McHugh PJ. Human SNM1A and XPF-ERCC1 collaborate to initiate DNA interstrand cross-link repair. Genes Dev. 2011;25(17):1859–70. doi: 10.1101/gad.15699211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crossan GP, van der Weyden L, Rosado IV, Langevin F, Gaillard PH, McIntyre R.E.; Sanger Mouse Genetics Project, Gallagher, F., Kettunen MI, Lewis DY, Brindle K, Arends MJ, Adams DJ, Patel KJ. Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat. Genet. 2011 doi: 10.1038/ng.752. doi:10.1038/ng.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y, Lach FP, Desetty R, Hanenberg H, Auerbach AD, Smogorzewska A. Mutations of the SLX4 gene in Fanconi anemia. Nat. Genet. 2011 doi: 10.1038/ng.750. doi:10.1038/ng.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoepker C, Hain K, Schuster B, Hilhorst-Hofstee Y, Rooimans MA, Steltenpool J, Oostra AB, Eirich K, Korthof ET, Nieuwint AW, Jaspers NG, Bettecken T, Joenje H, Schindler D, Rouse J, de Winter JP. SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat. Genet. 2011 doi: 10.1038/ng.751. doi:10.1038/ng.751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.