Abstract
The Mendelsohn maneuver, voluntary prolongation of laryngeal elevation during the swallow, has been widely used as a compensatory strategy to improve upper esophageal sphincter (UES) opening and bolus flow. Recent research suggests that when used as a rehabilitative exercise, it significantly improves durations of hyoid movement and positively impacts duration of UES opening. The current data were derived from that same prospective crossover study of 18 participants with dysphagia post-stroke evaluated with videofluoroscopy after treatment using the Mendelsohn maneuver versus no-treatment. Results demonstrate gains in extent of hyoid movement and UES opening and improvements in coordination of structural movements with each other as well as with bolus flow.
Keywords: Deglutition, Deglutition Disorders, Stroke, Rehabilitation, Mendelsohn Maneuver
Introduction
The Mendelsohn Maneuver is a voluntary prolongation of hyolaryngeal elevation at the peak of the swallow1. During normal swallowing, the hyoid bone rises superiorly and anteriorly, thereby pulling on the thyroid cartilage and cricoid cartilage. The pull on the cricoid creates traction on the upper esophageal sphincter (UES), allowing for about a 6 mm opening2–3. Timing, coordination, and muscle strength are critical for success of the swallow and are often impaired post-stroke3. When the UES does not open sufficiently, the bolus cannot pass efficiently into the esophagus. A common cause of this is decreased hyoid movement4. Research suggests use of the Mendelsohn maneuver as a compensatory strategy increases laryngeal elevation and maximal hyoid superior displacement and provides an immediate effect in prolonging the duration of UES opening but not the diameter5,6. Bolus passage through the pharynx improves. One problem with the use of the Mendelsohn maneuver as a compensatory strategy is that the individuals most likely to use it are elderly patients with musculature susceptible to fatigue in our predominating Type II muscle fibers of the oropharynx7. Adding strenuous compensation to the swallow of a compromised individual for the duration of a meal may exacerbate rather than ameliorate the problem. But can the Mendelsohn be used as a rehabilitative exercise, meaning it impacts either strength or coordination of the swallow in a manner that leads to permanent changes in the physiologic substrates of swallowing?
Whereas several studies have demonstrated the potential of the Mendelsohn maneuver for improving swallowing physiology when used as part of a larger treatment regimen3,8–9, a recent study reported effects of the Mendelsohn maneuver on measures of swallowing duration when used as a rehabilitative exercise in isolation10. Improvements in duration of hyoid maximum elevation (DOHME) and duration of hyoid maximum anterior excursion (DOHMAE) were significant (p = .011 and .009 respectively) at two weeks post-treatment compared to two weeks of no-treatment, and improvements were more substantive after two weeks of treatment than one week. Duration of upper esophageal sphincter opening (DOUESO) increased/improved, though not significantly. These results reinforced early findings regarding the immediate effects of the maneuver and demonstrated its potential to more permanently change the physiological substrates of swallowing through exercise.
Reductions in hyoid movement have been reported in patients with dysphagia post-stroke along with increases in aspiration11–12. Even small changes in the durations and/or extents of such movements can impact the timing and coordination of the swallow. It is, therefore, important to consider extent of hyoid movement and extent of UES opening in conjunction with—as well as in relation to—durations of hyoid movements and duration of UES opening. The purpose of this study is to provide data regarding effects of the Mendelsohn maneuver on the distance the hyoid travels superiorly (maximum hyoid elevation/MHE) and anteriorly (maximum hyoid anterior excursion (MHAE), and the impact on the mean width of UES opening (MWUESO).
METHODS
Participants
Participants were recruited through advertising and referrals at a university medical center, as well as word of mouth via area speech-language pathologists. All participants provided written consent and all procedures were approved by the hospital’s Institutional Review Board. Eighteen individuals, age 21 years and older, who suffered a stroke and were dysphagic participated in this investigation. Each was between six weeks and 22 months post stroke (9.5 months average) at the time of participation. None had experienced a prior stroke and none had a previous history of dysphagia or treatment for dysphagia. All participants scored 75 or higher on the Modified Mini-Mental State Examination. Individuals with current/history of tracheotomy or other structural alteration to the swallowing mechanism, history of swallowing problems prior to the stroke, progressive neurologic disease, gastroesophageal reflux disease, or cognitive and/or physical problems which would have impeded understanding or completion of the therapeutic tasks were excluded. A history questionnaire and a cranial nerve/oral motor screen helped determine the exact nature of the stroke and further define overall impairment. MRIs or CT Scans were obtained when available. In the absence of neuroimaging information, a neurological examination was performed by the study physician.
After written consent was obtained, each participant underwent an initial VFSS to ensure physiologic fit with the study as well as a baseline of swallowing function. If swallowing function appeared to be normal or did not meet the above inclusion criteria related to swallow physiology the participant was withdrawn from the study. Physiological fit means participants who had pharyngeal dysphagia characterized by any apparent reductions in hyolaryngeal elevation and or UES opening and evidence of some type of residue in the pharynx. Each participant also had to be on a restricted diet, defined by need for a nasogastric, jejunostomy, or percutaneous endoscopic gastrostomy tube, or an oral diet that was altered in any way due to swallowing difficulty. Individuals with an absent swallow were not included in the study. All individuals had to demonstrate at least a minimal functional swallow with some material passing through the UES. Aspiration was not required for participation.
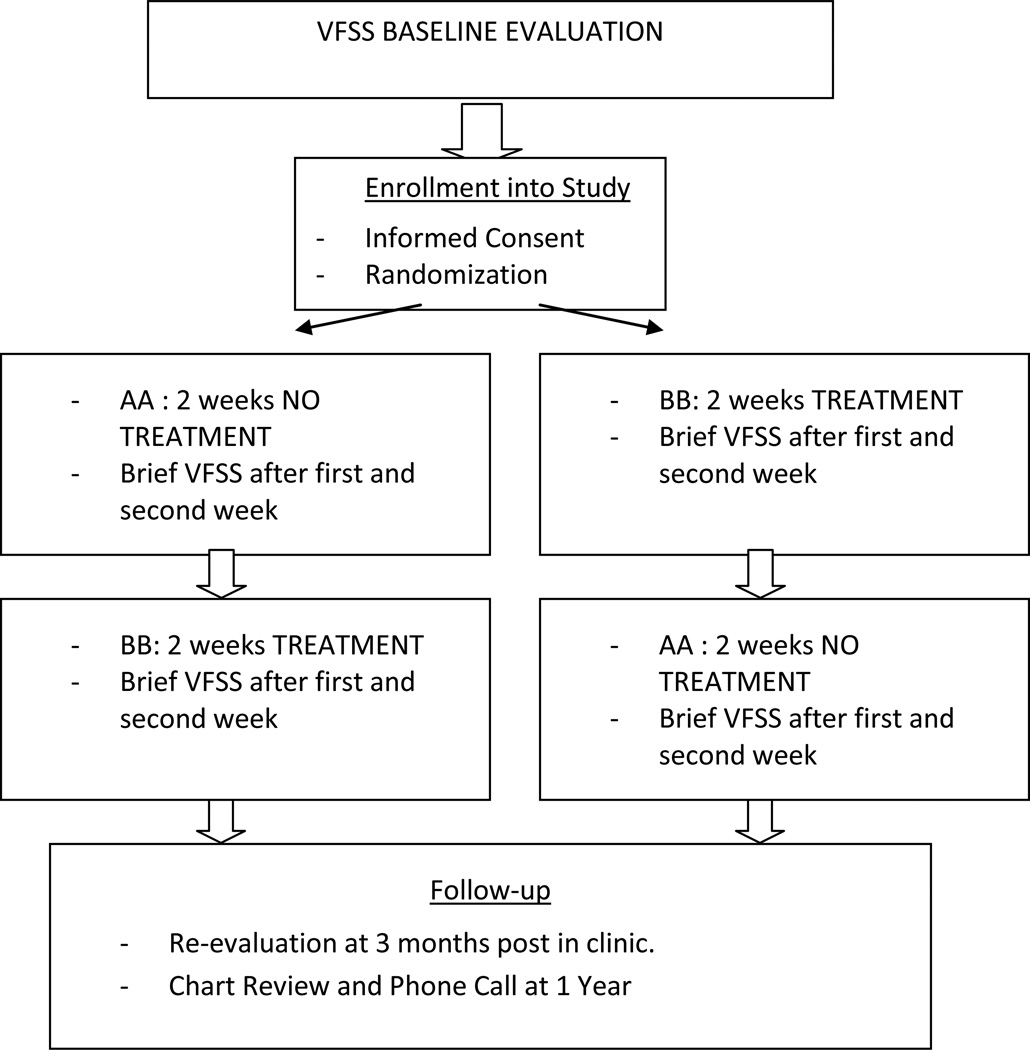
Each qualifying individual was randomized, via pre-study blinded number drawing, into one of two groups: Group A received two weeks of treatment followed by two weeks of no-treatment (BBAA) and Group B received two weeks of no treatment followed by two weeks of treatment (AABB). VFSSs were conducted at the end of each week of the study (A or B) to allow for dose-response comparisons of baseline measures of swallowing with measures at 1 and 2 weeks post treatment and no-treatment.
A schematic of the study design is provided in Figure 1.
Figure 1.
Videofluoroscopic Swallowing Studies (VFSS)
Participants swallowed three 3 mL thin liquids (E-Z-HD Barium sulfate powder for suspension and water/50–50; approximately 14 centipoise) and three 3 mL purees (3 parts applesauce to 1 part barium powder) for each study. All swallows were viewed in the lateral plane with a view of the entire oropharynx from the lips anteriorly to the posterior pharyngeal wall and from the hard palate superiorly to below the UES. Participants were evaluated on a Digital Fluoroscope (Shimadzu Model F100-02 for most), and results were transferred to Sony S-VHS through a FOR-A (Fort Lee, NJ) 100 millisecond videotimer (model VTG 33).
Treatment Sessions
During treatment weeks (B weeks), participants were seen twice a day for sessions lasting roughly 45 minutes with a 2–3 hour break in between sessions depending on participants’ schedules and availability. Each participant was taught the Mendelsohn maneuver, the process of squeezing and holding the larynx at the peak of the swallow, using surface electromyography (SEMG) biofeedback. SEMG biofeedback was provided via a two channel Pathway MR-20 (Prometheus Group, Dover, NH). The electrode pad was placed submentally at midline halfway between the mental symphysis and the tip of the hyoid bone. The signal derived from muscle activity was rectified and low pass filtered to produce a smooth signal. SEMG tracings were used only for participant biofeedback. Treatment sessions were administered primarily by the PI with some assistance from a study clinician once participants were well-trained with the treatment protocol.
In treatment, the PI demonstrated the maneuver and provided visual feedback from the computer as well as tactile feedback via laryngeal palpation (the participant feeling the rise, squeeze, and fall of the PIs larynx and then his own). Prior to each swallow, dental swabs were dipped in ice water and delivered to the mouth to provide a small amount of water to facilitate swallowing throughout the session.
Beginning with session 2, each participant began the standard regimen of treatment of 30 to 40 swallows per session utilizing the Mendelsohn maneuver. Participants were first baselined—meaning they were asked to swallow hard without looking at the computer. An SEMG target line for amplitude was then set at 5 microvolts above their mean established from three baseline swallows. This was to ensure the swallows were made with sufficient effort. The clinician then asked the participant to face the screen and swallow “long and strong” with a squeeze at the peak of the swallow for 3 to 4 seconds. The dental swab was delivered to the participant’s mouth by the clinician and the participant watched the video monitor and performed the maneuver. The clinician froze the video frame after each swallow and provided visual and verbal feedback regarding the strength (amplitude) and duration (seconds) of the SEMG tracing. Forty swallows per session were targeted, but participants were allowed to stop at a minimum of 30 if they showed signs of uncomfortable fatigue. At least 30 Mendelsohn swallows were completed during each treatment session. A successful Mendelsohn swallow meant the participant was able to swallow and sustain laryngeal elevation for approximately 2 seconds or greater. This duration was chosen based on prior investigations3,8–9.
Data Analysis
Primary Analysis
To analyze hyoid maximum anterior excursion (HMAE) and hyoid maximum elevation (HME) and the extent of UES opening (UESO), each SVHS tape from VFSS was digitized. Adobe Premiere Pro 1.5, a video editing program, was employed in conjunction with a Sony DVMC-DA1 Media Converter. Digitization provided clear images for analysis of structural movements. When structural movements of the hyoid or UES opening were not clearly visible, the data were not analyzed.
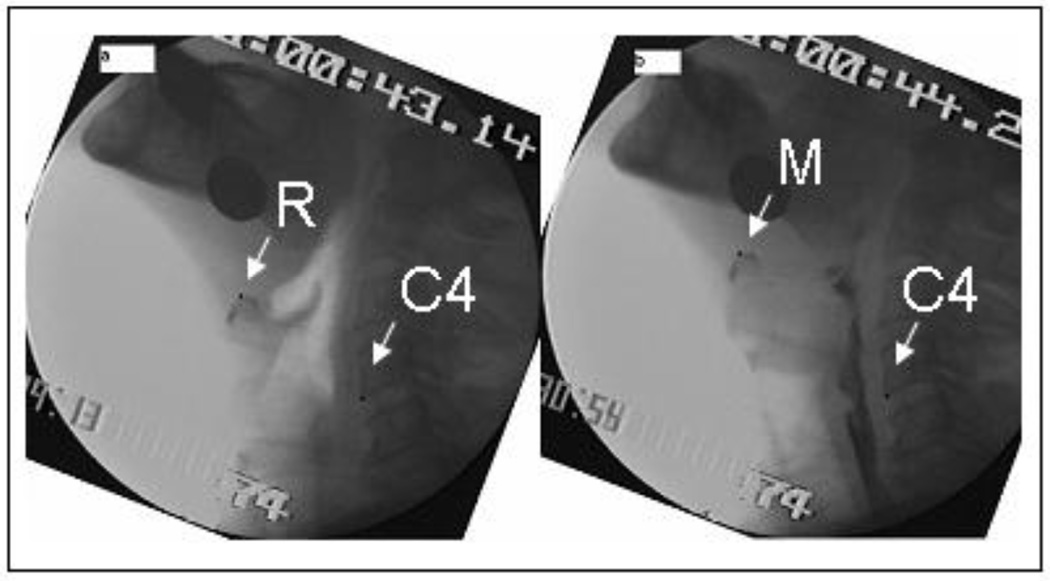
As described in previous works11,13, two frames were generated for hyoid movement, one at rest and one at maximum displacement (see Figure 2). Each picture was submitted to the ImageJ 1.32j program. Pictures were first rotated to a true 90 degrees to make calculations of anterior and vertical hyoid movements easier and assure calculation relative to the vertebral column. The distance between the anterior corners (superior and inferior) of C3 was used as the known length (15mm) to mark points to actual size. Fifteen millimeters was the average length of C3 found in cadavers in the physiology lab at Northwestern University11. The following points were marked on each digitized frame: (1) a point on the anterior-inferior corner of C4, which serves as an anchor point, and (2-a) a point on the resting position of the hyoid bone, or (2-b) a point on the most superior anterior position of the hyoid bone, which represented maximum displacement. The Image J program calculated the values of each point (x,y) for two frames: resting frame and maximum displacement frame. The following calculations were then made:
Anterior displacement: (x2-x1) – (C4x2 – C4x1)
Vertical displacement: (y2-y1) – (C4y2-C4y1)
where x1 and y1 are the starting (rest frame) coordinates of the structure of interest and x2 and y2 are the comparison image coordinates (e.g., maximum excursion coordinates). C4x1 and C4y1 are the coordinates of the anchor point in the rest frame, and C4x2 and C4y2 are the coordinates of the anchor point in the comparison frame. For mean width of UES opening (MWUESO), one picture frame showing maximum width of UES opening during the swallow was generated for each swallow. Each picture frame was then submitted to the ImageJ 1.32j program. MWUESO was measured at the narrowest point between C4 and C6 when bolus flows maximally in this zone during the swallow14. Some swallows were excluded from analysis because head tilting and movement created poor visibility of the hyoid bone and upper esophageal sphincter.
Figure 2.
A multivariate analysis of variance (MANOVA) was employed with HME, HMAE, and MWUESO as dependent variables and period (treatment v. no-treatment) as the independent variable. Consistency was included as a covariate.
In addition to measures of hyoid movement and UES opening, each swallow was rated on an 8-point penetration-aspiration scale15–16, a scale of oropharyngeal residue (0 = none, 1 = trace coating, 2 = pooling)17, and the DOSS18. The 8-point penetration-aspiration (P/A) scale rates P/A on the depth of the misdirected bolus into the airway (i.e., above, on, or below the vocal folds) and the participant’s response to it (i.e., coughed but did not clear, coughed and cleared, no cough). A rating of 1 is no P/A, 5 is penetration to the vocal folds, and 6 and greater are aspiration events. The DOSS is a seven point scale where 7 indicates normal swallowing and 1 and 2 indicate severe dysphagia where nothing is allowed by mouth or only therapeutic feedings. Scores in the 3–5 range indicate mild to moderate impairment where diets are adjusted and compensatory strategies are used.
Additional Analyses
Dose response was examined with univariate ANOVA with the three primary distance measures as dependent variables and study number as the independent variable. One month follow up analysis was conducted as a t-test between the baseline examination and the examination at 1 month. To examine changes in coordination between HME, HMAE, and MWUESO and measures of bolus flow, Pearsons correlations were employed.
RESULTS
Reliability
Intrajudge (rated twice by the second author) and intrajudge (rated by a second independent judge) reliability were established on the three primary measures based on approximately a 15% sampling. Pearson correlation coefficients were significant for both: interjudge (r=.85, p < .01) and intrajudge (r=.90, p < .01). Also for reliability, the median and interquartile range of the differences between judges (inter) and within a judge (intra) were measured. The median and interquartile range of the difference between judges was 0 and .40, respectively; and within a judge, .02 and .56, respectively.
Distance Measures
Of the original 18 subjects, only 17 were analyzed for this report due to loss of imaging quality in data collection for one subject. Table 1 provides results for the primary outcome measures of Hyoid Maximum Elevation (HME), Hyoid Maximum Anterior Excursion (HMAE), and mean width of UES opening (MWUESO) in millimeters. Results indicate all three measures increased more after a two-week treatment period than a two-week no treatment period, though results were not significant (F (3,206) = 1.875, p = .135; Wilk’s λ= .973; partial eta squared = .027). The covariate of consistency (liquid v. puree) significantly affected MWUESO (p=.03) (i.e., increasing consistency translated into increased width of UES opening). When consistency was held constant, the statistical impact of treatment versus no treatment was slightly stronger for MWUESO but still fell short of significance. The greatest improvement was observed for hyoid elevation (HME) in a follow-up univariate test (F(1,208) = 3.111, p=.079).
Table 1.
Distance measures in centimeters at 1 and 2 weeks post-treatment, 1 and 2 weeks post-no treatment, and 1 month follow-up.
| Duration Measure |
Mean Pre- Treatment |
Mean 1 Week Treatment |
Mean 2 Weeks Treatment |
Mean 1 Week No Treatment |
Mean 2 Weeks No Treatment |
1 Month Follow-up |
|---|---|---|---|---|---|---|
| Hyoid Maximum Anterior Excursion |
1.122 (.952–1.292) SE = .09 |
.947 (.695–1.268) SE = .13 |
1.225 (.977–1.974) SE = .13 |
1.189 (.975–1.450) SE = .12 |
1.066 (.887–1.274) SE = .09 |
.847 (.715–.979) SE = .06 |
| Hyoid Maximum Elevation |
1.473 (1.244–1.702) SE = .11 |
1.422 (1.03–1.926) SE = .21 |
1.990 (1.618–2.362) SE = .17 |
1.753 (1.40–2.132) SE = .17 |
1.678 (1.604–1.948) SE = .15 |
1.880 (1.667–2.093) SE = .10 |
| Mean Width UES Opening |
.890 (.820–.960) SE = .04 |
.806 (.647–.966) SE = .07 |
.919 (.764–1.076) SE = ..05 |
.937 (.854–1.020) SE = .04 |
.906 (.775–1.036) SE = .06 |
.932 (.772–1.091) SE = ..07 |
UES = Upper Esophageal Sphincter; SE = standard error of mean; numbers in parentheses = 95% confidence intervals for means
A separate MANOVA run from initial baseline to final VFSS was also not significant (F (3,137) = 2.171, p = .094; Wilk’s λ .955, partial eta squared = .019), but univariate follow up testing highlighted the relative strength of HME compared to changes in other measures (F (1,139) = 4.337, p = .02). HMAE and MWUESO also increased from baseline to final VFSS, though not significantly.
Dose Response
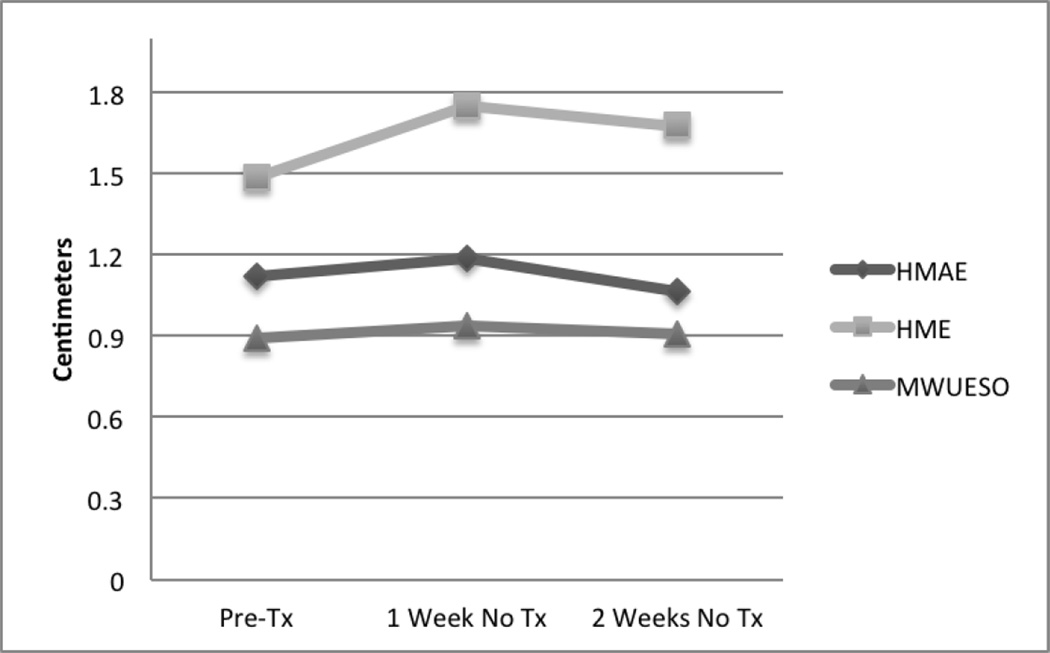
Dose response was examined with univariate ANOVA with the three primary distance measures as dependent variables and study number as the independent variable. Results indicated that whereas differences between no-treatment weeks and treatment weeks were not significant (HMAE, F=1.158, p = .33; HME, F=2.209, p = .07; MWUESO, F=2.138, p=.08), differences between the first and second weeks of treatment were significant (HMAE, F=2.44, p=.05; HME, F=3.396, p=.01; MWUESO, F=3.227, p=.016). Visual examination of Dose Response data are provided in Figures 3 and 4. Figure 3 provides visual comparison of pre-treatment values for distance measures compared with values after one week and two weeks of no treatment. All measures were observed to increase slightly after a week of no treatment and decrease after two weeks of no treatment. Only values for Hyoid Maximum Anterior Excursion remained above baseline value after two weeks of no treatment. The figure clearly indicates limited change in the no treatment conditions and worsening of values with two weeks of no treatment compared with one week of no treatment.
Figure 3.
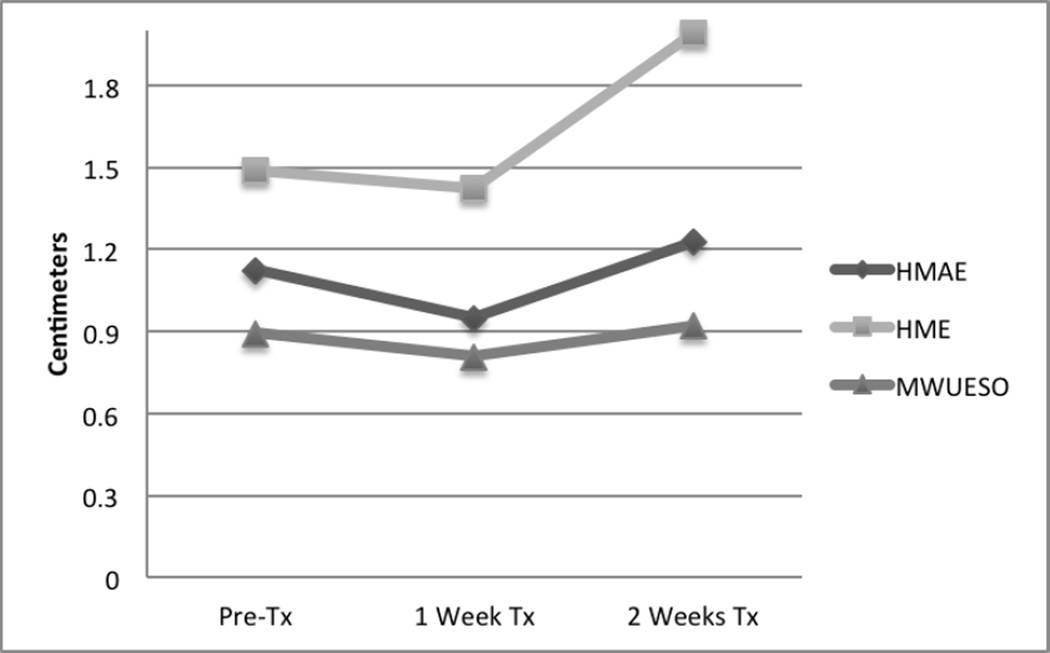
Figure 4.
Figure 4 provides the same comparisons of baseline values after one week and two weeks of treatment. Results are quite the opposite of the no treatment condition. All measures were observed to decrease (worsen) after one week of treatment and increase (improve) above baseline after two weeks of treatment. The figure clearly indicates that two weeks of intervention provides an increased benefit to one week of intervention.
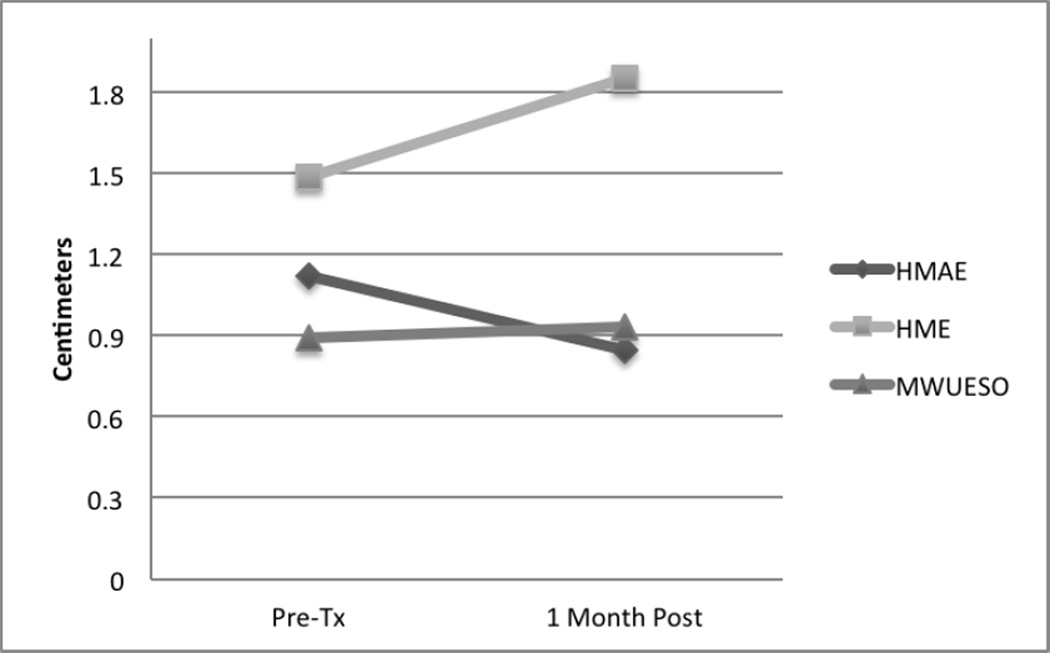
1 Month Follow-up
One month follow up analysis was conducted as a t-test between the baseline examination and the examination at 1 month. Values are provided in Table 1 and Figure 5 with comparisons to baseline, or pre-treatment, values. Results are as follows: HMAE, F=1.902, p=.07; HME, F=2.141, p=.04; MWUESO, F=.697, p=.693. Using a Bonferoni correction, non of the values were statistically significant. As Figure 5 shows, HMAE, or hyoid maximum anterior excursion, declined after the study treatments were stopped, but gains in HME, or hyoid maximum elevation, and MWUESO, or mean width of UES opening, were relatively maintained.
Figure 5.
Coordination of Movements and Bolus Flow
Pearsons Correlations were employed to examine whether any improvements in coordination of structural movements and bolus flow occurred. Results are provided in Table 2. Before treatment, only Dysphagia Outcome and Severity Scores (DOSS) were correlated with hyoid maximum anterior excursion (HMAE)—meaning greater hyoid anterior excursion was associated with less dysphagia severity/higher score (r=.419, p=.002). Post-treatment, however, a number of measures were correlated between structural movements and bolus flow. Correlations of movements with other movements are italicized and suggest some improvement in coordination of anterior and superior movements of the hyoid bone, as well as coordination of mean width of UES opening (MWUESO) with hyoid maximum elevation (r=.348, p=.003). Penetration/Aspiration scores (r= −.379, p=.001) decreased (improved) and DOSS severity scores (r=.613, p=.000) increased (improved) as hyoid maximum elevation (HME) increased; and they also improved with increases in mean width of UES opening (MWUESO) (P/A r=−.419, p=.000; DOSS r=.432, p=.000).
Table 2 .
Significant Pearsons’ correlations with primary measures before and after treatment.
| Measure | Pre-Treatment | Post-Treatment |
|---|---|---|
| Hyoid Maximum | DOSS (r=.419, .002) | Hyoid Maximum Elevation |
| Anterior Excursion | (r = .416, .000) | |
| DOSS | ||
| (r=.306, .010) | ||
| Hyoid Maximum | Mean Width UES Opening | |
| Elevation | (r=.348, .003) | |
| Hyoid Maximum Anterior Excursion | ||
| (r=.416, .000) | ||
| Penetration/Aspiration | ||
| (r=−.379, .001) | ||
| Pyriform Residue | ||
| (r=−.429, .000) | ||
| DOSS | ||
| (r=.613, .000) | ||
| Mean Width UES Opening | Hyoid Maximum Anterior Excursion | |
| (r=.256, .034) | ||
| Hyoid Maximum Elevation | ||
| (r=.348, .003) | ||
| DOSS | ||
| (r=.432, .000) | ||
| Penetration/Aspiration | ||
| (r=−.419, .000) | ||
| Pyriform Residue | ||
| (r=−.280, .020) |
UES = Upper Esophageal Sphincter; DOSS = Dysphagia Outcome and Severity Scale
Functional Outcomes & Follow-Up
As reported previously10, changes in overall dysphagia severity and oral intake were monitored using the Dysphagia Outcome and Severity Scale, a 7 point severity scale where 1 is no oral intake and 7 is full oral intake without restrictions. While participants did improve from an initial average of 3.92 to an average of 4.57, no significant differences were observed between dysphagia severity after treatment (mean = 4.49) versus no-treatment (mean = 4.61) weeks.
At 1 year all original 18 participants were recontacted by phone or, when possible, in person. Unfortunately, at 1 year post, 4 participants were deceased due to unrelated illnesses and 3 were unable to be located. Of the remaining 11, the two who were most severe remained largely NPO, three were maintaining full oral diets, and six were maintaining oral diets with minimal restrictions.
DISCUSSION
The Mendelsohn maneuver was intended to volitionally augment UES opening by prolonging elevation of the larynx for an extended period of time during the swallow1. Holding the hyoid bone and larynx in a maximally extended swallowing position creates a longer period of pull on the cricoid cartilage. As the pull on the cricoid creates traction on the upper esophageal sphincter (UES), it makes sense that UES opening could be prolonged2–3. Whereas the maneuver was initially intended as a compensatory strategy, and data have demonstrated immediate improvements in UES opening5–6, the reality of elderly individuals with dysphagia being able to use the maneuver every swallow of every meal should, perhaps, give us pause. Type II muscle fibers so prominent during swallowing are susceptible to fatigue7, and aging muscles with reduced numbers of functional motor units may prove even more susceptible. Several studies have reported the utility of the Mendelsohn maneuver as part of a rehabilitative program for improving swallow physiology3,8–9. These studies3,8–9 used the Mendelsohn maneuver as an exercise in conjunction with other exercises, including the effortful swallow— also used as a compensatory strategy—among others. All of those studies reported a majority of individuals with chronic dysphagia improved swallow physiology as a result of the exercises. Which specific exercises contributed which improvements in physiology could not be determined from the methodology.
The purpose of this study was to determine whether any lasting physiologic changes in swallowing function can occur from utilizing the Mendelsohn maneuver as an exercise. Our sample was relatively small (N=17) and served primarily to determine if larger studies were warranted and to refine a protocol for such investigation should it be warranted. Participants performed Mendelsohn maneuvers between 30 and 40 times in therapy sessions but did not utilize the Mendelsohn when swallowing during VFSS examinations or when eating at home. Our data from this exercise regimen do demonstrate some physiologic changes in the extent of hyoid movement during the swallow and concurrent opening of the UES. Hyoid maximum elevation (HME) was the only measure to demonstrate statistically significant gains, though hyoid maximum anterior excursion (HMAE) and mean width of UES opening (MWUESO) also improved. It is likely that with a larger sample, this trend would reach statistical significance. Anterior movement of the hyoid is considered to be more important than superior movement for affecting the UES19, which, potentially, explains why both were similarly non-significant. Results must be considered in light of a pilot sample. However, these are the first data to suggest use of the Mendelsohn maneuver as an exercise appears to have at least some lasting physiologic impact on both the extent of structural movements of the hyoid and the UES.
Perhaps more important are the statistically significant improvements in correlations among these structural movements (i.e., HME with HMAE and MWUESO) and consequent improvements in correlations with bolus flow (i.e., penetration/aspiration and residue), as well as the measure of dysphagia severity employed (DOSS). Lazarus and colleagues5 reported the Mendelsohn maneuver had an immediate positive impact on the timing of swallowing events in relation to one another—which is critical for successful bolus transit. Our results suggest these improvements are not only immediate but, when used as an exercise, create a lasting impact on swallowing coordination. Timing of swallowing events was the primary target of Crary and colleagues3 in their study utilizing the Mendelsohn maneuver as one of several rehabilitative strategies. The authors utilized surface electromyography to demonstrate changes in the organization and timing of swallowing-related events. Patients with chronic dysphagia made substantive clinical gains in their study, moving from tube feeds to oral diets and, in most cases, maintaining them. The results of the current investigation indicate that even in isolation, the Mendelosohn has a lasting impact on these same measures. Clinically functional gains in dysphagia severity (DOSS 3.92 to DOSS 4.57) were observed in this study post Mendelsohn exercise with 9 of 11 patients maintaining oral feeding with minimal or no restrictions at 1 year. Dysphagia is complex and multi-faceted, and it will certainly be a combination of approaches that heals the individual with swallowing impairment rather than a single exercise. Nevertheless, research must clarify what individual exercises offer to the greater good.
Duration of hyoid maximum elevation (DOHME) and duration of hyoid maximum anterior excursion (DOHMAE), along with duration of UES opening (DOUESO), can also substantively impact timing and coordination of the swallow. A prior report from this study focused on DOHME, DOHMAE, and DOUESO10. Results were statistically significant for increases in DOHME and DOHMAE post-treatment compared to no-treatment periods (see Table 3). Duration of UES opening also improved, but fell short of statistical significance. Comparing those data to the current data, it seems possible that using the Mendelsohn maneuver as a rehabilitative exercise may have a greater impact on swallowing durations than structural movements, though changes in the coordination of the structural movements with measures of duration requires further investigation. When the Mendelsohn was used as a compensatory strategy, duration measures also appeared to be more affected than measures of structural movements5–6.
Table 3.
Duration measures in seconds at 1 and 2 weeks post-treatment and 1 and 2 weeks post-no treatment compared with pre-treatment.
| Duration Measure |
Mean Pre- Treatment |
Mean 1 Week Treatment |
Mean 2 Weeks Treatment |
Mean 1 Week No Treatment |
Mean 2 Weeks No Treatment |
|---|---|---|---|---|---|
| DOHMAE | .222 (.200–.261) SE = .02 |
.223 (.199–.285) SE = .02 |
.250 (.196–.311) SE = .03 |
.227 (.181–.263) SE = .02 |
.220 (.186–.265) SE = .02 |
| DOHME | .213 (.184–.241) SE = .02 |
.196 (.164–.260) SE = .02. |
.233 (.171–.306) SE = .03 |
.212 (.145–.235) SE = .02 |
.210 (.173–.258) SE = .02 |
| DOUESO | .592 (.552–.643) SE = .02 |
.606 (.541–.660) SE = .03 |
.614 (.567–.677) SE = .03 |
.581 (.514–.624) SE = .03 |
.589 (.585–.578) SE = .02 |
DOHME – Duration of Hyoid Maximum Elevation; DOHMAE – Duration of Hyoid Maximum Anterior Excursion; DOUESO – Duration of Upper Esophageal Sphincter Opening; SE = standard error of mean; numbers in parentheses = 95% confidence interval for means; BOLD indicates significant at p = .01.
It is not entirely surprising that the most significant findings in the results reported in this study on movements of the hyoid and extent of UES opening relate to coordination of events and results of the prior data analysis on durations10 indicated improved coordination, as well. We know more about exercise physiology and how we might apply this knowledge to swallowing rehabilitation than when we first initiated this investigation. Based on other studies at the time3,8–9 we employed a very intensive period of rehabilitation over a short period of time. This type of intensive regimen should be more effective in reorganizing neural networks related to swallowing, though research suggests a period of four weeks would likely produce better results. In terms of muscle recruitment, a more protracted period of treatment using a work-rest model at levels that would definitively produce “overload” may provide more statistical significance in the measures of hyoid movement and UES opening, as such regimens are now believed to be more successful for strengthening muscles and improving endurance7.
Pharyngeal pressures were not collected in this study but could also provide valuable information for future research. Prior investigations utilizing pharyngeal manometry have shown immediate impacts of the Mendelsohn maneuver in the form of increased tongue base to posterior pharyngeal wall pressure6 and decreased peak UES contraction pressure5. Advances in high speed digital manometry since the time this study was initiated have made it possible to understand more about pharyngeal pressures in relation to the timing of swallowing events. Future studies should incorporate videomanometry to simultaneously investigate the timing and sequence of events in relation to the extent of structural movements, duration of those movements, and the pharyngeal pressures exerted to propel the bolus.
In conclusion, the data reported in this investigation support the use of the Mendelsohn maneuver as an exercise to improve swallow physiology. Significant improvements were observed after treatment in correlations between measures of hyoid movement, UES opening, and bolus flow. Improvements were observed, as well, in hyoid maximum anterior excursion, hyoid maximum elevation, and mean width of UES opening, as well, and improvements were more substantive after periods of treatment than no-treatment. Future studies should examine these measures when using a work-rest model over a longer period of time to potentially enhance muscle recruitment.
Study Limitations
The primary limitation of this study was that it began a long time ago and took too long to complete—as is the unfortunate case with most behavioral research investigations. Rapid advances in technology, including higher resolution digital VFSS and high resolution pharyngeal manometry, have occurred since this study began and will greatly enhance our ability to determine the effects of the Mendelsohn maneuver on overall swallowing strength and coordination. We also employed a very intensive period of rehabilitation based in part on other research reports at the time of study initiation. This type of intensive regimen appeared to be effective improving swallowing coordination but may not have been optimal in terms of muscle recruitment. Larger numbers of participants are needed in future investigations, as are variations in treatment regimens, duration, and intensity.
Footnotes
This research was funded in whole and in part by NIH NIDCD Grant R03 DC04942-01A2.
Conflict of Interest
The authors declare they have no conflict of interest.
References
- 1.Mendelsohn MS, McConnell FM. Function in the pharyngoesophageal segment. Laryngoscope. 1987;97(4):483–489. doi: 10.1288/00005537-198704000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. Am J Roentgenol. 1990;154:953–963. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- 3.Crary MA, Carnaby GD, Groher ME, Helseth E. Functional Benefits of Dysphagia Therapy Using Adjunctive sEMG Biofeedback. Dysphagia. 2004;19:160–164. doi: 10.1007/s00455-004-0003-8. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, McCullough GH. Maximal hyoid excursion in poststroke patients. Dysphagia. 2010;25(1):20–25. doi: 10.1007/s00455-009-9224-1. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus C, Logemann JA, Gibbons P. Effects of maneuvers on swallowing function in a dysphagic oral cancer patient. Head & Neck. 1993;15:419–424. doi: 10.1002/hed.2880150509. [DOI] [PubMed] [Google Scholar]
- 6.Lazarus C, Logemann JA, Song CW, Rademaker AW, Kahrilas PJ. Effects of voluntary maneuvers on tongue base function for swallowing. Folia Phon et Logoped. 2002;54:171–176. doi: 10.1159/000063192. [DOI] [PubMed] [Google Scholar]
- 7.Burkhead L, Sapienza CM, Rosenbek J. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22:251–265. doi: 10.1007/s00455-006-9074-z. [DOI] [PubMed] [Google Scholar]
- 8.Bryant M. Biofeedback in the treatment of a selected dysphagic patient. Dysphagia. 1991;6:140–144. doi: 10.1007/BF02493516. [DOI] [PubMed] [Google Scholar]
- 9.Huckabee ML, Cannito MP. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: a retrospective evaluation. Dysphagia. 1999;14:93–109. doi: 10.1007/PL00009593. [DOI] [PubMed] [Google Scholar]
- 10.McCullough GH, Kamarunas E, Mann GC, Schmidley JW, Robbins JA, Crary M. Effects of Mendelsohn maneuver on measures of swallowing duration post-stroke. Topics Stroke Rehabil. 2012;19:234–243. doi: 10.1310/tsr1903-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park T, Kim Y, McCullough GH. Oropharyngeal Transition of the Bolus in Poststroke Patients. Am J Phy Med Rehabil. doi: 10.1097/phm.0b013e318269d935. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Perlman AL, Booth BM, Grayhack JP. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia. 1994;9:90–95. doi: 10.1007/BF00714593. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, McCullough GH. Maximum hyoid displacement in normal swallowing. Dysphagia. 2008;23:274–279. doi: 10.1007/s00455-007-9135-y. [DOI] [PubMed] [Google Scholar]
- 14.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Sp Lang Hear Res. 2000;43:1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 16.McCullough GH, Rosenbek JC, Robbins JA, Coyle JL, Wood JL. Ordinality and intervality of a penetration-aspiration scale. J Med Sp-Lang Path. 1998;6:65–72. [Google Scholar]
- 17.Robbins JA, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, Taylor AJ. The effects of lingual exercise in stroke patients with dysphagia. Arch Phy Med Rehabil. 2007;88:150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 18.O’Neil KH, Purdy M, Falk J, Gallo L. The Dysphagia Outcome and Severity Scale. Dysphagia. 1999;14:139–145. doi: 10.1007/PL00009595. [DOI] [PubMed] [Google Scholar]
- 19.Nakane A, Tohara H, Ouchi Y, Goto S, Uematsu H. Videofluoroscopic kinesiologic analysis of swallowing: Defining a standard plane. J Med Dental Sci. 2006;53:7–15. [PubMed] [Google Scholar]