Abstract
Background
1,4-butanediol (1,4-BD) is a gamma-hydroxybutyrate (GHB) pro-drug, with multiple commercial uses, and a drug of abuse. Although there are case reports of a withdrawal syndrome following 1,4-BD use, no studies have evaluated the physical dependence potential of 1,4-BD and characterized the time course of withdrawal.
Methods
Vehicle and then 1,4-BD were administered continuously 24 h/day via intragastric catheters in male baboons (Papio anubis, n=3). Dosing was initiated at 100 mg/kg and increased by 100 mg/kg/day to 400 mg/kg. After a stabilization period, doses of 500 and then 600 mg/kg/day were each maintained for 3-4 weeks. Plasma levels of 1,4-BD and GHB were determined for each dose condition. Physical dependence was assessed via administration of a GABA-B antagonist (precipitated withdrawal test) during administration of the 600 mg/kg dose and via abrupt termination of chronic 1,4-BD administration (spontaneous withdrawal test). Outcome measures included the number of food pellets earned, performance on a fine-motor task, observed behaviors, and plasma levels of GHB and 1,4-BD.
Results
Following maintenance of 1,4-BD 600 mg/kg for 3 weeks, the number of food pellets earned was significantly decreased. At the end of chronic 1,4-BD dosing, the levels of GHB in plasma ranged from 1290- 2300 μmol/L and levels of 1,4-BD in plasma ranged from 13.1 -37.9 μmol/L. Signs of physical dependence were observed following precipitated and spontaneous withdrawal tests. Seizures were not observed.
Conclusions
These data indicate chronic 1,4-BD produced physical dependence in baboons and the withdrawal syndrome can be characterized as mild to intermediate.
Keywords: GHB, withdrawal, operant behavior, GABA-B receptor antagonists
1. INTRODUCTION
1,4-butanediol (1,4-BD) is used commercially as an industrial solvent as well as in the manufacturing of plastics, textiles, and other chemicals. 1,4-BD is also a pro-drug for gamma-hydroxybutyrate (GHB) as it is rapidly metabolized into GHB after ingestion via a two-step process. It is first converted via alcohol dehydrogenase into gamma-hydroxybutyraldehyde and then into GHB (Roth and Giarman, 1965; Snead et al., 1989). It is also a drug of abuse that gained popularity after legal restrictions were placed on the production and sale of GHB (Carter et al., 2009; Palmer, 2004; Wojtowicz et al., 2008; Wood et al., 2011). Users report that both GHB and 1,4-BD possess anesthetic properties and produce similar behavioral effects including feelings of euphoria, relaxation, drowsiness, and disinhibition, nausea, vomiting, anxiety, dizziness, agitation, respiratory depression, bradycardia, and hypotension (Galloway et al., 2000; Schep et al., 2012; Teter and Guthrie, 2001; Wood et al., 2011).
Users ingest 1,4-BD or convert it into GHB prior to ingestion. Instructions for this conversion process can be found on the Internet, where 1,4-BD may also be purchased for illicit use (Andresen et al., 2011; Hillebrand et al., 2010; Jones, 2010; Karila and Reynaud, 2010). Although the mechanism of action of 1,4-BD is still unclear, it is likely that the behavioral and pharmacological effects of 1,4-BD are produced due to its conversion to GHB (Duer et al., 2001; Nicholson and Balster, 2001; Roth and Giarman, 1968). Previously, we examined pharmacokinetic parameters and behavioral effects of acute doses of 1,4-BD in baboons in comparison to GHB (Goodwin et al., 2009, 2005). There were differences between drugs in the latency to on-set of behavioral effects, and duration of action. Importantly, the observed behavioral effects were related to plasma levels of GHB and not plasma levels of 1,4-BD. Likewise, rodent studies suggest GHB and 1,4-BD may differentially effect locomotor behavior (de Fiebre et al., 2004) and have different durations to the on-set of action (Carter et al., 2003; McMahon et al., 2003).
GHB, GBL and 1,4-BD have all been shown to maintain self-administration in baboons(Goodwin et al., 2011, 2012), which is consistent with an abuse liability of these drugs. Both GHB and the GHB pro-drug gamma-butyrolactone (GBL) produced physical dependence in baboons in previous studies, and administration of a GABA-B antagonist precipitated a withdrawal syndrome (Goodwin et al., 2006; Weerts et al., 2005). Withdrawal symptoms included vomiting, retching, tremors, jerks, postural changes, increased self-directed behavior, increased aggression, decreased operant responding for food pellets, and increased duration to complete a fine-motor task. Peak spontaneous withdrawal effects occurred in the first 6-72 hrs following cessation of chronic administration of GHB or GBL and returned to low levels within 2 weeks (Goodwin et al., 2006; Weerts et al., 2005). A similar time course of withdrawal effects is reported in humans following cessation of chronic GHB and GBL use (Barker et al., 2007; Carter et al., 2009; de Jong et al., 2012; McDonough et al., 2004; Miotto et al., 2001; Rosenberg et al., 2003).
Although there are case reports in humans of a withdrawal syndrome from 1,4-BD, which is similar to that reported for GHB (Schneir et al., 2001; Wojtowicz et al., 2008; Wood et al., 2011; Zvosec et al., 2001), there are limited studies which have directly examined the physical dependence potential of 1,4-BD. In addition, it is important to consider that many of the behavioral effects of 1,4-BD are similar to signs typically included in withdrawal symptoms (e.g., decrease in appetite/food intake, gastrointestinal symptoms, tremors/jerks) and need to be evaluated when assessing 1,4-BD withdrawal. We are aware of only two studies that have evaluated1,4-BD physical dependence under repeated dosing conditions in rats selectively bred for alcohol preference or sensitivity to GHB (Carai et al., 2005; Quang et al., 2006). Thus, the current study evaluated the behavioral effects and physical dependence potential of 1,4-BD in baboons.
2. METHODS
2.1 Subjects
Three adult male baboons (Papio anubis; Primate Imports, New York, NY and South West Foundation for Biomedical Research, San Antonio, TX) weighing 23-39 kg at the beginning of the study served as subjects. Prior to the start of the current study, baboons (designated HA, WL and PY) had intra-gastric (IG) catheters implanted using methods described previously (Lukas et al., 1982). The catheter was protected by a tether/harness/vest system that permitted free movement inside the cage. Baboons HA and WL had participated in previous studies evaluating the acute and chronic effects of GHB and GBL, as well as the acute effects of 1,4-BD. Baboon PY had previous experience with oral ethanol self-administration, intravenous (IV) self-administration of cocaine and several other sedative/anxiolytic compounds, and acute intragastric (IG) administration of (±)-3,4-methylenedioxymethamphetamine (MDMA). HA, PY, and WL had not received any study drugs, other than ketamine for conducting routine body checks, for at least 8 months when the current study began.
Baboons were anesthetized with ketamine hydrochloride (preceded by atropine to control secretions) every 2-4 weeks in order to perform physical examinations, routine catheter maintenance, and to collect blood samples. Days on which a baboon was anesthetized were not included in the data analysis. This protocol was approved by the Johns Hopkins University Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Facilities were maintained in accordance with USDA and AALAC standards.
2.2 Apparatus
Baboons were individually housed in standard primate cages that also served as the experimental chambers. Cages contained a Lindsley pull lever (Med-Associates, Georgia, VT, USA), a food hopper, and stimulus lights. A strain relief mount (Model SR-750B, Instech-Soloman, Plymouth Meeting, PA) was attached at the top of the cage and connected to a peristaltic pump (Harvard Model 1201 or 1203, Harvard Apparatus, S. Natick, MA) and to the catheter via a custom 18-gauge liquid swivel. The overhead lights in the room were illuminated for 13-h/day (06:00 – 19:00 h) and were dimly illuminated for the remaining 11-h/day. Experimental sessions were controlled using IBM compatible personal computers with Med-PC software and instrumentation (MED Associates, Inc., East Fairfield, VT).
2.3 Drugs
The stock solution of 1,4-BD (1.17 g/ml) was obtained from Sigma-Aldrich (St. Louise, MO, USA) and diluted with distilled water to concentrations that resulted in the administration of 100-600 mg/kg; all doses were administered at a constant volume (e.g., 550 mls) for each baboon. SGS742 (3-Aminopropyl-n-butyl-phosphinic acid; formerly CGP36742) was provided by Novartis Pharma, Basel, Switzerland and Saegis Pharmaceuticals, Inc., Half Moon Bay, CA . SGS742 (56 mg/kg) was dissolved in sterile water and injected intramuscularly (IM) at a volume of 2-2.5 ml.
2.4 Behavioral Measures
2.4.1 Food-maintained operant behavior procedure
An unlimited number of food pellets (1 g banana flavored, Bio-SERV, Ins., Frenchtown, NJ; or P.J. Noyes, Lancaster, NH) were available under a fixed-ratio (FR) schedule of reinforcement during daily 2-hr sessions that began at the same time every day (8:00 am). Pellet availability was signaled by a jewel light above the operandum. A pull and release of the Lindsley lever constituted one response. Baboons HA and WL worked a FR 10 schedule of reinforcement and baboon PY on an FR 5. Monkey biscuits (150-200 g to maintain stable weight), 1 piece of fruit/vegetable, and 1 multi-vitamin were supplied 1-2 hrs after the daily sessions. Any biscuits remaining in the food hopper were removed prior to the initiation of pellet sessions.
2.4.2 Behavioral Observations
The behavioral effects of chronically administered 1,4-BD were characterized using systematic 15-min behavioral observations that occurred during each condition (i.e., vehicle and each dose of 1,4-BD). During these observations, postures and behaviors (based on definitions in Weerts and Griffiths, 1998) were entered and recorded using laptop computers with The Observer software (Noldus Information Technology, Wageningen, The Netherlands). One group of mutually exclusive postures included: normal, sleep, moderately sedated, deeply sedated, rigidly braced, head below torso, convulsing, standing, or locomotion. A second group of mutually exclusive postures included lip droop or normal lip. Individual behaviors were: aggress/bruxism, ataxia, defecate, drink, forage in pan, grunt/bark, jerk, lip smack, masturbate, nose rub/wipe, scratch/groom, tremor, vomit/retch, wet dog shake, and yawn. Similar behaviors and postures have been used previously to successfully characterize the behavioral effects of GHB, GBL, and 1,4-BD (Goodwin et al., 2009, 2005, 2006, 2011, 2012; Weerts et al., 2005).
The overall reliability for the occurrence/non-occurrence and sequence of behaviors and postures among all observers was greater than 90% prior to the start of the present study. Observations began at the same time of day (e.g., 10 a.m.), after the food pellet sessions had concluded, and were 15-min in duration. The occurrence of each posture and behavior was recorded in 1-min intervals. The occurrence or nonoccurrence of each posture and behavior across all 15 intervals was tabulated and used for data analysis. Thus, the maximum score during a single observation for each behavior and posture was 15. Observers were also able to enter additional comments at any time during an observation.
2.4.3 Fine motor task
A fine motor task was conducted each morning at approximately the same time (e.g., 8:30 am). On days when behavioral observations occurred, an additional fine motor task was conducted at the end of the observation. In this procedure, a peanut (HA and WL) or M&M® (PY) was placed in each of six equally spaced 3-cm cups with 1-cm rims that were mounted on a custom made Plexiglas tray and placed in front of the cage for retrieval. The tray was presented for a maximum of 120-s or until all six items were retrieved. The duration (s) to complete the task and the number of food items retrieved and dropped were recorded, and a behavioral checklist was completed in order to document the occurrence of tremors, lip droop, and incoordination during the task.
2.5 Experimental Design
2.5.1 Phase 1: Baseline
Reverse osmosis (RO) water (1,4-BD vehicle) was continuously infused through the IG catheter at a rate of 0.38 ml/min such that approximately 550 ml of fluid was infused over each 24-h period. The actual volume (ml) infused was recorded each morning at the same time (e.g., 8:30 a.m.) and then the aspirator bottle was refilled with either RO water or 1,4-BD. RO water was infused for two weeks and baseline levels of operant responding for food pellets, performance on the fine motor task, and behavioral observations were characterized. Eight (PY) or ten (HA and WL) behavioral observations were conducted to characterize behaviors and postures under vehicle (i.e., baseline) conditions.
2.5.2 Phase 2: Chronic 1,4-BD Dosing
After baseline, 1,4-BD dosing was initiated at a dose of 100 mg/kg and increased by 100 mg/kg each day until a dose of 400 mg/kg was reached. A dose of 400 mg/kg was maintained for 14 days, followed by an increase to 500 mg/kg, which was maintained for 31-34 days. The final dose, 600 mg/kg/day, was maintained for 26 days. The total length of 1,4-BD dosing for each baboon was 85 days for HA, 80 days for PY, and 78 days for WL.
2.5.2 Phase 3: Precipitated Withdrawal Test
All three subjects competed a precipitated withdrawal test after 2 weeks of chronic administration of 600 mg/kg 1,4-BD. SGS742 (56 mg/kg) or its vehicle (saline) were administered IM 5-min before the pellet session was to begin. The vehicle test was on day 13 and the SGS742 test was on day 14 of chronic 600 mg/kg 1,4-BD administration. A 56 mg/kg dose of SGS742 has previously been shown to precipitate withdrawal in baboons chronically treated with 750 mg/kg GHB (Weerts et al., 2005) and 400-600 mg/kg GBL (Goodwin et al., 2006). The 600 mg/kg dose of 1,4-BD was then maintained an additional two weeks after the precipitated withdrawal test before proceeding to phase 4 (Spontaneous withdrawal test).
2.5.3 Phase 4: Spontaneous Withdrawal Test
Baseline levels of behavior while maintained on 600 mg/kg 1,4-BD were measured during behavioral observations completed over the four days preceding the spontaneous withdrawal test. For the spontaneous withdrawal test, chronic 1,4-BD dosing was abruptly discontinued by substitution of chronic RO water (vehicle) for 1,4-BD at 8 a.m. A behavioral observation and fine-motor task was conducted 6-h after and 24 hr after 1,4-BD administration was discontinued, and then at the same time each day for 14 days.
2.6 Evaluation of 1,4-BD and GHB in blood
Blood samples were collected as described previously (Goodwin et al., 2006) during regular medical checks while baboons were maintained on 500 mg/kg and 600 mg/kg 1,4 BD; and days 15-16 following cessation of 1,4-BD administration. Actual days for each sample are shown in table 1. Samples were shipped overnight on dry ice for analysis and analyzed by stable-isotope dilution gas chromatography-mass spectrometry as previously described (Gibson et al., 1990; Goodwin et al., 2009).
Table 1.
Amount of GHB (μmol/L) and 1,4-BD (μmol/L) in plasma during chronic IG administration of 1,4-BD in individual baboons. Body weights (BW) shown were obtained at the same time as blood samples. ND indicates a non-detectable level.
| Baboon | BW (kg) | Condition | GHB (μmol/L) | 1,4-BD (μmol/L) |
|---|---|---|---|---|
| HA | 36.8 | 500 mg/kg; Day 7 | 2240 | 9.0 |
| PY | 21.7 | 500 mg/kg; Day 6 | 690 | 17.3 |
| WL | 34.3 | 500 mg/kg; Day 7 | 1500 | 16.6 |
| HA | 36.1 | 500 mg/kg; Day 17 | 2010 | 20.9 |
| PY | 21.6 | 500 mg/kg; Day 21 | 603 | 6.9 |
| WL | 34.4 | 500 mg/kg; Day 17 | 924 | 16.8 |
| HA | 36.4 | 500 mg/kg; Day 31 | 2000 | 22.0 |
| PY | 21.3 | 500 mg/kg; Day 33 | 638 | 19.3 |
| WL | 34.3 | 500 mg/kg; Day 31 | 782 | 10.1 |
| HA | 36.1 | 600 mg/kg; Day 7 | 1750 | 11.5 |
| PY | sample missing | |||
| WL | 34.1 | 600 mg/kg; Day 9 | 1470 | 10.3 |
| HA | 35.2 | 600 mg/kg; Day 20 | 2300 | 37.9 |
| PY | 21.2 | 600 mg/kg; Day 20 | 1290 | 13.1 |
| WL | 33.8 | 600 mg/kg; Day 20 | 1950 | 18.3 |
| HA | 35.3 | WD; Day 16 | 18.3 | ND |
| PY | 21.8 | WD; Day 15 | 32.1 | ND |
| WL | 34 | WD; Day 16 | 12.3 | ND |
2.7 Data Analysis
A one-way repeated measures analysis of variance (ANOVA) with 1,4-BD dosing condition as the repeated measure was used to compare the mean number of food pellets earned across 1,4-BD dosing conditions; dosing conditions included the first 7 days of vehicle and 400-600 mg/kg 1,4-BD, and 7 days during weeks 2-3 of dosing with 400-600 mg/kg 1,4-BD. When a significant effect (p<0.05) was found, a Bonferroni multiple comparison posttest was used to compare the mean number of food pellets earned during the first 7 days of 400-600 mg/kg 1,4-BD with 1) the mean number of food pellets earned during the first 7 days of vehicle and 2) the mean number of food pellets earned across 7 days during weeks 2-3 of each respective 1,4-BD dose. To evaluate the effects of spontaneous withdrawal on the number of food pellets earned, a second ANOVA compared the mean number of pellets earned during the vehicle baseline, the last week of 1,4-BD dosing, and across each of the first two weeks of spontaneous withdrawal. The mean duration to complete the fine-motor task was analyzed in the same manner.
For behavioral observations, 95% confidence intervals for the number of intervals in which each behavior occurred were calculated based on the vehicle baseline condition for each baboon (n=8 for PY; n=10 for HA and WL). Changes in behavior during 1,4-BD dosing were judged significant when they fell outside the 95% confidence interval for the vehicle control condition. One-tailed t tests were used for behaviors and postures that were predicted to increase (e.g., lying down, head-below-torso posture, vomit/retch) and two-tailed t tests were used for behaviors and postures that were predicted to increase or decrease (e.g., self-directed behaviors, locomotion). Behaviors that never occurred under vehicle baseline conditions (e.g., tremors and jerks) were judged as significantly increased if they occurred during chronic 1,4-BD dosing.
Withdrawal categorizations of mild, intermediate or severe originally developed for barbiturate physical dependence (Yanagita and Takahashi, 1970) were assigned during precipitated and spontaneous withdrawal tests. Such categorizations and the development of a withdrawal scoring system has been used previously in baboons for evaluation of benzodiazepine (Kaminski et al., 2003; Weerts et al., 1998), GBL (Goodwin et al., 2006), and GHB dependence (Weerts et al., 2005). The nine categories of behavioral and postural differences used to determine the withdrawal score were: (1) pellets per day decreased, (2) increased time to complete the fine motor task, (3) increase in time spent in a sleeping posture, head-lower-than-torso posture, and/or a withdrawn posture, (4) locomotion increased or decreased, (5) self-directed behaviors (nose rub, nose wipe, masturbation, scratch and wet dog shake) increased, (6) aggressive threat, bruxism or yawn increased, (7) tremor (limb/body), jerk, ataxia, and/or rigidly braced posture increased, (8) vomit and/or retch increased, (9) seizures increased. For the food pellet and fine-motor task data, the incidence of precipitated withdrawal behaviors scored following SGS742 administration was judged as significant if the frequency of behavior exceeded the 95% confidence intervals for the vehicle baseline, and the 95% confidence intervals calculated using the previous 4 days of 600 mg/kg 1,4-BD dosing (including an IM vehicle administration the day before the IM SGS742 administration). For other behaviors, the incidence of precipitated withdrawal behaviors scored following SGS742 administration was judged as significant if the frequency of behavior exceeded the 95% confidence intervals for the vehicle baseline and the 95% confidence intervals calculated using days 3-5 and 12-13 (including an IM vehicle administration on day 13) of 600 mg/kg 1,4-BD administration. The means of two 15-min IM SGS742 observations on day 14 of 600 mg/kg, and the means of two 15-min IM vehicle observations on day 13 were used for calculating the 95% confidence intervals for subjects HA and WL. The incidence of spontaneous withdrawal behaviors scored following termination of drug treatment was judged as significant if the observed frequency of the behavior exceeded the 95% confidence intervals for both the vehicle baseline and the last 4 days of chronic 600 mg/kg 1,4-BD administration. Behaviors indicative of a withdrawal syndrome were those that were greater than the effects of vehicle and chronic 1,4-BD. Using these criteria, behaviors that were significantly changed when compared to vehicle, but not when compared to chronic GBL dosing, would not be considered as significant during withdrawal tests. Thus, both time-related and drug-produced changes in behavior were accounted for in the analysis.
3. RESULTS
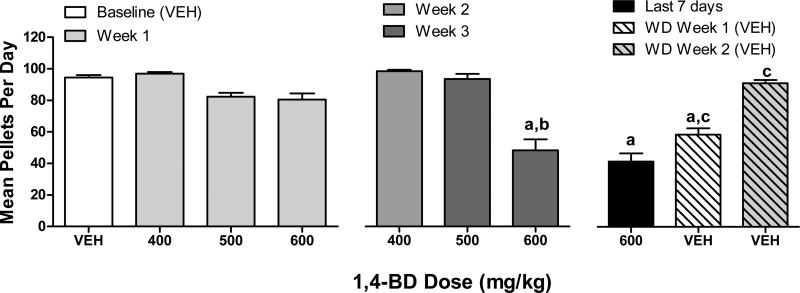
Effects of chronic administration of 1,4-BD on mean number of pellets earned per day are shown in figure 1. ANOVA revealed a significant main effect for 1,4-BD dose condition on the mean number of food pellets earned (F(6, 36)=24.95, p<0.0001). Post-hoc tests indicated the mean number of pellets per day during the first week of dosing with 400-600 mg/kg did not differ from vehicle. After 3 weeks of chronic administration, 600 mg/kg 1,4-BD significantly decreased (p<0.05) the mean number of pellets when compared to vehicle and when compared to the first week of dosing in post-hoc tests. An analysis of the mean number of food pellets earned during weeks 1 and 2 of the spontaneous withdrawal phase (compared to the last week of 600 mg/kg 1,4-BD and vehicle baseline) also revealed a significant main effect (F(6, 36)=55.52, p<0.0001). Post-hoc tests indicated the mean number of pellets per day during the last week of 600 mg/kg 1,4-BD was significantly different from vehicle baseline (p<0.05), as was week 1 of spontaneous withdrawal (p<0.05). The mean number of pellets earned during week 1 and week 2 of spontaneous withdrawal were both significantly different from the last week of 600 mg/kg 1,4-BD in post-hoc tests (p<0.05).
Figure 1.
Effects of 1,4-BD on food-maintained behavior across dosing conditions. Data shown are the mean (SEM) number of pellets delivered during daily 2 hr sessions during 7 days of the vehicle (“VEH”) baseline, during the first 7 days of 400-600 mg/kg 1,4-BD, across 7 seven days during week 2 (400 mg/kg), week 3 (500 and 600 mg/kg), and week 4 (600 mg/kg) of chronic 1,4-BD, and during weeks 1 and 2 of the spontaneous withdrawal phase. a indicates significantly different from vehicle baseline, b indicates significantly different from the first week of chronic 400-600 mg/kg 1,4-BD dosing for that respective dose, and c indicates significantly different from week 4 of chronic 600 mg/kg 1,4-BD dosing (for spontaneous withdrawal comparisons only).
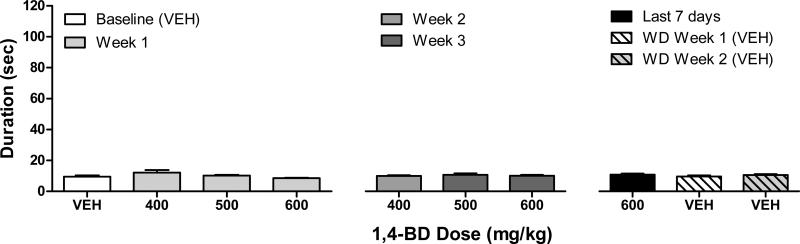
Chronic administration of 1,4-BD did not alter performance on the fine-motor task across 1,4-BD doses (figure 2; F(6,36)=1.56, p=0.19). Likewise, performance was not altered during the spontaneous withdrawal phase (F(6,36)=0.83, p=0.5). The effects of chronic 1,4-BD administration on behaviors recorded during observations were variable (data not shown). Ataxia, tremor/jerks, and increased self-directed behaviors (e.g., masturbation, nose rub/wipe, scratch/groom, wet dog shake) were all observed intermittently in all three subjects during various 1,4-BD dosing conditions. Vomiting was only observed in one subject (HA).
Figure 2.
Effects of 1,4-BD on duration to complete the fine-motor task across dosing conditions. Data shown are the mean (SEM) durations to complete the task (maximum of 120 sec) across daily presentations during 7 days of the vehicle (“VEH”) baseline, during the first 7 days of 400-600 mg/kg 1,4-BD, across 7 seven days during week 2 (400 mg/kg), week 3 (500 and 600 mg/kg), and week 4 (600 mg/kg) of chronic 1,4-BD, and during weeks 1 and 2 of the spontaneous withdrawal phase. a indicates significantly different from vehicle baseline, b indicates significantly different from the first week of chronic 400-600 mg/kg 1,4-BD dosing for that respective dose, and c indicates significantly different from week 4 of chronic 600 mg/kg 1,4-BD dosing (for spontaneous withdrawal comparisons only).
An analysis of GHB (μmol/L) and 1,4-BD (μmol/L) in plasma during chronic 1,4-BD administration is shown in table 1. Plasma levels of GHB were variable across subjects with the highest levels of GHB observed in the largest subject (HA). GHB levels were similar between week 1 and later weeks of dosing. Two weeks after cessation of 1,4-BD dosing, GHB levels were low but remained detectable (12.3 μmol/L to 32.1 μmol/L; i.e., slow clearance). Levels of 1,4-BD in plasma were also relatively stable across dosing conditions (see table 1), though much lower levels of 1,4-BD in plasma were detected when compared to GHB, and 1,4-BD was no longer detected two weeks after cessation of chronic 1,4-BD administration.
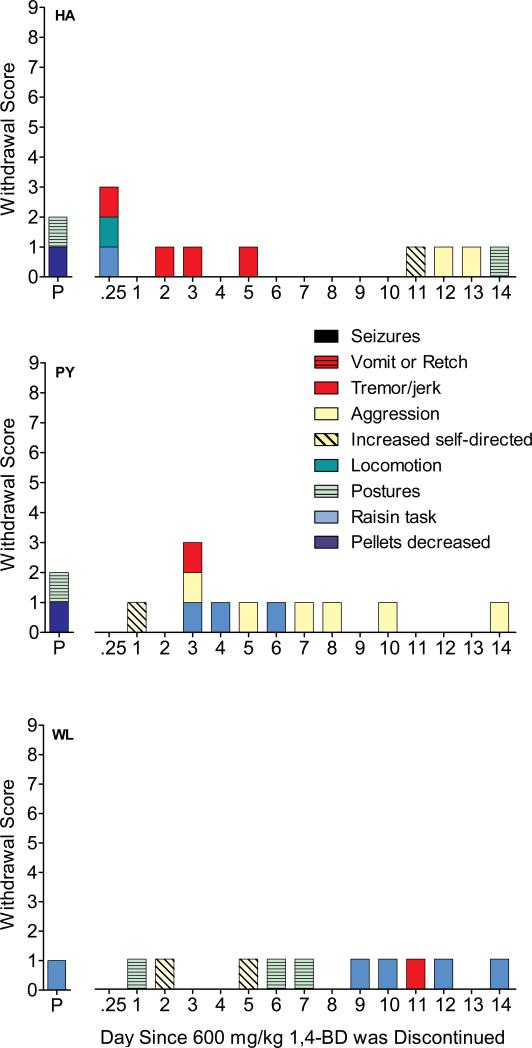
Administration of SGS742 during chronic administration of 600 mg/kg 1,4-BD (HA, PY, WL) increased mild signs of withdrawal (figure 3). Two baboons earned significantly fewer food pellets following administration of SGS742 (HA=6, PY=73) when compared to the means (SD) for vehicle baseline (HA=104.6±4.8, PY=87.4 ±4.2) and the previous 4 days of 600 mg/kg 1,4-BD, which included an IM vehicle administration the day before the IM SGS742 administration (HA=51.8±9.0, PY=89.0±6.0). The number of food pellets earned by baboon WL did not differ between vehicle baseline (92.4±14.4), chronic 600 mg/kg 1,4-BD (99.5±3.0), and precipitated withdrawal (100). Figure 3 also illustrates withdrawal scores across 14 days following cessation of chronic 600 mg/kg 1,4-BD. The highest number of withdrawal scores observed during any single day was 3, and occurred at 6-hr post-cessation (HA) and on day 3 following cessation (PY). Baboon WL never exhibited more than one sign of withdrawal on a daily basis. Tremor/jerks were observed in all 3 baboons on one or more day of spontaneous withdrawal. Seizures were not observed in any of the baboons during any time.
Figure 3.
Withdrawal scores for the precipitated (P) withdrawal test during chronic 600 mg/kg 1,4-BD administration; and over the first 14 days of spontaneous withdrawal following discontinuation of chronic 600 mg/kg 1,4-BD administration in individual baboons. The maximum possible score that could be obtained was 9, and points were assigned as follows: 1 point for each of seven categories of behavior recorded during observation sessions, 1 point for increased duration to complete the fine-motor task, and 1 point if the number of food pellets delivered was significantly decreased. Points for withdrawal scores were assigned for categories in which there was a significant change from both the vehicle control condition and chronic 600 mg/kg 1,4-BD administration.
4. DISCUSSION
The primary findings of the current study were 1) chronic administration of 1,4-BD produced high levels of GHB and 1,4-BD in plasma, 2) during chronic administration,1,4-BD decreased the number of food pellets earned and 3) precipitated and spontaneous withdrawal confirmed that 1,4-BD produced physical dependence.
Previously, we have shown that chronic administration of GHB, and another GHB pro-drug GBL, produced a dose-dependent decrease in the number of food pellets earned, increased duration to complete the fine-motor task, increased signs of sedation, muscle relaxation, gastro-intestinal effects, and tremor/jerks (Goodwin et al., 2006; Weerts et al., 2005). In the current study, chronically administered 1,4-BD primarily affected food-maintained operant behavior. Signs associated with muscle relaxation, sedation or gastrointestinal effects, previously observed with GHB and GBL were only observed intermittently during chronic administration of 1,4-BD.
Like GBL, the behavioral and pharmacological effects of 1,4-BD are thought to result from its conversion to GHB, although via different metabolic pathways (Duer et al., 2001; Nicholson and Balster, 2001; Roth and Giarman, 1968; Schep et al., 2012). There was significant variability between subjects in plasma levels of 1,4-BD, and this is likely related to metabolic differences. In humans, there has also been significant inter-individual variation in the rate of 1,4-BD metabolism to GHB (Thai et al., 2006).
Regardless of individual differences, the plasma levels of GHB obtained during 1,4-BD dosing the current study were well within range previously associated with overt behavioral effects and physical dependence. Specifically, after 3 weeks of 600 mg/kg 1,4-BD, plasma levels of GHB ranged from 1290-2300 μmol/L, compared to 486-2080 μmol/L after 4 weeks of 750 mg/kg GHB (Weerts et al., 2005). Higher plasma levels of GHB (2430-3785 μmol/L) were found with 600 mg/kg GBL (week 5) (Goodwin et al., 2006). Levels of 1,4-BD in plasma ranged from 13.1-37.9 μmol/L during week 3 of the highest dose tested in the present study. Individual variations in the metabolism of 1,4-BD to GHB in vivo was reported in our previous study of the acute effects of 1,4-BD in baboons (Goodwin et al., 2005). It is possible that different levels of the enzymes responsible for converting 1,4-BD into GHB (i.e., alcohol dehydrogenase and aldehyde dehydrogenase) may account for some differences in effects between subjects (WHO, 2012). It is important to note that in a previous study examining the acute effects of 1,4-BD administration, we found that the behavioral effects of 1,4-BD were associated with plasma levels of GHB and not 1,4-BD (Goodwin et al., 2005).
Behavioral signs of physical dependence and classifications of the severity of withdrawal in laboratory nonhuman primates have been well characterized. Classifications of “mild”, “intermediate” or “severe” were originally developed by Yanagita and Takahashi for comparison of barbiturates and novel drugs (Yanagita and Takahashi, 1970) and have been widely used by our lab and others for a range of sedative/hypnotic drugs, including GHB and GBL, and allows cross comparisons across drugs and laboratories (Goodwin et al., 2006; Kaminski et al., 2003; Weerts et al., 2005, 1998; Woods et al., 1987). In baboons signs of mild withdrawal would include decreases in food intake, increases in aggression, self-directed behaviors, abnormal postures associated with gastrointestinal discomfort (head lower than torso posture) or changes in activity (withdrawn posture, locomotion). Intermediate withdrawal includes tremors/jerks, postures associated with muscle rigidity (braced posture), impaired motor activity and vomit/retching. Classification of severe withdrawal includes seizures and convulsions. Using these classifications, the withdrawal syndrome observed following discontinuation of chronic 1,4-BD can be characterized as mild to intermediate. The number of withdrawal symptoms ranged from 0-3 across the first 14 days of withdrawal from 1,4-BD, with peak effects observed in the first 3 days. The only intermediate sign of withdrawal observed was an increase in tremors/jerks. Seizures were not observed in any of the baboons at any time during the withdrawal period. Our previous studies examining the dependence potential of GHB and GBL found intermediate levels of withdrawal symptoms following cessation of chronic administration of GBL (Goodwin et al., 2006) and GHB (Weerts et al., 2005). Although signs of intermediate withdrawal were observed for 1,4-BD, the number of symptoms were lower across days, and observed symptoms were generally mild when compared to GHB and GBL. In rats, precipitated (via GABA-B antagonist) and spontaneous withdrawal syndromes (i.e., occurrence of audiogenic seizures) from 1,4-BD (administered for 6-9 days) occurred only in selectively bred Sardinian alcohol-preferring rats but was not detected in other rat lines (Carai et al., 2005; Quang et al., 2006).
Human case studies suggest that the withdrawal syndrome produced by cessation of 1,4-BD use is similar to that for GHB (Schep et al., 2012; Schneir et al., 2001; Wojtowicz et al., 2008; Wood et al., 2011; Zvosec et al., 2001). Case studies, however, rely on self-report of drug use by patients. Thus, patients may believe they were ingesting 1,4-BD but it is possible they were actually ingesting GHB or GBL. Indeed, samples collected via “amnesty bins” at dance venues in Europe suggest limited use and availability of 1,4-BD (EMCDDA, 2008; Wood et al., 2011).
In our previous studies, we demonstrated that many of the behavioral effects of GHB and 1,4-BD, including physical dependence, were modulated by the GABA-B receptor (Goodwin et al., 2005, 2006; Weerts et al., 2005). Similar findings have been reported in other laboratories for the discriminative stimulus effects and cataleptic effects of GBL (Carai et al., 2008; Koek et al., 2007, 2009; Towiwat et al., 2013). One study, however, reported that a GABA-B antagonist failed to block the discriminative stimulus effects of GBL in rats (Baker et al., 2005). Administration of the GABA-B antagonist SGS742 during chronic 1,4-BD dosing produced behavioral changes (i.e., suppression of pellets, impairment of fine motor task performance and increased sleep postures) consistent with a mild precipitated withdrawal syndrome. The magnitude of SGS742-precipitated withdrawal was lower when compared to results observed during chronic GBL and GHB administration (Goodwin et al., 2006; Weerts et al., 2005). Specifically, fewer and less severe signs of withdrawal were observed for 1,4-BD than for GBL and GHB. There are some caveats in comparing the current study findings with those reported in previous GHB and GBL studies in baboons (Goodwin et al., 2006; Weerts et al., 2005). Effects on food maintained behavior were determined in the present study via a 2-hr operant session, versus a 20-hr session in previous studies. The current study also used an interval scoring system for the behavior observations instead of simple frequency. It is also possible that subjects previous experience with GHB and GBL (HA and WL), and other sedatives (PY) resulted in cross-tolerance to 1,4-BD.
In summary, chronic administration of 1,4-BD in baboons produced high levels of GHB in plasma, and reduced food-maintained operant behavior. Signs consistent with the development of 1,4-BD physical dependence were observed following precipitated and spontaneous withdrawal tests. The most severe sign observed was tremors/jerks. Seizures were not observed. Thus, the 1,4-BD withdrawal syndrome in the current study can be characterized as mild to intermediate.
Acknowledgments
Funding for this study was provided by NIH/NIDA grant R01 DA 014919 (Weerts), and NS40270 and HD58553 (Gibson). The contributions of S. Womack and E. Taylor for veterinary technical assistance, and Kelley Lane and Erwin EW Jansen in determination of blood GHB and 1,4-BD levels, are gratefully acknowledged.
Role of Funding Source
The National Institutes of Health had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Drs. Goodwin and Weerts designed the study and Dr. Weerts wrote the protocol. Dr. Goodwin managed the literature searches and summaries of previous related work, undertook the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Authors declare that they have no conflicts of interest.
REFERENCES
- Andresen H, Aydin BE, Mueller A, Iwersen-Bergmann S. An overview of gamma-hydroxybutyric acid: pharmacodynamics, pharmacokinetics, toxic effects, addiction, analytical methods, and interpretation of results. Drug Test Anal. 2011;3:560–568. doi: 10.1002/dta.254. [DOI] [PubMed] [Google Scholar]
- Baker LE, Van Tilburg TJ, Brandt AE, Poling A. Discriminative stimulus effects of gamma-hydroxybutyrate (GHB) and its metabolic precursor, gamma-butyrolactone (GBL) in rats. Psychopharmacol. (Berl.) 2005;181:458–466. doi: 10.1007/s00213-005-0003-x. [DOI] [PubMed] [Google Scholar]
- Barker JC, Harris SL, Dyer JE. Experiences of gamma hydroxybutyrate (GHB) ingestion: a focus group study. J. Psychoactive Drugs. 2007;39:115–129. doi: 10.1080/02791072.2007.10399870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carai MA, Lobina C, Maccioni P, Cabras C, Colombo G, Gessa GL. Gamma-aminobutyric acidB (GABAB)-receptor mediation of different in vivo effects of gamma-butyrolactone. J. Pharmacol. Sci. 2008;106:199–207. doi: 10.1254/jphs.fp0071487. [DOI] [PubMed] [Google Scholar]
- Carai MA, Quang LS, Atzeri S, Lobina C, Maccioni P, Orru A, Gessa GL, Maher TJ, Colombo G. Withdrawal syndrome from gamma-hydroxybutyric acid (GHB) and 1,4-butanediol (1,4-BD) in Sardinian alcohol-preferring rats. Brain Res. Brain Res. Protoc. 2005;15:75–78. doi: 10.1016/j.brainresprot.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABA-B receptors in the discriminative stimulus effects of gamma-hydroxybutyrate in rats: time course and antagonism studies. J. Pharmacol. Exp. Ther. 2003;305:668–674. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- Carter LP, Pardi D, Gorsline J, Griffiths RR. Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse. Drug Alcohol Depend. 2009;104:1–10. doi: 10.1016/j.drugalcdep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fiebre CM, de Fiebre NE, Coleman SL, Forster MJ. Comparison of the actions of gamma-butyrolactone and 1,4-butanediol in Swiss-Webster mice. Pharmacol. Biochem. Behav. 2004;77:705–710. doi: 10.1016/j.pbb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- de Jong CA, Kamal R, Dijkstra BA, de Haan HA. Gamma-hydroxybutyrate detoxification by titration and tapering. Eur. Addict. Res. 2012;18:40–45. doi: 10.1159/000333022. [DOI] [PubMed] [Google Scholar]
- Duer WC, Byers KL, Martin JV. Application of a convenient extraction procedure to analyze gamma-hydroxybutyric acid in fatalities involving gamma-hydroxybutyric acid, gamma-butyrolactone, and 1,4-butanediol. J. Anal. Toxicol. 2001;25:576–582. doi: 10.1093/jat/25.7.576. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) GHB and its Precursor GBL: An Emerging Trend Case Study. Lisbon: 2008. [Google Scholar]
- Galloway GP, Frederick-Osborne SL, Seymour R, Contini SE, Smith DE. Abuse and therapeutic potential of gamma-hydroxybutyric acid. Alcohol. 2000;20:263–269. doi: 10.1016/s0741-8329(99)00090-7. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Aramaki S, Sweetman L, Nyhan WL, DeVivo DC, Hodson AK, Jakobs C. Stable isotope dilution analysis of 4-hydroxybutyric acid: an accurate method for quantification in physiological fluids and the prenatal diagnosis of 4-hydroxybutyric aciduria. Biomed. Environ. Mass. Spectrom. 1990;19:89–93. doi: 10.1002/bms.1200190207. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Brown PR, Jansen EE, Jakobs C, Gibson KM, Weerts EM. Behavioral effects and pharmacokinetics of gamma-hydroxybutyrate (GHB) precursors gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) in baboons. Psychopharmacol. (Berl.) 2009;204:465–476. doi: 10.1007/s00213-009-1477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AK, Froestl W, Weerts EM. Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacol. (Berl.) 2005;180:342–351. doi: 10.1007/s00213-005-2165-y. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM, Weerts EM. Chronic intragastric admistration of gamma-butyrolactone (GBL) produces physical dependence in baboons. Psychopharmacol. (Berl.) 2006;189:71–82. doi: 10.1007/s00213-006-0534-9. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Kaminski BJ, Griffiths RR, Ator NA, Weerts EM. Intravenous self-administration of gamma-hydroxybutyrate (GHB) in baboons. Drug Alcohol Depend. 2011;114:217–224. doi: 10.1016/j.drugalcdep.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AK, Kaminski BJ, Weerts EM. Self-administration of gamma-hydroxybutyric acid (GHB) precursors gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD) in baboons. Psychopharmacol. (Berl.) 2013;225:637–646. doi: 10.1007/s00213-012-2851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand J, Olszewski D, Sedefov R. Legal highs on the Internet. Subst. Use Misuse. 2010;45:330–340. doi: 10.3109/10826080903443628. [DOI] [PubMed] [Google Scholar]
- Jones AL. Legal ‘highs’ available through the Internet-implications and solutions? QJM. 2010;103:535–536. doi: 10.1093/qjmed/hcq066. [DOI] [PubMed] [Google Scholar]
- Kaminski B, Sannerud C, Weerts E, Lamb R, Griffiths R. Physical dependence in baboons chronically treated with low and high doses of diazepam. Behav. Pharmcol. 2003;14:331–342. doi: 10.1097/01.fbp.0000082131.08343.0e. [DOI] [PubMed] [Google Scholar]
- Karila L, Reynaud M. GHB and synthetic cathinones: clinical effects and potential consequences. Drug Test Anal. 2010;3:552–559. doi: 10.1002/dta.210. [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A. Cataleptic effects of gamma-hydroxybutyrate (GHB), its precursor gamma-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348. Psychopharmacol. (Berl.) 2007;192:407–414. doi: 10.1007/s00213-007-0718-y. [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A, France CP. Behavioral effects of gamma-hydroxybutyrate, its precursor gamma-butyrolactone, and GABA(B) receptor agonists: time course and differential antagonism by the GABA(B) receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348). J. Pharmacol. Exp. Ther. 2009;330:876–883. doi: 10.1124/jpet.109.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR, Bradford LD, Brady JV, Daley L. A tethering system for intravenous and intragastric drug administration in the baboon. Pharmacol. Biochem. Behav. 1982;17:823–829. doi: 10.1016/0091-3057(82)90366-5. [DOI] [PubMed] [Google Scholar]
- McDonough M, Kennedy N, Glasper A, Bearn J. Clinical features and management of gamma-hydroxybutyrate (GHB) withdrawal: a review. Drug Alcohol Depend. 2004;75:3–9. doi: 10.1016/j.drugalcdep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Coop A, France CP, Winger G, Woolverton WL. Evaluation of the reinforcing and discriminative stimulus effects of 1,4-butanediol and gamma-butyrolactone in rhesus monkeys. Eur. J. Pharmacol. 2003;466:113–120. doi: 10.1016/s0014-2999(03)01486-9. [DOI] [PubMed] [Google Scholar]
- Miotto K, Darakjian J, Basch J, Murray S, Zogg J, Rawson R. Gamma-hydroxybutyric acid: patterns of use, effects and withdrawal. Am. J. Addict. 2001;10:232–241. doi: 10.1080/105504901750532111. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals (8th edition). The National Academies Press; Washington D.C.: 2011. [Google Scholar]
- Nicholson K, Balster R. GHB: A new and novel drug of abuse. Drug Alcohol Depend. 2001;63:1–22. doi: 10.1016/s0376-8716(00)00191-5. [DOI] [PubMed] [Google Scholar]
- Palmer R. Gamma-butyrolactone and 1,4-butanediol: abused analogues of gamma-hydroxybutyrate. Toxicol. Rev. 2004;23:21–31. doi: 10.2165/00139709-200423010-00003. [DOI] [PubMed] [Google Scholar]
- Quang LS, Colombo G, Lobina C, Maccioni P, Orru A, Gessa GL, Maher TJ, Carai MA. Evaluation for the withdrawal syndrome from gamma-hydroxybutyric acid (GHB), gamma-butyrolactone (GBL), and 1,4-butanediol (1,4-BD) in different rat lines. Ann. N.Y. Acad. Sci. 2006;1074:545–558. doi: 10.1196/annals.1369.055. [DOI] [PubMed] [Google Scholar]
- Rosenberg MH, Deerfield LJ, Baruch EM. Two cases of severe gamma-hydroxybutyrate withdrawal delirium on a psychiatric unit: recommendations for management. Am. J. Drug Alcohol Abuse. 2003;29:487–496. doi: 10.1081/ada-120020529. [DOI] [PubMed] [Google Scholar]
- Roth RH, Giarman NJ. Preliminary report on the metabolism of γ-butyrolactone and γ-hydroxybutyric acid. Biochem. Pharmacol. 1965;14:177–178. doi: 10.1016/0006-2952(65)90073-0. [DOI] [PubMed] [Google Scholar]
- Roth RH, Giarman NJ. Evidence that central nervous system depression by 1,4-butanediol is mediated through a metabolite, gammahydroxybutyrate. Biochem. Pharmacol. 1968;17:735–739. doi: 10.1016/0006-2952(68)90010-5. [DOI] [PubMed] [Google Scholar]
- Schep LJ, Knudsen K, Slaughter RJ, Vale JA, Megarbane B. The clinical toxicology of gamma-hydroxybutyrate, gamma-butyrolactone and 1,4-butanediol. Clin. Toxicol. (Phila.) 2012;50:458–470. doi: 10.3109/15563650.2012.702218. [DOI] [PubMed] [Google Scholar]
- Schneir AB, Ly BT, Clark RF. A case of withdrawal from the GHB precursors gamma-butyrolactone and 1,4-butanediol. J. Emerg. Med. 2001;21:31–33. doi: 10.1016/s0736-4679(01)00324-9. [DOI] [PubMed] [Google Scholar]
- Snead OC, Furner R, Liu CC. In vivo conversion of gamma-aminobutyric acid and 1,4-butanediol to gamma-hydroxybutyric acid in rat brain. Studies using stable isotopes. Biochem. Pharmacol. 1989;38:4375–4380. doi: 10.1016/0006-2952(89)90645-x. [DOI] [PubMed] [Google Scholar]
- Teter CJ, Guthrie SK. A comprehensive review of MDMA and GHB: two common club drugs. Pharmacotherapy. 2001;21:1486–1513. doi: 10.1592/phco.21.20.1486.34472. [DOI] [PubMed] [Google Scholar]
- Thai D, Dyer JE, Jacob P, Haller CA. Clinical pharmacology of 1,4-butanediol and gamma-hydroxybutyrate after oral 1,4-butanediol administration to healthy volunteers. Clin. Pharmacol. Ther. 2006;81:178–184. doi: 10.1038/sj.clpt.6100037. [DOI] [PubMed] [Google Scholar]
- Towiwat P, Phattanarudee S, Maher TJ. Comparative study of equimolar doses of gamma-hydroxybutyrate (GHB), 1,4-butanediol (1,4-BD) and gamma-butyrolactone (GBL) on catalepsy after acute and chronic administration. Food Chem. Toxicol. 2013;51:337–342. doi: 10.1016/j.fct.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Weerts E, Goodwin A, Griffiths R, Brown P, Froestl W, Jakobs C, Gibson K. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacol. (Berl.) 2005;179:678–687. doi: 10.1007/s00213-004-2079-0. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR. Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J. Pharmacol. Exp. Ther. 1998;285:41–53. [PubMed] [Google Scholar]
- Weerts EM, Griffiths RR. Zolpidem self-injection with concurrent physical dependence under conditions of long-term continuous availability in baboons. Behav. Pharmacol. 1998;9:285–297. [PubMed] [Google Scholar]
- World Health Organization (WHO) 1,4-Butanediol (1,4-BD) pre-review report. In: E.C.D.D., editor. Dependence. World Health Organization; Hammamet, Tunisia: 2012. [Google Scholar]
- Wojtowicz J, Yatema M, Wax P. Withdrawal from gamma-hydroxybutyrate, 1,4-butanediol and gamma-butyrolactone: a case report and systematic review. Can. J. Emerg. Med. 2008;10:69–74. doi: 10.1017/s1481803500010034. [DOI] [PubMed] [Google Scholar]
- Wood DM, Brailsford AD, Dargan PI. Acute toxicity and withdrawal syndromes related to gamma-hydroxybutyrate (GHB) and its analogues gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD). Drug Test Anal. 2011;3:417–425. doi: 10.1002/dta.292. [DOI] [PubMed] [Google Scholar]
- Woods JH, Katz JL, Winger G. Abuse liability of benzodiazepines. Pharmacol. Rev. 1987;39:251–413. [PubMed] [Google Scholar]
- Yanagita T, Takahashi S. Development of tolerance to and physical dependence on barbiturates in rhesus monkeys. J. Pharmacol. Exp. Ther. 1970;172:163–169. [PubMed] [Google Scholar]
- Zvosec DL, Smith SW, McCutcheon JR, Spillane J, Hall BJ, Peacock EA. Adverse Events, Including Death, Associated with the Use of 1,4- Butanediol. N. Engl. J. Med. 2001;344:87–94. doi: 10.1056/NEJM200101113440202. [DOI] [PubMed] [Google Scholar]