Abstract
The ventral subiculum (vSub) of the hippocampus is critically involved in mediating the forebrain’s response to stress, particularly with regard to psychogenic stressors. Stress, in turn, is known to aggravate many psychiatric conditions including schizophrenia, depression, anxiety, and drug abuse. Pathological alterations in hippocampal function have been identified in all these disorders; thus, it is of interest to understand how stress affects this brain region. The vSub receives dense projections from the stress-related locus coeruleus (LC); however, it is not known what role this input plays in signaling stressful stimuli. In this study, the direct LC innervation of the vSub was investigated as a potential mediator of stress responses in this region. To examine responses to an acute stressor, the effect of footshock on single vSub neurons was tested in rats. Footshock inhibited 13%, and activated 48% of neurons in this region. Importantly, responses to footshock were correlated with LC stimulation-evoked responses in single neurons, and LC inactivation blocked these responses. Furthermore, prazosin, an alpha-1 antagonist, reversed footshock-evoked inhibition, revealing an underlying activation. Inactivation of the basolateral amygdala (BLA) did not block phasic footshock-evoked activation; however, it reduced tonic activity in the vSub. These results suggest that the LC NE system plays an important role in mediating stress responses in the vSub. Footshock evokes both inhibition and excitation in the vSub, by activating noradrenergic inputs from the LC. These responses may contribute to stress adaptation; while an imbalance of this system may lead to pathological stress responses in mental disorders.
Keywords: stress, norepinephrine, footshock, ventral subiculum, locus coeruleus, rat
Introduction
Stress is defined as any stimulus that disrupts an animal’s ability to maintain homeostasis. The organism’s stress response is a spectrum of neuroendocrine, cardiovascular, immune and autonomic changes precipitated by aversive stimuli or contexts (Kopin, 1995). Recent advances have broadened our understanding of the stress response to include changes in brain function mediated by the central monoamine system, including norepinephrine (Sullivan et al., 1999; Liu and Nakamura, 2006; Joca et al., 2007).
The locus coeruleus-norepinephrine system is activated by a variety of stressors, including restraint, tailshock, footshock, hypotension, immune challenge, swim, water avoidance stress and social stress (Mana and Grace, 1997; Jedema and Grace, 2003; Valentino and Van Bockstaele, 2008). Furthermore, the behavioral effects of LC activation mimic the effects of acute stress exposure, and lesions of the LC block or attenuate neuroendocrine and behavioral stress responses (Ziegler et al., 1999). Given that noradrenergic neurons of the LC are potently activated by stressful stimuli, that the vSub receives among the densest innervations from the LC (Oleskevich et al., 1989; Schroeter et al., 2000), and that the vSub is considered to be a central component in the stress response (Mueller et al., 2004; Herman and Mueller, 2006), the way in which the vSub processes NE input is critical to understanding its role in the brain’s stress response. However, it is not known what role the NE system plays in altering neuronal activity in the vSub in response to stressful stimuli, and surprisingly few studies have addressed this question.
The vSub is also implicated in processing contextual information, which is significant since stress is a context-dependent phenomenon; i.e., the context in which the stressor is administered plays a major role in the adaptive response of the organism (Bouton and Bolles, 1979). In addition, the vSub potently influences dopaminergic neuron activity (Floresco et al., 2001, 2003), and prolonged stress is known to trigger maladaptive responses to acute stress involving dopamine dysregulation, such as relapse in drug addiction, schizophrenia and depression.
In this experiment, neural pathways involved in stress responses were investigated. First, the response of single vSub neurons to acute footshock and electrical LC stimulation was tested and the potential underlying noradrenergic mechanisms were examined. Given that the LC projects to the basolateral amygdala (BLA) a stress-reactive structure that also innervates the vSub (Pitkänen et al., 2000), the relative contribution of the LC and the BLA was then assessed by testing the effect of inactivating these two structures on footshock responses.
Experimental procedures
Surgery
All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male Sprague Dawley rats (300–400 g) were housed 2 per cage with food and water available ad libidum. Rats were anesthetized with urethane (1.5 g/kg i.p.) and placed in a stereotaxic device (David Kopf Instruments, Tujunga, CA). The skull was exposed, and holes were drilled in the skull overlaying the vSub, the LC and/or the BLA. Coordinates (rostral from bregma; lateral from midline) were determined using a stereotaxic atlas (Paxinos and Watson, 2007): vSub: −6.0, 4.6; BLA: −3.5, 5.0; LC (see LC stimulation below).
Extracellular single unit recording
Recordings were performed using microelectrodes constructed from borosilicate glass tubing using a vertical microelectrode puller (Narishige PE-2, Tokyo, Japan) as previously described (Goto & Anthony A Grace 2006). Briefly, microelectrodes were filled with 2% pontamine sky blue dye dissolved in 2 M NaCl. The recording electrode impedance measured in situ ranged between 6 and 14 MOhms. Electric potentials were amplified using an extracellular amplifier (Fintronics Inc., Orange, CT), and monitored on an oscilloscope. Data were fed to a PC and recorded using LabChart software (ADInstruments, Inc., Colorado Springs, CO). Following recording, dye was ejected from the electrode by applying a constant negative current in order to mark the electrode placement.
Effects of footshock on vSub neuron activity
The sciatic nerve was activated indirectly using current injection (5 s trains; 8 mA; 20 Hz; 0.25 ms pulse duration), by two 26-gauge needles inserted into the medial aspect of the contralateral hind paw contralateral to the neuronal recordings (Laviolette et al., 2005). At least 5 footshock trials were delivered with 60 s inter-trail intervals.
LC stimulation
The LC (A: −12.6, M/L: +1.1, D/V: −6.0 from bregma, at 10°) was stimulated using current pulses (0.25 ms duration) delivered via a bipolar concentric electrode at current amplitudes between 500–800 uA. Train stimulation (4 pulses at 20 Hz) was delivered to mimic bursts of spikes typical of phasically activated LC responses (Akaike, 1982; Florin-Lechner et al., 1996; Devilbiss and Waterhouse, 2000; Berridge and Waterhouse, 2003). Responses were characterized in terms of firing rate within 4 sec of LC stimulation, relative to a 4 sec pre-stimulation baseline. Stimulation pulses were delivered every 10 sec. At the end of LC stimulation experiments, stimulation electrode locations were marked by ejection of constant current (150 uA, 10 seconds) through the stimulation electrode.
Dopamine beta hydroxylase (DBH) immunocytochemistry
In a subset of LC-stimulation experiments (N=5), brain tissue was processed with DBH immunocytochemistry to visualize DBH-expressing NE neurons within the LC, relative to stimulation electrode placements. Following electrophysiology, rats were perfused transcardially with 4 % paraformaldehyde and the brains were fixed in 4 % paraformaldehyde for 24 hours. Brains were sliced at 40 um, blocked with normal goat serum, and incubated in a monoclonal mouse anti-DBH antibody, clone 4F10.2 (Millipore, Billerica, MA) for 24 hours (Mody et al., 2011). DBH binding was visualized using a biotinylated anti-mouse IgG secondary antibody (Vector Laboratories, Inc., Burlingame, CA) followed by binding of the avidin-biotin peroxidase complex (Vectastain ABC, Vector Laboratories) and nickel-enhanced 3,3′-diaminobenzidine (DAB).
LC inactivation
In order to determine if LC activity is required for footshock-evoked responses in the vSub, LC neuron firing was suppressed by local infusion of the alpha-2 adrenergic agonist clonidine (4.8 μg/0.5μl) into the LC, at doses effective at reducing hippocampal NE levels as measured by microdialysis (Mair et al., 2005). A guide cannula (Plastics One, Roanoke, VA) was lowered into the LC (A: −12.6, M/L: +1.1, D/V: −6.0 from bregma, at 10°), and spontaneously active neurons were isolated in the vSub. A cannula was inserted into the guide, so that the tip of the cannula extended 1 mm past the tip of the guide cannula. After baseline responses to footshock were established, clonidine was infused over 30 seconds using a Hamilton syringe. Two minutes following the infusion, the response to footshock stimulation was again tested.
BLA inactivation
In a subset of experiments, a guide cannula (Plastics One, Roanoke, VA) was lowered into the BLA (from bregma: −3.5 caudal, 5.0 lateral) of urethane-anesthetized rats. Once baseline responses to footshock stimulation were established, the effect of BLA inactivation by tetrodotoxin (TTX, Sigma-Aldrich) infusion was tested. A cannula was inserted into the guide, so that the tip of the cannula extended 1 mm past the tip of the guide cannula. TTX (1.0 μM; 0.5 μL) was infused over 30 seconds using a Hamilton syringe (Belujon and Grace, 2008). Two minutes following the infusion, the response to footshock stimulation was again tested.
Results
Effects of footshock on vSub neuron activity in anesthetized rats
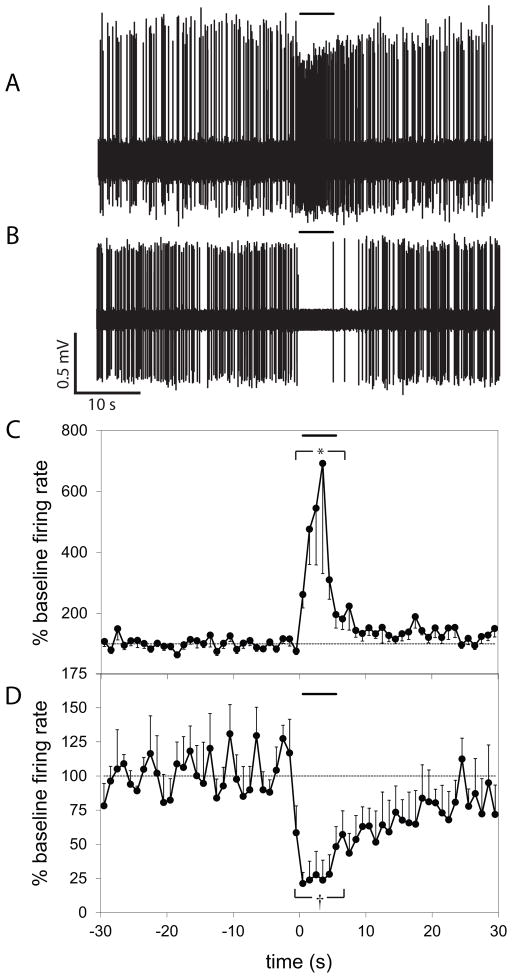
Spontaneously active neurons were isolated in the vSub of urethane anesthetized rats. Recording placements were confirmed histologically, and were located primarily within the pyramidal layer, but also within the molecular layer of the vSub (Figure 1A–B). After a stable baseline recording was obtained, the response to at least 5 trials of footshock was tested. Neurons were categorized based on their firing rate (FR) during the 5 second footshock relative to baseline FR. Of 38 neurons tested, 18 (48%) were activated (footshock-activated (FA); baseline FR = 3.1±0.7 Hz, footshock FR 7.0±0.9 Hz, p<0.001, t-test), 5 (13%) were inhibited (footshock-inhibited (FI); baseline FR = 6.9±2.3 Hz, footshock FR = 2.8±1.7 Hz, p=0.008, t-test), while 15 (39%) did not show a significant change in firing (baseline FR = 1.8±0.4 Hz, footshock FR = 1.9±0.4 Hz, p=0.326) to footshock (Figure 2).
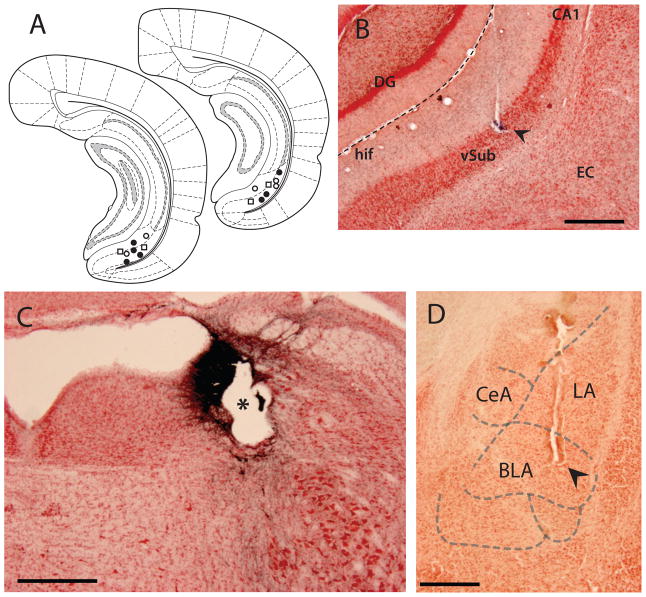
Figure 1.
Histological confirmation of electrode placements. A. Recording electrode placements within the vSub. ● marks locations of neurons activated by LC stimulation and footshock; ○ marks locations of neurons inhibited by LC stimulation and footshock; □ marks the location of neurons not showing a response to these stimuli. B. Representative example of a dye deposit (arrowhead) marking recording electrode placement within the vSub in a Nissl-stained section. C. Electrode placement (*) within the LC in a section immunolabeled for dopamine beta-hydroxylase (DBH) and Nissl-stained; blue-black DDH staining of noradrenergic neurons indicates location of the LC. D. Example of cannula placement (arrowhead) within the BLA in a Nissl-stained section. CeA – central nucleus of the amygdala; LA – lateral nucleus of the amygdala; DG – dentate gyrus of the hippocampus; hif – hippocampal fissure; EC – entorhinal cortex. Scale bar = 0.5 mm.
Figure 2.
Footshock resulted in activation or inhibition of neurons in the vSub. Representative voltage traces from extracellular single unit recording showing activation (A) and inhibition (B) of vSub neurons by footshock. Overall, footshock stimulation resulted in activation in 48% (C), and inhibition in 13% (D) of vSub neurons tested. Each plot shows the average firing rate of the neurons in response to a 5 second footshock (delivered at t = 0), represented as a percentage of the 30 second baseline. (* indicates significant difference (p<0.001) between baseline FR and footshock FR in FA neurons; † indicates significant difference (p=0.008) between baseline FR and footshock FR in FI neurons.)
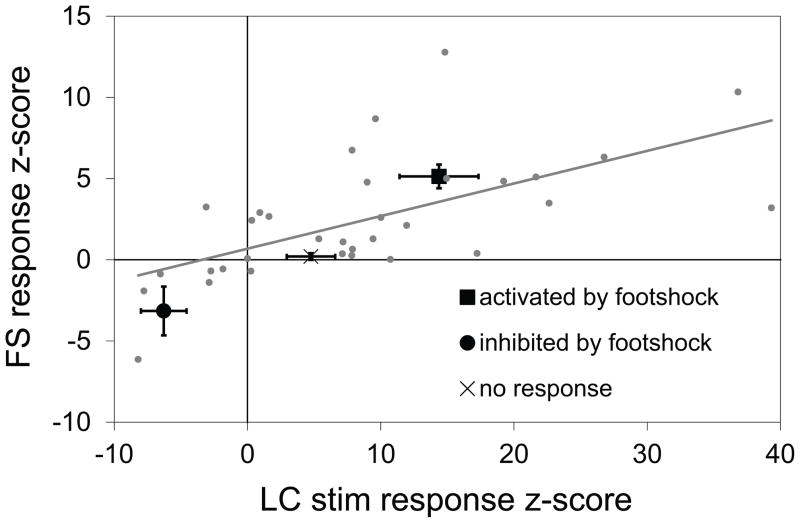
Footshock response was correlated with response to LC stimulation
In a subset of the neurons tested for response to footshock, response to electrical stimulation of the LC was also examined. The response of every neuron to each stimulus was represented as a single measure, calculated as a z-score based on the neurons’ average firing rate during the 5 second footshock, and during one second beginning at the start of LC stimulation, relative to each pre-stimulus baseline. When the responses of the vSub neurons to footshock were regressed against responses to LC stimulation, a significant positive correlation emerged (Figure 3; N = 33, constant coefficient = 4.0±1.9; slope coefficient = 1.9±0.4; R2 = 0.39, p < 0.001). Thus, FI neurons tended also to be inhibited by LC stimulation; and, conversely, FA neurons tended to be activated by LC stimulation. Histological analysis confirmed that stimulation electrode placements were located within or adjacent to the noradrenergic neuron cell bodies and fibers of the LC, which were labeled using DBH immunocytochemistry (Figure1C).
Figure 3.
Responses of individual vSub neurons to LC stimulation and to footshock are correlated. The response to footshock was correlated with the response to LC stimulation in vSub neurons tested with both stimuli. Response to each stimulus is represented as z-score based on the firing rate of the neuron during the 5 second footshock, and 1 second beginning at the start of LC stimulation, relative to baseline.
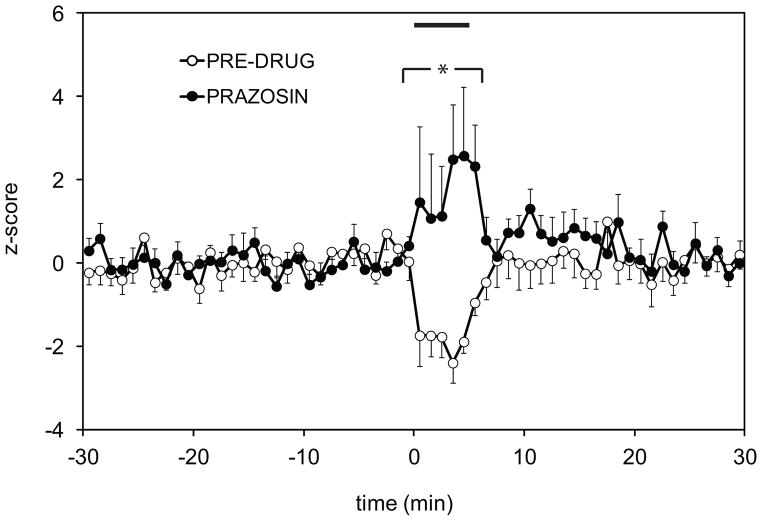
Alpha-1 receptor blockade reversed the inhibitory responses to footshock
In a separate population of 10 neurons inhibited by footshock, the effect of the alpha-1 noradrenergic antagonist prazosin on footshock-induced inhibition was examined. After establishing a pre-drug response, 1 mg/kg prazosin was injected i.v. and the response to footshock was re-tested. Prazosin reversed the footshock-induced inhibition, revealing instead an activating response (Figure 4). A two way repeated measure ANOVA revealed a significant effect of prazosin versus the pre-drug condition (p=0.0497), and the Holm-Sidak post-hoc analysis showed a statistically significant difference in the footshock response between prazosin and the pre-drug baseline (p=0.006).
Figure 4.
Footshock-evoked inhibition is alpha-1 receptor-dependent. Systemic application of alpha-1 noradrenergic receptor antagonist prazosin (1 mg/kg) reversed the inhibitory effect of footshock, revealing an underlying fooshock-evoked excitation. Response is expressed as z-score relative to the 30 second baseline. 5 second footshock was delivered at t = 0. (* indicates significant difference in firing rate following prazosin injection (two-way RM ANOVA, Holm-Sidak test, p=0.006), compared with the pre-drug condition.)
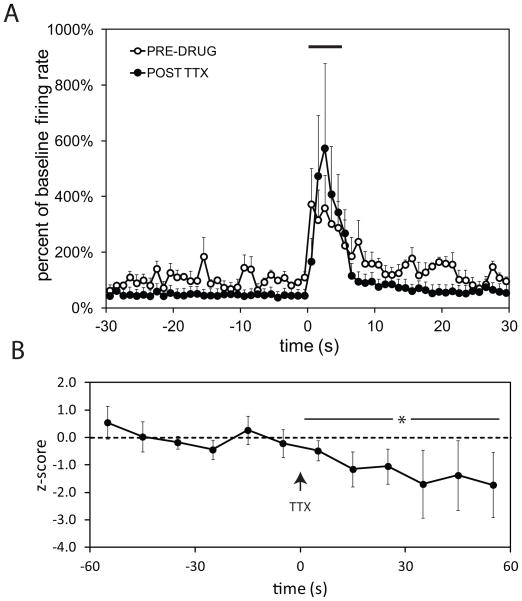
BLA drives tonic activity in the vSub, but is not required for footshock-evoked activation
The BLA is activated by footshock (Rosenkranz and Grace, 2002) and is capable of driving vSub neurons (Lipski and Grace, 2009). In order to determine whether the footshock-evoked activation observed in the vSub was mediated via the BLA, the response to footshock was tested before and after TTX infusion into the BLA. Histological analysis confirmed that cannula placements were confined to the BLA (Figure1D). TTX infusion into the BLA did not significantly affect the activation of vSub neurons by footshock (Figure 5A). A two way repeated measure ANOVA did not show an effect of TTX infusion on the evoked response (N=5; p = 0.130). However, the spontaneous firing rate of vSub neurons was significantly reduced following TTX infusion into the BLA (Figure 5B; N =10, baseline FR = 5.1±1.6; FR after TTX infusion = 1.4±0.3; paired t-test, p < 0.05). Thus, BLA inactivation reduced tonic activity within the vSub without affecting phasic responses to footshock.
Figure 5.
Inactivation of the BLA with TTX did not block footshock-induced activation of vSub neurons (A), but significantly reduced spontaneous firing (B). ( * indicates difference between pre-drug baseline and TTX conditions, p<0.05)
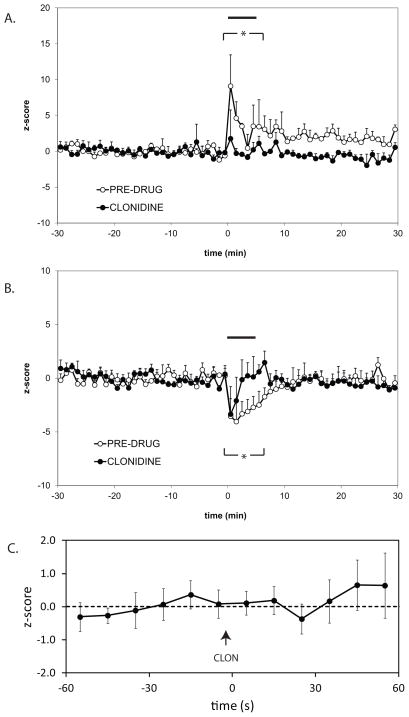
Footshock-evoked responses are mediated via the LC
In order to determine if LC activity is required for footshock-evoked responses in the vSub, LC neuron firing was suppressed by cannula infusion of the alpha-2 adrenergic agonist clonidine into the LC. Footshock responses were tested before and after clonidine infusion. In neurons activated by footshock before clonidine infusion, LC inactivation blocked footshock-evoked responses (Figure 6A; N=5; Two-way repeated ANOVA, Holm-Sidak test, significant difference in footshock response between TTX and pre-drug baseline, p<0.05). On the other hand, in neurons inhibited by footshock before clonidine infusion, LC inactivation only partially suppressed footshock-evoked responses (Figure 6B; N=5; Two-way repeated ANOVA, Holm-Sidak test, significant difference in footshock response between TTX and pre-drug baseline, p<0.05). Spontaneous firing was also recorded at least 60 s after clonidine infusion; clonidine did not have a significant effect on spontaneous activity of vSub neurons (Figure 6C; N=7). Thus, LC inactivation suppressed phasic responses to footshock without affecting the tonic activity in the vSub.
Figure 6.
Footshock-evoked responses in vSub neurons require LC activity. Inactivation of the LC by local clonidine infusion suppressed footshock-induced activation (A) and partially suppressed inhibition (B) in two separate populations of vSub neurons, without affecting spontaneous firing (C). (* indicates difference between pre-drug baseline and clonidine conditions, p<0.05)
Discussion
The ventral subiculum is a key forebrain structure in regulating the behavioral response of an animal to environmental challenges (O’Mara et al., 2001; Herman and Mueller, 2006), and contains one of the densest NE innervations of the brain (Oleskevich et al., 1989). In this manuscript, we examined the responses of single vSub neurons to footshock, and the relative contribution of the LC and the BLA in mediating these responses.
Footshock activated 48% (FA), and suppressed 13% (FI) neurons in the vSub. Furthermore, responses to footshock were correlated with responses to LC stimulation in single neurons. This suggests that footshock responses may be mediated by the LC. Indeed, inactivation of the LC with clonidine infusion blocked footshock-evoked activation in FA neurons and partially suppressed footshock-evoked inhibition in FI neurons, without affecting baseline activity. At least two factors could account for the incomplete block of footshock-evoked inhibition. First, there may be a NE-independent component to the inhibitory footshock responses in the vSub. Second, although doses of clonidine used in this experiment have been shown to effectively reduce hippocampal NE levels as measured by microdialysis (Mair et al., 2005), inhibition of LC neurons via alpha-2 autoreceptors may be insufficient to completely override the activating effect of footshock on these neurons.
Blockade of alpha-1 noradrenergic receptors with systemic prazosin injection reversed the inhibitory responses in FI neurons, revealing an underlying footshock-induced activation. This result suggests that FA and FI neurons may not represent static populations, but rather that the state of alpha-1 activation may determine the type of response a neuron shows to footshock. One possibility would be that FI neurons are selectively innervated by a population of alpha-1 expressing GABA interneurons. Alpha-1 receptors are generally known to increase neuronal excitability via the Gq/11 class of G proteins and activation of phospholipase C beta, which leads to an inositol triphosphate-mediated Ca++ release from intracellular stores, as well as activation of protein kinase C (Wozniak M et al., 2000). mRNAs of the alpha-1A/D receptor subtype are expressed throughout the hippocampal formation including the dentate gyrus, Ammon’s horn, and the subiculum (McCune et al., 1993; Pieribone et al., 1994), particularly on interneurons (Hillman et al., 2007). Activation of alpha-1 receptors has been reported to depolarize GABA interneurons, thereby increasing inhibitory input onto pyramidal neurons in the vSub (Bergles et al., 1996). It is noteworthy that somatostatin-expressing interneurons in area CA1 that also express alpha-1 receptors (Hillman et al., 2005a, 2005b) correspond to a population of GABAergic interneurons targeting both the basal and apical dendrites of pyramidal cells (Somogyi and Klausberger, 2005). While it is not known whether the same interneuron type exists in the subiculum (O’Mara et al., 2001), it is likely that footshock-induced inhibition of pyramidal neurons in the vSub may be mediated by alpha-1 receptors located on GABAergic interneurons. By increasing firing of the interneurons, alpha-1 activation would, in turn, suppress pyramidal neuron activity.
Importantly, footshock responses may also be mediated by glutamatergic input to the vSub. One possible glutamatergic afferent pathway originates in the BLA, a brain region that also receives a strong LC projection, and that is known to be activated during stress (Cullinan et al., 1995; Rosen et al., 1998; Chowdhury et al., 2000; Akirav et al., 2001; Dayas et al., 2001; Rosenkranz and Grace, 2002). Importatly, neurons in the vSub are activated by BLA inputs (Lipski and Grace, 2011). In order to evaluate whether part of the excitatory response to footshock in the vSub was mediated by the BLA, the BLA was inactivated by TTX microinfusion. BLA inactivation reduced tonic baseline firing of vSub neurons, but did not block phasic activation by footshock. This result is not surprising, since in unstressed rats NE release from the LC into the BLA has been reported to primarily inhibit neurons in this region, with only a small proportion of neurons being activated through a beta adrenergic mechanism (Buffalari and Grace, 2009). However, chronic cold stress was reported to increase the excitatory effects of NE in the BLA, suggesting this input to the vSub may play a role in responses to acute footshock under chronic stress conditions.
In addition to the BLA, the response to footshock in the vSub may be occurring through other afferents. Indeed, one function of the hippocampal formation is to continually encode episodic memories based on multimodal sensory input. Accordingly, various sensory cortical areas send projections to this structure, most notably to the entorhinal cortex (EC) (Amaral et al., 1991; Witter et al., 2000; Kloosterman et al., 2004). The EC projects directly to the vSub, as well as indirectly through the multisynaptic hippocampal circuit (Witter et al., 2000; Kloosterman et al., 2004). This sensory input, modulated by the LC noradrenergic signal, could be involved in mediating footshock responses. One possible scenario is that this activating sensory signal is facilitated by beta adrenergic receptors on FA neurons and suppressed by alpha-1 receptors on interneurons projecting to FI neurons, as discussed above. Further experiments investigating the noradrenergic pharmacology – including alpha-2 and beta receptors – is needed to fully understand the underlying mechanisms of footshock responses in the vSub.
In this study, we demonstrate that LC activity is required for the full expression of footshock-evoked phasic responses in both FA and FI neurons in the vSub, without affecting tonic firing. On the other hand, the BLA drives tonic firing in the vSub without affecting phasic responses to footshock. These data are consistent with the BLA providing a biasing input to make the vSub more responsive to inputs under stressful conditions, whereas the LC only activates the vSub phasically to discrete stimuli. With chronic stressors, the activity of these components are known to change, with the BLA showing increased excitation by NE from the LC (Buffalari and Grace, 2009), and the LC showing increased responses to inputs (Mana and Grace, 1997; Jedema et al., 2001; Jedema and Grace, 2003), which would substantially augment stressor responses in this region. The balance between baseline excitability by the BLA and phasic input from the LC and how it is altered during maintained presentation of a stressor is likely to play a major role in stress adaptation. In contrast, an imbalance of this system may lead to pathological stress responses in mental disorders.
Acknowledgments
Role of the Funding Source
Funding for this study was provided by NIH Grants MH57440 and DA15408; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
We thank Dr. Pauline Belujon who has provided helpful comments during preparation of the manuscript, and Nicole MacMurdo for technical assistance.
Footnotes
Contributors
Dr. Lipski and Dr. Grace designed the study and collaborated on writing of the manuscript. Dr. Lipski performed the experiments and the statistical analyses. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Dr. Grace declares that he has received compensation from Johnson & Johnson, Lundbeck, Pfizer, GalaxoSmithKline, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo and Otsuka. Dr. Lipski declares that he has no conflicts of interest.
References
- Akaike T. Periodic bursting activities of locus coerulleus neurons in the rat. Brain research. 1982;239:629–633. doi: 10.1016/0006-8993(82)90540-6. [DOI] [PubMed] [Google Scholar]
- Akirav I, Sandi C, Richter-Levin G. Differential activation of hippocampus and amygdala following spatial learning under stress. Eur J Neurosci. 2001;14:719–725. doi: 10.1046/j.0953-816x.2001.01687.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Doze VA, Madison DV, Smith SJ. Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons. J Neurosci. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research Brain research reviews. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. The International Journal of Neuropsychopharmacology. 2009;12:95–107. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Fujioka T, Nakamura S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 2000;52:171–182. doi: 10.1016/s0361-9230(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. The European journal of neuroscience. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse (New York, NY) 2000;37:273–282. doi: 10.1002/1098-2396(20000915)37:4<273::AID-SYN4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain research. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biological psychiatry. 2006;60:1259–1267. doi: 10.1016/j.biopsych.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Doze VA, Porter JE. Functional characterization of the beta-adrenergic receptor subtypes expressed by CA1 pyramidal cells in the rat hippocampus. J Pharmacol Exp Ther. 2005a;314:561–567. doi: 10.1124/jpet.105.084947. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Doze VA, Porter JE. Alpha1A-adrenergic receptors are functionally expressed by a subpopulation of cornu ammonis 1 interneurons in rat hippocampus. J Pharmacol Exp Ther. 2007;321:1062–1068. doi: 10.1124/jpet.106.119297. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Knudson CA, Carr PA, Doze VA, Porter JE. Adrenergic receptor characterization of CA1 hippocampal neurons using real time single cell RT-PCR. Brain Res Mol Brain Res. 2005b;139:267–276. doi: 10.1016/j.molbrainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Finlay JM, Sved AF, Grace AA. Chronic cold exposure potentiates CRH-evoked increases in electrophysiologic activity of locus coeruleus neurons. Biol Psychiatry. 2001;49:351–359. doi: 10.1016/s0006-3223(00)01057-x. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Chronic exposure to cold stress alters electrophysiological properties of locus coeruleus neurons recorded in vitro. Neuropsychopharmacology. 2003;28:63–72. doi: 10.1038/sj.npp.1300020. [DOI] [PubMed] [Google Scholar]
- Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, van Haeften T, Lopes da Silva FH. Two reentrant pathways in the hippocampal-entorhinal system. Hippocampus. 2004;14:1026–1039. doi: 10.1002/hipo.20022. [DOI] [PubMed] [Google Scholar]
- Kopin IJ. Definitions of stress and sympathetic neuronal responses. Ann N Y Acad Sci. 1995;771:19–30. doi: 10.1111/j.1749-6632.1995.tb44667.x. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski WJ, Grace AA. Abstract Program No 815.10. Chicago, IL: Society for Neuroscience; 2009. Locus coeruleus stimulation and norepinephrine application produce opposite effects on accumbens-projecting neurons in the ventral subiculum. [Google Scholar]
- Lipski WJ, Grace AA. Abstract Program No 284.10. Washington, DC: Society for Neuroscience; 2011. Restraint stress activates c-fos in nucleus accumbens-projecting neurons of the hippocampal ventral subiculum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Nakamura S. Stress-induced plasticity of monoamine axons. Front Biosci. 2006;11:1794–1801. doi: 10.2741/1923. [DOI] [PubMed] [Google Scholar]
- Mair RD, Zhang Y, Bailey KR, Toupin MM, Mair RG. Effects of clonidine in the locus coeruleus on prefrontal- and hippocampal-dependent measures of attention and memory in the rat. Psychopharmacology (Berl) 2005;181:280–288. doi: 10.1007/s00213-005-2263-x. [DOI] [PubMed] [Google Scholar]
- Mana MJ, Grace AA. Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience. 1997;81:1055–1064. doi: 10.1016/s0306-4522(97)00225-x. [DOI] [PubMed] [Google Scholar]
- McCune SK, Voigt MM, Hill JM. Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience. 1993;57:143–151. doi: 10.1016/0306-4522(93)90116-w. [DOI] [PubMed] [Google Scholar]
- Mody P, Rukhadze I, Kubin L. Rats subjected to chronic-intermittent hypoxia have increased density of noradrenergic terminals in the trigeminal sensory and motor nuclei. Neuroscience letters. 2011;505:176–179. doi: 10.1016/j.neulet.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2004;145:3763–3768. doi: 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Descarries L, Lacaille JC. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci. 1989;9:3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara SM, Commins S, Anderson M, Gigg J. The subiculum: a review of form, physiology and function. Prog Neurobiol. 2001;64:129–155. doi: 10.1016/s0301-0082(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Stereotaxic Coordinates. 6. Elsevier Inc; 2007. The Rat Brain. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biological psychiatry. 1999;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. European Journal of Pharmacology. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Schramm NL, LEL . The noradrenergic receptor subtypes. In: Bloom FE, DJK, editors. Psychopharmacology: the fourth generation of progress. New York: Raven; 2000. [Google Scholar]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]