Summary
Presenilins (PSs) are catalytic components of the γ-secretase complex that produces Aβ peptides. Substrates of γ-secretase are membrane-bound protein fragments deriving from the cleavage of extracellular sequence of cell surface proteins. APP-derived γ-secretase substrates are cleaved at gamma (γ) sites to produce Aβ while cleavage at the epsilon (ε) site produces AICD proposed to function in transcription. In addition to APP, γ-secretase promotes the ε-cleavage of a large number of cell surface proteins producing cytosolic peptides shown to function in cell signaling.
A common hypothesis suggests that Alzheimer's disease (AD) is caused by Aβ peptides or their products. Treatment of patients with inhibitors of Aβ production however, showed no therapeutic benefits while inducing cytotoxicity. Similarly, treatments with anti-Aβ antibodies yielded disappointing results. Importantly, recent evidence shows that PS FAD mutations cause a loss of γ-secretase cleavage activity at ε site of substrates thus inhibiting production of biologically important cell signaling peptides while promoting accumulation of membrane-bound cytotoxic substrates. These data support a hypothesis that FAD mutations may increase neurotoxicity by inhibiting the γ-secretase-catalyzed ε cleavage of substrates thus interfering with cell signaling while also promoting accumulation of cytotoxic peptides. Similar mechanisms may explain γ-secretase inhibitor-associated toxicities observed in clinical trials. Here we discuss evidence that FAD neurodegeneration may be caused by loss of γ-secretase cleavage function at ε sites of substrates.
Introduction
AD is a progressive neurodegenerative disorder of the central nervous system (CNS) leading to the most common form of age-associated dementia. Clinical symptoms of AD include loss of recent memory, faulty judgment, personality changes and progressive loss of reasoning. The neuropathology of AD is characterized by large numbers of neuritic plaques (NPs) and neurofibrillary tangles (NFTs) in the brain striatum and neocortex (Newell et al., 1999). It is now believed that dementia is caused by neuronal and synapse losses in the affected areas of the CNS. NPs are extracellular structures containing a core of amyloid depositions of aggregated fibrillar A protein surrounded by dystrophic neuritis, astrocytes and reactive microglia. In contrast to NPs, NFTs are intracellular aggregates of paired helical filaments of hyper-phosphorylated tau protein. In addition, AD patients often display high levels of Aβ amyloid depositions on the walls of cerebral blood vessels, a condition termed cerebrovascular amyloidosis (CVA) (Glenner and Wong, 1987). Most AD cases are sporadic affecting patients over 65 or 70 years old but about 5% are classified as familial (FAD) because they are due to specific genetic mutations. FADs occur mostly at younger ages and follow a more aggressive clinical course than sporadic AD. The brain neuropathology however, is similar in sporadic and familial AD, suggesting the involvement of common cellular mechanisms in both forms of the disease. Currently, a definite diagnosis of the disease is made after clinical symptoms are combined with post-mortem examination of brain tissue for detection of plaques and tangles, the pathological hallmarks of the disease.
Despite intense research efforts, the cause of the accelerated neuronal degeneration that causes dementia remains unclear although it is now believed that the disease is driven by both environmental and genetic factors. Presently, age and the apolipoprotein allele E4 are the most important risk factors for sporadic AD. In contrast, FAD is driven by specific genetic mutations localized in at least three genetic loci including the genes for the amyloid precursor protein (APP) (Chartier-Harlin et al., 1991), presenilin1 (PS1), (Sherrington et al., 1995) and PS2 (Levy-Lahad et al., 1995). APP is important to all forms of AD because in addition to its contribution to FAD, it is the precursor of the Aβ peptides that form the amyloid depositions used to define the disorder (Robakis et al., 1987a). APP is also important to Down syndrome (DS) as almost all patients over the age of 40 develop brain neuropathologies similar to AD including amyloid depositions (Wisniewski et al., 1985). Localization of the APP gene on chromosome 21 revealed a direct genetic linkage between these two disorders (Robakis et al., 1987b). It is important to note however, that neither NPs nor NFTs are specific to AD as both pathological structures are also found in normal aged people, usually at lower numbers compared to AD. In addition, NFTs are found in other neurodegenerative disorders including frontotemporal dementia (FTD) and Parkinson's disease. The presence of NFTs in neurodegenerative disorders of distinct etiology suggests that tau heperphosphorylation and aggregation may represent a reaction to neurotoxicity induced by genetic lesions or environmental factors such as oxidative and excitotoxic stresses.
The etiology of AD
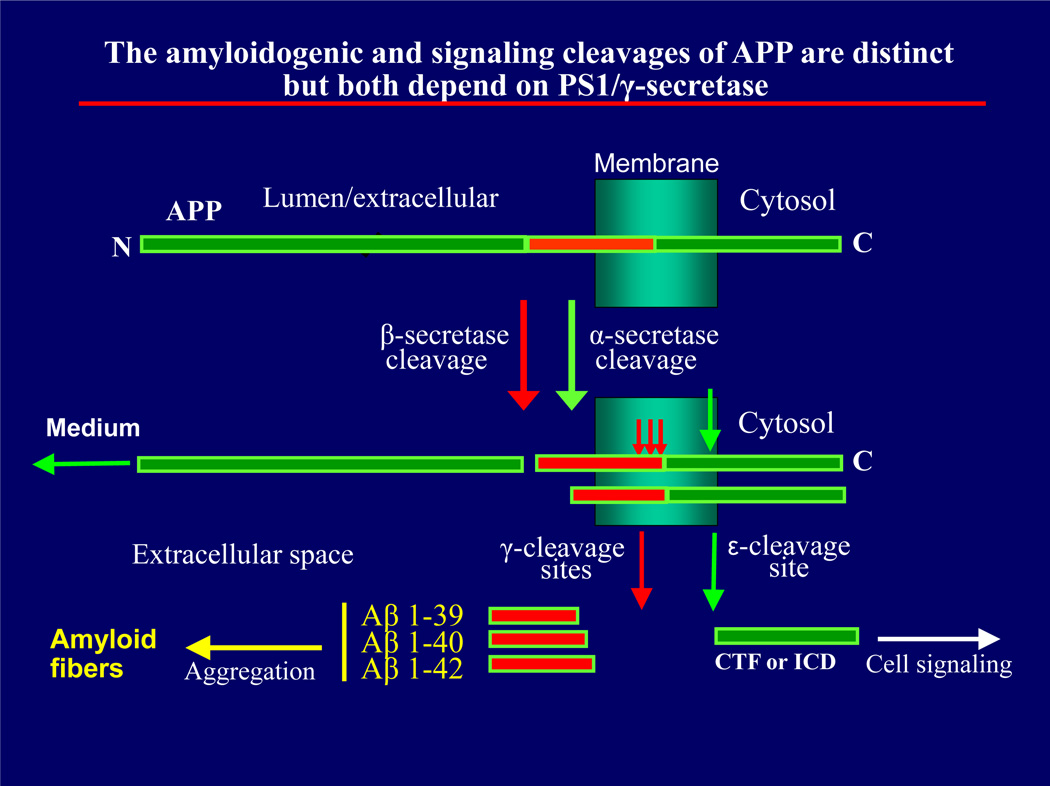
Following molecular characterization of brain amyloid depositions and NFTs, the two pathological hallmarks of AD, research focused mainly on Aβ peptides and their soluble and insoluble derivatives as the main causative agents of dementia. Aβ peptides are a family of small proteins with heterogenous ends containing 35 to 43 amino acids with Aβ40 and 42 being the predominant species (Miller et al., 1993; Mori et al., 1992). Under conditions that favor aggregation, Aβ peptides aggregate to form amyloid fibrils that precipitate in NPs found in interneuronal spaces and in blood vessels as CVA. Aβ peptides are derived from the amyloidogenic processing of APP, a type I transmemebrane protein, through the combined action of β (beta) and γ (gamma) secretases (reviewed by Robakis 2011). β-secretase (Vassar et al., 1999) acts on extracellular APP to produce peptide C99 that is then processed by the PS/γ-secretase complex (Wolfe et al., 1999) at γ sites to produce the Aβ peptides found in amyloid depositions (Fig.1). In the non-amyloidogenic processing, APP is cleaved by α-secretase, usually an ADAM, within the Aβ sequence thus inhibiting production of Aβ peptides (Anderson et al., 1991). α-Secretase-derived APP fragments are also processed by PS/γ-secretase at the ε site (Fig. 1), a process that results in the production of intracellular peptides containing the carboxyl-terminal fragment (CTF) of APP. Recent work revealed a number of cell surface proteins and receptors that, similar to APP, are processed by γ-secretase at ε sites producing intracellular CTF peptides (also called intracellular domains, ICDs). Importantly, a number of these peptides have been shown to act as signal transduction and gene expression factors (Marambaud and Robakis, 2005).
Figure 1.
The amyloidogenic (red arrows) and signaling (green arrows) processing of APP and other transmembrane proteins. The α-secretase cleavage is common for many cell surface proteins including APP shown here and are usually mediated by ADAM metalloproteinases. The β-secretase cleavage is specific to APP and produces the precursors of Aβ peptides. The transmembrane peptides resulting from the α-cleavage are usually referred to as CTF1s (Marambaud et al., 2003). Products of both α and β cleavages are processed by γ-secretase. Cleavage at γ sites produces Aβ-like peptides. Cleavage at ε-site produces the soluble cytosolic CTF2 (or ICD for APP) peptides shown to function in signal transduction (for more details see Barthet et al., 2012). N and C; protein N- and C-termini respectively.
Despite extensive research efforts in the last two decades there is no agreement on the proposed neurotoxicity of NPs and many workers doubt these structures are the main causative agents of AD (Neve and Robakis, 1998; Smith et al., 2000). Although amyloid depositions may contribute secondarily to neuronal dysfunction, it now seems unlikely that these are the main cause of the AD neurodegeneration as studies have failed to show significant correlations between brain NPs and degree of dementia or neuronal loss (Bouras et al., 2006; Crystal et al., 1988; Davis et al., 1999). Importantly, amyloid depositions at levels similar to those seen in AD are often detected in normal aged individuals (Crystal et al., 1988; Davis et al., 1999), and transgenic (Tg) animals with high levels of brain amyloid deposits show no significant neurodegeneration (Hsia et al., 1999). Studies in humans showed that clearance of NPs resulted neither in cognitive improvement nor in decreased rate of mental deterioration (Holmes et al., 2008) suggesting that amyloid deposits are not the driving force of neurodegeneration and cognitive decline in AD. It seems therefore unlikely that clearance of brain amyloid depositions will result in significant improvements of AD patients.
The inability of amyloid-based theories to explain AD prompted the development of the soluble Aβ oligomer theories that posit soluble oligomers of extracellular or intracellular Aβ42 represent the neurotoxic forms of Aβ. Indeed, evidence suggests that soluble oligomeric Aβ may interfere with synaptic function in vitro or memory function in experimental animal models (Cleary et al., 2005; Walsh et al., 2005). Most of these models however, are based on overexpression of exogenous APP, an artificial condition that does not apply to AD where there is no APP overxpression (Robakis et al., 1987a). In addition, behavioral abnormalities in animal models overexpressing APP cannot be unambiguously assigned to soluble Aβ or oligomers as APP is metabolized to a large number of derivatives some of which, such as C-terminal fragments, have been shown to be cytotoxic. It is thus unclear whether the behavioral abnormalities detected in Tg APP mice are due to specific Aβ species or to toxicities associated with the overexpression of exogenous APP. Furthermore, there is no evidence for the existence of soluble toxic Aβ oligomeric species specific to AD. Regarding the in vitro neurotoxicity of Aβ42, it is important to note that this neurotoxicity becomes detectable when Aβ is used at concentrations which are at least ten thousand times higher than the peptide concentrations found in vivo (usually less than 500pM). Presently there is little evidence of AD-associated increases in the levels of soluble Aβ or its oligomers. Absence of data supporting disease-associated increases in soluble toxic Aβ species is a serious weakness to the theory that such oligomers cause the neurodegeneration of AD.
FAD, Presenilins and Aβ
Mutations in three distinct genes, presenilin-1 (PS-1), PS2 and APP, have been implicated as causative agents of FAD, with mutations in the PS1 gene responsible for most cases. To date more than 150 FAD mutations have been linked to PS1 gene. Although early reports suggested that FAD mutations increase production of neurotoxic Aβ42 by causing a gain of γ-secretase function, the activity involved in its production (Borchelt et al., 1996; Scheuner et al., 1996; Citron et al., 1997), recent work (Shioi et al., 2007; Bentahir et al., 2006) showed that many PS1 FAD mutants do not increase Aβ. Similarly, reports that FAD mutations increase the Aβ42/40 ratio may need further examination because many FAD mutants fail to increase this ratio (Shioi et al., 2007) and although the APP Swedish FAD mutation causes a robust increase in both Aβ42 and Aβ40 due to increases in β-secretase cleavage, it does not alter the Aβ42/40 ratio (Duering et al., 2005). Additional evidence indicate that affected individuals carrying PS1 FAD mutants have no abnormalities in neither the in vivo levels of soluble Aβ nor the Aβ 42/40 ratio (Batelli et al., 2008).
That many FAD mutations have no significant effect on Aβ supports the hypothesis that these mutations promote neurodegeneration independent of Aβ peptides (Shioi et al., 2007). The neurodegeneration caused by FAD mutants suggests the WT proteins play critical roles in neuronal survival. FAD mutations may interfere with these neuronal survival activities, thus promoting neuronal cell death. Although the autosomal dominant mode of FAD transmission seems consistent with the hypothesis that FAD mutants cause gain of a toxic function, such a specific gain is unexpected for a large number of FAD mutations distributed throughout the PS1 polypeptide chain. A more parsimonious explanation is the suggestion that the FAD mutant alleles cause a dominant negative loss of function. The protein product of the mutant allele could for example physically interact with and inactivate the product of the wild type allele. This mechanism of "allelic interference in FAD" (Robakis 2011) is supported by recent evidence that FAD mutations inhibit biological functions of PS and that PSs, as well as APP, form dimmers (Schroeter et al., 2003; Scheuermann et al., 2001). Such a mechanism would be consistent with both the autosomal dominant transmission of FAD neurodegeneration and absence of haploinsufficiency mutations in FAD.
Role of γ-secretase-dependent ε cleavage of proteins in cell signaling and AD
Recent evidence shows that in addition to amyloidogenic γ-cleavages of APP, the PS/γ-secretase system promotes the ε-cleavage of a number of type I transmembrane proteins, including APP, cadherins, Notch1 and EphB receptors and CD44. The ε-cleavage occurs downstream from the γ-cleavages releasing soluble cytosolic peptides containing the cytosolic carboxyl-terminal fragments of cleaved substrates (Fig. 1). To date more than 30 cell surface transmembrane proteins have been shown to be cleaved at the ε-site by γ-secretase, producing soluble peptides shown to migrate to the nucleus where they act as regulators of gene expression while others remain in the cytoplasm where they regulate metabolism of transcription factors (Marambaud and Robakis, 2005). Together, these data revealed that in addition to producing Aβ, the γ-secretase system plays central roles in the production of peptides with important biological functions in signal transduction and gene expression.
Recent work reveals that many PS1 FAD mutants inhibit production of CTF2 peptides indicating these mutants cause a loss of γ-secretase cleavage function at the ε site of substrates such as N-cadherin, efnB2, and EphB2 (Georgakopoulos et al., 2006; Litterst et al., 2007; Marambaud et al., 2003; Wiley et al., 2005). These data support the hypothesis that PS FAD mutations contribute to neurotoxicity by inhibiting production of peptides with useful cellular functions (Fortini, 2003; Marambaud et al., 2003). Furthermore, by reducing the ε-cleavage, PS FAD mutations promote accumulation of membrane-bound CTF1 fragments, the substrates of γ-secretase (Litterst et al., 2007; Marambaud et al., 2003). Recent reports indicate that increased levels of CTF1s, such as those derived from APP and netrin, are associated with increased cytotoxicity (Bai et al., 2011; Jiang et al., 2010; Lu et al., 2000) and their accumulation may contribute to the neurodegeneration associated with FAD mutations. Presently, it is unclear why CTF1s are toxic. It is possible that accumulation of high levels of these transmembrane peptides interferes with the movements of receptors and other factors on the plane of the membrane with toxic consequences (Barthet et al., 2012). Thus, PS FAD mutations may promote neurotoxicity by both, reducing production of biologically active CTF2 peptides and increasing accumulation of toxic CTF1 fragments. Both effects can result from a reduction of γ-secretase cleavage activity at the ε site of substrates suggesting that increasing γ-secretase cleavage activity may be of therapeutic interest in PS FAD cases (Barthet et al., 2012). Interestingly, recent clinical trials showed that inhibition of Aβ production by γ-secretase inhibitors (GSI) was associated with toxicity in treated patients (Cummings, 2010), an observation consistent with the reduced production of CTF2 peptides and increased accumulation of toxic CTF1 fragments expected from the inhibition of γ-secretase activity by the GSIs (Barthet et al., 2011; see also Fig.1). Thus, GSIs may act in vivo similar to PS FAD mutations. Both may cause toxicities by a double hit, reducing production of functionally important CTF2 peptides and increasing the levels of toxic CTF1 protein fragments.
Finally, there is evidence that in addition to their role in γ-secretase proteolysis, PSs have γ-secretase-independent functions including stimulation of the PI3K/Akt cell survival signaling, regulation of GSK-3 kinase (Baki et al., 2004) and calcium homeostasis (Tu et al., 2006.). Importantly, a number of PS1 FAD mutations have been reported to interfere with γ-secretase-independent functions of PS1, revealing additional mechanisms by which these mutations may promote neurodegeneration and tau overphosphorylation (Kang et al., 2005; Pigino et al., 2003; Baki et al., 2008).
In summary, the main mechanisms responsible for the neurodegeneration of AD are still poorly understood. It is unclear for example why certain neuronal populations such as cholinergic neurons are more vulnerable to AD than other neurons and how risk factors like the apoE4 allele, the process of aging, and genetic FAD mutations promote specific degeneration of these neuronal populations. There are additional indications that environmental factors such as oxidative stress and inflammatory processes may also contribute to the neuronal cell death of AD (Pappolla et al., 1992; HWeggen et al., 2007.). Thus although it is clear that FAD is caused by specific genetic legions, it is reasonable to assume that in the majority of cases such as sporadic AD, the final outcome is determined by both genetic and environmental factors. Presently the FAD mutations are the only identifiable causative agents of AD and these mutations may offer the best available models for the study of the cellular and molecular mechanisms involved in the development of the more common sporadic disease. Since the clinical manifestations and neuropathology are similar in both sporadic and familial AD, lessons learned from studying the mechanisms of FAD should also be applicable to sporadic AD.
Acknowledgements
supported by a grant from the AP Slaner family and NIH grant R37AG017926
References
- Anderson JP, Esch FS, Keim PS, Sambamurti K, Lieberburg I, Robakis NK. Exact cleavage site of Alzheimer amyloid precursor in neuronal PC-12 cells. Neurosci. Lett. 1991;128:126–128. doi: 10.1016/0304-3940(91)90775-o. [DOI] [PubMed] [Google Scholar]
- Bai G, Chivatakarn O, Bonanomi D, Lettieri K, Franco L, Xia C, Stein E, Ma L, Lewcock JW, Pfaff SL. Presenilin-dependent receptor processing is required for axon guidance. Cell. 2011;144:106–118. doi: 10.1016/j.cell.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, Neve RL, Shao Z, Shioi J, Georgakopoulos A, Robakis NK. Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling. J. Neurosci. 2008;28:483–490. doi: 10.1523/JNEUROSCI.4067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthet G, et al. Inhibitors of gamma-secretase stabilize the complex and differentially affect processing of amyloid precursor protein and other substrates. Faseb J. 2011;25:2937–2946. doi: 10.1096/fj.11-183806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthet G, Georgakopoulos A, Robakis NK. Cellular mechanisms of γ-secretase substrate selection, processing and toxicity. Progress in Neurobiology. 2012;98:166–175. doi: 10.1016/j.pneurobio.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batelli S, Albani D, Prato F, Polito L, Franceschi M, Gavazzi A, Forloni G. Early-onset Alzheimer disease in an Italian family with presenilin-1 double mutation E318G and G394V. Alzheimer Dis. Assoc. Disord. 2008;22:184–187. doi: 10.1097/WAD.0b013e31815a9dec. [DOI] [PubMed] [Google Scholar]
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, Wiltfang J, Esselmann H, De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J. Neurochem. 2006;96:732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Bouras C, Kovari E, Herrmann FR, Rivara CB, Bailey TL, von Gunten A, Hof PR, Giannakopoulos P. Stereologic analysis of microvascular morphology in the elderly: Alzheimer disease pathology and cognitive status. J. Neuropathol. Exp. Neurol. 2006;65:235–244. doi: 10.1097/01.jnen.0000203077.53080.2c. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat. Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- Cummings J. What can be inferred from the interruption of the semagacestat trial for treatment of Alzheimer's disease? Biol Psychiatry. 2010;68:876–878. doi: 10.1016/j.biopsych.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J. Neuropathol. Exp. Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- Duering M, Grimm MO, Grimm HS, Schroder J, Hartmann T. Mean age of onset in familial Alzheimer's disease is determined by amyloid beta 42. Neurobiol. Aging. 2005;26:785–788. doi: 10.1016/j.neurobiolaging.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Neurobiology: double trouble for neurons. Nature. 2003;425:565–566. doi: 10.1038/425565a. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Banbury Report 27: Mol. Neuropath of Aging. Cold Spring Harbor: Cold Spring Harbor Press; 1987. Amyloidogenesis in Alzheimer’s disease and Down’s syndrome; pp. 253–265. [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Pro.c Natl. Acad. Sci. U. S. A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A, Ginsberg SD, Cataldo AM, Mathews PM, Nixon RA. Alzheimer's-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1630–1635. doi: 10.1073/pnas.0908953107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Yoon IS, Repetto E, Busse T, Yermian N, Ie L, Koo EH. Presenilins mediate phosphatidylinositol 3-kinase/AKT and ERK activation via select signaling receptors. Selectivity of PS2 in platelet-derived growth factor signaling. J. Biol. Chem. 2005;280:31537–31547. doi: 10.1074/jbc.M500833200. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK. Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J. Biol. Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Rabizadeh S, Chandra S, Shayya RF, Ellerby LM, Ye X, Salvesen GS, Koo EH, Bredesen DE. A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat Med. 2000;6:397–404. doi: 10.1038/74656. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Robakis NK. Genetic and molecular aspects of Alzheimer's disease shed light on new mechanisms of transcriptional regulation. Genes Brain. Behav. 2005;4:134–146. doi: 10.1111/j.1601-183X.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Miller DL, Papayannopoulos IA, Styles J, Bobin SA, Lin YY, Biemann K, Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch. Biochem. Biophys. 1993;301:41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J. Biol. Chem. 1992;267:17082–17086. [PubMed] [Google Scholar]
- Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Neve RL, Robakis NK. Alzheimer's disease: a re-examination of the amyloid hypothesis. Trends Neurosci. 1998;21:15–19. doi: 10.1016/s0166-2236(97)01168-5. [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Omar RA, Kim KS, Robakis NK. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am. J. Pathol. 1992;140:621–628. [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J. Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc. Natl. Acad. Sc.i U. S. A. 1987a;84:4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis NK, Wisniewski HM, Jenkins EC, Devine-Gage EA, Houck GE, Yao XL, Ramakrishna N, Wolfe G, Silverman WP, Brown WT. Chromosome 21q21 sublocalisation of gene encoding beta-amyloid peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet. 1987b;1:384–385. doi: 10.1016/s0140-6736(87)91754-5. [DOI] [PubMed] [Google Scholar]
- Scheuermann S, Hambsch B, Hesse L, Stumm J, Schmidt C, Beher D, Bayer TA, Beyreuther K, Multhaup G. Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer's disease. J. Biol. Chem. 2001;276:33923–33929. doi: 10.1074/jbc.M105410200. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Ilagan MX, Brunkan AL, Hecimovic S, Li YM, Xu M, Lewis HD, Saxena MT, De Strooper B, Coonrod A, Tomita T, Iwatsubo T, Moore CL, Goate A, Wolfe MS, Shearman M, Kopan R. A presenilin dimer at the core of the gamma-secretase enzyme: insights from parallel analysis of Notch 1 and APP proteolysis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13075–13080. doi: 10.1073/pnas.1735338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Shioi J, Georgakopoulos A, Mehta P, Kouchi Z, Litterst CM, Baki L, Robakis NK. FAD mutants unable to increase neurotoxic Abeta 42 suggest that mutation effects on neurodegeneration may be independent of effects on Abeta. J. Neurochem. 2007;101:674–681. doi: 10.1111/j.1471-4159.2006.04391.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Joseph JA, Perry G. Arson. Tracking the culprit in Alzheimer's disease. Ann. N. Y. Acad. Sci. 2000;924:35–38. doi: 10.1111/j.1749-6632.2000.tb05557.x. [DOI] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. The role of cell-derived oligomers of Abeta in Alzheimer's disease and avenues for therapeutic intervention. Biochem. Soc. Trans. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- Weggen S, Rogers M, Eriksen J. NSAIDs: small molecules for prevention of Alzheimer's disease or precursors for future drug development? Trends Pharmacol. Sc.i. 2007;28:536–543. doi: 10.1016/j.tips.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Wiley JC, Hudson M, Kanning KC, Schecterson LC, Bothwell M. Familial Alzheimer's disease mutations inhibit gamma-secretase-mediated liberation of beta-amyloid precursor protein carboxy-terminal fragment. J. Neurochem. 2005;94:1189–1201. doi: 10.1111/j.1471-4159.2005.03266.x. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann. Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]