Abstract
Purpose
Red and processed meat intake is convincingly associated with colorectal cancer (CRC) incidence, but its impact on prognosis after CRC diagnosis is unknown. We examined associations of red and processed meat consumption, self-reported before and after cancer diagnosis, with all-cause and cause-specific mortality among men and women with invasive, nonmetastatic CRC.
Patients and Methods
Participants in the Cancer Prevention Study II Nutrition Cohort reported information on diet and other factors at baseline in 1992-1993, 1999, and 2003. Participants with a verified CRC diagnosis after baseline and up to June 30, 2009, were observed for mortality through December 31, 2010.
Results
Among 2,315 participants diagnosed with CRC, 966 died during follow-up (413 from CRC and 176 from cardiovascular disease [CVD]). In multivariable-adjusted Cox proportional hazards regression models, red and processed meat intake before CRC diagnosis was associated with higher risks of death as a result of all causes (top v bottom quartile, relative risk [RR], 1.29; 95% CI, 1.05 to 1.59; Ptrend = .03) and from CVD (RR, 1.63; 95% CI, 1.00 to 2.67; Ptrend = .08) but not CRC (RR, 1.09; 95% CI, 0.79 to 1.51; Ptrend = 0.54). Although red and processed meat consumption after CRC diagnosis was not associated with mortality, survivors with consistently high (median or higher) intakes before and after diagnosis had a higher risk of CRC-specific mortality (RR, 1.79; 95% CI, 1.11 to 2.89) compared with those with consistently low intakes.
Conclusion
This study suggests that greater red and processed meat intake before diagnosis is associated with higher risk of death among patients with nonmetastatic CRC.
INTRODUCTION
As a result of successes in early detection and treatment, there are now more than 1.1 million colorectal cancer (CRC) survivors in the United States.1 Cancer survivors are often motivated to learn how food choices, dietary supplements, and complementary nutritional therapies can improve response to treatment and reduce risk of cancer recurrence and cancer-specific mortality.2,3 This population is also at higher risk of other chronic diseases, including cardiovascular disease (CVD), compared with general populations,3,4 so understanding the potential role of diet in both cancer and noncancer outcomes in this patient group has strong clinical and population health relevance.5–8
There is convincing evidence that diets high in red and processed meat are associated with increased risk of incident CRC,9 and public health guidelines recommend limited red and processed meat consumption for primary cancer prevention.9,10 In contrast, evidence for a role of diet in CRC survival is limited,8 and current dietary recommendations for CRC survivors are based largely on data from incidence studies.2 No study to date has specifically examined red and processed meat intake in relation to CRC survival, although three studies have examined related dietary measures.11–13 A prediagnostic diet high in meat (defined as including fish and chicken),11 and pre-13 and postdiagnostic12 diet patterns partly characterized by high red and processed meat consumption have been associated with increased mortality among patients with CRC. Red and processed meat consumption may contribute to higher mortality risk among CRC survivors through promotion of micrometastases, N-nitrosation, oxidative damage,14,15 and effects on circulating markers of inflammation and endothelial dysfunction.16
We evaluated the associations of red and processed meat intake reported before and after CRC diagnosis with overall and cause-specific (eg, CRC and CVD) mortality among 2,315 CRC survivors in the American Cancer Society's Cancer Prevention Study II (CPS-II) Nutrition Cohort.
PATIENTS AND METHODS
Study Cohort
Men and women in this study were identified from among the 184,000 participants in the CPS-II Nutrition Cohort, a prospective study of cancer incidence initiated in 1992.17 At enrollment in 1992 or 1993, Nutrition Cohort participants were age 40 to 93 years and completed a 10-page, self-administered questionnaire that included questions on usual diet, physical activity, body size, other lifestyle factors, and medical history. Follow-up questionnaires were sent to cohort members in 1997 and biennially thereafter to ascertain newly diagnosed cancers and to update exposure information. The CPS-II Nutrition Cohort has been approved by the institutional review board at Emory University.
Among the 181,293 Nutrition Cohort participants with no personal history of a CRC diagnosis at baseline, 3,826 men and women were diagnosed with invasive colon or rectal cancer by the end of incidence follow-up on June 30, 2009. Among these 3,826 patients, 3,047 were initially identified by self-report and subsequently verified through acquisition of medical records (n = 2,185) or through linkage with state cancer registries (n = 862). An additional 779 patients with CRC were initially identified as cancer deaths through linkage with the National Death Index (NDI).18 Data on diagnosis date and stage of 531 of these 779 patients were obtained through subsequent linkage with state cancer registries (n = 529) or through medical record acquisition (n = 2). Of the 3,826 participants with verified CRC, the following exclusions were made: patients linked with the NDI but whose diagnosis date and stage could not be obtained through medical records or cancer registry linkage (n = 248), patients with history of a different cancer reported at baseline (n = 386), implausible diagnosis date (n = 11), missing stage information (n = 136), distant metastatic SEER stage or TNM summary stage IV at diagnosis (n = 421), non–adenocarcinoma histology (n = 50), diagnosis and death dates occurred on the same day (n = 2), and poor dietary reporting at baseline (n = 257). The decision to exclude patients with metastatic disease was made a priori and is consistent with other recent CRC survival studies from this cohort.19–21 The 5-year survival rate is poor for patients with distant stage disease (approximately 12%), and the likelihood that red meat intake would materially influence long-term mortality in this group is small.
After exclusions, 1,282 men and 1,033 women were included in this analysis. Participants were observed until death or December 31, 2010. Among the 2,315 men and women with CRC, 1,711 were diagnosed with colon cancer (International Classification of Diseases for Oncology [ICD-O]: C18.0, C18.2 to C18.9), and 604 were diagnosed with rectal cancer (ICD-O: C19.9, C20.9). When stratified by stage, 1,167 patients had localized disease as defined by the SEER program (invasive tumors confined to the colorectum), and 1,148 had SEER regional disease (tumors that extend through the bowel wall to adjacent tissue or to regional lymph nodes).
Study Outcomes
Vital status of participants was determined through December 31, 2010, by linkage to the NDI.18 Cause of death has been obtained for 99.3% of all known deaths in the Nutrition Cohort. The primary outcome in this study was all-cause mortality. Secondary outcomes were mutually exclusive and were defined according to the singular underlying cause of death in the NDI records: CRC-specific mortality (ICD Ninth Revision [ICD-9]: 153,154; ICD Tenth Revision [ICD-10]: C18, C19, C20) and CVD-specific mortality (ICD-9: 390-459; ICD-10: I00-I99).
Pre- and Postdiagnosis Diet
Diet was assessed at baseline by using a validated, modified brief Block food frequency questionnaire (FFQ)17,22,23 and updated in 1999 and 2003 by using modified Willett FFQs.17,24,25 On all FFQs, participants were asked to report average frequency of consumption over the previous year. Comparable questions on red and processed meats were included on all FFQs (see Appendix Table A1, online only, for a list of foods included). Self-reported diet at baseline (1992-1993) was used to characterize prediagnostic red and processed meat intake. Of the 2,315 patients included in the prediagnostic diet analysis, information on postdiagnostic diet was available for 1,186 (51%). Postdiagnosis red and processed meat consumption was calculated from the first FFQ returned after the participant's diagnosis of colon or rectal cancer (ie, the 1999 FFQ was used for individuals diagnosed between baseline and the date of completion for the 1999 questionnaire, and the 2003 FFQ was used for patients with CRC who were diagnosed between completion of the 1999 and 2003 questionnaires). Participants who were diagnosed with CRC after completion of the 2003 questionnaire were included only in analyses of prediagnosis diet.
Statistical Analysis
Sex-specific quartiles of red and processed meat intakes were created for analyses of pre- and postdiagnostic meat intake. Cox proportional hazards regression models were used to calculate relative risks (RR) and 95% CIs for the associations of meat intake with mortality from all causes, CRC, CVD, and all other causes combined. Time from diagnosis to death or end of follow-up was used as the underlying time axis for all analyses. For analyses of prediagnosis diet, follow-up time began on the date of CRC diagnosis. For the postdiagnosis assessment of diet, delayed entry Cox proportional hazards regression models were used in which entry occurred on the date of the first FFQ completed after CRC diagnosis.
We included age at diagnosis, sex, and tumor stage at diagnosis (SEER stage: local or regional) in all Cox models of pre- and postdiagnostic diet and mortality. Prediagnostic diet models also included prediagnostic body mass index (BMI: underweight [< 18.5 kg/m2], normal [18.5- < 25.0 kg/m2], overweight [25- < 30 kg/m2], obese [30+ kg/m2], missing), history of myocardial infarction (yes/no), history of diabetes (yes/no), and prediagnostic energy intake. Other potential covariates that were considered but that did not change RR estimates were race/ethnicity; education; smoking; history of hypertension; physical activity; alcohol intake; nonsteroidal anti-inflammatory drug use; multivitamin use; postmenopausal hormone use; family history of CRC; tumor grade; type of treatment; history of high cholesterol, stroke, or lung disease; total folate; dietary folate; total calcium; dietary calcium; and fruit, vegetables, whole grains, and fish/poultry consumption. Sensitivity analyses excluded the first 2 years of follow-up after diagnosis and excluded patients with a history of heart attack, stroke, or lung disease (because of the potential for reverse causation). Covariates in postdiagnostic meat intake models also included postdiagnostic energy intake and red and processed meat intake from baseline. We controlled for weight change between baseline and postdiagnosis surveys as a proxy for illness-related weight loss. Sensitivity analyses excluded individuals diagnosed within 1 year before postdiagnostic FFQ administration, because diet may be highly variable during active treatment. We examined consistency of pre- and postdiagnosis meat intake in relation to cause-specific mortality according to median red and processed meat intake cut points before and after diagnosis. These models were adjusted for age at diagnosis, sex, stage, and pre- and postdiagnosis energy intakes.
Likelihood ratio tests26 were used to test for violation of the Cox proportional hazards assumption and for statistical interactions between meat and BMI, sex, family history of CRC, tumor stage, CRC subsite, and mortality. All analyses were conducted by using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Participants were on average age 64 years (standard deviation [SD], 5.8 years) at baseline in 1992 or 1993, and on average age 73 years (SD, 6.7 years) at CRC diagnosis. Table 1 depicts the distribution of clinical, sociodemographic, and other characteristics within frequency of red and processed meat intake at baseline (ie, prediagnosis). The majority of participants were white. No differences were noted in clinical characteristics across categories of meat intake. Frequent red and processed meat consumption was more common among patients reporting a history of diabetes, but no differences were observed for history of hypertension, myocardial infarction, stroke, or lung disease (data not shown). Frequent meat eaters were more likely to be less educated, current smokers, heavy drinkers, overweight or obese, and to have a less healthy overall diet than those consuming less meat. Patterns of nonsteroidal anti-inflammatory drug use and postmenopausal hormone use did not differ according to meat intake level (data not shown).
Table 1.
Baseline Characteristics Among 2,315 Patients With CRC (1,282 men and 1,033 women) in the Cancer Prevention Study II Nutrition Cohort, by Quartiles of Prediagnostic (baseline) Red and Processed Meat Intake
| Characteristic | Quartile of Red and Processed Meat Intake (servings/week)* |
P† | |||
|---|---|---|---|---|---|
| Q1 (%) (n = 576) | Q2 (%) (n = 578) | Q3 (%) (n = 581) | Q4 (%) (n = 580) | ||
| Age at CRC diagnosis, years | .09 | ||||
| < 65 | 9.0 | 12.8 | 14.5 | 10.9 | |
| 65- < 70 | 20.0 | 19.9 | 19.3 | 22.9 | |
| 70- < 75 | 27.1 | 28.9 | 25.6 | 26.7 | |
| 75- < 80 | 24.7 | 24.6 | 26.3 | 24.5 | |
| 80+ | 19.3 | 13.8 | 14.3 | 15.0 | |
| Year of CRC diagnosis | .42 | ||||
| 1992-1996 | 20.5 | 26.1 | 23.4 | 22.1 | |
| 1997-2000 | 28.8 | 29.8 | 30.8 | 29.5 | |
| 2001-2004 | 27.1 | 25.8 | 25.1 | 26.7 | |
| 2005-2009 | 23.6 | 18.3 | 20.7 | 21.7 | |
| Sex | 1.00 | ||||
| Male | 55.6 | 55.0 | 55.6 | 55.3 | |
| Female | 44.4 | 45.0 | 44.4 | 44.7 | |
| Race/ethnicity | .25 | ||||
| White/white-Hispanic | 98.6 | 97.2 | 98.6 | 97.4 | |
| Black/black-Hispanic | 0.9 | 1.0 | 0.9 | 1.6 | |
| Other/missing | 0.5 | 1.7 | 0.5 | 1.0 | |
| Education | < .01 | ||||
| Less than high school | 3.5 | 5.7 | 6.9 | 11.4 | |
| High school degree | 20.0 | 27.3 | 30.1 | 34.5 | |
| Some college/trade school | 30.0 | 31.3 | 30.1 | 27.4 | |
| College graduate | 45.8 | 35.1 | 32.4 | 26.6 | |
| Clinical characteristics | |||||
| SEER summary stage | .26 | ||||
| Local | 53.5 | 48.4 | 48.5 | 51.2 | |
| Regional | 46.5 | 51.6 | 51.5 | 48.8 | |
| Tumor grade at diagnosis | .75 | ||||
| Well differentiated | 11.1 | 13.0 | 13.3 | 10.9 | |
| Moderately differentiated | 63.5 | 60.6 | 59.6 | 60.5 | |
| Poorly differentiated | 17.2 | 16.8 | 16.9 | 17.1 | |
| Undifferentiated | 1.2 | 1.6 | 0.9 | 1.4 | |
| CRC diagnosis site | .49 | ||||
| Colon | 75.0 | 73.5 | 71.8 | 75.3 | |
| Rectum | 25.0 | 26.5 | 28.2 | 24.7 | |
| First course of cancer treatment | |||||
| Surgery | .09 | ||||
| No | 1.0 | 1.6 | 3.1 | 2.6 | |
| Yes | 72.9 | 76.1 | 71.1 | 71.2 | |
| Chemotherapy | .34 | ||||
| No | 44.3 | 43.6 | 42.2 | 45.5 | |
| Yes | 29.7 | 34.1 | 32.0 | 28.3 | |
| Radiation | .39 | ||||
| No | 67.2 | 69.7 | 65.7 | 67.9 | |
| Yes | 6.8 | 8.0 | 8.4 | 5.9 | |
| Family history of CRC in 1982 | .05 | ||||
| No | 94.6 | 94.8 | 94.5 | 91.4 | |
| Yes | 5.4 | 5.2 | 5.5 | 8.6 | |
| History of diabetes | < .01 | ||||
| No | 93.8 | 90.7 | 90.5 | 87.6 | |
| Yes | 6.3 | 9.3 | 9.5 | 12.4 | |
| Physical activity (MET hours/week)‡ | .04 | ||||
| Q1 | 9.4 | 9.9 | 11.7 | 14.0 | |
| Q2 | 30.4 | 33.4 | 36.0 | 32.8 | |
| Q3 | 30.0 | 29.2 | 26.7 | 30.0 | |
| Q4 | 29.5 | 25.8 | 23.8 | 22.1 | |
| BMI, kg/m2 | < .01 | ||||
| < 18.5 | 1.7 | 1.6 | 0.3 | 0.7 | |
| 18.5- < 25 | 48.3 | 39.8 | 35.5 | 26.6 | |
| 25- < 30 | 36.6 | 39.6 | 45.8 | 46.6 | |
| 30+ | 12.3 | 18.2 | 16.7 | 23.4 | |
| Cigarette smoking status | < .01 | ||||
| Never | 36.6 | 38.1 | 39.9 | 41.2 | |
| Current | 4.7 | 6.6 | 10.5 | 10.9 | |
| Former | 57.5 | 54.5 | 49.4 | 47.9 | |
| Dietary characteristics | |||||
| Alcohol intake, drinks per day | .02 | ||||
| Nondrinker | 41.1 | 38.4 | 40.6 | 40.2 | |
| < 1 | 39.6 | 39.4 | 32.0 | 35.0 | |
| ≥ 1 | 17.5 | 19.7 | 25.8 | 23.4 | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|---|---|---|---|---|---|---|---|---|---|
| Red and processed meat intake (servings/week) | 1.5 | 1.0 | 3.8 | 1.1 | 6.0 | 1.3 | 10.4 | 3.4 | |
| Prediagnostic energy intake (kcal/day)§ | 1310 | 459.0 | 1440.8 | 490.5 | 1635.1 | 558.9 | 1959.8 | 652.9 | < .01 |
| Dietary folate intake (μg/day) | 302.3 | 105.7 | 260.5 | 90.4 | 235.8 | 78.9 | 229.7 | 76.6 | < .01 |
| Total folate intake (μg/day) | 492.3 | 342.6 | 394.8 | 260.7 | 377.4 | 260.0 | 342.0 | 248.3 | < .01 |
| Dietary calcium intake (mg/day) | 851.1 | 356.1 | 781.8 | 364.4 | 725.4 | 319.1 | 652.5 | 271.6 | < .01 |
| Total calcium intake (mg/day) | 1046.1 | 577.2 | 949.8 | 552.5 | 865.2 | 458.3 | 772.4 | 442.2 | < .01 |
| Fruit intake (servings/week) | 10.6 | 7.8 | 10.1 | 7.2 | 8.8 | 6.6 | 9.0 | 6.6 | < .01 |
| Vegetable intake (servings/week) | 13.4 | 7.7 | 12.7 | 6.3 | 12.7 | 6.7 | 13.7 | 6.5 | .85 |
NOTE. On average, 7.7 years before diagnosis; some percentages do not add up to 100% due to missing data or rounding.
Abbreviations: BMI, body mass index; CRC, colorectal cancer; MET, metabolic equivalent; Q, quartile; SD, standard deviation.
Quartiles in men: < 3.39, 3.39- < 5.77, 5.77- < 8.29, ≥ 8.29; quartiles in women: < 1.99, 1.99- < 3.72, 3.72- < 5.83, ≥ 5.83.
χ2 test for differences in frequencies across meat strata for categorical predictors; t test for continuous predictors and continuous meat intake.
METs are defined for each type of exercise-related physical activity as a multiple of metabolic equivalent of sitting quietly for 1 hour in quartiles based on distribution in each sex. Quartiles in men: < 3.5, 3.5- < 7.5, 7.5- < 19.5, ≥ 19.5; quartiles in women: < 3.5, 3.5- < 7.5, 7.5- < 18.0, ≥ 18.0.
Energy intake from the brief Block food frequency questionnaire is estimated to be approximately 80% of total.
In the analysis of prediagnostic diet, a total of 966 patients with CRC died during an average 7.5-year (± 4.6-year) follow-up period (350 from colon cancer, 63 from rectal cancer, 176 from CVD, and 377 from all other causes combined). The average time between completing the baseline questionnaire and diagnosis was 7.7 years (SD, 4.4 years). As depicted in Table 2, red and processed meat consumption in the highest compared with the lowest quartile at baseline was associated with a 29% higher risk (RR, 1.29; 95% CI, 1.05 to 1.59) of all-cause death, a 63% higher risk (RR, 1.63; 95% CI, 1.00 to 2.67) of CVD-specific death, and a 39% higher risk (RR, 1.39; 95% CI, 1.00 to 1.92) of death as a result of other causes combined. No association was observed between prediagnostic red and processed meat consumption and CRC-specific death (RR, 1.09; 95% CI, 0.79 to 1.51). Results were not materially different when the first 2 years of follow-up after diagnosis were excluded, or when patients with a history of myocardial infarction, stroke, or lung disease were excluded (data not shown). Results were somewhat stronger for consumption of processed meat than for consumption of fresh red meat (Appendix Tables A2 and A3, online only).
Table 2.
Associations Between 1992 Baseline (prediagnostic) Red and Processed Meat Intake and Mortality Among 2,315 Patients With CRC (1,282 men and 1,033 women) in the Cancer Prevention Study II Nutrition Cohort
| Outcome | Prediagnostic Red and Processed Meat Intake (servings/week)* |
Continuous Meat Intake (servings/week) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1† |
Q2 |
Q3 |
Q4 |
Ptrend‡ | |||||||||||||||
| Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | RR | 95% CI | ||
| All-cause mortality | 212 | 4,332 | 260 | 4,502 | 242 | 4,434 | 252 | 4,197 | |||||||||||
| Base model§ | 1.00 | — | 1.23 | 1.03 to 1.48 | 1.16 | 0.96 to 1.40 | 1.31 | 1.09 to 1.58 | .01 | 1.02 | 1.00 to 1.04 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.18 | 0.98 to 1.43 | 1.13 | 0.92 to 1.37 | 1.29 | 1.05 to 1.59 | .03 | 1.02 | 1.00 to 1.04 | ||||||||
| CRC mortality | 91 | 4,332 | 110 | 4,502 | 119 | 4,434 | 93 | 4,197 | |||||||||||
| Base model§ | 1.00 | — | 1.17 | 0.89 to 1.56 | 1.29 | 0.98 to 1.70 | 1.06 | 0.79 to 1.42 | .69 | 1.00 | 0.97 to 1.03 | ||||||||
| Multivariable model‖ | 1.00 | — | 1.13 | 0.85 to 1.50 | 1.28 | 0.96 to 1.71 | 1.09 | 0.79 to 1.51 | .54 | 1.00 | 0.97 to 1.03 | ||||||||
| Cardiovascular disease mortality | 39 | 4,332 | 52 | 4,502 | 37 | 4,434 | 48 | 4,197 | |||||||||||
| Base model§ | 1.00 | — | 1.42 | 0.92 to 2.18 | 1.05 | 0.66 to 1.68 | 1.62 | 1.04 to 2.51 | .06 | 1.04 | 1.00 to 1.08 | ||||||||
| Multivariable model‖ | 1.00 | — | 1.35 | 0.87 to 2.11 | 0.99 | 0.60 to 1.63 | 1.63 | 1.00 to 2.67 | .08 | 1.04 | 1.00 to 1.09 | ||||||||
| All other causes of mortality | 82 | 4,332 | 98 | 4,502 | 86 | 4,434 | 111 | 4,197 | |||||||||||
| Base model§ | 1.00 | — | 1.23 | 0.91 to 1.65 | 1.05 | 0.77 to 1.44 | 1.48 | 1.11 to 1.99 | .02 | 1.03 | 1.01 to 1.06 | ||||||||
| Multivariable model‖ | 1.00 | — | 1.14 | 0.84 to 1.55 | 0.98 | 0.71 to 1.36 | 1.39 | 1.00 to 1.92 | .08 | 1.03 | 1.00 to 1.06 | ||||||||
Abbreviations: CRC, colorectal cancer; Q, quartile; RR, relative risk.
Quartiles in men: < 3.39, 3.39- < 5.77, 5.77- < 8.29, ≥ 8.2; quartiles in women: < 1.99, 1.99- < 3.72, 3.72- < 5.83, ≥ 5.83.
Reference quartile.
Ptrend calculated by using the median meat intake in each quartile, specific to sex.
Base model adjusted for age at diagnosis and sex.
Multivariable model adjusted for age at diagnosis, sex, tumor stage at diagnosis, 1992 prediagnostic energy intake (sex-specific quartiles), 1992 body mass index, history of diabetes, and history of myocardial infarction.
In the analysis of postdiagnostic red and processed meat intake, 472 deaths occurred during a mean follow-up of 7.6 years (± 3.4 years; 146 deaths from CRC, 110 from CVD, and 216 from other causes; Table 3). The mean follow-up from postdiagnostic diet reporting until death was 4.6 years (SD, 3.0 years). Postdiagnostic meat intake was not independently associated with all-cause or cause-specific mortality. Adjustment for weight change (model 2) increased the RRs slightly, and when baseline red and processed meat intake was added (model 3), associations were further attenuated. Excluding individuals who completed an FFQ within 1 year after diagnosis did not change results (data not shown). Results were similar when examining postdiagnosis intake of red and processed meats separately (Appendix Tables A4 and A5, online only).
Table 3.
Associations Between Postdiagnostic Red and Processed Meat Intake and Mortality Among 1,186 Patients With CRC (663 men and 523 women) in the Cancer Prevention Study II Nutrition Cohort
| Outcome | Postdiagnostic Red and Processed Meat Intake (servings/week)* |
Continuous Meat Intake (servings/week) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1† |
Q2 |
Q3 |
Q4 |
Ptrend‡ | |||||||||||||||
| Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | RR | 95% CI | ||
| All-cause mortality | 112 | 2,140 | 131 | 2,423 | 119 | 2,198 | 110 | 2,213 | |||||||||||
| Base model§ | 1.00 | — | 1.13 | 0.87 to 1.47 | 1.23 | 0.94 to 1.61 | 1.03 | 0.78 to 1.36 | .97 | 1.00 | 0.97 to 1.03 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.13 | 0.87 to 1.47 | 1.25 | 0.94 to 1.64 | 1.02 | 0.76 to 1.38 | .90 | 1.00 | 0.97 to 1.03 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.24 | 0.95 to 1.62 | 1.26 | 0.95 to 1.67 | 1.09 | 0.81 to 1.48 | .91 | 1.00 | 0.97 to 1.03 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.17 | 0.89 to 1.55 | 1.13 | 0.84 to 1.52 | 0.94 | 0.68 to 1.30 | .36 | 0.98 | 0.95 to 1.01 | ||||||||
| CRC mortality | 27 | 2,140 | 42 | 2,423 | 35 | 2,198 | 42 | 2,213 | |||||||||||
| Base model§ | 1.00 | — | 1.42 | 0.86 to 2.35 | 1.34 | 0.80 to 2.25 | 1.55 | 0.94 to 2.57 | .11 | 1.03 | 0.98 to 1.08 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.31 | 0.79 to 2.17 | 1.12 | 0.66 to 1.91 | 1.28 | 0.74 to 2.21 | .46 | 1.01 | 0.95 to 1.06 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.38 | 0.83 to 2.30 | 1.09 | 0.63 to 1.86 | 1.34 | 0.77 to 2.33 | .42 | 1.00 | 0.95 to 1.06 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.28 | 0.76 to 2.15 | 0.93 | 0.53 to 1.64 | 1.10 | 0.61 to 1.98 | .91 | 0.98 | 0.92 to 1.04 | ||||||||
| Cardiovascular disease mortality | 31 | 2,140 | 30 | 2,423 | 27 | 2,198 | 22 | 2,213 | |||||||||||
| Base model§ | 1.00 | — | 1.01 | 0.60 to 1.70 | 1.09 | 0.63 to 1.88 | 0.88 | 0.50 to 1.57 | .50 | 0.99 | 0.93 to 1.05 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.06 | 0.62 to 1.81 | 1.18 | 0.67 to 2.08 | 0.88 | 0.47 to 1.64 | .48 | 0.98 | 0.92 to 1.05 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.17 | 0.68 to 2.02 | 1.21 | 0.68 to 2.16 | 0.96 | 0.51 to 1.83 | .63 | 0.99 | 0.92 to 1.05 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.09 | 0.62 to 1.91 | 1.10 | 0.60 to 2.02 | 0.86 | 0.43 to 1.71 | .42 | 0.97 | 0.91 to 1.05 | ||||||||
| All other causes of mortality | 54 | 2,140 | 59 | 2,423 | 57 | 2,198 | 46 | 2,213 | |||||||||||
| Base model§ | 1.00 | — | 1.05 | 0.72 to 1.53 | 1.26 | 0.86 to 1.86 | 0.84 | 0.56 to 1.27 | .39 | 0.99 | 0.95 to 1.03 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.11 | 0.75 to 1.62 | 1.42 | 0.95 to 2.11 | 0.94 | 0.60 to 1.46 | .76 | 1.00 | 0.96 to 1.04 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.21 | 0.82 to 1.78 | 1.44 | 0.96 to 2.15 | 0.98 | 0.63 to 1.53 | .79 | 1.00 | 0.96 to 1.04 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.18 | 0.79 to 1.77 | 1.33 | 0.87 to 2.04 | 0.87 | 0.54 to 1.41 | .38 | 0.98 | 0.94 to 1.03 | ||||||||
Abbreviations: CRC, colorectal cancer; Q, quartile; RR, relative risk.
Quartiles in men and women vary based on which survey year postdiagnostic meat intake is from.
Reference quartile.
Ptrend calculated by using the median meat intake in each quartile, specific to sex, and vary based on which survey year post-diagnostic meat intake is from.
Base model adjusted for age at diagnosis and sex.
Multivariable model 1 adjusted for age at diagnosis, sex, tumor stage at diagnosis, and postdiagnostic energy intake (sex-specific quartiles).
Multivariable model 2 additionally adjusted for weight change between 1992 prediagnostic and postdiagnostic questionnaires.
Multivariable model 3 additionally adjusted for 1992 prediagnostic meat intake (sex-specific quartiles).
Consistency or change in meat intake before and after diagnosis in relation to mortality is provided in Table 4. Compared with patients who consistently consumed below the median (referent group), those with consistently median or higher intakes had a higher risk of death as a result of CRC (RR, 1.79; 95% CI, 1.11 to 2.89). Patients with increased intakes had a greater risk of death as a result of other causes (RR, 1.62; 95% CI, 1.06 to 2.48). Individuals with decreased intakes had a significantly greater risk of all-cause mortality (RR, 1.37; 95% CI, 1.02 to 1.85).
Table 4.
Associations Between Combinations of Low (less than median) and High (median or higher) Pre- and Postdiagnostic Red and Processed Meat Intake and Mortality Among 1,186 Patients With CRC (663 men and 523 women) in the Cancer Prevention Study II Nutrition Cohort
| Outcome | Category of Red and Processed Meat Intake (servings/week)* |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Prediagnostic/Low Postdiagnostic† |
Low Prediagnostic/High Postdiagnostic |
High Prediagnostic/Low Postdiagnostic |
High Prediagnostic/High Postdiagnostic |
|||||||||||||
| Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | |
| All-cause mortality | 140 | 3,171 | 81 | 1,552 | 82 | 1,276 | 169 | 2,975 | ||||||||
| Base model‡ | 1.00 | — | 1.24 | 0.93 to 1.65 | 1.40 | 1.05 to 1.86 | 1.27 | 1.00 to 1.62 | ||||||||
| Multivariable model§ | 1.00 | — | 1.25 | 0.93 to 1.67 | 1.37 | 1.02 to 1.85 | 1.28 | 0.98 to 1.67 | ||||||||
| CRC mortality | 40 | 3,171 | 23 | 1,552 | 20 | 1,276 | 63 | 2,975 | ||||||||
| Base model‡ | 1.00 | — | 1.12 | 0.66 to 1.91 | 1.30 | 0.75 to 2.25 | 1.70 | 1.11 to 2.61 | ||||||||
| Multivariable model§ | 1.00 | — | 0.96 | 0.55 to 1.66 | 1.43 | 0.80 to 2.57 | 1.79 | 1.11 to 2.89 | ||||||||
| Cardiovascular disease mortality | 37 | 3,171 | 17 | 1,552 | 20 | 1,276 | 36 | 2,975 | ||||||||
| Base model‡ | 1.00 | — | 1.07 | 0.58 to 1.95 | 1.25 | 0.70 to 2.23 | 1.15 | 0.69 to 1.91 | ||||||||
| Multivariable model§ | 1.00 | — | 1.06 | 0.57 to 1.97 | 1.25 | 0.68 to 2.32 | 1.18 | 0.69 to 2.04 | ||||||||
| All other causes of mortality | 63 | 3,171 | 41 | 1,552 | 42 | 1,276 | 70 | 2,975 | ||||||||
| Base model‡ | 1.00 | — | 1.42 | 0.94 to 2.13 | 1.52 | 1.01 to 2.28 | 1.09 | 0.76 to 1.56 | ||||||||
| Multivariable model§ | 1.00 | — | 1.62 | 1.06 to 2.48 | 1.36 | 0.88 to 2.09 | 1.09 | 0.73 to 1.62 | ||||||||
Abbreviations: CRC, colorectal cancer; RR, relative risk.
Categories based on median meat intake: 4.74 servings/week for prediagnostic (1992) and 4.13 servings/week for postdiagnostic diet; low refers to less than median intake; high refers to median intake or higher.
Reference group.
Base model adjusted for age at diagnosis and sex.
Multivariable model adjusted for age at diagnosis, sex, tumor stage at diagnosis, 1992 prediagnostic energy intake, and postdiagnostic energy intake (both sex-specific quartiles).
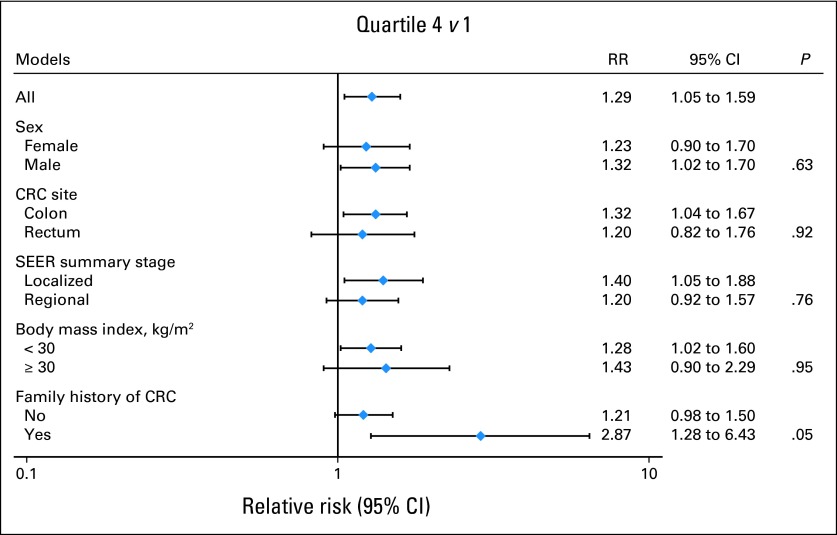
No statistically significant interactions were found between prediagnostic (Fig 1) or postdiagnostic (data not shown) red and processed meat intake and total mortality stratified by sex, tumor stage, subsite in the colon or rectum, or BMI, although a borderline significant interaction was observed with family history of CRC (Pinteraction = .05). In a post hoc analysis of our data, red and processed meat intake did not predict survival among patients with metastatic disease (data not shown).
Fig 1.
1992 prediagnostic meat intake and all-cause mortality among survivors of colorectal cancer (CRC), stratified by sex, CRC site, stage, body mass index, and family history of CRC. RR, relative risk.
DISCUSSION
In this cohort of 2,315 CRC survivors, men and women who reported consuming the highest amount of red and processed meat before CRC diagnosis had a 29% higher risk of all-cause mortality compared with those consuming the least amount in statistical models that included age at diagnosis, disease stage, BMI, energy intake, and other factors. Meat consumption reported after CRC diagnosis was not independently associated with any mortality outcome. However, patients who consistently reported eating at or above the median of red and processed meat before and after diagnosis had a 79% higher risk of death as a result of CRC compared with those who consistently reported eating less than the median.
This study adds to the limited evidence on the role of diet and survival among individuals diagnosed with CRC.8 Our findings of a higher risk of overall mortality with high prediagnostic consumption of red and processed meat, predominantly from causes other than CRC, is generally consistent with findings from epidemiologic studies of more general populations,27,28 in which greater red and/or processed meat intakes were associated with approximately 20% to 40% increased risk of CVD mortality and 20% to 30% greater risk of all-cause mortality. Our results are consistent with two studies that assessed risk of mortality in patients with CRC in relation to prediagnostic meat intake11 and dietary patterns high in processed meat.13 The first study included 511 patients with CRC who were asked to recall meat intake from 1 year before diagnosis: “meat” included red meat, poultry, and fish.11 Among those with a family history of CRC, individuals with the highest meat consumption had a greater than two-fold higher risk of death compared with those with lower intakes, whereas no associations were observed among patients without a family history of CRC.11 Our study also observed higher risks of all-cause mortality among patients with CRC who had a family history of CRC compared with those who did not have a family history of CRC (Pinteraction = .05). Genetic polymorphisms in detoxification pathways for heterocyclic amines and polycyclic aromatic hydrocarbons—mutagens formed during high-temperature cooking of meat—have been reported to modify the association between red and processed meat intake and CRC incidence.29–31 Individuals with a positive family history of CRC appear to have a higher prevalence of high-risk genotypes in these detoxification pathways.32 However, heritable differences would likely need to affect multiple outcomes to measurably impact overall mortality risk. Because the number of deaths among those with a positive family history of CRC in this study was relatively small (n = 57), these findings may be due to chance. In the second study, a cohort of 529 patients with CRC, Zhu et al13 reported a two-fold higher risk of total mortality among colon cancer survivors whose recalled diet 1 year before diagnosis conformed to a high processed meat dietary pattern (also high in red meat, fish, and processed fish).
In contrast to findings on prediagnosis diet, consumption of red and processed meat reported after CRC diagnosis was not independently associated with mortality. Several factors can temporarily influence dietary intake after a CRC diagnosis, including adverse effects from surgery and adjuvant treatment. The CPS-II Nutrition cohort did not collect information on these potentially important variables. In addition, reduced meat consumption might result from CRC recurrence or other illnesses associated with higher risk of mortality. Thus, our risk estimates for postdiagnosis meat intake may be biased toward the null because of reverse causation. In addition, long-term meat consumption may be a more relevant measure and may be better represented by prediagnostic diet than by postdiagnostic diet.
In a study of postdiagnostic diet among 1,009 patients with stage III colon cancer who were enrolled onto a randomized, controlled trial of adjuvant chemotherapy,12 participants who reported having a high “Western” dietary eating pattern had a nearly three-fold higher risk of colon cancer recurrence and a 2.3-fold higher risk of all-cause mortality compared with individuals who scored low on the Western pattern.12 Although red and processed meats were strong contributors to the Western pattern, additional (and approximately equal) contributors included dairy products, refined grains, condiments, and desserts, which may also have contributed to the associations observed.33 Because diet was not measured before diagnosis, it is not clear whether the reported intake after diagnosis represented long-term diet or recent changes in diet.
In the subgroup of CPS-II patients with information on pre- and postdiagnostic diet, men and women who consistently ate the most red and processed meat before and after diagnosis had a statistically significant higher risk of death as a result of CRC compared with those who consistently ate the least red and processed meat. Future research in large prospective studies with repeated measures of dietary intake is needed to clarify the relationship between consistency of meat intake and CRC-specific death in patients with CRC.
Strengths of this study include representation of patients with both localized and regionally staged CRC who provided detailed information on diet, lifestyle, and other CRC risk factors collected before and after diagnosis. Limitations of the study include missing treatment information on 25% of the cohort and lack of information on treatment-related complications. In addition, no information was available on CRC recurrence. Participants were primarily white and middle-class. Recalled diet, especially postdiagnosis, may be subject to bias. Finally, we cannot rule out other sources of unmeasured confounding.
In conclusion, high red and processed meat intake before a diagnosis of CRC was associated with a greater risk of death, a finding driven mainly by death as a result of causes other than CRC. Our findings, which underscore the importance of a long-term healthy diet with limited red and processed meat intake, are relevant because cancer survivors in general are at a greater risk of chronic diseases such as heart disease compared with the general population.8,34 Future studies should continue to identify modifiable lifestyle factors associated with CRC survival.
Acknowledgment
We thank all CPS-II study participants and the Study Management Group. We acknowledge contributions by the central cancer registries supported by the Centers for Disease Control and Prevention's National Program of Cancer Registries and by the National Cancer Institute's SEER Program.
Appendix
Table A1.
Red and Processed Meat* Line Items on Each FFQ, Cancer Prevention Study II Nutrition Cohort
| Meat | 1992 | 1999 | 2003† |
|---|---|---|---|
| Hamburger | Hamburgers, cheeseburger, meatloaf, casserole with ground beef | Hamburger, regular | Hamburger, regular |
| Hamburger, lean or extra-lean | Hamburger, lean or extra-lean | ||
| Beef | Beef steaks, roasts, including on sandwiches | Beef or lamb as a main dish (steak or roast) | Beef steak |
| Beef, pork, or lamb roast | |||
| Beef, other | Beef stew or pot pie | Beef, pork, or lamb as a sandwich or mixed dish (stew, casserole, lasagna) | Beef, pork, or lamb as sandwich or in a stew, casserole, lasagna, frozen dinner, etc. |
| Pork | Pork, including chops, roast | Pork as a main dish (eg, ham or chops) | Pork chops |
| Baked ham | |||
| Liver | Liver, including chicken liver | Liver: beef, calf, or pork | N/A‡ |
| Liver: chicken or turkey | N/A‡ | ||
| Hot dogs | Hot dogs | Beef or pork hot dogs | Beef or pork hot dogs |
| Lunch meat | Ham, bologna, salami, lunchmeat | Salami, bologna, or other processed meat sandwiches | Salami, bologna, or other processed meat sandwiches |
| Sausage | Sausage | Sausage, kielbasa | Sausage, kielbasa |
| Bacon | Bacon | Bacon | Bacon |
Abbreviations: FFQ, food frequency questionnaire; N/A, not applicable.
Red meat included all items listed, shown as listed on the FFQs. “Processed meat” included hot dogs, lunch meats, sausage, and bacon only. “Fresh” red meat included all items listed, excluding processed meats.
The 2003 FFQ included questions on meat preparation and therefore separated some questions from the similar 1999 survey.
< 1% of participants reported consuming liver weekly in 1999; therefore, the question was omitted from the 2003 FFQ.
Table A2.
Associations Between 1992 Prediagnostic “Fresh” Red Meat Intake and Mortality Among 2,315 Patients With CRC (1,282 men and 1,033 women) in the Cancer Prevention Study II Nutrition Cohort
| Outcome | Quartile of Prediagnostic “Fresh” Red Meat Intake (servings/week)* |
Continuous Meat Intake (servings/week) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1† |
Q2 |
Q3 |
Q4 |
Ptrend‡ | |||||||||||||||
| Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | RR | 95% CI | ||
| All-cause mortality | 219 | 4,168 | 250 | 4,676 | 249 | 4,315 | 248 | 4,307 | |||||||||||
| Base model§ | 1.00 | — | 1.05 | 0.87 to 1.26 | 1.16 | 0.97 to 1.40 | 1.15 | 0.95 to 1.38 | .11 | 1.03 | 1.00 to 1.06 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.03 | 0.85 to 1.24 | 1.14 | 0.94 to 1.38 | 1.12 | 0.92 to 1.38 | .21 | 1.03 | 0.99 to 1.06 | ||||||||
| CRC mortality | 89 | 4,168 | 107 | 4,676 | 115 | 4,315 | 102 | 4,307 | |||||||||||
| Base model§ | 1.00 | — | 1.08 | 0.81 to 1.44 | 1.24 | 0.93 to 1.64 | 1.11 | 0.83 to 1.48 | .41 | 1.02 | 0.97 to 1.07 | ||||||||
| Multivariable model‖ | 1.00 | — | 1.03 | 0.77 to 1.38 | 1.24 | 0.92 to 1.66 | 1.16 | 0.84 to 1.58 | .24 | 1.03 | 0.98 to 1.09 | ||||||||
| Cardiovascular disease mortality | 46 | 4,168 | 44 | 4,676 | 47 | 4,315 | 39 | 4,307 | |||||||||||
| Base model§ | 1.00 | — | 0.92 | 0.60 to 1.41 | 1.20 | 0.79 to 1.83 | 0.96 | 0.62 to 1.50 | .88 | 1.02 | 0.95 to 1.09 | ||||||||
| Multivariable model‖ | 1.00 | — | 0.88 | 0.56 to 1.36 | 1.15 | 0.74 to 1.80 | 0.90 | 0.56 to 1.46 | .90 | 1.01 | 0.93 to 1.10 | ||||||||
| All other causes of mortality | 84 | 4,168 | 99 | 4,676 | 87 | 4,315 | 107 | 4,307 | |||||||||||
| Base model§ | 1.00 | — | 1.08 | 0.80 to 1.45 | 1.07 | 0.79 to 1.46 | 1.29 | 0.96 to 1.73 | .12 | 1.04 | 1.00 to 1.10 | ||||||||
| Multivariable model‖ | 1.00 | — | 1.03 | 0.76 to 1.39 | 1.02 | 0.74 to 1.40 | 1.19 | 0.87 to 1.64 | .33 | 1.03 | 0.98 to 1.09 | ||||||||
Abbreviations: CRC, colorectal cancer; Q, quartile; RR, relative risk.
Quartiles in men: < 1.81, 1.81- < 3.05, 3.05- < 4.57, ≥ 4.57; quartiles in women: < 1.27, 1.27- < 2.32, 2.32- < 3.58, ≥ 3.58.
Reference quartile.
Ptrend calculated by using the median meat intake in each quartile, specific to sex.
Base model adjusted for age at diagnosis and sex.
Multivariable model adjusted for age at diagnosis, sex, tumor stage at diagnosis, 1992 prediagnostic energy intake (sex-specific quartiles), 1992 body mass index, history of diabetes, and history of myocardial infarction.
Table A3.
Associations Between 1992 Prediagnostic Processed Meat Intake and Mortality Among 2,315 Patients With CRC (1,282 men and 1,033 women) in the Cancer Prevention Study II Nutrition Cohort
| Outcome | Quartile of Prediagnostic Processed Meat Intake (servings/week)* |
Ptrend‡ | Continuous Meat Intake (servings/week) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1† |
Q2 |
Q3 |
Q4 |
||||||||||||||||
| Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | RR | 95% CI | ||
| All-cause mortality | 199 | 3,802 | 265 | 4,931 | 237 | 4,545 | 265 | 4,187 | |||||||||||
| Base model§ | 1.00 | — | 1.13 | 0.94 to 1.37 | 1.03 | 0.85 to 1.25 | 1.25 | 1.04 to 1.52 | .04 | 1.02 | 1.00 to 1.05 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.15 | 0.95 to 1.39 | 1.02 | 0.83 to 1.24 | 1.21 | 0.99 to 1.48 | .14 | 1.02 | 0.99 to 1.04 | ||||||||
| CRC mortality | 86 | 3,802 | 122 | 4,931 | 98 | 4,545 | 107 | 4,187 | |||||||||||
| Base model§ | 1.00 | — | 1.17 | 0.88 to 1.55 | 0.97 | 0.73 to 1.31 | 1.10 | 0.83 to 1.47 | .97 | 0.99 | 0.95 to 1.03 | ||||||||
| Multivariable model‖ | 1.00 | — | 1.20 | 0.90 to 1.60 | 0.98 | 0.72 to 1.33 | 1.09 | 0.80 to 1.48 | .92 | 0.98 | 0.94 to 1.03 | ||||||||
| Cardiovascular disease mortality | 38 | 3,802 | 43 | 4,931 | 45 | 4,545 | 50 | 4,187 | |||||||||||
| Base model§ | 1.00 | — | 1.08 | 0.68 to 1.70 | 1.09 | 0.69 to 1.71 | 1.48 | 0.95 to 2.30 | .04 | 1.06 | 1.01 to 1.11 | ||||||||
| Multivariable model‖ | 1.00 | — | 1.08 | 0.68 to 1.71 | 1.07 | 0.67 to 1.70 | 1.47 | 0.92 to 2.33 | .06 | 1.07 | 1.01 to 1.12 | ||||||||
| All other causes of mortality | 75 | 3,802 | 100 | 4,931 | 94 | 4,545 | 108 | 4,187 | |||||||||||
| Base model§ | 1.00 | — | 1.12 | 0.82 to 1.52 | 1.07 | 0.78 to 1.46 | 1.35 | 0.99 to 1.82 | .06 | 1.04 | 1.00 to 1.07 | ||||||||
| Multivariable model‖ | 1.00 | — | 1.10 | 0.81 to 1.50 | 1.02 | 0.74 to 1.40 | 1.27 | 0.92 to 1.75 | .17 | 1.03 | 0.99 to 1.07 | ||||||||
Abbreviations: CRC, colorectal cancer; Q, quartile; RR, relative risk.
Quartiles in men: < 1.04, 1.04- < 2.23, 2.23- < 4.16, ≥ 4.16; quartiles in women: < 0.23, 0.23- < 1.04, 1.04- < 2.22, ≥ 2.22.
Reference quartile.
Ptrend calculated by using the median meat intake in each quartile, specific to sex.
Base model adjusted for age at diagnosis and sex.
Multivariable model adjusted for age at diagnosis, sex, tumor stage at diagnosis, 1992 prediagnostic energy intake (sex-specific quartiles), 1992 body mass index, history of diabetes, and history of myocardial infarction.
Table A4.
Associations Between Postdiagnostic “Fresh” Red Meat Intake and Mortality Among 1,186 Patients With CRC (663 men and 523 women) in the Cancer Prevention Study II Nutrition Cohort
| Outcome | Quartile of Postdiagnostic “Fresh” Red Meat Intake (servings/week)* |
Ptrend‡ | Continuous Meat Intake (servings/week) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1† |
Q2 |
Q3 |
Q4 |
||||||||||||||||
| Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | RR | 95% CI | ||
| All-cause mortality | 120 | 1,969 | 132 | 2,389 | 115 | 2,458 | 105 | 2,158 | |||||||||||
| Base model§ | 1.00 | — | 0.91 | 0.71 to 1.18 | 0.84 | 0.64 to 1.09 | 0.89 | 0.68 to 1.17 | .41 | 0.98 | 0.94 to 1.02 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 0.92 | 0.71 to 1.19 | 0.82 | 0.63 to 1.09 | 0.85 | 0.63 to 1.14 | .28 | 0.97 | 0.93 to 1.02 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 0.96 | 0.74 to 1.24 | 0.85 | 0.64 to 1.12 | 0.88 | 0.66 to 1.18 | .38 | 0.97 | 0.93 to 1.02 | ||||||||
| Multivariable model 3# | 1.00 | — | 0.88 | 0.67 to 1.15 | 0.75 | 0.56 to 1.00 | 0.75 | 0.55 to 1.03 | .09 | 0.95 | 0.91 to 1.00 | ||||||||
| CRC mortality | 23 | 1,969 | 47 | 2,389 | 37 | 2,458 | 39 | 2,158 | |||||||||||
| Base model§ | 1.00 | — | 1.63 | 0.97 to 2.73 | 1.31 | 0.76 to 2.25 | 1.54 | 0.90 to 2.64 | .31 | 1.03 | 0.95 to 1.11 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.58 | 0.94 to 2.67 | 1.15 | 0.66 to 2.00 | 1.26 | 0.71 to 2.24 | .87 | 0.99 | 0.91 to 1.08 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.68 | 0.99 to 2.83 | 1.17 | 0.67 to 2.05 | 1.33 | 0.75 to 2.35 | .78 | 0.99 | 0.91 to 1.08 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.52 | 0.89 to 2.61 | 1.01 | 0.57 to 1.82 | 1.13 | 0.62 to 2.06 | .79 | 0.96 | 0.88 to 1.05 | ||||||||
| Cardiovascular disease mortality | 37 | 1,969 | 25 | 2,389 | 23 | 2,458 | 25 | 2,158 | |||||||||||
| Base model§ | 1.00 | — | 0.57 | 0.34 to 0.98 | 0.60 | 0.35 to 1.03 | 0.81 | 0.48 to 1.39 | .61 | 0.98 | 0.89 to 1.08 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 0.58 | 0.34 to 0.99 | 0.62 | 0.35 to 1.09 | 0.82 | 0.46 to 1.45 | .65 | 0.98 | 0.88 to 1.08 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 0.58 | 0.34 to 1.01 | 0.62 | 0.35 to 1.09 | 0.82 | 0.46 to 1.48 | .69 | 0.98 | 0.89 to 1.09 | ||||||||
| Multivariable model 3# | 1.00 | — | 0.52 | 0.30 to 0.91 | 0.53 | 0.30 to 0.96 | 0.71 | 0.38 to 1.31 | .49 | 0.97 | 0.87 to 1.08 | ||||||||
| All other causes of mortality | 60 | 1,969 | 60 | 2,389 | 55 | 2,458 | 41 | 2,158 | |||||||||||
| Base model§ | 1.00 | — | 0.83 | 0.58 to 1.20 | 0.80 | 0.55 to 1.17 | 0.68 | 0.45 to 1.03 | .09 | 0.95 | 0.89 to 1.02 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 0.86 | 0.59 to 1.25 | 0.86 | 0.58 to 1.29 | 0.73 | 0.47 to 1.14 | .22 | 0.96 | 0.89 to 1.03 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 0.86 | 0.59 to 1.26 | 0.88 | 0.59 to 1.31 | 0.74 | 0.47 to 1.15 | .25 | 0.96 | 0.89 to 1.03 | ||||||||
| Multivariable model 3# | 1.00 | — | 0.81 | 0.55 to 1.20 | 0.79 | 0.52 to 1.20 | 0.64 | 0.40 to 1.03 | .11 | 0.94 | 0.87 to 1.01 | ||||||||
Abbreviations: CRC, colorectal cancer; Q, quartile; RR, relative risk.
Quartiles in men and women vary based on which survey year postdiagnostic meat intake is from.
Reference quartile.
Ptrend calculated by using the median meat intake in each quartile, specific to sex, and vary based on which survey year postdiagnostic meat intake is from.
Base model adjusted for age at diagnosis and sex.
Multivariable model 1 adjusted for age at diagnosis, sex, tumor stage at diagnosis, and postdiagnostic energy intake (sex-specific quartiles).
Multivariable model 2 additionally adjusted for weight change between 1992 prediagnostic and postdiagnostic questionnaires.
Multivariable model 3 additionally adjusted for 1992 prediagnostic meat intake (sex-specific quartiles).
Table A5.
Associations Between Postdiagnostic Processed Meat Intake and Mortality Among 1,186 Patients With CRC (663 men and 523 women) in the Cancer Prevention Study II Nutrition Cohort
| Outcome | Quartile of Postdiagnostic Processed Meat Intake (servings/week)* |
Ptrend‡ | Continuous Meat Intake (servings/week) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1† |
Q2 |
Q3 |
Q4 |
||||||||||||||||
| Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | Total Deaths | Person-Years | RR | 95% CI | RR | 95% CI | ||
| All-cause mortality | 109 | 2,137 | 119 | 2,407 | 124 | 2,192 | 120 | 2,238 | |||||||||||
| Base model§ | 1.00 | — | 1.04 | 0.80 to 1.37 | 1.21 | 0.92 to 1.57 | 1.16 | 0.88 to 1.52 | .33 | 1.01 | 0.97 to 1.05 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.07 | 0.82 to 1.41 | 1.20 | 0.91 to 1.57 | 1.18 | 0.89 to 1.57 | .30 | 1.01 | 0.97 to 1.05 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.13 | 0.86 to 1.49 | 1.25 | 0.95 to 1.64 | 1.23 | 0.93 to 1.64 | .26 | 1.01 | 0.97 to 1.06 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.07 | 0.81 to 1.41 | 1.14 | 0.86 to 1.52 | 1.11 | 0.82 to 1.49 | .71 | 1.00 | 0.96 to 1.04 | ||||||||
| CRC mortality | 28 | 2,137 | 36 | 2,407 | 41 | 2,192 | 41 | 2,238 | |||||||||||
| Base model§ | 1.00 | — | 1.18 | 0.71 to 1.97 | 1.44 | 0.87 to 2.36 | 1.46 | 0.88 to 2.41 | .12 | 1.04 | 0.97 to 1.12 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.18 | 0.70 to 1.99 | 1.23 | 0.74 to 2.05 | 1.27 | 0.75 to 2.14 | .37 | 1.02 | 0.95 to 1.09 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.18 | 0.70 to 2.00 | 1.21 | 0.73 to 2.02 | 1.25 | 0.74 to 2.11 | .44 | 1.01 | 0.94 to 1.09 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.09 | 0.64 to 1.85 | 1.05 | 0.62 to 1.80 | 1.06 | 0.61 to 1.84 | .84 | 0.99 | 0.92 to 1.07 | ||||||||
| Cardiovascular disease mortality | 31 | 2,137 | 29 | 2,407 | 24 | 2,192 | 26 | 2,238 | |||||||||||
| Base model§ | 1.00 | — | 1.03 | 0.60 to 1.75 | 0.93 | 0.53 to 1.62 | 1.03 | 0.59 to 1.78 | .81 | 0.99 | 0.90 to 1.08 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.06 | 0.62 to 1.81 | 0.99 | 0.56 to 1.75 | 1.05 | 0.59 to 1.86 | .82 | 0.99 | 0.90 to 1.08 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.14 | 0.66 to 1.98 | 1.05 | 0.59 to 1.88 | 1.12 | 0.63 to 2.01 | .94 | 0.99 | 0.90 to 1.09 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.08 | 0.62 to 1.89 | 0.98 | 0.54 to 1.79 | 1.05 | 0.57 to 1.93 | .81 | 0.98 | 0.89 to 1.09 | ||||||||
| All other causes of mortality | 50 | 2,137 | 54 | 2,407 | 59 | 2,192 | 53 | 2,238 | |||||||||||
| Base model§ | 1.00 | — | 0.99 | 0.66 to 1.47 | 1.22 | 0.83 to 1.79 | 1.06 | 0.71 to 1.59 | .72 | 1.00 | 0.95 to 1.06 | ||||||||
| Multivariable model 1‖ | 1.00 | — | 1.04 | 0.70 to 1.55 | 1.32 | 0.89 to 1.96 | 1.20 | 0.79 to 1.82 | .39 | 1.02 | 0.96 to 1.08 | ||||||||
| Multivariable model 2¶ | 1.00 | — | 1.10 | 0.73 to 1.64 | 1.38 | 0.93 to 2.05 | 1.24 | 0.82 to 1.89 | .38 | 1.02 | 0.96 to 1.08 | ||||||||
| Multivariable model 3# | 1.00 | — | 1.05 | 0.70 to 1.58 | 1.32 | 0.87 to 1.99 | 1.14 | 0.73 to 1.77 | .69 | 1.01 | 0.95 to 1.08 | ||||||||
Abbreviations: CRC, colorectal cancer; Q, quartile; RR, relative risk.
Quartiles in men and women vary based on which survey year postdiagnostic meat intake is from.
Reference quartile.
Ptrend calculated by using the median meat intake in each quartile, specific to sex, and vary based on which survey year postdiagnostic meat intake is from.
Base model adjusted for age at diagnosis and sex.
Multivariable model 1 adjusted for age at diagnosis, sex, tumor stage at diagnosis, and postdiagnostic energy intake (sex-specific quartiles).
Multivariable model 2 additionally adjusted for weight change between 1992 prediagnostic and postdiagnostic questionnaires.
Multivariable model 3 additionally adjusted for 1992 prediagnostic meat intake (sex-specific quartiles).
Footnotes
See accompanying editorial on page 2763
Supported by the American Cancer Society.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Marjorie L. McCullough, Peter T. Campbell
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Colorectal Cancer Facts & Figures 2011-2013. Atlanta, GA: 2011. [Google Scholar]
- 2.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 3.Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 2006;7:1017–1026. doi: 10.1016/S1470-2045(06)70976-7. [DOI] [PubMed] [Google Scholar]
- 4.Irwin ML, Mayne ST. Impact of nutrition and exercise on cancer survival. Cancer J. 2008;14:435–441. doi: 10.1097/PPO.0b013e31818daeee. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhardt JA. Beyond standard adjuvant therapy for colon cancer: Role of nonstandard interventions. Semin Oncol. 2011;38:533–541. doi: 10.1053/j.seminoncol.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin North Am. 2008;22:319–342. doi: 10.1016/j.hoc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167–178. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: A review of the literature. Am J Clin Nutr. 2010;92:471–490. doi: 10.3945/ajcn.2010.29005. [DOI] [PubMed] [Google Scholar]
- 9.World Cancer Research Fund/American Institute for Cancer Research (AICR) Food, Nutrition, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 10.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 11.Zell JA, Ignatenko NA, Yerushalmi HF, et al. Risk and risk reduction involving arginine intake and meat consumption in colorectal tumorigenesis and survival. Int J Cancer. 2006;120:459–468. doi: 10.1002/ijc.22311. [DOI] [PubMed] [Google Scholar]
- 12.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Wu H, Wang PP, et al. Dietary patterns and colorectal cancer recurrence and survival: A cohort study. BMJ Open. 2013;3:e002270. doi: 10.1136/bmjopen-2012-002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugimura T. Carcinogenicity of mutagenic heterocyclic amines formed during the cooking process. Mutat Res. 1985;150:33–41. doi: 10.1016/0027-5107(85)90098-3. [DOI] [PubMed] [Google Scholar]
- 15.Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: A meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila) 2011;4:177–184. doi: 10.1158/1940-6207.CAPR-10-0113. [DOI] [PubMed] [Google Scholar]
- 16.Fung TT, McCullough ML, Newby PK, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 17.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: Rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 18.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among Cancer Prevention Study II participants. Am J Epidemiol. 1993;137:235–241. doi: 10.1093/oxfordjournals.aje.a116664. [DOI] [PubMed] [Google Scholar]
- 19.Dehal AN, Newton CC, Jacobs EJ, et al. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: The Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:53–59. doi: 10.1200/JCO.2011.38.0303. [DOI] [PubMed] [Google Scholar]
- 20.Campbell PT, Newton CC, Dehal AN, et al. Impact of body mass index on survival after colorectal cancer diagnosis: The Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 21.Campbell PT, Patel AV, Newton CC, et al. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 22.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: Development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Flagg EW, Coates RJ, Calle EE, et al. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11:462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbaum DG, Kupper LL, Muller KE. Applied Regression Analysis and Other Multivariable Methods. Belmont, CA: Duxbury Press; 1988. [Google Scholar]
- 27.Sinha R, Cross AJ, Graubard BI, et al. Meat intake and mortality: A prospective study of over half a million people. Arch Intern Med. 2009;169:562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and mortality: Results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–563. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Marchand L, Hankin JH, Pierce LM, et al. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutat Res. 2002;506-507:205–214. doi: 10.1016/s0027-5107(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 30.Girard H, Butler LM, Villeneuve L, et al. UGT1A1 and UGT1A9 functional variants, meat intake, and colon cancer, among Caucasians and African-Americans. Mutat Res. 2008;644:56–63. doi: 10.1016/j.mrfmmm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan AT, Tranah GJ, Giovannucci EL, et al. Prospective study of N-acetyltransferase-2 genotypes, meat intake, smoking and risk of colorectal cancer. Int J Cancer. 2005;115:648–652. doi: 10.1002/ijc.20890. [DOI] [PubMed] [Google Scholar]
- 32.Keku TO, Millikan RC, Martin C, et al. Family history of colon cancer: What does it mean and how is it useful? Am J Prev Med. 2003;24:170–176. doi: 10.1016/s0749-3797(02)00590-1. [DOI] [PubMed] [Google Scholar]
- 33.Meyerhardt JA, Sato K, Niedzwiecki D, et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Natl Cancer Inst. 2012;104:1702–1711. doi: 10.1093/jnci/djs399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown BW, Brauner C, Minnotte MC. Noncancer deaths in white adult cancer patients. J Natl Cancer Inst. 1993;85:979–987. doi: 10.1093/jnci/85.12.979. [DOI] [PubMed] [Google Scholar]