Abstract
Background
Numerous studies have supported the effectiveness of recombinant activated factor VII (rFVIIa) for the control of bleeding after cardiac procedures; however safety concerns persist. Here we report the novel use of intraoperative low-dose rFVIIa in thoracic aortic operations, a strategy intended to improve safety by minimizing rFVIIa exposure.
Methods
Between July 2005 and December 2010, 425 consecutive patients at a single referral center underwent thoracic aortic operations with cardiopulmonary bypass (CPB); 77 of these patients received intraoperative low-dose rFVIIa (≤60 μg/kg) for severe coagulopathy after CPB. Propensity matching produced a cohort of 88 patients (44 received intraoperative low-dose rFVIIa and 44 controls) for comparison.
Results
Matched patients receiving intraoperative low-dose rFVIIa got an initial median dose of 32 μg/kg (interquartile range [IQR], 16–43 μg/kg) rFVIIa given 51 minutes (42–67 minutes) after separation from CPB. Patients receiving intraoperative low-dose rFVIIa demonstrated improved postoperative coagulation measurements (partial thromboplastin time 28.6 versus 31.5 seconds; p = 0.05; international normalized ratio, 0.8 versus 1.2; p < 0.0001) and received 50% fewer postoperative blood product transfusions (2.5 versus 5.0 units; p = 0.05) compared with control patients. No patient receiving intraoperative low-dose rFVIIa required postoperative rFVIIa administration or reexploration for bleeding. Rates of stroke, thromboembolism, myocardial infarction, and other adverse events were equivalent between groups.
Conclusions
Intraoperative low-dose rFVIIa led to improved postoperative hemostasis with no apparent increase in adverse events. Intraoperative rFVIIa administration in appropriately selected patients may correct coagulopathy early in the course of refractory blood loss and lead to improved safety through the use of smaller rFVIIa doses. Appropriately powered randomized studies are necessary to confirm the safety and efficacy of this approach.
Off-label use of recombinant activated factor VII (rFVIIa) has proved effective for the management of hemorrhage after cardiovascular operations [1–9]. However the safety of rFVIIa and optimal dosing strategy remain controversial. Although multiple studies have supported the safety of rFVIIa in cardiac operations [1–5, 7–13], randomized data and a recent meta-analysis of all studies with patient matching suggest an increased rate of stroke with rFVIIa therapy [6, 14]. Clouding the interpretation of these data is the wide variation in treatment protocols between centers. Reported rFVIIa dosages have ranged from 11 to 100 μg/kg [4, 7], and thresholds for administration have ranged from prophylactic use in the operating room after reversal of heparin [1] to salvage use in the intensive care unit (ICU) after an initial surgical reexploration for bleeding [4].
Beginning in 2005, we developed guidelines for intraoperative low-dose rFVIIa (ILD-rFVIIa) administration for patients demonstrating severe coagulopathy after cardiopulmonary bypass (CPB) during complex thoracic aortic operations. This strategy was intended to achieve therapeutic effect with smaller rFVIIa doses by intervening early in the pathogenesis of coagulopathic bleeding [15] and thereby reduce costs and adverse events associated with rFVIIa exposure. Here we report our experience with ILD-rFVIIa in thoracic aortic operations using a conservative propensity-matching approach designed to assess the safety of ILD-rFVIIa therapy compared with control patients with severe coagulopathy after CPB who were treated successfully by conventional measures.
Patients and Methods
Patient Population and Data Collection
This study was approved by the Institutional Review Board of Duke University, and the need for individual patient consent was waived. A query of the Duke Thoracic Aortic Surgery Database [16, 17] identified 425 consecutive thoracic aortic operations using CPB performed between July 2005 and December 2010. Anesthesia records were retrospectively reviewed to identify patients who received ILD-rFVIIa (initial dose of <60 μg/kg; less than two thirds of the standard US Food and Drug Administration approved dose for patients with hemophilia with inhibitors [15]) during the procedure. Fourteen patients who received an initial intraoperative rFVIIa dose of 60 μg/kg or more were excluded from the study. Detailed data on intraoperative and postoperative hemorrhage, transfusions, and use of hemostatic adjuncts were ascertained from anesthesia, pharmacy, and blood bank records. Direct hospital costs exclusive of physician fees were obtained from the Duke Hospital finance department and were adjusted for inflation to 2010 US dollar values based on the US Bureau of Labor and Statistics Consumer Price Index (http://www.blsgov/data/inflation_calculator.htm). Blood product costs were estimated for all patients using the Duke Transfusion Service 2011 Price Book. Comorbid conditions and postoperative complications were defined using the Society of Thoracic Surgeons' definitions (www.sts.org).
Guidelines for ILD-rFVIIa Use
Thoracic aortic operations and transfusion practices were performed as previously described [16,17]. If hemostasis was unsatisfactory after separation from CPB and routine transfusion procedures, additional fresh frozen plasma, platelets, cryoprecipitate, and red blood cells were administered guided by point-of-care testing (Fig 1). If hemostasis remained unsatisfactory after correction of coagulation measurements and exclusion of a surgical source of bleeding rFVIIa was given on conferral between the surgeon and the anesthesiologist. Initially, doses of 40 to 80 μg/kg rFVIIa were used empirically based on published reports. However with the availability of 1-mg rFVIIa vials and reports of efficacy with lower rFVIIa doses [4, 5, 18], our practice evolved to the initial administration of 10 to 20 μg/kg (1-2 mg) rFVIIa, with the dose repeated if bleeding continued after a minimum of 15 minutes. Sternal closure and transfer to the ICU were not performed until hemostasis was achieved.
Fig 1.
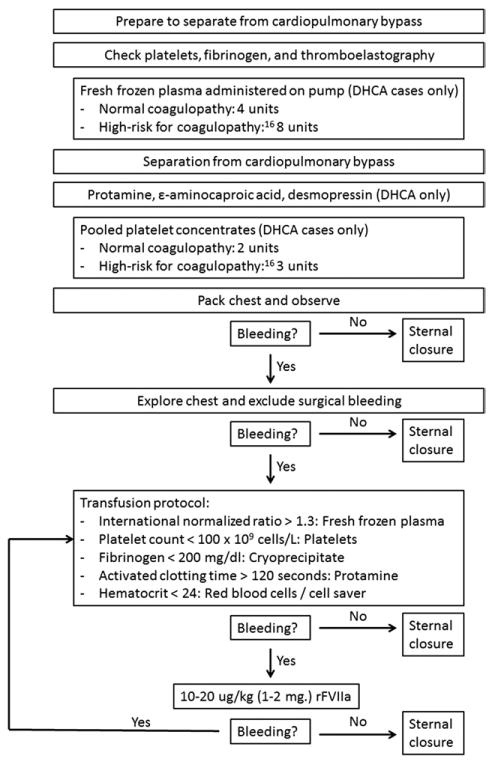
Guidelines for intraoperative low-dose recombinant activated factor VII use during complex thoracic aortic operations. (DHCA = deep hypothermic circulatory arrest.)
Statistical Analysis
All comparisons were made between patients who did (ILD-rFVIIa group) or did not (control group) receive ILD-rFVIIa during operation based on an intention-to-treat analysis of the initial rFVIIa dose administered. Group status did not change regardless of the total cumulative rFVIIa dose received or the postoperative administration of rFVIIa.
Given the significant differences in preoperative risk factors for bleeding, case acuity, case complexity, and degree of intraoperative hemorrhage between groups (Table 1), control and ILD-rFVIIa patients were propensity matched to produce 2 patient cohorts with a similar propensity for receiving ILD-rFVIIa. Propensity scores for receiving ILD-rFVIIa were calculated by multivariable conditional logistic regression, including the following characteristics: age, sex, race, height, weight, body mass index, preoperative anticoagulant use, baseline laboratory values, case status, American Society of Anesthesiologists class, redo sternotomy, principal procedure performed, concomitant procedure performed, use of deep hypothermic circulatory arrest, CPB time, nadir hemoglobin level on CPB, and the total volume of intraoperative red blood cells, fresh frozen plasma, platelets, cryoprecipitate, and cellsaving device transfusions. The propensity model had a fair discriminatory power (C-index, 0.81) and patients and controls were matched on propensity scores within a 0.1 margin using the Greedy match algorithm in a 1:1 ratio. All further comparisons and analyses were performed in this matched cohort. The parametric χ2 test and the Kruskal-Wallis test were used to compare normally and nonnormally distributed continuous variables. Binary variables were compared using the Cochran-Armitage trend test. p values < 0.05 were considered statistically significant. Calculations were performed using SAS software, version 9.2 (SAS Institute, Cary, NC).
Table 1.
Prematching Characteristics
| Variable | Control (n = 334) | ILD-rFVIIa (n = 77) | p Value |
|---|---|---|---|
| Age (years) | 59 (46–67) | 59 (48–70) | 0.24 |
| Male sex | 233 (70%) | 52 (68%) | 0.70 |
| Race | 0.009 | ||
| White | 256 (77%) | 51 (66%) | |
| Black | 66 (20%) | 17 (22%) | |
| Other | 12 (4%) | 9 (12%) | |
| Height (cm) | 175 (168–183) | 175 (165–183) | 0.23 |
| Weight (kg) | 86 (73–99) | 76 (65–92) | 0.002 |
| Body mass index (kg/m2) | 27.6 (24.4–31.7) | 26.4 (21.6–29.8) | 0.005 |
| Preoperative anticoagulants | |||
| Aspirin | 145 (43%) | 34 (44%) | 0.91 |
| Clopidogrel | 16 (5%) | 4 (5%) | 0.88 |
| Warfarin | 41 (12%) | 16 (21%) | 0.05 |
| Baseline laboratory values | |||
| Hemoglobin (g/dL) | 13.6 (12.2–14.8) | 12.8 (11.4–13.7) | 0.0006 |
| Platelets (109 cells/L) | 213 (179–253) | 180 (163–226) | 0.001 |
| International normalized ratio | 1.0 (1.0–1.1) | 1.0(1.0–1.1) | 0.29 |
| Partial thromboplastin time (sec) | 30.5 (28.4–33.6) | 31.1 (27.9–33.1) | 0.89 |
| Creatinine (mg/dL) | 1.0 (0.9–1.2) | 1.0 (0.8–1.2) | 0.83 |
| Case status | 0.0004 | ||
| Elective | 259 (78%) | 44 (57%) | |
| Urgent | 39 (12%) | 16 (21%) | |
| Emergent | 36 (11%) | 17 (22%) | |
| ASA class | 0.009 | ||
| II | 5 (2%) | 0 | |
| III | 203 (61%) | 36 (47%) | |
| IV | 126 (38%) | 41 (53%) | |
| Redo sternotomy | 59 (18%) | 24 (31%) | 0.008 |
| Principal procedure | 0.93 | ||
| Root or ascending replacement, or both | 63 (19%) | 16 (21%) | |
| Root or ascending, or both, + hemiarch | 228 (68%) | 48 (62%) | |
| Root or ascending, or both + total arch | 16 (5%) | 9 (12%) | |
| Descending or thoracoabdominal | 27 (8%) | 4 (5%) | |
| Concomitant procedure | 92 (28%) | 31 (40%) | 0.03 |
| Deep hypothermic circulatory arrest | 263 (79%) | 62 (81%) | 0.73 |
| Cardiopulmonary bypass time (min) | 204 (183–232) | 222 (195–264) | 0.0005 |
| Nadir hemoglobin on bypass (g/dL) | 7.8 (7.0–8.7) | 7.3 (6.7–8.0) | 0.0003 |
| Total intraoperative transfusions | |||
| Red blood cells (mL) | 700 (0–1,400) | 1400 (700–2100) | <0.0001 |
| Fresh frozen plasma (mL) | 1000 (540–1,750) | 2000 (1,500–2,750) | <0.0001 |
| Pooled platelet concentrates (mL) | 489 (300–600) | 800 (600–863) | <0.0001 |
| Cryoprecipitate (mL) | 0 (0–0) | 100 (0–100) | <0.0001 |
| Cell saving device return (mL) | 500 (250–700) | 750 (500–1,200) | <0.0001 |
Values expressed as median (interquartile range) or number (percent).
ASA = American Society of Anesthesiologists; ILD-rFVIIa = intraoperative low-dose recombinant activated factor VII.
Results
Prematching characteristics revealed that ILD-rFVIIa use was restricted to a high-risk patient cohort with severe coagulopathy after CPB, as evidenced by more preoperative risk factors for bleeding [16], higher case acuity and complexity, and greater intraoperative transfusion requirements (Table 1). Propensity matching produced a cohort of 44 patients who received ILD-rFVIIa and 44 high-risk control patients with balanced covariates (Table 2).
Table 2.
Postmatching Characteristics
| Variable | Control (n = 44) | ILD-rFVIIa (n = 44) | p Value |
|---|---|---|---|
| Age (years) | 60 (46–68) | 59 (50–70) | 0.54 |
| Male sex | 28 (64%) | 27 (61%) | 0.83 |
| Race | 0.20 | ||
| White | 29 (66%) | 33 (75%) | |
| Black | 11 (25%) | 10 (23%) | |
| Other | 4 (9%) | 1 (2%) | |
| Height (cm) | 175 (167–182) | 175 (166–182) | 0.77 |
| Weight (kg) | 80 (70–95) | 80 (68–92) | 0.63 |
| Body mass index (kg/m2) | 26.1 (23.7–29.1) | 26.0 (22.7–30.3) | 0.97 |
| Preoperative anticoagulants | |||
| Aspirin | 17 (39%) | 22 (50%) | 0.28 |
| Clopidogrel | 2 (5%) | 2 (5%) | 1 |
| Warfarin | 6 (14%) | 9 (21%) | 0.40 |
| Baseline laboratory values | |||
| Hemoglobin (g/dL) | 13.1 (11.3–14.0) | 12.7 (11.4–13.7) | 0.87 |
| Platelets (109 cells/L) | 202 (161–233) | 192 (161–228) | 0.70 |
| International normalized ratio | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 0.83 |
| Partial thromboplastin time (sec) | 29.9 (28.5–31.0) | 30.5 (28.4–32.3) | 0.49 |
| Creatinine level (mg/dL) | 1.0 (0.9–1.2) | 1.0 (0.8–1.3) | 0.72 |
| Case status | 0.16 | ||
| Elective | 24 (55%) | 29 (66%) | |
| Urgent | 7 (16%) | 8 (18%) | |
| Emergent | 13 (27%) | 7 (16%) | |
| ASA class | 0.56 | ||
| II | 2 (5%) | 0 | |
| III | 18 (41%) | 25 (57%) | |
| IV | 24 (55%) | 19 (43%) | |
| Redo sternotomy | 11 (25%) | 13 (30%) | 0.63 |
| Principal procedure | 0.67 | ||
| Root or ascending replacement, or both | 7 (16%) | 8 (18%) | |
| Root or ascending, or both + hemiarch | 28 (64%) | 27 (61%) | |
| Root or ascending, or both + total arch | 5 (11%) | 7 (16%) | |
| Descending or thoracoabdominal | 4 (9%) | 2 (5%) | |
| Concomitant procedure | 14 (32%) | 16 (36%) | 0.65 |
| Deep hypothermic circulatory arrest | 38 (86%) | 37 (84%) | 0.76 |
| Cardiopulmonary bypass time (min) | 208 (188–244) | 220 (191–247) | 0.70 |
| Nadir hemoglobin on bypass (g/dL) | 7.7 (7.0–8.5) | 7.4 (6.9–8.1) | 0.30 |
| Total intraoperative transfusions | |||
| Red blood cells (mL) | 825 (0–1488) | 1050 (700–1750) | 0.22 |
| Fresh frozen plasma (mL) | 1553 (1000–2000) | 2000 (1000–2313) | 0.38 |
| Pooled platelet concentrates (mL) | 600 (400–800) | 600 (598–800) | 0.17 |
| Cryoprecipitate (mL) | 100 (0–100) | 100 (0–100) | 0.96 |
| Cell saving device return (mL) | 540 (425–900) | 713 (463–880) | 0.50 |
Values expressed as median (interquartile range) or number (percent).
ASA = American Society of Anesthesiologists; ILD-rFVIIa = intraoperative low-dose recombinant activated factor VII.
Initial rFVIIa doses received by the matched ILD-rFVIIa patients ranged from 8 to 57 μg/kg (median, 32 μg/kg interquartile range [IQR], 16–43 μg/kg) and were administered at a median of 51 minutes (IQR, 42–67 minutes) after separation from CPB. Nine of 44 (20%) patients who received ILD-rFVIIa got a second intraoperative rFVIIa dose (median second dose, 28 μg/kg IQR, 11–39 μg/kg). The median time between doses was 37 minutes (range, 15–50 minutes).
On arrival in the ICU, patients who received ILD-rFVIIa were warmer (36.5°C versus 36.2°C; p = 0.03) and had improved coagulation indicators (INR, 0.8 versus 1.2; p < 0.0001; partial thromboplastin time, 28.6 versus 31.5; p = 0.05) compared with control patients (Table 3). Four of 44 (9%) control patients required postoperative rFVIIa administration, compared with none of the patients who received ILD-rFVIIa (p = 0.12) (Table 4). Patients who received ILD-rFVIIa had 50% fewer total postoperative blood product transfusions (2.5 versus 5.0 units; p = 0.05). When stratified by postoperative day, reductions in postoperative transfusions were restricted to reduced fresh frozen plasma and platelet transfusions on postoperative day 0 (Table 4).
Table 3.
Immediate Postoperative Laboratory Data
| Variable | Control (n = 44) | ILD-rFVIIa (n = 44) | p Value |
|---|---|---|---|
| Temperature (°C) | 36.2 (35.6–36.7) | 36.5 (36.0–37.0) | 0.03 |
| pH | 7.39 (7.35–7.45) | 7.43 (7.36–7.46) | 0.22 |
| Hemoglobin (g/dL) | 9.7 (9.4–10.1) | 9.7 (9.4–10.7) | 0.74 |
| Platelets (109 cells/L) | 132 (105–154) | 139 (115–165) | 0.15 |
| International normalized ratio | 1.2 (1.2–1.3) | 0.8 (0.7–0.8) | <0.0001 |
| Partial thromboplastin time (seconds) | 31.5 (27.4–47.3) | 28.6 (25.8–34.6) | 0.05 |
| Fibrinogen (mg/dL) | 247 (181–303) | 253 (216–276) | 0.60 |
Values expressed as median (interquartile range).
ILD-rFVIIa = intraoperative low-dose recombinant activated factor VII.
Table 4.
Postoperative Bleeding, Recombinant Activated Factor VII Exposures, and Transfusions
| Variable | Control (n = 44) | ILD-rFVIIa (n = 44) | P Value |
|---|---|---|---|
| 12-hour chest tube drainage (mL) | 550 (348–923) | 570 (398–846) | 0.96 |
| Postoperative rFVIIa | 4 (9%) | 0 | 0.12 |
| Dose (μg/kg) | 70 (49–89) | ⋯ | |
| Transfusions: postoperative day 0 | |||
| Packed red blood cells (units) | 1.3 (2.1) | 0.7 (1.0) | 0.89 |
| Fresh frozen plasma (units) | 1.3 (2.0) | 0.4 (0.9) | 0.01 |
| Pooled platelet concentrates (units) | 0.5 (0.8) | 0.2 (0.5) | 0.02 |
| Cryoprecipitate (units) | 0.1 (0.3) | 0.1 (0.2) | 0.40 |
| Transfusions: Total postoperative | |||
| Packed red blood cells (units) | 2.2 (2.7) | 1.5 (1.9) | 0.29 |
| Fresh frozen plasma (units) | 1.6 (2.9) | 0.7 (1.9) | 0.02 |
| Pooled platelet concentrates (units) | 1.0 (2.0) | 0.3 (0.8) | 0.02 |
| Cryoprecipitate (units) | 0.2 (0.6) | 0.1 (0.2) | 0.39 |
| Total (units) | 5.0 (7.2) | 2.5 (4.1) | 0.05 |
| Discharge hemoglobin (g/dL) | 9.6 (1.3) | 9.6 (1.1) | 1 |
Values expressed as median (interquartile range), average (standard deviation), or number (percent).
ILD-rFVIIa = intraoperative low-dose recombinant activated factor VII.
All postoperative complications were equivalent between groups (Table 5). The 1 stroke in the ILD-rFVIIa group was caused by an air embolus in a patient undergoing total arch replacement, and the 1 myocardial infarction was caused by creation of a significant left main coronary artery stenosis after mobilization of a left coronary button during a Bentall procedure. These complications were related to technical issues and did not appear to be caused by thromboembolism or rFVIIa administration. One patient (2.3%) in the ILD-rFVIIa group presented with a small segmental pulmonary embolus diagnosed on readmission for 2 weeks after hospital discharge.
Table 5.
Adverse Events
| Complication | Control (n = 44) | ILD-rFVIIa (n = 44) | p Value |
|---|---|---|---|
| Reoperation for bleeding | 1 (2.3%) | 0 | 0.31 |
| Delayed sternal closure | 2 (4.5%) | 2 (4.5%) | 1 |
| Deep sternal wound infection | 0 | 0 | 1 |
| New-onset arrhythmia | 15 (34.1%) | 19 (43.2%) | 0.51 |
| Pacemaker placement | 3 (6.8%) | 2 (4.5%) | 0.65 |
| Peak postoperative creatinine (mg/dL) | 1.4 (1.2–1.7) | 1.4 (1.1–2.0) | 0.80 |
| Acute renal failure (Cr > 2.0 and > 2× baseline) | 3 (6.8%) | 5 (11.4%) | 0.46 |
| New-onset dialysis | 1 (2.3%) | 1 (2.3%) | 1 |
| Prolonged ventilation (> 24 hours) | 11 (25%) | 7 (15.9%) | 0.43 |
| Tracheostomy | 4 (9.1%) | 0 | 0.12 |
| Myocardial infarction | 0 | 1 (2.3%) | 0.31 |
| Pulmonary embolism | 1 (2.3%) | 1 (2.3%) | 1 |
| Mesenteric ischemia | 1 (2.3%) | 0 | 0.31 |
| Any stroke (neurologic deficit lasting > 72 hours) | 3 (6.8%) | 1 (2.3%) | 0.62 |
| Embolic stroke | 2 (4.5%) | 0 | 0.49 |
| Permanent paraparesis/paraplegia | 1 (2.3%) | 0 | 0.31 |
| Postoperative length of stay (days) | 6 (5–9) | 7 (5–11) | 0.33 |
| 30-day/in-hospital death | 1 (2.3%) | 1 (2.3%) | 1 |
Values expressed as median (interquartile range) or number (percent).
Cr = creatinine; ILD-rFVIIa = intraoperative low-dose recombinant activated factor VII.
Total direct hospital costs were $2,008 (6%) higher in the ILD-rFVIIa group. The cost of intraoperative rFVIIa appeared partially offset by savings in postoperative rFVIIa use and postoperative blood product transfusions (Table 6).
Table 6.
Inflation-Adjusted Direct Hospital Costs
| Variable | Control (n = 44) | ILD-rFVIIa (n = 44) | p Value |
|---|---|---|---|
| Intraoperative rFVIIa ($) | 0 | 3,032 (2,205) | <0.0001 |
| Postoperative rFVIIa ($) | 269 (1,140) | 0 | <0.0001 |
| All pharmacy ($) | 3,947 (2,959) | 7,164 (3,692) | <0.0001 |
| Total blood bank costs ($) | 5,891 (4,305) | 5,607 (2,586) | 0.98 |
| Intraoperative blood products ($) | 4,092 (2,534) | 4,719 (1,650) | 0.03 |
| Postoperative blood products ($) | 1,799 (2,582) | 888 (1,392) | 0.04 |
| Surgical ($) | 12,056 (7,658) | 13,301 (7,577) | 0.11 |
| Respiratory ($) | 1,051 (2,014) | 580 (606) | 0.49 |
| Nursing ($) | 8,521 (9,731) | 7,306 (4,382) | 0.61 |
| Total costs ($) | 34,878 (23,492) | 36,886 (13,945) | 0.02 |
Values expressed as mean (standard deviation).
ILD-rFVIIa = intraoperative low-dose recombinant activated factor VII.
Comment
We report the results of ILD-rFVIIa administration during complex thoracic aortic operations, a strategy intended to treat severe coagulopathy after CPB with reduced blood product transfusions and improved safety through the early targeted administration of small rFVIIa doses. Our findings suggest that ILD-rFVIIa led to improved postoperative hemostasis, as evidenced by fewer postoperative transfusions and no requirement for postoperative rFVIIa administration or reexploration for bleeding among patients who received ILD-rFVIIa when compared with propensity-matched control patients. The safety profile of ILD-rFVIIa appeared promising, with few overall adverse events observed in the ILD-rFVIIa group and no apparent increase in adverse events compared with control patients. The cost of ILD-rFVIIa averaged $3,032 per patient; however these costs were partially offset by savings in postoperative care and were less than in protocols using full-dose rFVIIa.
The utility of rFVIIa for the control of hemorrhage after cardiovascular procedures appears well established. In addition to multiple case reports and nonrandomized single-institution and large database studies, 2 randomized trials using rFVIIa doses between 40 and 90 μg/kg have documented the efficacy of rFVIIa in controlling hemorrhage when used in either the intraoperative or postoperative setting [1, 6]. In 2005, we proposed that low-dose rFVIIa (20–40 μg/kg) may be adequate to achieve hemostasis in patients with intact hemostatic systems [15], and other reports have since supported this approach [4, 5, 18–20]. The rationale for early rFVIIa dosing appears further supported by the findings of a large Canadian review of rFVIIa use in cardiac procedures, in which the authors concluded the efficacy of rFVIIa may be improved if administered early in the setting of adequate coagulation factors [11]. Intraoperative dosing further allows for careful exclusion of surgical bleeding before drug administration, as opposed to blind postoperative dosing in which rFVIIa use may be futile if administered in the setting of unrecognized surgical bleeding. The improvements in postoperative hemostasis observed in the present study suggest that rFVIIa remains effective when used intraoperatively at initial doses as low as 10 to 20 μg/kg. Although we believe ILD-rFVIIa also leads to reductions in intraoperative transfusions and time to sternal closure, the intraoperative benefits of ILD-rFVIIa therapy were unable to be assessed in our study given that patients who received ILD-rFVIIa and control patients were matched on intraoperative variables, including intraoperative blood product requirements.
Safe use of rFVIIa in adult cardiac operations has been suggested by multiple small, nonrandomized single-institution reports [1–4, 7, 8, 10, 13]. Although patients receiving “rescue” rFVIIa are uniformly high risk and generally display poor postoperative outcomes, 2 large registry reviews failed to identify rFVIIa exposure as an independent risk factor for stroke or other complications [11, 12]. Nonetheless, routine use of rFVIIa in cardiac operations has been discouraged because of anecdotal reports of catastrophic thromboembolic events after rFVIIa administration [15, 21], a nonsignificant increase in the rate of stroke and serious adverse events in the only multicenter randomized placebo-controlled trial of postoperative rFVIIa administration [6], and a metaanalysis of all studies with patient matching that reported a statistically significant increase in the rate of stroke in patients who received rFVIIa [14]. The interpretation of these results is challenged by the small sample size used by most isolated studies and the pooling of highly variable treatment protocols in the cumulative studies. Further, the application of these findings to thoracic aortic surgery may not be justified. Virchow's triad describes stasis, abnormal vasculature, and hypercoagulability as risk factors for thrombosis. The population undergoing aortic procedures generally has robust cardiac function leading to a hyperdynamic circulation rather than stasis, vascular abnormality limited to large vessels with high flow, and profound coagulopathy. In our study, overall rates of adverse events were low, and only 1 thromboembolic event (2.3%) was appreciated in the ILD-rFVIIa group, compared with 4 events (9.1%) in the control group (p = 0.36). Although our study is underpowered to draw firm conclusions regarding safety, the rationale for ILD-rFVIIa therapy coupled with these early promising results suggest that ILD-rFVIIa may be the optimal strategy for rFVIIa use with thoracic aortic operations and warrants further rigorous study.
Limitations
Our study represents a small, observational, single-institution report and is therefore limited by the constraints of sample size, selection bias, confounding by indication, and the presence of unmeasured confounders. Although the decision to administer ILD-rFVIIa was directed by institutional guidelines, ILD-rFVIIa administration was ultimately at the discretion of the operative team. This decision likely evolved throughout the study period as comfort and experience with ILD-rFVIIa increased. In addition, despite propensity matching, selection bias may favor administration of ILD-rFVIIa to sicker or more apparently coagulopathic patients. Evidence that the ILD-rFVIIa group represents a higher risk cohort than the control group is supported by the fact that the ILD-rFVIIa group received intraoperative transfusions similar to those of the control group. ILD-rFVIIa should theoretically reduce intraoperative transfusions if matched patients were truly equivalent. Also, pretreatment transfusions may detract from potential postoperative benefits by overestimating adverse events and increasing overall hospital costs in the ILD-rFVIIa group; this could be elucidated only by further prospective study that has previously shown promising results in the thoracic aortic surgical population [1].
Conclusion
We describe a novel strategy for ILD-rFVIIa administration during thoracic aortic operations intended to improve perioperative hemostasis and reduce blood product transfusions while avoiding the safety concerns of full-dose rFVIIa. Between propensity-matched patient groups, this strategy led to improved postoperative hemostasis with no apparent increase in adverse events. Appropriately powered randomized studies are necessary to confirm the safety and efficacy of ILD-rFVIIa in the population undergoing thoracic aortic procedures before these methods are routinely adopted.
Acknowledgments
Support was received by a Thoracic Surgery Foundation for Research and Education Research Fellowship to Dr Andersen and National Institutes of Health grants T32-CA093245 to Dr Bhattacharya and T32-HL069749 and U01-HL088953 to Dr Williams. The authors thank Jennifer Lietzke for assistance procuring financial data.
Footnotes
Presented at the Fifty-eighth Annual Meeting of the Southern Thoracic Surgical Association, San Antonio, TX, November 9–12, 2011.
References
- 1.Diprose P, Herbertson MJ, O'Shaughnessy D, Gill RS. Activated recombinant factor VII after cardiopulmonary bypass reduces allogeneic transfusion in complex non-coronary cardiac surgery: randomized double-blind placebo-controlled pilot study. Br J Anaesth. 2005;95:596–602. doi: 10.1093/bja/aei244. [DOI] [PubMed] [Google Scholar]
- 2.Karkouti K, Beattie WS, Wijeysundera DN, et al. Recombinant factor VIIa for intractable blood loss after cardiac surgery: a propensity score-matched case-control analysis. Transfusion. 2005;45:26–34. doi: 10.1111/j.1537-2995.2005.04216.x. [DOI] [PubMed] [Google Scholar]
- 3.Tritapepe L, De Santis V, Vitale D, et al. Recombinant activated factor VII for refractory bleeding after acute aortic dissection surgery: a propensity score analysis. Crit Care Med. 2007;35:1685–90. doi: 10.1097/01.CCM.0000269033.89428.B3. [DOI] [PubMed] [Google Scholar]
- 4.Gelsomino S, Lorusso R, Romagnoli S, et al. Treatment of refractory bleeding after cardiac operations with low-dose recombinant activated factor VII (NovoSeven): a propensity score analysis. Eur J Cardiothorac Surg. 2008;33:64–71. doi: 10.1016/j.ejcts.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Masud F, Bostan F, Chi E, et al. Recombinant factor VIIa treatment of severe bleeding in cardiac surgery patients: a retrospective analysis of dosing, efficacy, and safety outcomes. J Cardiothorac Vasc Anesth. 2009;23:28–33. doi: 10.1053/j.jvca.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Gill R, Herbertson M, Vuylsteke A, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21–7. doi: 10.1161/CIRCULATIONAHA.108.834275. [DOI] [PubMed] [Google Scholar]
- 7.Tatoulis J, Theodore S, Meswani M, et al. Safe use of recombinant activated factor VIIa for recalcitrant postoperative haemorrhage in cardiac surgery. Interact Cardiovasc Thorac Surg. 2009;9:459–62. doi: 10.1510/icvts.2009.204735. [DOI] [PubMed] [Google Scholar]
- 8.Uber WE, Toole JM, Stroud MR, et al. Administration of recombinant activated factor VII in the intensive care unit after complex cardiovascular surgery: clinical and economic outcomes. J Thorac Cardiovasc Surg. 2011;141:1469–77. e1462. doi: 10.1016/j.jtcvs.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Hacquard M, Durand M, Lecompte T, et al. Off-label use of recombinant activated factor VII in intractable haemorrhage after cardiovascular surgery: an observational study of practices in 23 French cardiac centres (2005–7) Eur J CardiothoracSurg. 2011;40:1320–7. doi: 10.1016/j.ejcts.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 10.von Heymann C, Redlich U, Jain U, et al. Recombinant activated factor VII for refractory bleeding after cardiac surgery—a retrospective analysis of safety and efficacy. Crit Care Med. 2005;33:2241–6. doi: 10.1097/01.ccm.0000181527.47749.57. [DOI] [PubMed] [Google Scholar]
- 11.Karkouti K, Beattie WS, Arellano R, et al. Comprehensive Canadian review of the off-label use of recombinant activated factor VII in cardiac surgery. Circulation. 2008;118:331–8. doi: 10.1161/CIRCULATIONAHA.108.764308. [DOI] [PubMed] [Google Scholar]
- 12.Mitra B, Phillips L, Cameron PA, Billah B, Reid C. The safety of recombinant factor VIIa in cardiac surgery. Anaesth Intensive Care. 2010;38:671–7. doi: 10.1177/0310057X1003800409. [DOI] [PubMed] [Google Scholar]
- 13.Chapman AJ, Blount AL, Davis AT, Hooker RL. Recombinant factor VIIa (NovoSeven RT) use in high risk cardiac surgery. Eur J Cardiothorac Surg. 2011;40:1314–8. doi: 10.1016/j.ejcts.2011.03.048. discussion 1318–9. [DOI] [PubMed] [Google Scholar]
- 14.Ponschab M, Landoni G, Biondi-Zoccai G, et al. Recombinant activated factor VII increases stroke in cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2011;25:804–10. doi: 10.1053/j.jvca.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Welsby IJ, Monroe DM, Lawson JH, Hoffmann M. Recombinant activated factor VII and the anaesthetist. Anaesthesia. 2005;60:1203–12. doi: 10.1111/j.1365-2044.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- 16.Williams JB, Phillips-Bute B, Bhattacharya SD, et al. Predictors of massive transfusion with thoracic aortic procedures involving deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141:1283–8. doi: 10.1016/j.jtcvs.2010.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima B, Williams JB, Bhattacharya SD, et al. Results of proximal arch replacement using deep hypothermia for circulatory arrest: is moderate hypothermia really justifiable? Am Surg. 2011;77:1438–44. [PMC free article] [PubMed] [Google Scholar]
- 18.van de Garde EM, Bras LJ, Heijmen RH, et al. Low-dose recombinant factor VIIa in the management of uncontrolled postoperative hemorrhage in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2006;20:573–5. doi: 10.1053/j.jvca.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Willis C, Bird R, Mullany D, Cameron P, Phillips L. Use of rFVIIa for critical bleeding in cardiac surgery: dose variation and patient outcomes. Vox Sang. 2010;98:531–7. doi: 10.1111/j.1423-0410.2009.01276.x. [DOI] [PubMed] [Google Scholar]
- 20.Altman R, Scazziota A, de Lourdes Herrera M, Gonzalez CD. The hemostatic profile of recombinant activated factor VII. Can low concentrations stop bleeding in off-label indications? Thromb J. 2010;8:8. doi: 10.1186/1477-9560-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syburra T, Lachat M, Genoni M, Wilhelm MJ. Fatal outcome of recombinant factor VIIa in heart transplantation with extracorporeal membrane oxygenation. Ann Thorac Surg. 2010;89:1643–5. doi: 10.1016/j.athoracsur.2009.09.039. [DOI] [PubMed] [Google Scholar]


