Abstract
Objective
Hybrid repair of the transverse aortic arch may allow for aortic arch repair with reduced morbidity in patients who are suboptimal candidates for conventional open surgery. Here, we present our results with an algorithmic approach to hybrid arch repair, based upon the extent of aortic disease and patient comorbidities.
Methods
Between August 2005 and January 2012, 87 patients underwent hybrid arch repair by three principal procedures: zone 1 endograft coverage with extra-anatomic left carotid revascularization (zone 1, n=19), zone 0 endograft coverage with aortic arch debranching (zone 0, n=48), or total arch replacement with staged stented elephant trunk completion (stented elephant trunk, n=20).
Results
The mean patient age was 64 years and the mean expected in-hospital mortality rate was 16.3% as calculated by the EuroSCORE II. 22% (n=19) of operations were non-elective. Sternotomy, cardiopulmonary bypass, and deep hypothermic circulatory arrest were required in 78% (n=68), 45% (n=39), and 31% (n=27) of patients, respectively, to allow for total arch replacement, arch debranching, or other concomitant cardiac procedures, including ascending ± hemi-arch replacement in 17% (n=8) of patients undergoing zone 0 repair. All stented elephant trunk procedures (n=20) and 19% (n=9) of zone 0 procedures were staged, with 41% (n=12) of patients undergoing staged repair during a single hospitalization. The 30-day/in-hospital rates of stroke and permanent paraplegia/paraparesis were 4.6% (n=4) and 1.2% (n=1), respectively. Three of 27 (11.1%) patients with native ascending aorta zone 0 proximal landing zone experienced retrograde type A dissection following endograft placement. The overall in-hospital mortality rate was 5.7% (n=5), however, 30-day/in-hospital mortality increased to 14.9% (n=13) due to eight 30-day out-of-hospital deaths. Native ascending aorta zone 0 endograft placement was found to be the only univariate predictor of 30-day/in-hospital mortality (odds ratio, 4.63; 95% confidence interval, 1.35-15.89; P=0.02). Over a mean follow-up of 28.5 ± 22.2 months, 13% (n=11) of patients required reintervention for type 1A (n=4), type 2 (n=6), or type 3 (n=1) endoleak. Kaplan-Meier estimates of survival at 1, 3, and 5 years were 73%, 60%, and 51%, respectivel.
Conclusions
Hybrid aortic arch repair can be tailored to patient anatomy and comorbid status to allow complete repair of aortic pathology, frequently in a single stage, with acceptable outcomes. However, endograft placement in the native ascending aorta is associated with high rates of retrograde type A dissection and 30-day/in-hospital mortality and should be approached with caution.
INTRODUCTION
Conventional open repair of thoracic aortic pathology involving the transverse aortic arch is associated with significant morbidity and mortality, and many patients are not candidates for conventional open operation. A strategy that incorporates thoracic endovascular aortic repair (TEVAR) into “hybrid” open/endovascular aortic arch procedures may allow for aortic arch repair with reduced morbidity and allow for repair in patients who are suboptimal candidates for open surgery. Specifically, hybrid arch procedures can be used for multi-segment aortic arch repair while avoiding morbidity associated with thoracotomy or thoracosternotomy incisions, cardiopulmonary bypass (CPB), hypothermic circulatory arrest, and interstage attrition in patients requiring staged open repair.
Despite the potential advantages of less-invasive hybrid arch procedures, outcomes following hybrid arch repair remain unclear. Limitations of existing data include small patient numbers, diverse operations performed for varying indications, and imprecise terminology.1-20 We therefore sought to review our results with hybrid arch repair using an algorithmic approach to patient and operative selection and standardized operative techniques.
METHODS
Patient Population and Data Collection
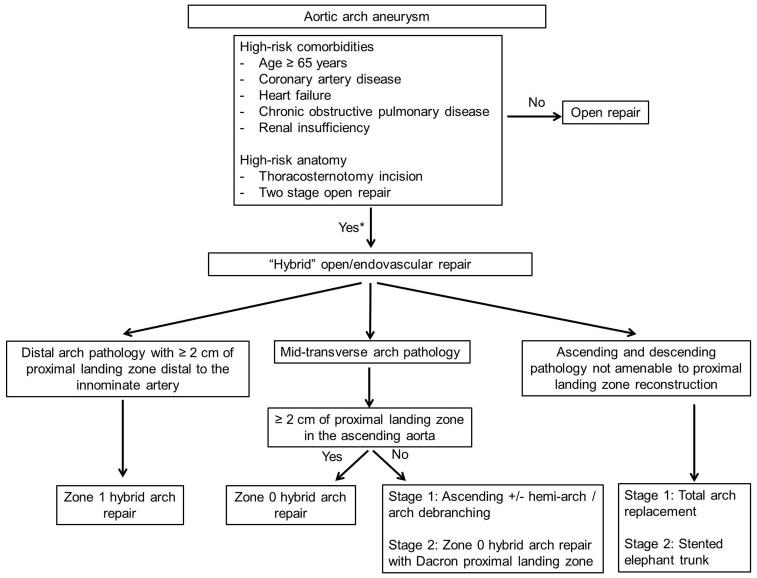
This study was approved by the Institutional Review Board of Duke University and the need for individual patient consent was waived. The Duke Thoracic Aortic Surgery Database is a prospectively maintained electronic clinical registry of all patients who have undergone thoracic aortic surgery at Duke University Medical Center (Durham, NC) since 2005.21, 22 A query of the database identified 387 consecutive TEVAR operations performed between March 2005 and January 2012; of these, 87 (22%) were hybrid arch repairs and form the basis of this report. General criteria regarding patient selection for hybrid arch repair are listed in Figure 1. Patients with advanced age, extensive comorbidities, or with high-risk anatomic features are preferentially selected for less invasive hybrid repairs at our institution.
Figure 1.
Algorithm for hybrid aortic arch repair. *Note that these criteria are relative factors in the decision-making process but not absolute indications/contraindications. Ideally, the decision for conventional versus hybrid repair should be made by a surgical team with expertise in both techniques. Institutional results with each approach should further influence the decision making process.
Aortic arch landing zones were defined using the Ishimaru classification.23 Hybrid arch repairs were defined as those involving endograft coverage of the innominate artery and/or the left common carotid artery (LCCA; zone 0 or zone 1 proximal landing zone) or endovascular completion following total arch replacement (stented elephant trunk). Operations involving coverage of the left subclavian artery (LSCA) only (zone 2 proximal landing zone) were excluded. Comorbid conditions and postoperative complications were defined using Society of Thoracic Surgeons definitions (www.sts.org). European System for Cardiac Operative Risk Evaluation (EuroSCORE) II scores were calculated at http://euroscore.org/calc.html.24 Aortic diameter measurements were obtained using the centerline method with a TeraRecon Aquarius iNtuition workstation (TeraRecon Inc, San Mateo, CA).
Indications for surgery, techniques of device delivery and deployment, and postoperative surveillance have been described previously.3-5 Indications for operation were classified as degenerative aneurysm (fusiform, saccular, or penetrating atherosclerotic ulcers), acute dissection, or chronic dissection. All commercially available endografts available during the study period were used and included the Gore TAG and C-TAG (W.L Gore & Associates, Flagstaff, AZ), Medtronic Talent (Medtronic, Inc, Santa Rosa, CA), and Zenith TX2 (Cook Medical Incorporated, Bloomington, IN) devices. Primary technical success was defined according to Society of Vascular Surgery reporting standards.25
Statistical Analysis
Continuous and categorical variables were compared between groups using the Kruskal-Wallis test and the Chi-squared test, respectively. Survival was estimated using the Kaplan-Meier method. For patients who underwent staged repair, the date of the completion procedure was used to calculate survival. Survival was compared between groups using the log rank test. Calculations were performed using STATA 11.1 (StataCorp, College Station, TX).
OPERATIVE TECHNIQUES
Patient Preparation and Monitoring
Preoperative coronary angiography was performed for previously established indications.26 All cases were performed with continuous transesophageal echocardiographic (TEE) monitoring. Intraoperative neurophysiologic monitoring of spinal cord somatosensory and motor evoked potentials was performed for elective cases, and when available for non-elective cases (78 cases monitored; 90%).27 Electroencephalographic (EEG) neurocerebral monitoring was used in all elective zone 0 hybrid arch repairs (37 of 48; 77%) to assess for cerebral ischemia during arch debranching.3, 5 Adjunctive intravascular ultrasound (IVUS) was used in all dissection cases as previously described.28 Routine inspection of the ascending aorta for retrograde type A dissection was performed at case completion by TEE, as well as IVUS when utilized.29
Zone 1 Hybrid Arch Repair
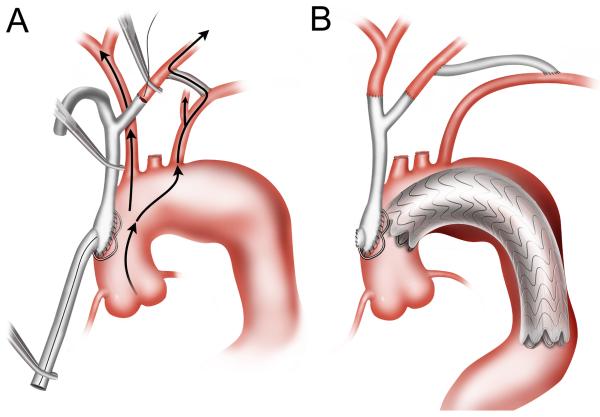
Zone 1 hybrid arch repair was utilized for patients with distal arch pathology but with two or more centimeters of proximal landing zone distal to the innominate artery.3, 5 First, an 8-mm polytetrafluoroethylene carotid-carotid bypass was performed via an anterior subplatysmal plane. The graft was extended to revascularize the LSCA, when indicated.30 The proximal LCCA was ligated to prevent type II endoleak. The proximal LSCA was similarly ligated or occluded to prevent endoleak, when necessary. The endograft was then delivered via retrograde access and deployed with a zone 1 proximal landing site (Figure 2).
Figure 2.
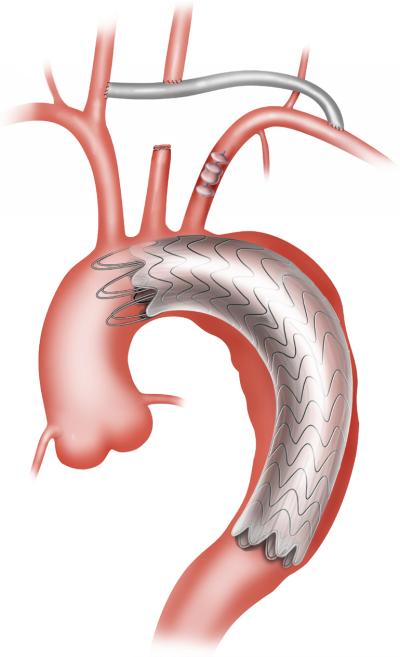
Zone 1 hybrid arch repair. In the case depicted, the carotid-carotid bypass graft was extended to revascularize the LSCA, and the proximal LSCA was occluded with an Amplatzer vascular plug (St. Jude Medical Inc., St. Paul, MN) to prevent type II endoleak.
Zone 0 Hybrid Arch Repair
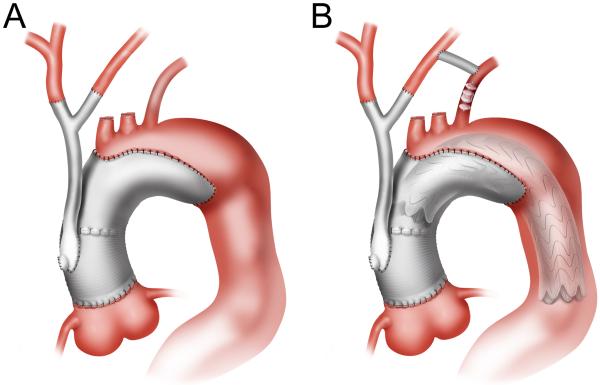
Zone 0 hybrid arch repair was utilized for patients with mid transverse arch pathology but with greater than two centimeters of proximal landing zone in the ascending aorta. Ascending aorta-based arch debranching was performed using a custom-designed Dacron “hybrid antegrade arch graft” (Vascutek USA, Ann Arbor, MI) to debranch the innominate artery and LCCA and allow for antegrade endograft delivery.3-5 First, a left carotid-subclavian bypass was performed in the neck, followed by median sternotomy. The ascending aorta debranching graft inflow anastomosis was then performed without CPB (pump on standby) using a partial occlusion clamp technique. The LCCA was debranched first, with full left cerebral blood flow supplied by retrograde flow in the left carotid-subclavian bypass (Figure 3A). The innominate artery was then debranched following a 3 minute test clamp to assess for EEG ischemic changes as previously described.3-5 The endograft was then delivered antegrade or retrograde across the arch with a zone 0 proximal landing site (Figure 3B). The decision to deliver the endograft antegrade or retrograde was determined by the device utilized, as only the Gore devices are capable of antegrade delivery. In patients with prior ascending aorta replacement, the artificial Dacron ascending aorta served as the inflow site for the arch debranching graft as well as proximal landing zone.
Figure 3.
Native zone 0 hybrid arch repair. (A) Initial construction of a left carotid-subclavian bypass allows uninterrupted left cerebral blood flow during LCCA debranching. (B) Following arch debranching, the endograft is deployed with native ascending aorta proximal landing zone. The arch debranching graft utilized incorporates an integral limb just above the inflow anastomosis to allow for antegrade endograft delivery without the need for femoral exposure. This limb is oversewn after completion of the procedure.
For patients with native ascending aorta not suitable for proximal landing zone, generally due to a diameter ≥ 40 mm, ascending aorta with or without hemi-arch replacement was first performed together with arch debranching on CPB, as previously described,21 with the arch debranching graft anastomosed to the newly constructed Dacron ascending aorta (Figure 4A). For patients requiring hemi-arch replacement, deep hypothermic circulatory arrest (DHCA) with antegrade cerebral perfusion via right axillary cannulation was used during hemi-arch replacement and arch debranching.21 The distal arch and descending aortic pathologies were then definitively treated by endografting with Dacron zone 0 proximal landing zone, typically during a second stage procedure (Figure 4B). We favor staged repair in this scenario given the competing postoperative management strategies with regards to blood pressure following open proximal versus endovascular distal aortic repair. In the former scenario, the patient is frequently coagulopathic following CPB +/− DHCA and lower mean arterial pressures (MAPs) are preferred in the early postoperative period to reduce bleeding, whereas in the latter scenario, where bleeding is generally not an issue, higher MAPs are favored for spinal cord protection. Further, similar to our findings with hybrid thoracoabdominal repair, staged repair reduces the nephrotoxic insult of prolonged surgery followed by contrast administration, reduces blood loss by limiting the period of heparinization, and allows for patient recovery and medical optimization prior to the second stage procedure.31 The only exception to this rule is in the setting of rupture (n=1 in this series), where the proximal aortic replacement using CPB and the endovascular portion of the repair must be done as a single stage.
Figure 4.
Dacron zone 0 hybrid arch repair with ascending/hemi-arch replacement. (A) In the first stage procedure, ascending aorta and hemi-arch replacement are performed followed by arch debranching from the newly constructed Dacron ascending aorta. (B) Endografting is performed during a second procedure with the Dacron ascending aorta serving as proximal landing zone. In the case depicted, a left carotid-subclavian bypass was performed during the second stage procedure to revascularize the LSCA, and the proximal LSCA was occluded with a vascular plug to prevent type II endoleak.
Total Arch Replacement with Staged Stented Elephant Trunk Completion
Total arch replacement with staged stented elephant trunk completion was utilized for patients with aneurysms of the ascending and descending aorta (“mega aorta”) not amenable to proximal landing zone reconstruction. The first stage involved total arch replacement with a collared elephant trunk graft (Vascutek) with use of CPB and DHCA, using a modified Mt. Sinai technique as previously described (Figure 5A).5 The graft utilized incorporates radiographic markers which facilitate second stage repair. Further, four large hemoclips are placed circumferentially around the distal end of the elephant trunk graft to assist with identification under fluoroscopy. Pacing wires (#0) are also secured to the distal end of the elephant trunk and allowed to trail distally a few centimeters into the descending thoracic aorta. The second stage involved completion endografting with at least 10 centimeters of proximal landing zone in the Dacron elephant trunk (Figure 5B).3, 5 At the time of endograft deployment, either of the wires is snared to provide counter traction and prevent graft intussusception as the endograft is advanced retrograde into the Dacron elephant trunk. Staged repair is performed in all cases for the same reasons as described above.
Figure 5.
Total arch replacement with staged stented elephant trunk completion. (A) The first stage procedure involves total arch replacement using a modified Mt. Sinai technique. (B) The second stage procedure involves endovascular completion with the elephant trunk graft serving as proximal landing zone. Note the radiographic markers, hemoclips, and pacing wires on the elephant trunk graft.3
RESULTS
OPERATIVE DETAILS AND 30-DAY/IN-HOSPITAL OUTCOMES
Patient demographics are shown in Table 1, operative characteristics are shown in Table 2, arch vessel bypass grafts are shown in Table 3, and 30-day/in-hospital outcomes are shown in Table 4.
Table 1.
Demographics
| Variable | Total (N=87) |
Zone 1 (n=19) |
Zone 0 (n=48) |
Total Arch + SET (n=20) |
P Value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| - Age (years) | 64 ± 13 | 67 ± 14 | 65 ± 12 | 59 ± 12 | 0.07 |
| - Male gender | 45 (52%) | 8 (42%) | 26 (54%) | 11 (55%) | 0.64 |
| - White race | 60 (69%) | 14 (74%) | 34 (71%) | 12 (60%) | 0.60 |
| - Body mass index (kg/m2) | 26.3 ± 5.7 | 25.6 ± 4.5 | 26.7 ± 6.3 | 26.1 ± 5.3 | 0.76 |
| Patient comorbidities | |||||
| - Hypertension | 79 (91%) | 15 (79%) | 44 (92%) | 20 (100%) | 0.07 |
| - Hyperlipidemia | 54 (62%) | 11 (58%) | 33 (69%) | 10 (50%) | 0.32 |
| - History of tobacco use | 60 (69%) | 12 (63%) | 33 (69%) | 15 (75%) | 0.73 |
| - Diabetes | 9 (10%) | 4 (21%) | 4 (8%) | 1 (5%) | 0.21 |
| - Coronary artery disease | 30 (34%) | 7 (37%) | 18 (38%) | 5 (25%) | 0.60 |
| - History of stroke | 18 (21%) | 4 (21%) | 9 (19%) | 5 (25%) | 0.84 |
| -COPD | 33 (38%) | 4 (21%) | 18 (38%) | 11 (55%) | 0.09 |
| - Baseline creatinine > 1.5 mg/dl | 24 (28%) | 3 (16%) | 16 (33%) | 5 (25%) | 0.34 |
| - Peripheral vascular disease | 24 (28%) | 6 (32%) | 15 (31%) | 3 (15%) | 0.36 |
| - Connective tissue disease | 6 (7%) | 0 | 3 (6%) | 3 (15%) | 0.18 |
| - Prior aortic surgery | 42 (48%) | 9 (47%) | 23 (48%) | 10 (50%) | 0.98 |
| - EuroSCORE II (%)24 | 16.3 ± 11.0 | 17.8 ± 11.6 | 17.3 ± 11.8 | 12.5 ± 7.8 | 0.27 |
Values expressed as mean ± standard deviation or number (percent). COPD = chronic obstructive pulmonary disease. SET = stented elephant trunk.
Table 2.
Operative characteristics
| Variable | Total (N=87) |
Zone 1 (n=19) |
Zone 0 (n=48) |
Total Arch + SET (n=20) |
P Value |
|---|---|---|---|---|---|
| Indication | 0.09 | ||||
| - Aneurysm | 54 (62%) | 14 (74%) | 30 (63%) | 10 (50%) | |
| - Acute dissection | 3 (3%) | 2 (11%) | 1 (2%) | 0 | |
| - Chronic dissection | 30 (34%) | 3 (16%) | 17 (35%) | 10 (50%) | |
| Maximum aortic diameter (cm) | 6.5 ± 1.5 | 6.0 ± 1.9 | 6.3 ± 1.4 | 7.2 ± 1.2 | 0.02 |
| Case status | 0.49 | ||||
| - Elective | 68 (78%) | 13 (68%) | 37 (77%) | 18 (90%) | |
| - Urgent | 14 (16%) | 5 (26%) | 8 (17%) | 1 (5%) | |
| - Emergent | 5 (6%) | 1 (5%) | 3 (6%) | 1 (5%) | |
| Concomitant procedures | 29 (33%) | 1 (5%) | 16 (33%) | 12 (60%) | 0.001 |
| - Coronary artery bypass grafting | 12 (14%) | 1 (5%) | 7 (15%) | 4 (20%) | |
| - AVR | 5 (6%) | 2 (4%) | 3 (15%) | ||
| - Aortic valve repair | 3 (3%) | 1 (2%) | 2 (10%) | ||
| - Sinus of valsalva aneurysm repair | 1 (1%) | 1 (5%) | |||
| - Root replacement | 2 (2%) | 2 (10%) | |||
| - Valve sparing root replacement | 3 (3%) | 1 (2%) | 2 (10%) | ||
| - Ascending aorta replacement | 1 (1%) | 1 (2%) | |||
| - Ascending + hemi-arch | 5 (6%) | 5 (10%) | |||
| - AVR + ascending + hemi-arch | 1 (1%) | 1 (2%) | |||
| - Root + hemi-Arch | 1 (1%) | 1 (2%) | |||
| - Hybrid TAAA | 3 (3%) | 1 (2%) | 2 (10%) | ||
| Open procedure | |||||
| - Sternotomy | 68 (78%) | 1 (5%) | 47 (98%) | 20 (100%) | <0.0001 |
| - Redo sternotomy | 25 (29%) | 0 | 17 (35%) | 9 (40%) | 0.007 |
| -Use of CPB | 39 (45%) | 1 (5%) | 18 (38%) | 20 (100%) | <0.0001 |
| - Cross-clamp time (min) | 120 ± 58 | 46 | 87 ± 53 | 151 ± 45 | 0.003 |
| - CPB time (min) | 214 ± 65 | 122 | 178 ± 69 | 252 ± 29 | 0.0001 |
| -Use of DHCA | 27 (31%) | 0 | 7 (15%) | 20 (100%) | <0.0001 |
| - Cerebral DHCA time (min) | 7.3 ± 5.2 | n/a | 4.9 ± 5.0 | 8.3 ± 5.1 | 0.07 |
| - Antegrade CP time (min) | 21.6 ± 7.5 | n/a | 13.7 ± 4.2 | 24.5 ± 6.3 | 0.0009 |
| - Systemic DHCA time (min) | 49.0 ± 23.3 | n/a | 18.6 ± 6.5 | 60.2 ± 15.7 | 0.0002 |
| Endovascular procedure | |||||
| - Staged procedure | 29 (33%) | 0 | 9 (19%) | 20 (100%) | <0.0001 |
| - Staged same hospitalization | 12 (41%) | n/a | 4 (44%) | 8 (40%) | 0.82 |
| - Duration between stages (days) | 68 [8, 151] | n/a | 63 [9, 154] | 72 [8, 143] | 0.69 |
| - Number of stents implanted | 2.1 ± 1.0 | 1.6 ± 0.7 | 2.1 ± 1.0 | 2.6 ± 1.1 | 0.01 |
| - Antegrade stent deployment | 32 (37%) | 0 | 32 (67%) | 0 | <0.0001 |
| - Native aorta proximal landing zone | 46 (53%) | 19 (100%) | 27 (56%) | 0 | <0.0001 |
| - Native zone 0 proximal landing zone | 27 (31%) | 0 | 27 (56%) | 0 | <0.0001 |
| - Native ascending aorta ≥ 4.0 cm | 12 (14%) | 3 (16%) | 9 (19%) | 0 | 0.12 |
| - Native aorta distal landing zone | 79 (91%) | 18 (95%) | 45 (94%) | 16 (80%) | 0.16 |
| Proximal device | 0.57 | ||||
| -TAG / C-TAG | 58 (67%) | 11 (58%) | 33 (69%) | 14 (70%) | |
| - Talent | 10 (11%) | 2 (11%) | 7 (15%) | 1 (5%) | |
| - TX2 | 19 (22%) | 6 (32%) | 8 (17%) | 5 (25%) | |
| - Exposed proximal springs or barbs | 29 (33%) | 8 (42%) | 15 (31%) | 6 (30%) | 0.65 |
Values expressed as mean ± standard deviation, median [interquartile range], or number (percent). AVR = aortic valve replacement. CP = cerebral perfusion. n/a = not applicable. TAAA = thoracoabdominal aneurysm repair.
Table 3.
Arch vessel bypass grafts
| Bypass grafts | Total (N=87) |
Zone 1 (n=19) |
Zone 0 (n=48) |
Total Arch + SET (n=20) |
|---|---|---|---|---|
| 4 vessel head graft (Aorta – RSCA, RCCA, LCCA, LSCA) |
1 (1%) | 1 (2%) | ||
| Classic trifurcated head graft (Aorta – IA, LCCA, LSCA) |
15 (17%) | 1 (2%) | 14 (70%) | |
| Modified trifurcated head graft (Aorta – R axillary artery, RCCA, LCCA |
1 (1%) | 1 (2%) | ||
| Modified trifurcated head graft (Aorta – RCCA, LCCA, LSCA) |
2 (2%) | 2 (4%) | ||
| Classic bifurcated head graft (Aorta – IA, LCCA) |
44 (51%) | 39 (81%) | 5 (25%) | |
| Modified bifurcated head graft (Aorta – IA, LSCA) |
1 (2%) | 1 (2%) | ||
| Aorta – IA | 2 (2%) | 2 (4%) | ||
| Aorta – LCCA | 1 (5%) | 1 (5%) | ||
| RCCA – LCCA | 20 (23%) | 17 (89%) | 3 (6%) | |
| RSCA – LCCA | 1 (1%) | 1 (5%) | ||
| LCCA – LSCA* | 29 (33%) | 10 (53%) | 18 (38%) | 1 (5%) |
| LCCA – L axillary artery | 1 (1%) | 1 (5%) | ||
| RSCA – LSCA | 1 (1%) | 1 (5%) | ||
| RSCA – RCCA | 1 (1%) | 1 (2%) | ||
| RCCA – R axillary artery | 1 (1%) | 1 (2%) | ||
| L vertebral – LSCA transposition | 1 (1%) | 1 (2%) | ||
| L vertebral – LCCA transposition | 1 (1%) | 1 (2%) | ||
| Total number of grafts | 123 | 29 | 72 | 22 |
| Total vessels bypassed | 207 | 29 | 123 | 55 |
Data expressed as number (percent).
Includes five patients who underwent delayed LCCA-LSCA bypass for left upper extremity ischemia (n=3), vertebrobasilar insufficiency (n=1), or left upper extremity dialysis access placement (n=1). IA = innominate artery. L = left. R = right.
Table 4.
30-day/in-hospital outcomes
| Complication | Total (N=87) |
Zone 1 (n=19) |
Zone 0 (n=48) |
Total Arch + SET (n=20) |
P Value |
|---|---|---|---|---|---|
| In-hospital death | 5 (5.7%) | 1 (5.3%) | 4 (8.3%) | 0 | 0.40 |
| 30-day/in-hospital death | 13 (14.9%) | 1 (5.3%) | 10 (20.8%) | 2 (10%) | 0.21 |
| Stroke | 4 (4.6%) | 0 | 2 (4.2%) | 2 (10%) | 0.32 |
| (neurologic deficit lasting > 72 hours) | |||||
| Permanent paraplegia/paraparesis | 1 (1.2%) | 1 (5.3%) | 0 | 0 | 0.16 |
| Retrograde type A dissection | 3 (3.4%) | 0 | 3 (6.3%) | 0 | 0.28 |
| Myocardial infarction | 1 (1.2%) | 1 (5.3%) | 0 | 0 | 0.16 |
| Acute renal failure | 6 (6.9%) | 0 | 3 (6.3%) | 3 (15%) | 0.18 |
| (Cr > 2.0 and > 2x baseline) | |||||
| New onset dialysis | 3 (3.5%) | 0 | 2 (4.2%) | 1 (5%) | 0.64 |
| Tracheostomy | 6 (6.9%) | 0 | 4 (8.3%) | 2 (10%) | 0.39 |
| Length of stay (days) | 8 [5, 16] | 4 [3, 7] | 7 [5, 15] | 19 [12, 30] | <0.0001 |
| Primary technical success | 84 (97%) | 18 (95%) | 46 (96%) | 20 (100%) | 0.61 |
Includes cumulative morbidity for stage 1 and stage 2 procedures for staged patients. Values expressed as median [interquartile range] or number (percent). Cr = creatinine.
Zone 1 Hybrid Arch Repair
Zone 1 hybrid arch repair was performed in 19 patients with an average age of 67 years. The expected in-hospital mortality rate was 17.8% as calculated by the EuroSCORE II.24 Six (32%) patients underwent non-elective operation due to symptomatic aneurysm (n=4) or complicated acute type B dissection (n=2). One patient required median sternotomy and CPB to allow for concomitant coronary artery bypass grafting, and this patient underwent aorta-LCCA bypass for LCCA revascularization. A second patient underwent LCCA revascularization via right subclavian artery (RSCA)-LCCA bypass due to an enlarged right-sided thyroid goiter which precluded right common carotid artery (RCCA) exposure. The LSCA was revascularized in 53% (n=10) of patients for previously described indications.30
One (5.3%) patient experienced delayed onset permanent paraplegia on postoperative day 1 following urgent repair of a complicated acute type B dissection involving distal endograft coverage to the celiac axis. In this patient, the LSCA was revascularized at the time of operation due to a patent left internal mammary artery coronary bypass graft. One (5.3%) patient died intraoperatively due to myocardial infarction following an endograft induced inferior vena cava injury. The primary technical success rate was 95% (18 of 19) as a result of the intraoperative death.
Zone 0 Hybrid Arch Repair
Zone 0 hybrid arch repair was performed in 48 patients with an average age of 65 years. The expected in-hospital mortality rate was 17.3% as calculated by the EuroSCORE II.24 Twenty-three percent (n=11) of patients underwent non-elective operation due to contained ruptured aneurysm (n=2), symptomatic aneurysm (n=2), coronary artery disease with recent myocardial infarction and concomitant arch aneurysm (n=2), mycotic aneurysm with aorta-innominate vein fistula (n=1), aberrant RSCA aneurysm with vascular-esophageal fistula (n=1), acute type A dissection (n=1), or type IA endoleak following zone 2 TEVAR.
98% (n=47) of patients required median sternotomy for arch debranching. Arch debranching was completed without sternotomy in one patient who had previously undergone type A dissection repair at another institution including ascending aorta-RSCA bypass graft for concomitant innominate artery aneurysm. In this patient, arch vessel revascularization was accomplished via RSCA-RCCA-LCCA bypass.
CPB was utilized in 38% (n=18) of patients. Sixteen patients required CPB for concomitant cardiac operations, and one required CPB for repair of a pulmonary artery injury in the redo-sternotomy setting. The remaining patient was placed on CPB for cooling given persistent EEG ischemic changes with innominate artery test clamping. The EEG changes with test clamping resolved after cooling to 27 degrees Celsius, at which time innominate artery debranching was performed. Despite these maneuvers, the patient experienced an ischemic right-hemispheric stroke. DHCA was required in 15% (n=7) of patients to accommodate hemi-arch replacement. Nineteen percent (n=9) of patients underwent staged open/endovascular repair, of whom 44% (n=4) underwent completion of both stages during a single hospitalization.
56% (n=27) of patients underwent arch debranching and zone 0 endograft placement with arch debranching inflow and proximal landing zone in native ascending aorta, whereas the remaining 44% (n=21) underwent arch debranching and zone 0 endograft placement using artificial Dacron ascending aorta due to prior ascending aorta replacement (n=13) or concomitant ascending aorta replacement at the time of arch debranching (n=8). The LSCA was revascularized in 46% (n=22) of patients as part of the native zone 0 arch debranching procedure or for previously described indications.30
4.2% percent (n=2) of zone 0 patients experienced stroke. The first was the aforementioned patient with EEG changes during innominate artery test clamping. The second patient experienced a thromboembolic stroke on postoperative day 7 after uneventful hospital discharge.
Retrograde type A dissection occurred in three zone 0 patients for an overall rate of 6.3%, but a true rate of 11.1% (3 of 27) when including only patients with native ascending aorta who were at risk for this complication. The first patient died intraoperatively from right ventricular failure following emergent open repair. The second patient died suddenly on postoperative day 3 and was diagnosed with retrograde type A dissection at autopsy. The third patient underwent successful open repair following intraoperative identification of the dissection by TEE following completion of the TEVAR procedure.
8.3% (n=4) of patients died prior to hospital discharge. The causes of death were retrograde type A dissection (n=2), atheroembolic syndrome (n=1) and sepsis (n=1). Six additional patients died after hospital discharge but within 30 days of surgery, yielding a 30-day/in-hospital mortality rate of 20.8%. The causes of death were known for three of the six patients who died out-of-hospital and included respiratory failure after long-term care facility transfer on mechanical ventilation (n=2) and upper gastrointestinal bleeding (n=1). The remaining three patients died of unknown causes 6, 7, and 14 days, respectively, after uneventful discharge to home. Thirty-day/in-hospital mortality was 29.6% (8 of 27) for patients with native zone 0 proximal landing zone, compared to 9.5% (2 of 21) for patients with Dacron zone 0 proximal landing zone (P=0.15). The primary technical success rate was 96% (46 of 48) as a result of two patient deaths within 24 hours of operation (retrograde type A dissection and atheroembolic syndrome).
Total Arch Replacement with Staged Stented Elephant Trunk Completion
Total arch replacement with staged stented elephant trunk completion was performed in 20 patients with an average age of 59 years. The expected in-hospital mortality rate was 12.5% as calculated by the EuroSCORE II.24 10% (n=2) of patients underwent non-elective operation due to symptomatic aneurysm (n=1), or contained ruptured descending thoracic aortic aneurysm following stage 1 repair (n=1).
All patients required median sternotomy, CPB, and DHCA for total arch replacement. 95% (n=19) of patients underwent total arch replacement via individual aortic arch vessel reimplantation (modified Mt. Sinai technique) and one (5%) patient underwent total arch replacement at an outside hospital via the island technique. All patients underwent staged open/endovascular repair, of whom 40% (n=8) underwent completion of both stages during a single hospitalization. The LSCA was revascularized in 90% (n=18) of patients.
All 20 patients survived the stage 1 procedure and underwent stage 2 endovascular completion. Including both stages, two (10%) patients experienced stroke. The first was an embolic perioperative stroke during total arch replacement. The second patient experienced severe antibiotic-induced hemolytic anemia following readmission within 30 days for pneumonia after uneventful stage 2 endovascular completion. He suffered a cardiac arrest due to profound acute anemia resulting in anoxic brain injury and death.
All patients survived to hospital discharge following the stage 2 procedure. However, two (10%) patients died after hospital discharge but within 30-days of operation, yielding a 30-day/in-hospital mortality rate of 10%. The causes of death were the aforementioned case of hemolytic anemia (n=1) and pulmonary embolism (n=1).
Univariate Predictors of 30-Day/In-Hospital Mortality
All variables contained within Tables 1 and 2 were tested for univariate association with 30-day/in-hospital mortality. Only native zone 0 proximal landing zone was associated with 30-day/in-hospital mortality when compared to all other hybrid arch cases (odds ratio, 4.63; 95% confidence interval, 1.35-15.89; P=0.02). All other variables, including age (P=0.61), age ≥ 75 (P=0.40), EuroSCORE II (P=0.18), principal procedure type (P= 0.21), dissection indication (P=0.24), preoperative cardiac catheterization (P=0.87), and non-elective operation (P=0.41), were not associated with 30-day/in-hospital mortality on univariate analysis.
LONG-TERM FOLLOW-UP
Reinterventions and Graft Patency
Reinterventions and graft patency during follow-up are shown in Table 5. Over a mean follow-up of 28.5 ± 22.2 months, 13% (n=11) of patients required reintervention for endoleak. One (1%) additional zone 1 hybrid arch patient underwent carotid-carotid thrombectomy for partial thrombosis of the bypass graft noted on surveillance imaging, and five (6%) patients required reintervention for new aortic pathology. Three of the 207 (1%) bypassed arch vessels were found to be occluded during follow-up. Two involved the left carotid limbs of bifurcated or trifurcated head grafts, and one involved an occluded carotid-carotid bypass. All three occluded grafts were identified incidentally on imaging and were clinically silent.
Table 5.
Reinterventions and graft patency
| Variable | Total (N=87) |
Zone 1 (n=19) |
Zone 0 (n=48) |
Total Arch + SET (n=20) |
P Value |
|---|---|---|---|---|---|
| Duration of follow-up (mos) | 28.5 ± 22.2 | 33.7 ± 23.0 | 28.4 ± 21.5 | 23.4 ± 23.3 | 0.33 |
| Reintervention for endoleak | 11 (13%) | 2 (11%) | 8 (17%) | 1 (5%) | 0.40 |
| - TypeIA | 4 (5%) | 0 | 4 (8%) | 0 | 0.18 |
| - Type IB | 0 | 0 | 0 | 0 | 1 |
| - Type II | 6 (7%) | 2 (11%) | 4 (8%) | 0 | 0.36 |
| - Type III | 1 (1%) | 0 | 0 | 1 (5%) | 0.18 |
| Arch vessel bypass graft revision | 1 (1%) | 1 (5%) | 0 | 0 | 0.16 |
| Patency of bypassed arch vessels | 204/207 (99%) |
28/29 (97%) |
122/123 (99%) |
54/55 (98%) |
1 |
| Aortic reintervention for new disease | 5 (6%) | 0 | 3 (6%) | 2 (10%) | 0.40 |
Values expressed as mean ± standard deviation or number (percent).
Survival
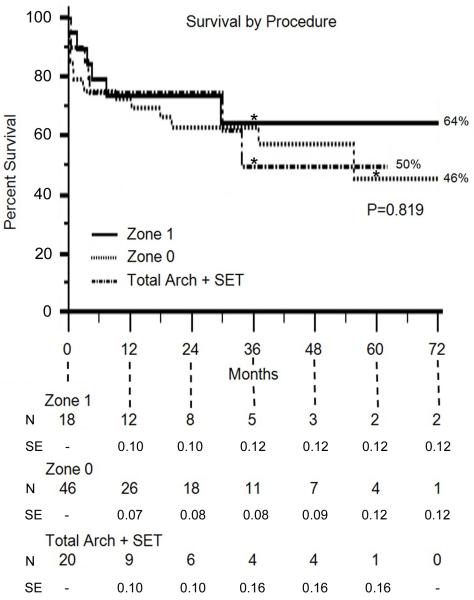
Kaplan-Meier estimates of survival are shown in Figure 6. Overall survival for the entire hybrid arch cohort at 1, 3, and 5 years was 73%, 60%, and 51%, respectively, and was equivalent between procedures (P=0.819)
Figure 6.
Kaplan-Meier estimates of survival by procedure type. N = number at risk. SE = standard error. *denotes the time point at which standard error exceeds 10%.
DISCUSSION
This report presents our algorithmic approach to the use of TEVAR for aortic arch repair in high-risk patients by way of three complimentary procedures. The overall rates of stroke (4.6%), paraplegia (1.2%), in-hospital mortality (5.7%), and 30-day mortality (14.9%) appear favorable and suggest that hybrid procedures are important in the armamentarium of treatment options for arch pathology in select patients. However, the data reveal some important limitations to the application of hybrid arch operations that warrant discussion.
Perioperative mortality following hybrid arch repair is frequently reported as in-hospital mortality,10-12, 20 as opposed to the conventional 30-day/in-hospital mortality definition used in cardiovascular surgery. In the current study, eight patients died after hospital discharge but within 30-days of surgery, yielding a clinically significant difference between in-hospital (5.7%) and 30-day/in-hospital (14.9%) mortality. This finding confuses the comparison of operative mortality between reports and suggests studies which only report in-hospital mortality may significantly underestimate 30-day/in-hospital mortality. Nonetheless, the procedure-specific 30-day/in-hospital mortality rates in this study are comparable to prior reports of zone 1 hybrid arch repair (0%-15.6%),11, 12, 17, 18 zone 0 hybrid arch repair (8.5%-29.6%),9-12, 14, 17, 20 stented elephant trunk completion (4.5%-11%),1, 2, 6, 7, 13 and total arch replacement with stented elephant trunk completion (17%).7
The observation of eight (9.2%) patient deaths shortly after hospital discharge is concerning and requires scrutiny. The causes of death for five of the patients are known, and appear to be related to comorbid conditions or unpredictable and highly unfortunate circumstances. The causes of death for the remaining three patients are unknown. However, two of these patients underwent zone 0 hybrid arch repair with native ascending aorta landing zone. Given the high incidence of retrograde type A dissection in this group (11.1%) we speculate that early unexplained death following native zone 0 repair may be due to unrecognized or spontaneously occurring retrograde type A dissection in some patients.
Despite acceptable procedural mortality, we posit that patients surviving less than 1-year after surgery are unlikely to derive a survival benefit from the procedure, and hence that 1-year survival rates are a more relevant metric of success than procedural mortality.32 One-year survival in the current study was 73% and was equivalent between procedures (Zone 1, 73%; Zone 0, 72%; stented elephant trunk, 74%). These 1-year survival rates following hybrid arch repair are similar to other reports (65%-84.2%),2,6,10,11,14,15,17-20 but are somewhat lower than the 81%-82% 1-year survival experienced by all TEVAR patients in the Duke database or other large TEVAR registries.32-34 Correspondingly, Desai and colleagues found patients undergoing hybrid arch procedures to be at greater risk of 30-day mortality (odds ratio, 2.7) and late mortality (odds ratio, 2.1) when compared to patients undergoing other TEVAR procedures.33 These data likely reflect the increased operative complexity of hybrid arch operations as well as the preferential use of hybrid arch repair in high-risk patients who are deemed unfit for conventional open surgery. Regardless, patient selection for hybrid arch repair may require further refinement given that approximately a quarter of patients may not experience a survival benefit from the procedure using current selection criteria.
Retrograde type A dissection appears to be a frequent and often lethal complication of hybrid arch repair. Although retrograde type A dissection may occur following any TEVAR procedure, the incidence appears especially high following endograft placement in the ascending aorta. In the present series, three patients experienced retrograde type A dissection, for an overall incidence of 3.4%. However, given that patients with artificial Dacron ascending aorta are not at risk for this complication, the rate of retrograde type A dissection in patients with native ascending aorta was 6.5% (3 of 46). In patients with native ascending aorta who underwent zone 0 endograft placement the rate was 11.1% (3 of 27). These data appear similar to a recent report by Czerny and colleagues of 66 zone 0 hybrid arch repairs performed at five institutions.20 Of the 66 patients, two experienced early retrograde type A dissection and three experienced late retrograde type A dissection, for an overall rate of 7.6%. However, the report includes five patients with previous type A dissection repair and 15 patients who underwent ascending aorta replacement at the time of arch debranching. Thus, the rate of retrograde type A dissection in patients with native ascending aorta was likely at least as high as 10.8% (5 of 46), which is nearly identical to our study.
The causes of retrograde type A dissection in zone 0 patients suggested by Czerny and colleagues include clamp injury to the ascending aorta during performance of the proximal debranching anastomoses, compliance mismatch between the rigid endograft and the ascending aorta, or alterations in blood flow caused by the debranching graft itself.20 In addition, our group previously found retrograde type A dissection after TEVAR was more likely to occur in patients treated for a dissection indication, in patients with an ascending aorta ≥ 4 cm in diameter, and with the use of devices with exposed proximal barbs or springs.29 As a result of this prior publication and the present findings, we now aim to replace the ascending aorta whenever feasible in patients undergoing zone 0 hybrid arch repair who harbor these risk factors for retrograde type A dissection.
The high incidence of retrograde type A dissection appears to represent a major technical limitation of the zone 0 hybrid arch operation. Total endovascular aortic arch repair through the use of branched endografts or chimney grafts may lead to improved results and ultimately replace the zone 0 hybrid arch operation if the incidence of retrograde type A dissection is reduced.35,36 Alternatively, retrograde type A dissection may occur uniformly whenever endografts are placed within the ascending aorta despite the avoidance of clamping and arch debranching. In this case, conventional open arch repair, or prophylactic ascending aorta replacement prior to endograft placement, may prove superior to all native ascending aorta-based endovascular repair options in patients who can tolerate the open procedures. Additional work is needed to investigate this possibility.
Limitations
The present report describes a large cohort of patients undergoing hybrid arch repair and represents the largest single-institution series of zone 0 hybrid arch repairs in the literature. However, the study remains limited by the constraints of sample size, which limits the comparison of findings between procedures and precludes the use of multivariable statistics for risk factor analysis. The operations were performed by two principal co-surgeons using standardized techniques, and therefore results may not be generalizable to other practitioners treating different patient populations in different arenas.
Conclusion
Patients with transverse arch aneurysms selected for hybrid arch repair represent a high-risk patient cohort, as evidenced by an expected in-hospital mortality rate of 16.3% as calculated by the EuroSCORE II. Despite this limitation, acceptable outcomes are possible when operations are tailored to patient anatomy and comorbid status. However, 30-day and 1-year mortality remain considerable, and we suspect patient selection may benefit from further refinement. Lastly, the native ascending aorta appears to be a hostile location for endograft placement and is associated with high rates of retrograde type A dissection and 30-day/in-hospital mortality. As a result, we recommend caution with native zone 0 stent graft placement and now aim to replace the ascending aorta when feasible in scenarios previously shown to be at high risk for retrograde type A dissection.
ACKNOWLEDGEMENTS
Support was received by a Thoracic Surgery Foundation for Research and Education Research Fellowship to Dr. Andersen and National Institutes of Health grants T32-HL069749 and U01-HL088953 to Dr. Williams. The authors thank Stan Coffman for providing illustrations.
Footnotes
DISCLOSURES
G. Chad Hughes is a consultant for W.L. Gore and Associates and Vascutek; a member of the speaker’s bureau for Vascutek; and received unrestricted research grants from W.L. Gore and Associates and Vascutek.
REFERENCES
- 1.Carroccio A, Spielvogel D, Ellozy SH, Lookstein RA, Chin IY, Minor ME, et al. Aortic arch and descending thoracic aortic aneurysms: experience with stent grafting for second-stage “elephant trunk” repair. Vascular. 2005;13(1):5–10. doi: 10.1258/rsmvasc.13.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg RK, Haddad F, Svensson L, O’Neill S, Walker E, Lyden SP, et al. Hybrid approaches to thoracic aortic aneurysms: the role of endovascular elephant trunk completion. Circulation. 2005;112(17):2619–26. doi: 10.1161/CIRCULATIONAHA.105.552398. [DOI] [PubMed] [Google Scholar]
- 3.Hughes GC, Sulzer CF, McCann RL, Swaminathan M. Endovascular approaches to complex thoracic aortic disease. Semin Cardiothorac Vasc Anesth. 2008;12(4):298–319. doi: 10.1177/1089253208328667. [DOI] [PubMed] [Google Scholar]
- 4.Hughes GC, Nienaber JJ, Bush EL, Daneshmand MA, McCann RL. Use of custom Dacron branch grafts for “hybrid” aortic debranching during endovascular repair of thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2008;136(1):21–8. 8 e1–6. doi: 10.1016/j.jtcvs.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Hughes GC, Daneshmand MA, Balsara KR, Achneck HA, Sileshi B, Lee SM, et al. “Hybrid” repair of aneurysms of the transverse aortic arch: midterm results. Ann Thorac Surg. 2009;88(6):1882–7. doi: 10.1016/j.athoracsur.2009.07.027. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 6.Kawaharada N, Kurimoto Y, Ito T, Koyanagi T, Yamauchi A, Nakamura M, et al. Hybrid treatment for aortic arch and proximal descending thoracic aneurysm: experience with stent grafting for second-stage elephant trunk repair. Eur J Cardiothorac Surg. 2009;36(6):956–61. doi: 10.1016/j.ejcts.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Kim T, Martin TD, Lee WA, Hess PJ, Jr., Klodell CT, Tribble CG, et al. Evolution in the management of the total thoracic aorta. J Thorac Cardiovasc Surg. 2009;137(3):627–34. doi: 10.1016/j.jtcvs.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Koullias GJ, Wheatley GH., 3rd. State-of-the-art of hybrid procedures for the aortic arch: a meta-analysis. Ann Thorac Surg. 2010;90(2):689–97. doi: 10.1016/j.athoracsur.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Bavaria J, Milewski RK, Baker J, Moeller P, Szeto W, Pochettino A. Classic hybrid evolving approach to distal arch aneurysms: toward the zone zero solution. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S77–80. doi: 10.1016/j.jtcvs.2010.07.031. discussion S6-91. [DOI] [PubMed] [Google Scholar]
- 10.Milewski RK, Szeto WY, Pochettino A, Moser GW, Moeller P, Bavaria JE. Have hybrid procedures replaced open aortic arch reconstruction in high-risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2010;140(3):590–7. doi: 10.1016/j.jtcvs.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 11.Geisbusch P, Kotelis D, Muller-Eschner M, Hyhlik-Durr A, Bockler D. Complications after aortic arch hybrid repair. J Vasc Surg. 2011;53(4):935–41. doi: 10.1016/j.jvs.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 12.Kotelis D, Geisbusch P, Attigah N, Hinz U, Hyhlik-Durr A, Bockler D. Total vs hemi-aortic arch transposition for hybrid aortic arch repair. J Vasc Surg. 2011;54(4):1182–6 e2. doi: 10.1016/j.jvs.2011.02.069. [DOI] [PubMed] [Google Scholar]
- 13.Jim J, Moon MR, Rubin BG, Sicard GA, Sanchez LA. Hybrid repair of distal arch aortic aneurysms: endovascular elephant trunk completion. Ann Vasc Surg. 2011;25(5):598–604. doi: 10.1016/j.avsg.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Lee CW, Beaver TM, Klodell CT, Jr., Hess PJ, Jr., Martin TD, Feezor RJ, et al. Arch debranching versus elephant trunk procedures for hybrid repair of thoracic aortic pathologies. Ann Thorac Surg. 2011;91(2):465–71. doi: 10.1016/j.athoracsur.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ham SW, Chong T, Moos J, Rowe VL, Cohen RG, Cunningham MJ, et al. Arch and visceral/renal debranching combined with endovascular repair for thoracic and thoracoabdominal aortic aneurysms. J Vasc Surg. 2011;54(1):30–40. doi: 10.1016/j.jvs.2010.12.033. discussion-1. [DOI] [PubMed] [Google Scholar]
- 16.Murashita T, Matsuda H, Domae K, Iba Y, Tanaka H, Sasaki H, et al. Less invasive surgical treatment for aortic arch aneurysms in high-risk patients: a comparative study of hybrid thoracic endovascular aortic repair and conventional total arch replacement. J Thorac Cardiovasc Surg. 2012;143(5):1007–13. doi: 10.1016/j.jtcvs.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Vallejo N, Rodriguez-Lopez JA, Heidari P, Wheatley G, Caparrelli D, Ramaiah V, et al. Hybrid repair of thoracic aortic lesions for zone 0 and 1 in high-risk patients. J Vasc Surg. 2012;55(2):318–25. doi: 10.1016/j.jvs.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Canaud L, Joyeux F, Ziza V, Branchereau P, Marty-Ane C, Alric P. Hemi-aortic arch debranching for hybrid aortic arch repair by sequential transposition of the left common carotid and subclavian arteries. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Lotfi S, Clough RE, Ali T, Salter R, Young CP, Bell R, et al. Hybrid Repair of Complex Thoracic Aortic Arch Pathology: Long-Term Outcomes of Extra-anatomic Bypass Grafting of the Supra-aortic Trunk. Cardiovasc Intervent Radiol. 2012 doi: 10.1007/s00270-012-0383-3. [DOI] [PubMed] [Google Scholar]
- 20.Czerny M, Weigang E, Sodeck G, Schmidli J, Antona C, Gelpi G, et al. Targeting Landing Zone 0 by Total Arch Rerouting and TEVAR: Midterm Results of a Transcontinental Registry. Ann Thorac Surg. 2012 doi: 10.1016/j.athoracsur.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Lima B, Williams JB, Bhattacharya SD, Shah AA, Andersen N, Gaca JG, et al. Results of proximal arch replacement using deep hypothermia for circulatory arrest: is moderate hypothermia really justifiable? Am Surg. 2011;77(11):1438–44. [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen ND, Williams JB, Fosbol EL, Shah AA, Bhattacharya SD, Mehta RH, et al. Cardiac catheterization within 1 to 3 days of proximal aortic surgery is not associated with increased postoperative acute kidney injury. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishimaru S. Endografting of the aortic arch. J Endovasc Ther. 2004;11(Suppl 2):II62–71. doi: 10.1177/15266028040110S614. [DOI] [PubMed] [Google Scholar]
- 24.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–44. doi: 10.1093/ejcts/ezs043. discussion 44-5. [DOI] [PubMed] [Google Scholar]
- 25.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (TEVAR) J Vasc Surg. 2010;52(4):1022–33. 33–e15. doi: 10.1016/j.jvs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr., Faxon DP, Freed MD, et al. Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;2008;118(15):e523–661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 27.Husain AM, Swaminathan M, McCann RL, Hughes GC. Neurophysiologic intraoperative monitoring during endovascular stent graft repair of the descending thoracic aorta. J Clin Neurophysiol. 2007;24(4):328–35. doi: 10.1097/WNP.0b013e31811ebf6e. [DOI] [PubMed] [Google Scholar]
- 28.Parsa CJ, Schroder JN, Daneshmand MA, McCann RL, Hughes GC. Midterm results for endovascular repair of complicated acute and chronic type B aortic dissection. Ann Thorac Surg. 2010;89(1):97–102. doi: 10.1016/j.athoracsur.2009.09.029. discussion-4. [DOI] [PubMed] [Google Scholar]
- 29.Williams JB, Andersen ND, Bhattacharya SD, Scheer E, Piccini JP, McCann RL, et al. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J Vasc Surg. 2012;55(5):1255–62. doi: 10.1016/j.jvs.2011.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TC, Andersen ND, Williams JB, Bhattacharya SD, McCann RL, Hughes GC. Results with a selective revascularization strategy for left subclavian artery coverage during thoracic endovascular aortic repair. Ann Thorac Surg. 2011;92(1):97–102. doi: 10.1016/j.athoracsur.2011.03.089. discussion-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes GC, Barfield ME, Shah AA, Williams JB, Kuchibhatla M, Hanna JM, et al. Staged total abdominal debranching and thoracic endovascular aortic repair for thoracoabdominal aneurysm. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2011.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah AA, Craig DM, Andersen ND, Williams JB, Bhattacharya SD, Shah SH, et al. Risk factors for 1-year mortality after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Desai ND, Pochettino A, Szeto WY, Moser GW, Moeller PJ, Sodhi N, et al. Thoracic endovascular aortic repair: evolution of therapy, patterns of use, and results in a 10-year experience. J Thorac Cardiovasc Surg. 2011;142(3):587–94. doi: 10.1016/j.jtcvs.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 34.Goodney PP, Travis L, Lucas FL, Fillinger MF, Goodman DC, Cronenwett JL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation. 2011;124(24):2661–9. doi: 10.1161/CIRCULATIONAHA.111.033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gehringhoff B, Torsello G, Pitoulias GA, Austermann M, Donas KP. Use of chimney grafts in aortic arch pathologies involving the supra-aortic branches. J Endovasc Ther. 2011;18(5):650–5. doi: 10.1583/11-3504.1. [DOI] [PubMed] [Google Scholar]
- 36.Chuter TA, Schneider DB. Endovascular repair of the aortic arch. Perspect Vasc Surg Endovasc Ther. 2007;19(2):188–92. doi: 10.1177/1531003507304165. [DOI] [PubMed] [Google Scholar]