Abstract
We have previously demonstrated that (+)-morphine and (−)-morphine pretreated spinally for 45 min stereoselectively attenuates the tail-flick inhibition produced by (−)-morphine given spinally in the mouse. The present study is then undertaken to determine if the same phenomenon observed in the mouse spinal cord can also take place in the ventral periaqueductal gray of the rat. Pretreatment with (+)-morphine for 45 min at 0.3 to 3.3 fmol dose-dependently attenuated the tail-flick inhibition produced by (−)-morphine (9 nmol) given into the ventral periaqueductal gray. Likewise, pretreatment with (−)-morphine for 45 min at a higher dose (3-900 pmol), which given alone did not affect the baseline tail-flick latency, also dose-dependently attenuated the tail-flick inhibition produced by (−)-morphine. Thus, (+)-morphine is approximately 270,000-fold more potent than (−)-morphine in attenuating the (−)-morphine-produced tail-flick inhibition. The attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine was dose-dependently reversed by (+)-naloxone (27.5 to 110 pmol) pretreatment for 50 min given into the ventral periaqueductal gray. Pretreatment with the sigma receptor antagonist BD1047 (N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide) (11-45 nmol) for 45 min given into the ventral periaqueductal gray also reversed dose-dependently the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine, indicating that the effects are mediated by the activation of the sigma receptors. Since (+)-morphine, (−)-morphine and (+)-naloxone do not have any affinity for the naloxone-inaccessible sigma receptors, we therefore propose that (+)-morphine and (−)-morphine attenuate the (−)-morphine-produced tail-flick inhibition via the activation of the naloxone-sensitive sigma receptor originally proposed by Tsao and Su (1997).
Keywords: sigma receptors, analgesia, opioid, morphine, periaqueductal gray, rat
1. Introduction
The naturally occurring morphine, which is isolated from the juice of the opium poppy, papaver somniferum, is stereochemically identified as a levorotatory form. (−)-Morphine produces analgesia and other major pharmacological effects, which are mainly mediated by the stimulation of μ-opioid receptors. The dextrorotatory enantiomer of morphine, (+)-morphine, which is synthesized from sinomenine (Iijima et al., 1978a), does not have any affinity for μ-opioid receptors and therefore does not produce any μ-opioid receptor-mediated pharmacological effects (Jacquet et al., 1977). However, we have previously demonstrated that (+)-morphine or (−)-morphine given spinally for the pretreatment stereoselectively attenuates the spinally administered (−)-morphine-produced tail-flick inhibition in the mouse. The phenomenon has been defined as antianalgesia. The antianalgesia induced by (+)-morphine or (−)-morphine is blocked by the non-opioid (+)-naloxone, indicating that the antianalgesic effect induced by (+)-morphine or (−)-morphine is mediated by the stimulation of a non-opioid receptor (Wu et al., 2004, 2005).
The ventral periaqueductal gray area of the mesencephalon is a primary site sensitive to opioid agonists for producing analgesia (Pavlovic and Bodnar, 1998; Smith et al., 1988; Yaksh et al., 1988). The analgesia produced by opioid agonists from the ventral periaqueductal gray is mediated by the activation of the spinopetal descending pain control pathways, which are initiated from the ventral periaqueductal gray via the rostral ventromedial medulla projecting to the spinal and trigeminal dorsal horns (Basbaum and Fields, 1984). The present study is then undertaken to determine if the antianalgesia induced by (+)-morphine or (−)-morphine against (−)-morphine-produced analgesia observed in the mouse spinal cord (Wu et al., 2004, 2005, 2006) can also take place in the ventral periaqueductal gray of the rat.
The type of the receptor to be stimulated by (+)-morphine and (−)-morphine for inducing antianalgesia against (−)-morphine-produced analgesia is not clear. Other dextrorotatory opiates such as (+)-pentazocine and (+)-N-ally-normetazocine stereoselectively interact with sigma receptors, which are distinct from classical opioid receptors. Sigma receptors have been reported to play an important role in the modulation of analgesia produced by μ-, δ- or κ-opioid receptor agonists (Mei and Pasternak, 2002; Marrazzo et al, 2006; Chien and Pasternak, 1993, 1994). Activation of sigma receptors by intracerebroventricular administration of sigma receptor agonist (+)-pentazocine or 1,3-di-o-tolylguanidine attenuates the (−)-morphine-produced analgesia. The attenuation of the (−)-morphine analgesia is reversed by sigma receptor antagonist haloperidol (Chien and Pasternak, 1994). The experiment was then undertaken to determine if the sigma receptors are involved in the (+)-morphine- and (−)-morphine-induced antianalgesia in the rat ventral periaqueductal gray. We now report that the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine pretreatment is reversed by the sigma receptor antagonist BD1047 and by (+)-naloxone. The finding provides the evidence that the antianalgesia induced by (+)-morphine or (−)-morphine against (−)-morphine-produced analgesia is mediated by the activation of the naloxone-sensitive sigma receptor in the ventral periaqueductal gray of the rat, originally proposed by Tsao and Su (1997).
2. Materials and Methods
2.1. Animals
Male CD rats (Charles River Laboratories Inc., Wilmington, MA) weighing between 300 to 350 g at the time of surgery were housed in pairs before and after surgery. They were maintained in a room at 22 ± 0.5°C with an alternating 12 h light/dark cycle. Food and water were available ad libitum. All experiments were approved by and conformed to the guidelines of the Animal Care Committee of the Medical College of Wisconsin.
2.2. Surgical procedures
Rats were pretreated with methylatropine bromide (5 mg/kg, i.p.), anesthetized with pentobarbital sodium (50 mg/kg, i.p.) and then mounted in a stereotaxic apparatus (David Kopf Instrument, Tujinga, CA). A 23-gauge stainless steel guide cannula 12 mm in length was then implanted unilaterally 3 mm down from the surface of the skull and anchored to the skull with three stainless screws and dental cement. The coordinate for placement of the cannula for ventral periaqueductal gray microinjection was AP 1.20 mm anterior to interaural point and 0.7 mm lateral to the midline (Paxinos and Watson, 1997). After a recovery period of at least 5 days, animals were used for the experiments.
2.3. Assessment of Analgesia
Analgesic responses were measured with the tail-flick test (D'Amour and Smith, 1941). To measure the latency of the tail-flick response, rats were gently held by hand and their tail positioned on the apparatus (Model TF6; EMDIE Instrument Co., Maidens, VA). The tail-flick response was elicited by applying radiant heat to the dorsal surface of the tail. The intensity of the heat stimulus was set to provide a pre-drug tail-flick response time of 3 to 4 s. The cutoff time was set at 10 s to minimize tissue damage. The tail-flick latencies were measured at 1) before pretreatment with (+)-morphine, (−)-morphine and BD1047; 2) 45 min after pretreatment i.e. right before (−)-morphine (9 nmol) challenge; and 3) different time points after (−)-morphine challenge.
2.4. Experimental Protocol
Groups of rats were pretreated with (+)-morphine, (−)-morphine, (+)-naloxone, N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide (BD1047), or vehicle given into ventral periaqueductal gray 45 min (Wu et al., 2004; 2005) before the injection of (−)-morphine sulfate (9 nmol) given into the same ventral periaqueductal gray site (Terashvili et al., 2005) and the tail-flick response was measured at different times thereafter. The following three experiments were performed; 1) determine the dose-response effects of (+)-morphine and (−)-morphine in the attenuation of the tail-flick inhibition produced by (−)-morphine, 2) determine the effects of (+)-naloxone on the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine and (−)-morphine pretreatment and 3) determine the effects of the sigma receptor antagonist BD1047 on the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine and (−)-morphine pretreatment.
2.5. Drugs and Drug Administration
(−)-Morphine sulfate, (+)-morphine (base) and (+)-naloxone HCl were obtained from National Institute on Drug Abuse (Baltimore, MD). N-[2-(3,4-Dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide (BD1047) was purchased from Tocris (Ellisville, MO). (−)-Morphine sulfate, (+)-naloxone HCl and BD1047 were dissolved in sterile saline solution (0.9% NaCl solution). (+)-Morphine was dissolved in10 N hydrochloric acid and then titrated with 1 N sodium hydroxide to pH 6-7, which then diluted to intended dose in 0.9 % saline. Microinjections into the ventral periaqueductal gray were made by hand with a 30-gauge injection needle attached to a microsyringe via polyethylene tubing. The injection needle was inserted directly into the guide cannula. Injection volume for each microinjection was 0.5 μl and the solution was administered over a 30 s period. The injection needle was left in place for an additional 60 s to ensure complete distribution. The stereotaxic coordinate of the ventral periaqueductal gray injection site was aimed at AP 1.20 mm anterior to interaural point, 0.7 mm lateral to the midline and 5.8 mm down from the surface of the skull.
2.6. Histological Identification of the Injection Site
At the end of the experiments, 0.5 μl of methylene blue solution (2%) was injected into the ventral periaqueductal gray. The rats were then sacrificed with CO2 (100%) 10 to 20 min after injection. The brains were removed, frozen, and sectioned sagittally for microscopic identification of the injection sites. The stereotaxic atlas of rats by Paxinos and Watson (1997) was used as a guide for the identification of anatomical injection sites. Only the data obtained from rats in which the injection sites were accurately identified to be in ventral periaqueductal gray were used for further statistical analysis.
2.7. Statistical Analysis
The analgesic responses, tail-flick latency (s), were presented as the mean ± S.E.M. One-way analysis of variance (ANOVA) followed by Dunnett's post-test or two-way ANOVA followed by Bonferroni post-test was used to test the difference between groups. The “percent of maximum possible effect (%MPE)” was used to calculate the ED50 values. The % MPE was calculated as [(T1-T0) / (T2-T0)] X 100. T0 and T1 were the tail-flick latencies where T0 represented the tail-flick latency before (−)-morphine (9 nmol) challenge and T1 represented the tail-flick latency 20 min after (−)-morphine (9 nmol). T2 was the cutoff time, which was set at 10 s. Nonlinear regression model was used to fit the dose-response curve and calculate the ED50 values and 95% confidence intervals for (+)-morphine and (−)-morphine induced antianalgesia. The GraphPad Prism software was used to perform the statistics (version 4.1; GraphPad Software, Inc., San Diego, CA).
3. Results
3.1. (+)-Morphine or (−)-morphine pretreatment attenuates the tail-flick inhibition produced by (−)-morphine in the rat ventral periaqueductal gray
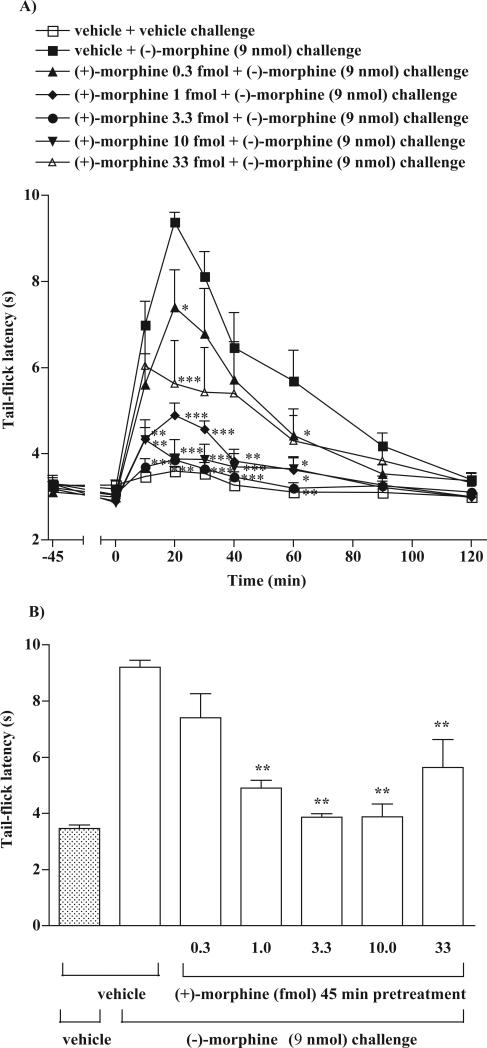
Groups of rats were microinjected with a various dose of (+)-morphine (0.3 to 10.0 fmol) or vehicle given into the ventral periaqueductal gray 45 min before microinjection of (−)-morphine (9 nmol) given into the ventral periaqueductal gray and the tail-flick response was measured at various times thereafter. (−)-Morphine (9 nmol) microinjected into the ventral periaqueductal gray produced the tail-flick inhibition; the tail-flick inhibition developed in 10 min, reached a maximal inhibition at 20 min and gradually returned to the pre-injection control level in 120 min in rats pretreated with vehicle for 45 min. Pretreatment with (+)-morphine at a dose 0.3 to 10.0 fmol dose-dependently attenuated the tail-flick inhibition produced by (−)-morphine. The (−)-morphine-produced tail-flick inhibition was almost completely abolished by the pretreatment with 3.3 or 10 fmol of (+)-morphine. However, a higher dose of (+)-morphine 33 fmol was found to be less effective in attenuating the (−)-morphine-produced tail-flick inhibition (Fig. 1A and 1B). Fig 1A also shows that pretreatment with these doses of (+)-morphine did not affect the baseline tail-flick latencies observed at 45 min after injection. The ED50 value for (+)-morphine given into the ventral periaqueductal gray for attenuation of the (−)-morphine-produced tail-flick inhibition was estimated to be 0.82 (95 % confidence intervals: 0.007 to 97.12) fmol.
Fig. 1.
Pretreatment with (+)-morphine attenuates the tail-flick inhibition produced by (−)-morphine in the rat ventral periaqueductal gray. Groups of rats were microinjected with different doses (0.3 to 10.0 fmol) of (+)-morphine or vehicle given into the ventral periaqueductal gray 45 min before microinjection of (−)-morphine (9 nmol) given into the ventral periaqueductal gray and the tail-flick response was measured at different time thereafter. (A) Two-way ANOVA followed by Bonferroni's post-test was used to test the difference between groups. Vehicle pretreatment group followed by vehicle challenge group and the points of −45 min were not included in the statistical test. F interaction, treatment, time = 14.23, 25.77, 27.40; * p < 0.01, ** p < 0.001 when compared with vehicle pretreatment followed by morphine challenge group. (B) The bar graph represented the tail-flick inhibition 20 min after (−)-morphine (9 nmol) challenge. One-way ANOVA followed by Dunnett's post-test was used to test the difference between groups; the F (5,40) = 30.9; The first column from the left represented control group, which did not include in the statistic analysis. * p < 0.01 compared with the vehicle injected group (the second column from the left). Each point or column represent the mean and vertical bar represents the S.E.M. with 6 to 8 rats in each group.
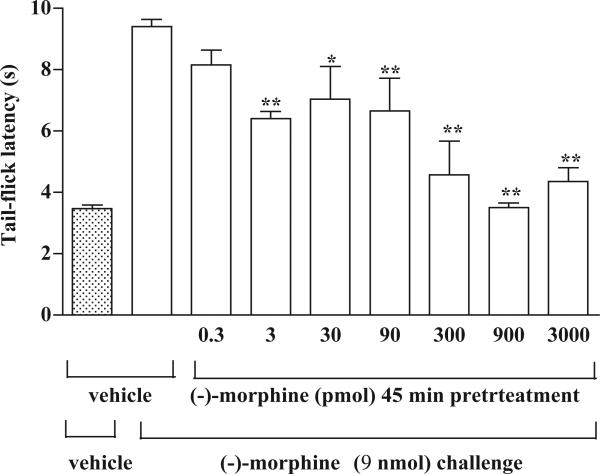
Similarly, pretreatment with (−)-morphine at a dose 3 to 3000 pmol for 45 min dose-dependently attenuated the tail-flick inhibition produced by (−)-morphine. The (−)-morphine-produced tail-flick inhibition was almost completely abolished by the pretreatment with 900 pmol of (−)-morphine (Fig. 2). Pretreatment with these doses of (−)-morphine did not affect the baseline tail-flick latencies observed at 45 min after injection (data not shown). The ED50 value for (−)-morphine given into the ventral periaqueductal gray for the attenuation of (−)-morphine-produced tail-flick inhibition was estimated to be 221.6 (95 % confidence intervals: 87.5 to 561.4) pmol. Thus, (+)-morphine is approximately 270,000-fold more potent than (−)-morphine in attenuating the (−)-morphine-produced tail-flick inhibition.
Fig 2.
Pretreatment with (−)-morphine given into ventral periaqueductal gray dose-dependently attenuates the tail-flick inhibition produced by (−)-morphine given into ventral periaqueductal gray in the rat. Groups of rats were microinjected with a various dose (0.3 to 3000 pmol) of (−)-morphine or vehicle given into the ventral periaqueductal gray 45 min before the microinjection of (−)-morphine (9 nmol) given into the ventral periaqueductal gray and the tail-flick response was measured 20 min after (−)-morphine challenge. Each column represents the mean and vertical bar represents the S.E.M. with 5-8 rats in each group. One-way ANOVA followed by Dunnett's post-test was used to test the difference between groups; the F (7,41) = 16.93; * p < 0.05, ** p < 0.01, compared with the vehicle pretreated rats challenged with (−)-morphine (the second column from the left).
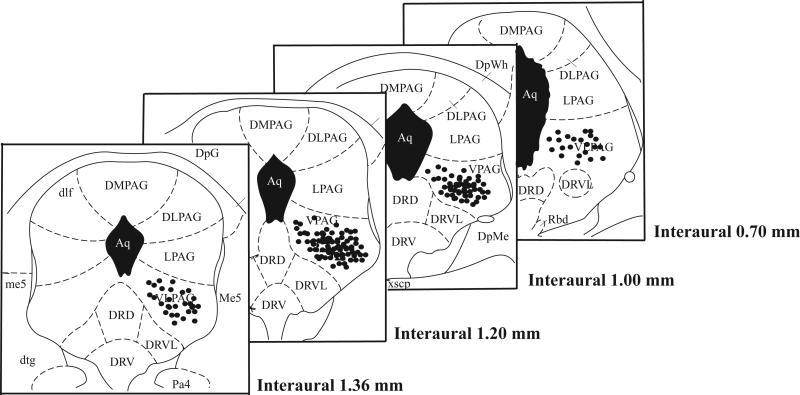
The microinjection sites for rats pretreated with (+)-morphine and (−)-morphine and challenged with (−)-morphine of experiments presented in Fig. 1 and Fig. 2 were verified to be accurately located in the ventral periaqueductal gray (Fig 3).
Fig. 3.
Coronal sections of the atlas of Paxinos and Watson (1997) showing the microjection sites for (+)-morphine and (−)-morphine given into ventral periaqueductal gray of the experiments shown in Fig. 1 and Fig. 2.
3.2. Pretreatment with (+)-naloxone reverses the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine in the rat ventral periaqueductal gray
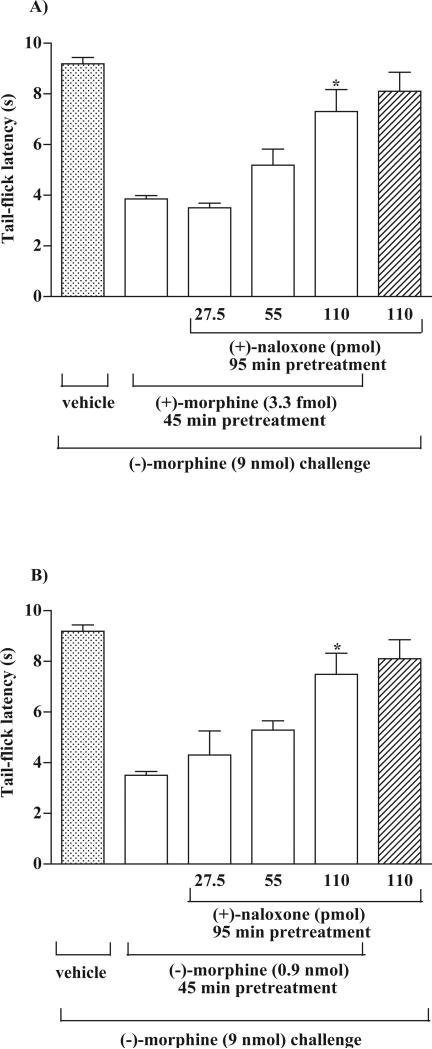
The non-opioid receptor antagonist (+)-naloxone was used to determine if the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine is mediated by the stimulation of a non-opioid mechanism. Preliminary studies indicated that pretreatment with (+)-naloxone at a dose up to 110 pmol given into ventral periaqueductal gray for 95 min did not affect the tail-flick inhibition produced by (−)-morphine. Groups of rats were microinjected with different doses of (+)-naloxone (27.5-110 pmol) 50 min (Terashvili et al., 2005) before microinjection of (+)-morphine (3.3 fmol) given into the ventral periaqueductal gray and were microinjected with (−)-morphine (9 nmol) given into the ventral periaqueductal gray 45 min thereafter. The tail-flick response was then measured 20 min after the last injection. Pretreatment with (+)-morphine 3.3 fmol markedly attenuated the tail-flick inhibition produced by (−)-morphine. The attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine was reversed dose-dependently by (+)-naloxone pretreatment (Fig. 4A).
Fig. 4.
Pretreatment with (+)-naloxone reverses the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine (A) or (−)-morphine (B) in the rat ventral periaqueductal gray. Groups of rats were pretreated with different doses (27.5, 55 and 110 pmol) of (+)-naloxone or vehicle for 95 min and (+)-morphine (3.3 fmol) or (−)-morphine (0.9 nmol) for 45 min given into ventral periaqueductal gray and were challenged with (−)-morphine (9 nmol) given into the ventral periaqueductal gray thereafter. The tail-flick response was measured 20 min after (−)-morphine challenge. Each column represents the mean and the vertical bar represents the S.E.M. with 6 -7 rats in each group. One-way ANOVA followed by Dunnett's post-test was used to test the difference between groups; the F (3,28) = 10.36 (A) and F (3,26) = 7.20 (B); * p < 0.01 compared with the (+)-morphine or (−)-morphine pretreated rats challenged with (−)-morphine (the second column from the left). The first column from the left and right represented control groups, which did not include in the statistical test.
Similarly, pretreatment with (−)-morphine (900 pmol) markedly attenuated the tail-flick inhibition produced by (−)-morphine (9 nmol). The attenuation of the (−)-morphine-produced tail-flick inhibition induced by (−)-morphine pretreatment was reversed dose-dependently by the (+)-naloxone (27.5-110 pmol) pretreatment (Fig. 4B).
3.3. Pretreatment with the sigma receptor antagonist BD1047 reverses the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine in the rat ventral periaqueductal gray
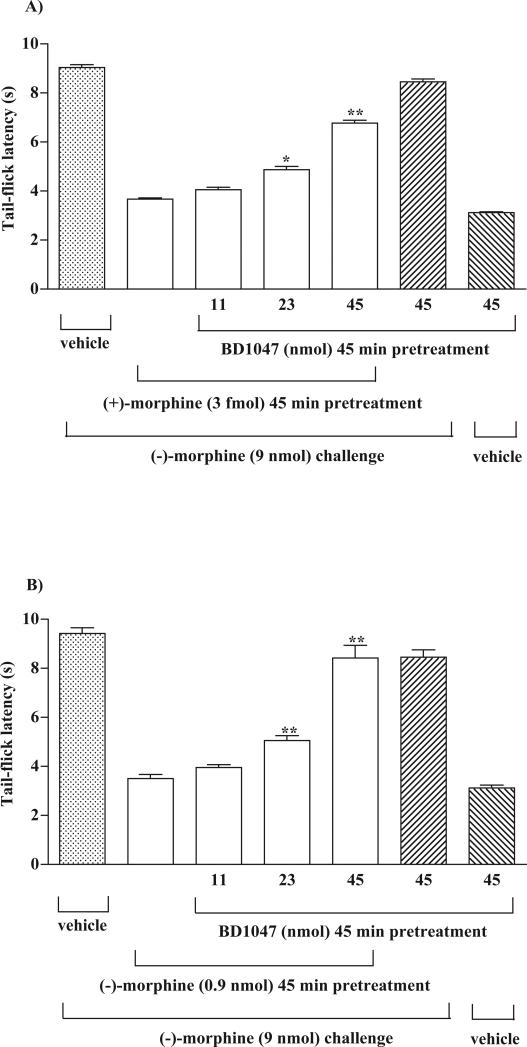
Sigma receptor antagonist BD1047 was used to determine if the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine pretreatment is mediated by the stimulation of sigma receptors. Groups of rats were co-administered with different doses (11, 23 and 45 nmol) of BD1047 and (+)-morphine (3.3 fmol) or (−)-morphine (900 pmol) given into the ventral periaqueductal gray and (−)-morphine (9 nmol) was then administered into the same ventral periaqueductal gray 45 min thereafter. The tail-flick responses were measured 20 min after last microinjection. As shown in Fig 5A, pretreatment with (+)-morphine for 45 min attenuated the tail-flick inhibition produced by (−)-morphine. The attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine was reversed dose-dependently by the BD1047 pretreatment. BD1047 (45 nmol) pretreated alone did not affect the (−)-morphine-produced tail-flick inhibition, nor did it affect the baseline tail-flick response in rats injected with saline vehicle (Fig. 5A).
Fig. 5.
Pretreatment with the sigma receptor antagonist BD1047 reverses the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine (A) or (−)-morphine (B) pretreatment in the rat ventral periaqueductal gray. Groups of rats were co-administered with different doses (11, 23 and 45 nmol) of BD1047 and (+)-morphine (3.3 fmol) or (−)-morphiine (0.9 nmol) given into the ventral periaqueductal gray and (−)-morphine (9 nmol) was then administered into the ventral periaqueductal gray 45 min thereafter. The tail-flick responses were measured 20 min after the last injection. Each column represents the mean and the vertical bar represents the S.E.M. with 6-10 rats in each group. One-way ANOVA followed by Dunnett's post-test was used to test the difference between groups; the F (3,26) = 26.32 (A) and F (3,27) = 61.24(B). * p < 0.05, ** p < 0.01 compared with the (+)-morphine or (−)-morphine pretreated rats challenged with (−)-morphine (the second column from the left). The first column from the left and first two columns from the right represented control groups, which did not include in the statistical test.
Similarly, pretreatment with (−)-morphine (900 pmol) for 45 min attenuated the tail-flick inhibition produced by (−)-morphine (9 nmol). The attenuation of the (−)-morphine-produced tail-flick inhibition induced by (−)-morphine pretreatment was reversed dose-dependently by the BD1047 (11, 23 and 45 nmol) pretreatment (Fig. 5B).
4. Discussion
4.1. (+)-Morphine and (−)-morphine induce antianalgesia against (−)-morphine-produced antinociception at the ventral periaqueductal gray of the rat
We found in the present study that pretreatment with (+)-morphine at a femtomolar dose range or (−)-morphine at a picomolar dose range given into the ventral periaqueductal gray dose-dependently attenuated the tail-flick inhibition produced by (−)-morphine from the ventral periaqueductal gray, indicating that the ventral periaqueductal gray is the site sensitive to (+)-morphine and (−)-morphine for the induction of antianalgesia against (−)-morphine-produced antinociception. Thus, the descending pain controls activated by (−)-morphine for producing analgesia is negatively regulated by (+)-morphine or (−)-morphine at the ventral periaqueductal gray. We have previously demonstrated that (+)-morphine or (−)-morphine pretreatment produces antianalgesia in the spinal cord of mice (Wu et al., 2004, 2005).
We found in the present studies that (+)-morphine at a dose range from 0.3 to 3.3 fmol dose-dependently attenuated the (−)-morphine-produced tail-flick inhibition. Paradoxically, (+)-morphine at a higher dose 33 fmol was less effective in attenuating (−)-morphine-produced tail-flick inhibition. Thus, (+)-morphine induces a U-shape dose-response curve with a maximal attenuation at 3.3 fmol for attenuating the (−)-morphine-produced tail-flick inhibition. Similarly, we have previously demonstrated that (+)-morphine attenuates the (−)-morphine-produced conditioned place preference in an U-shaped dose-response relationship manner (Wu et al., 2007). However, the neural mechanism for (+)-morphine to induce the U-shaped dose-response curves in attenuating the effects produced by (−)-morphine is not clear at this time. It is likely that (+)-morphine at a high dose desensitize the naloxone-sensitive sigma receptors and becomes less effective or not effective in attenuating the effects produced by (−)-morphine.
4.2. The antianalgesia induced by (+)-morphine and (−)-morphine is mediated by a non-opioid mechanism
We found in the present study that the dextrorotatory (+)-morphine is 270,000-fold more potent than the levorotatory (−)-morphine in attenuating the (−)-morphine-produced tail-flick inhibition in the ventral periaqueductal gray of the rat. Similarly, we have found in our previous study that (+)-morphine is 71,000-fold more potent than (−)-morphine in attenuating (−)-morphine-produced tail-flick inhibition in the spinal cord of the mouse (Wu et al., 2005). Since opioid receptors are stereospecific for (−)-morphine, but not (+)-morphine (Jacquet et al., 1977), the high stereoselective action of (+)-morphine over (−)-morphine in attenuating the (−)-morphine-produced antinociception strongly indicate that the antianalgesia of (+)-morphine and (−)-morphine is mediated by a non-opioid mechanism.
Unlike (−)-naloxone, which is an μ-opioid receptor antagonist, the optic enantiomer of naloxone, (+)-naloxone does not have any affinity for μ-opioid receptors and does not antagonize the analgesia produced by μ-opioid receptor agonists (Dunwoodie et al., 1982; Iijima et al., 1978b). We found in the present study that (+)-naloxone pretreatment reversed the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine and (−)-morphine in the rat ventral periaqueductal gray, indicating that the antianalgesia induced by (+)-morphine and (−)-morphine is mediated by a non-opioid mechanism in the ventral periaqueductal gray. Further, we have previously demonstrated that (+)-morphine and (−)-morphine are able to attenuate the tail-flick inhibition produced by opioid δ and κ agonists in the μ-opioid receptor knockout mice, providing an additional evidence that μ-opioid receptors are not involved in the antianalgesia induced by (+)-morphine and (−)-morphine (Wu et. al., 2006). This neural mechanism of antianalgesia induced by (+)-morphine and (−)-morphine is completely different from that of antianalgesia induced by the selective μ-opioid ligand endomorphin-1 or endomorphin-2. The antianalgesia induced by endomorphin-1 or endomorphin-2 is selectively blocked by (−)-naloxone, but not by (+)-naloxone, indicating that the endomorphin-1- and endomorphin-2-induced antianalgesia is mediated by an opioidergic mechanism (Terashvili et al., 2005). Thus, (+)-morphine and (−)-morphine are considered to be agonists for inducing antianalgesia and (+)-naloxone is an antagonist to reverse the antianalgesia induced by (+)-morphine or (−)-morphine.
4.3. The antianalgesia induced by (+)-morphine and (−)-morphine is mediated by activation of the naloxone-sensitive sigma receptor
We found in the present study that pretreatment with the sigma receptor antagonist BD1047 given into ventral periaqueductal gray blocked or reversed the attenuation of the (−)-morphine-produced antinociception induced by (+)-morphine or (−)-morphine in the rat ventral periaqueductal gray, indicating that the antianalgesia induced by (+)-morphine or (−)-morphine is mediated by the activation of the sigma receptors.
Sigma receptors were originally thought to be a type of opiate receptor (Martin et al., 1976), but two subsequent findings convincingly demonstrate that it is not: (a) whereas opiate receptor are stereoselective for the (−)-isomers of opium-derived opiate, opiate antagonists, and their congeners, sigma receptors are stereoselective for the (+)-isomers and (b) naloxone is ineffective against both the in vivo and the in vitro effects of the sigma ligands (Brady et al., 1982; Iwamoto, 1981; Katz et al., 1985; Slifer and Balster, 1983; Vaupel, 1983). Both optic enantiomers (−)-naloxone and (+)-naloxone and (−)-morphine and (+)-morphine bind poorly to [3H](+)-SKF10,047 ligand (Tam and Cook, 1984; Martin et al., 1984). Therefore, it is clear that the sigma receptor is not a type of opiate receptor (Walker et al., 1990) and has been defined as the naloxone-inaccessible sigma receptor (Tam, 1983). It is highly unlikely that the antianalgesia of (+)-morphine and (−)-morphine is mediated by the activation of the naloxone-inaccessible sigma receptor.
A naloxone-sensitive, haloperidol-sensitive, [3H](+)SKF-10047-binding protein has been purified from the rat brain membrane in an affinity chromatography originally designed to purify sigma receptors (Tsao and Su,1997). [3H](+)-SKF-10047 binding to the protein is inhibited by (+)-pentazocine, (−)-pentazocine, (−)-morphine, (−)-naloxone, haloperidol, (+)-SKF-10047 or (−)-SKF-10047. Further, the prototypic sigma receptor ligand, such as 1.3-di-o-tolylguanidine (DTG), (+)-3-PPP, and progesterone, bind poorly to the protein. The protein resembles the opiate sigma receptor originally proposed by Martin et al. (1976). This receptor is therefore tentatively defined as the naloxone-sensitive sigma receptor. We found in the present study that the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine is reversed by (+)-naloxone and by the sigma receptor antagonist BD1047 in the rat ventral periaqueductal gray. The finding is consistent with our previous findings that the attenuation of the (−)-morphine-produced tail-flick inhibition induced by (+)-morphine or (−)-morphine is reversed by (+)-naloxone or (−)-naloxone in the spinal cord of male CD-1 mice (Wu et al., 2005). The findings indicate that the antianalgesia induced by (+)-morphine or (−)-morphine against (−)-morphine-produced antinociception is mediated by the activation of the naloxone-sensitive sigma receptor.
Acknowledgements
This work was supported by grant DA12588 from the National Institute of Health, National Institute on Drug Abuse (PI: Leon F.Tseng) and Research Affair Committee, Medical College of Wisconsin (PI: Hsiang-En Wu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Brady KT, Balster RL, May EL. Stereoisomers of N-allylnormetazocine: phencyclidine-like behavioral effects in squirrel mondeys and rats. Science. 1982;215:178–180. doi: 10.1126/science.6274022. [DOI] [PubMed] [Google Scholar]
- Chien CC, Pasternak GW. Functional antagonism of morphine analgesia by (+)-pentazocine: evidence for an antiopioid sigma-1 system. Eur. J. Pharmacol. 1993;250:R7–R8. doi: 10.1016/0014-2999(93)90650-7. [DOI] [PubMed] [Google Scholar]
- Chien CC, Pasternak GW. Selective antagonism of opioid analgesia by a sigma system. J. Pharmacol. Exp. Ther. 1994;271:1583–1590. [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
- Dunwoodie TV, Peres-Reyes E, Rice KC. Stereospecificity of opiate antagonists in rat hippocampus and neocortex responses to (+)- and (−)-isomers of naloxone. Neuroscience. 1982;7:1691–1702. doi: 10.1016/0306-4522(82)90027-6. [DOI] [PubMed] [Google Scholar]
- Iijima I, Minamikawa JI, Jacobson AE, Brossi A, Rice KC. Studies in the (+)-morphinan series. 4. A markedly improved synthesis of (+)-morphine. J. Org. Chem. 1978a;43:1462–1463. doi: 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- Iijima I, Minamikawa JI, Jacobsen AE. Studies in the (+)-morphine series 5. Synthesis and biological properties of (+)-naloxone. J. Med. Chem. 1978b;21:398–400. doi: 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Locomotor activity and antinociception after putative μ, κ, and σ opioid receptor agonists in the rat: influence of dopaminergic agonists and antagonists. J. Pharmacol. Exp. Ther. 1981;217:451–460. [PubMed] [Google Scholar]
- Jacquet YF, Klee WA, Rice KC, Iijima I, Minamikawa J. Stereospecific and nonstereospecific effects of (+)- and (−)-morphine: evidence for a new class of receptors. Science. 1977;198:842–845. doi: 10.1126/science.199942. [DOI] [PubMed] [Google Scholar]
- Katz JL, Spealman RD, Clark RD. Stereoselective behavioral effects of N-allylnormetazocine in pigeon and squirrel monkeys. J. Pharmacol. Exp. Ther. 1985;232:452–461. [PubMed] [Google Scholar]
- Marrazzo A, Parenti C, Scavo V, Ronsisvalle S, Scoto GM, Ronsisvalle G. In vivo evaluation of (+)-MR200 as a selective sigma ligand modulating MOP, DOP and KOP supraspinal analgesia. Life Sci. 2006;78:2449–2553. doi: 10.1016/j.lfs.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Martin BR, Katzen JS, Woods JA, Tripathi HL, Harris LS, May EL. Stereoisomers of [3H]-N-allylnormetazocine bind to different sites in mouse brain. J. Pharmacol. Exp. Ther. 1984;231:539–544. [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Mei J, Pasternak GW. σ1 Receptor modulation of opioid analgesia in the mouse. J. Pharmacol. Exp. Ther. 2002;300:1070–1074. doi: 10.1124/jpet.300.3.1070. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. AcademicPress; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- Pavlovic ZW, Bodnar RJ. Opioid supraspinal analgesic synergy between the amygdala and periaqueductal gray in rat. Brain Res. 1998;779:158–169. doi: 10.1016/s0006-8993(97)01115-3. [DOI] [PubMed] [Google Scholar]
- Slifer BL, Balster RL. Reinforcing properties of stereoisomers of the putative sigma agonists N-allylnormetazocine and cyclazocine in Rheus monkeys. J. Pharmacol. Exp. Ther. 1983;225:522–528. [PubMed] [Google Scholar]
- Smith DJ, Perotti JM, Crisp T, Cabral MEY, Long JT, Scalziti JN. The mu receptor is responsible for descending pain inhibition originating in the periaqueductal gray region of the rat brain. Eur. J. Pharmacol. 1988;156:47–54. doi: 10.1016/0014-2999(88)90145-8. [DOI] [PubMed] [Google Scholar]
- Tam SW. Naloxone-inaccessible σ receptor in rat central nervous system. Proc. Natl. Acad. Sci. USA. 1983;80:6703–6707. doi: 10.1073/pnas.80.21.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SW, Cook L. σ opiates and certain antipsychotic drugs mutually inhibit (+)-[3H]SKF 10,047 and [3H]haloperidol binding in guinea pig brain membranes. Pro. Natl. Acad. Sci. USA. 1984;81:5618–5621. doi: 10.1073/pnas.81.17.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashvili M, Wu HE, Leitermann RJ, Sun HS, Clithero AD, Tseng LF. Differential mechanisms of antianalgesia induced by endomorphin-1 and endomorphin-2 in the ventral periaqueductal grey of the rat. J. Pharmacol. Exp. Ther. 2005;312:1257–1265. doi: 10.1124/jpet.104.076224. [DOI] [PubMed] [Google Scholar]
- Tsao LT, Su TP. Naloxone-sensitive, haloperidol-sensitive, [3H](+)-SKF-1047-binding protein partially purified from rat liver and rat brain membranes: an opioid/sigma receptor. Synapse. 1997;25:117–124. doi: 10.1002/(SICI)1098-2396(199702)25:2<117::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Vaupel DB. Naltrexone fails to antagonize the effects of PCP and SKF 10,047 in the dog. Eur. J. Pharmacol. 1983;92:269–274. doi: 10.1016/0014-2999(83)90297-2. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, de Coster B, Rice K. Sigma receptors: Biology and function. Pharmacol. Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Wu HE, Schwasinger ET, Terrashvili M, Tseng LF. dextro-Morphine attenuates the morphine-produced conditioned place preference via the sigma1 receptor activation in the rat. Eur. J. Pharmacol. 2007;562:221–226. doi: 10.1016/j.ejphar.2007.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HE, Thompson J, Sun HS, Leitermann RJ, Fujimoto JM, Tseng LF. Nonopioidergic mechanism mediating morphine-induced antianalgesia in the mouse spinal cord. J. Pharmacol. Exp. Ther. 2004;310:240–246. doi: 10.1124/jpet.104.065334. [DOI] [PubMed] [Google Scholar]
- Wu HE, Thompson J, Sun HS, Terashvili M, Tseng LF. Antianalgesia: stereoselective action of dextro-morphine over levo-morphine on glia in the mouse spinal cord. J. Pharmacol. Exp. Ther. 2005;314:1101–1108. doi: 10.1124/jpet.105.087130. [DOI] [PubMed] [Google Scholar]
- Wu HE, Sun HS, Terashivili M, Schwasinger E, Sora I, Hall FS, Uhl GR, Tseng LF. dextro-Morphine and levo-morphine induce antianalgesia against antinociception produced by opioid δ- and κ-agonists in μ-opioid receptor knockout mice. Eur. J. Pharmacol. 2006;531:103–107. doi: 10.1016/j.ejphar.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Al-Rodham NRF, Jensen TS. Sites of actions of opiates in production of analgesia. Prog. Brain Res. 1988;77:371–391. doi: 10.1016/s0079-6123(08)62803-4. [DOI] [PubMed] [Google Scholar]