Abstract
Many researchers have used local area practice style measures as instruments in instrumental variable analysis. What constitutes the size of a “local area” for measuring practice styles may affect the strength of the relationship between the instrument and treatment choice, and whether the instrument is related to unmeasured confounding factors. Among previous studies using local area practice style measures as instruments, only two reported whether their estimates were robust to changes in the local area size. There has been no discussion on how area size may affect IV estimates when local area practice style measures are used as instruments. The objective of this study is to discuss the tradeoffs inherent in choosing a local area size when using a measure of local area practice style as an instrument. We used the effectiveness of angiotensin converting-enzyme inhibitors and angiotensin receptor blockers on survival post acute myocardial infarction as an example. Across local area size definitions we contrasted treatment effect estimates in terms of (1) the strength of the relationship between local area practice styles and individual patient treatment choices; and (2) indirect assessments of the assumption of no correlation between local area practice style and unmeasured confounders.
Introduction
Instrumental variable (IV) estimators have been recognized as useful tools to assess the effectiveness of alternative treatments in healthcare using observational data.1, 2 The “instruments” required in IV studies must be measured factors that are strongly related to treatment choice but are unrelated to either study outcomes or other unmeasured factors related to study outcomes. Thus, instruments essentially provide an ex post randomization of treatment choice or exposure across patients.3–9 IV methods yield estimates of the average treatment effect for the subset of patients whose treatment choices were mutable to changes in the instrument variable or “instrument” values.4, 10–13 IV estimates have been labeled a local average treatment effect (LATE) and are thought most suitable to assess the effect of treatment rate changes in a population.11, 13–19 Many researchers have used local area practice style measures as instruments in IV analysis20–33 which conjectures that (1) patients residing in areas where physicians have stronger preferences for a particular treatment are more apt to receive that treatment; and (2) unmeasured confounding variables are unrelated to the differential patient access to physicians with distinct treatment preferences.
Only two studies that used a local area practice style measure as instruments have reported whether their estimates were robust to adjustments in the size of the local area used to measure practice style,28, 33 and there has been no discussion as to the potential effects of local area size on the properties of the resulting IV estimates. The size of the local area used to measure practice style may affect both the strength of the relationship between the instrument and treatment choice and whether the instrument is related to unmeasured confounding factors. One might expect that the larger the local area around a patient residence used to measure practice style, the weaker the relationship will be between the instrument and the treatment choices for individual patients. Confounding emerges when local area practice style measures are correlated with differences in average unmeasured patient characteristics or ecological factors across areas that are related to patient outcomes. A priori relationships between local area size patient and ecological factors that may confound estimates do not generally exist. Ecological factors have been categorized as aggregate attributes (e.g. smoking rates, average health behaviors), contagion factors (e.g. flu prevalence), environmental factors (e.g. pollution, weather, sunlight hours); patterns of interaction among area individuals (e.g. social networks); and global factors (e.g. local regulations or market structures).34 One can envision that smaller local area sizes could introduce correlations between practice style and unmeasured neighborhood-level cultural and health behavior-related confounders. In contrast, use of larger local area sizes may introduce correlations with regional unmeasured confounders related to regulatory structures, regional healthcare systems, and climate. It is true, however, that because relationships between individual treatment choice and local area practice style measures weaken as local area size increases, this increases the potential for unmeasured patient and ecological factors to confound the treatment effect estimates. However, the only possible approach to validate assumptions of no correlation between local area practice style measures and potential confounding factors for specific area sizes and is to obtain secondary data sources describing these factors and directly estimate the correlations by area size.
If treatment effectiveness is heterogeneous across patients, another source of confounding related to local area size needs to be considered. “Essential heterogeneity” occurs when treatment effects are heterogeneous across patients and providers make treatment recommendations based on patient characteristics that are related to expected treatment effectiveness24, 35–38 If the patient characteristics related to treatment effectiveness are unobserved by the researcher, we theorize that local area practice style measures will be positively correlated with average treatment effectiveness across areas causing LATE estimates to be biased toward positive treatment effectiveness. It will appear that higher treatment rates will yield better outcomes when in fact areas with higher treatment rates simply contained more patients apt to gain from treatment. However, as we discuss in Appendix A, as the number of patients used to define a local area increases, the variation in average treatment effectiveness across local areas diminishes as will the favorable bias in LATE estimates.
The objective of this study is to discuss the tradeoffs involved in choosing a local area size when using local area practice style as an instrument. We use IV methods to estimate the LATE of renin-angiotensin system antagonists including angiotensin converting-enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) on one-year patient survival among Medicare patients post acute myocardial infarction (AMI). ACE/ARB use post-AMI provides an interesting setting for this discussion because the benefits of ACE/ARBs are known to be heterogeneous across AMI patients with greater benefit for patients at higher risk of future cardiovascular events.39 ACE/ARB survival benefit estimates from randomized controlled trials (RCT) vary from 50 lives saved per 1000 high-risk patients to 5 lives saved per 1000 low-risk patients.40 In addition, evidence shows substantial variation in ACE/ARB prescribing as only 50% of Medicare patients from two states received an ACE/ARB post-AMI in 2004,41 and large geographic variation has been reported.42 If providers believe ACE/ARB benefits are heterogeneous across patients, and ACE/ARB treatments are sorted across AMI patients based on expected benefits -- “essential heterogeneity”24, 43 – we expect that local area ACE/ARB practice styles variation will reflect moderate risk AMI patients whose ACE/ARB choices are more discretionary than either high or low risk patients. As a result, IV estimates of LATE for ACE/ARB use on one-year survival for post-AMI patients should fall between the RCT estimates described above. In addition, all else equal, if essential heterogeneity is occurring we expect that LATE estimates will be biased high and that the bias will diminish as the size of the local area increases (see Appendix A). The consistency of our estimates is also conditional on the assumptions that local area ACE/ARB practice styles are unrelated to unmeasured average patient and ecological factors that affect cardiovascular patient outcomes such as general provider access,44, 45 characteristics of the healthcare delivery system,44, 45 area socioeconomic status and homogeneity,44–50 pollution,51 social environment and support,45, 52, 53 and area health behaviors and disease prevention.53
We used the driving area for clinical care (DACC) method to define local areas around Medicare AMI patient residence ZIP codes.28, 30, 33 The DACC method enables researchers to create local area practice style measures for alternative size definitions based on threshold numbers of patients living within a specified driving time of each ZIP code. Defining local areas based on the number of patients instead of distances alone helps account for urban/rural differences in healthcare access as rural patients routinely drive greater distances for healthcare. Across local area size definitions we contrasted ACE/ARB one-year survival effectiveness estimates in terms of (1) the strength of the relationship between local area practice styles and individual patient treatment choices; and (2) indirect assessments of the assumption of no correlation between local area practice style and unmeasured confounders.
Methods
Data and Sample
All Medicare claims files, enrollment information, and Part D prescription drug events for patients hospitalized for their first AMI in 2008 that did not have AMI in 2006 and 2007 were obtained. We applied the Chronic Care Warehouse definition of AMI as an inpatient stay with an ICD-9 code 410.xx, (excluding 410.x2) in the first or second diagnosis position of the claim.54 AMI stay admission and discharge dates were based on all Medicare institutional claims (acute, long term care hospital, inpatient rehabilitation facility, critical-access hospital, and short-term nursing facility) with overlapping admission and discharge dates following an initial acute hospital admission with an AMI diagnosis. We restricted our sample to patients discharged alive; with continuous Medicare Part A and B fee-for-service enrollment 12 months prior to their index AMI admission and 12 months post-index discharge or until death; and with continuous Part D enrollment 6 months prior to admission and 12 months post-index discharge or until death. To ensure that all Part D events were observable during a 30-day post index treatment observation period, we further excluded patients who utilized hospice or skilled nursing care; were readmitted to inpatient care; or died within the 30-days post-index discharge. Finally, because driving times between ZIP codes may have inconsistent meaning for geographically non-contiguous areas, we restricted our sample to patients living in the continental United States at AMI admission. The final sample size was 68,236.
Measures
Patients were designated as having been prescribed an ACE/ARB post-AMI if they filled a prescription for an ACE/ARB within 30 days post AMI discharge (1 if an ACE/ARB prescription was filled within 30 days post AMI discharge, 0 otherwise).55 The study outcome was a binary variable equaling 1 if the patient survived for one year post AMI discharge, 0 otherwise. Measured covariates included patient demographics; baseline medical conditions for both the year prior to the AMI admission and during the index AMI stay; medications used during the 180 days prior to the AMI admission; AMI diagnosis-type on admission; procedures during the AMI stay; complications during the AMI stay; other medications filled immediately post discharge (statins, beta blockers); Part D variables including premium levels and deductible phase at diagnosis; whether patients were Medicaid dual-eligible in their AMI index month; and socioeconomic characteristics for the patient residence zip code (per capita income, poverty rate, education level, English speaking percentage). Full definitions of these variables are included in Appendix B.
We defined local area practice style as the average intent of physicians in an area to prescribe ACE/ARBs for patients upon AMI discharge. Because measurement of treatment intent is less clear for patients already using ACE/ARBs when admitted for an AMI, to measure intent we used the patients who did not have ACE/ARBs available at home on their index AMI admission date based on previous prescription dates and days supplied figures on Part D claims (N=43,842). For these patients, we created a variable equaling 1 if the patient filled an ACE/ARB prescription within one day of their first prescription claim in the first 30 days post-AMI discharge, 0 otherwise. With these patients and this treatment definition we measured local area ACE/ARB practice style at the patient ZIP code-level using the DACC method. This method creates “local areas” around each patient residence ZIP code by consecutively adding patients from the next closest ZIP codes based on driving times between zip codes until a threshold number of patients is reached. Distinct local area sizes were created by varying the threshold number of patients from 10 to 200 patients in increments of 10. For the patients associated by the DACC method with each ZIP code for an area size definition, we calculated ZIP code-specific area treatment ratios (ATR) as the ratio of the number of patients receiving ACE/ARBs post-AMI over the sum of the probabilities of their receiving an ACE/ARB post-AMI. ACE/ARB probabilities were predicted using a multivariate logistic model of ACE/ARB choice over the patients with no ACE/ARBs available at index using the measured covariates described above as independent variables. Patients in our full sample (N=68,236) were then assigned local practice style ATR values for each of the 20 area size definitions based on their residence ZIP code.
Analytical Approach
We applied the linear two-stage least squares (2SLS) instrumental variable estimator that has been used in several previous IV studies.18, 23, 24, 26, 27, 33 Linear 2SLS yields consistent estimates that are robust to underlying error distributions unlike other estimators based on distributional assumptions that yield inconsistent estimates if the assumptions are wrong.56, 57 In the first stage of 2SLS, a linear probability model of ACE/ARB choice was estimated for the 68,236 patients in our sample. Independent variables in the first stage model were the measured covariates described above and the local area ACE/ARB practice style instrument. The instrument was specified empirically using nine binary variables that placed each patient residence ZIP code ATR within deciles of the distribution of ATRs across patients. In the second stage of 2SLS, a linear probability model of one-year survival was estimated. The second-stage model specified all the non-instrument covariates from the first-stage model as independent variables plus the predicted ACE/ARB choice probability from the first stage. Using this approach, the estimated parameter associated with the predicted ACE/ARB choice probability in the second stage model is the local average treatment effect (LATE). We repeated this 2SLS method using ACE/ARB ATR values across the 20 different local area sizes. For each local area size we estimated (1) the strength of the relationship between the instrument and ACE/ARB treatment choice via the Chow58 F-value which tests whether the local area practice style instrument describes a statistically significant portion of the variation in ACE/ARB choice; (2) the ACE/ARB 1-year survival LATE estimate with respective standard error; (3) the Hausman over-identification test statistic59 and (4) the correlation between ACE/ARB ATR and overall life expectancy for the county containing each patient ZIP code.60 Statistics (3) and (4) provide indirect assessments of whether the instruments are related to unmeasured confounding variables. The Hausman over-identification statistic tests the null hypothesis that the direct exclusion of the instrument from the second stage one-year survival equation was appropriate. A high Hausman statistic rejects the null hypothesis, suggesting a relationship between the instrument and survival.59 The correlation parameter between local area ACE/ARB practice style and local area life expectancy suggests whether ACE/ARB practice styles are related to unmeasured differences in baseline patient health at diagnosis.
Results
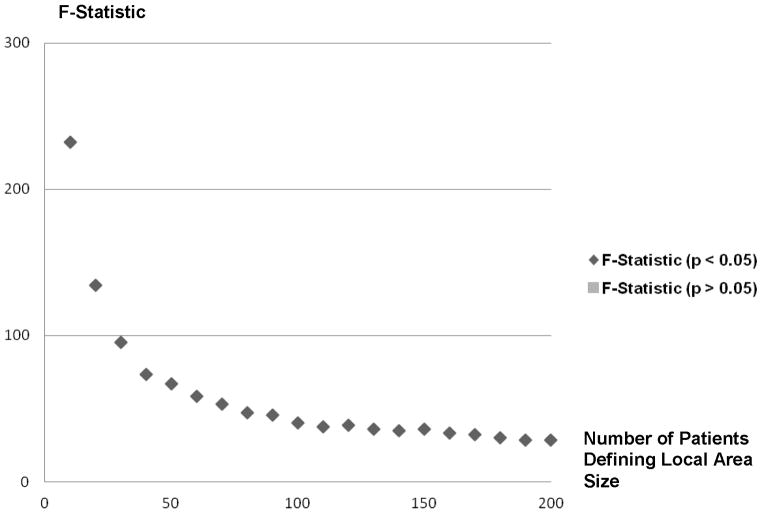
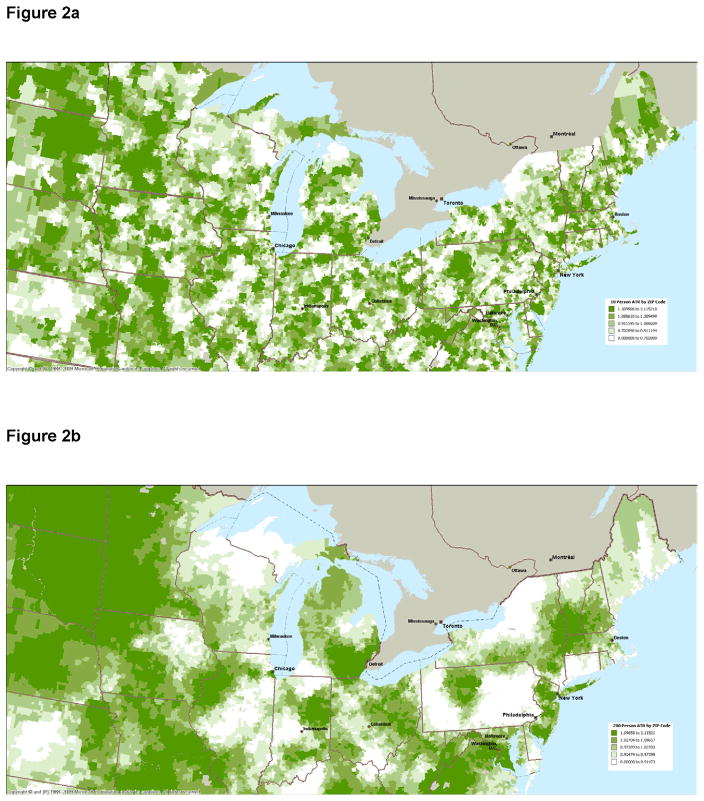
Table 1 describes the local areas defined using the DACC method by the number of threshold patients used. For 10-person local areas it took an average of 26.5 driving minutes from each ZIP code and 12.1 ZIP codes to find sufficient patients, whereas for the 200-person local areas it took an average of 87.5 driving minutes from each ZIP code and 201.2 ZIP codes to find sufficient patients. Figure 1 shows the relationship between the first-stage model Chow F-statistics assessing the strength of the local area practice style instrument and individual patient ACE/ARB choice by local area size. All F-statistics show statistically significant relationships between local area ACE/ARB practice styles and ACE/ARB choice but the values of the F-statistics fell (from 232 to 28) as the local area size increases. Figures 2a and 2b present maps of the northeastern United States illustrating the dispersion of ATR values for 10-patient and 200-patient areas, respectively. These figures show substantial geographic variation in the ACE/ARB use regardless of the local area size, but more practice style variation is revealed using the 10-patient sized local areas.
Table 1.
Statistics Describing ZIP Codes Within Local Areas Around Patient Residence ZIP Codes by the Threshold Number of Patient Defining a Local Area
| Driving Time in Minutes Required to Reach Patient Threshold Number | Number of ZIP Codes Required to Reach Patient Threshold Number | |||||
|---|---|---|---|---|---|---|
| Threshold Number of Patients in Local Area | Mean | Minimum | Maximum | Mean | Minimum | Maximum |
| 10 | 26.5 | 0.0 | 384.6 | 12.1 | 1 | 234 |
| 20 | 34.2 | 0.0 | 384.6 | 22.6 | 1 | 262 |
| 30 | 39.9 | 0.0 | 384.6 | 33.0 | 1 | 274 |
| 40 | 44.5 | 4.5 | 384.6 | 43.4 | 2 | 288 |
| 50 | 48.5 | 4.9 | 384.6 | 53.5 | 2 | 303 |
| 60 | 52.1 | 4.9 | 384.6 | 63.7 | 2 | 321 |
| 70 | 55.4 | 5.3 | 384.6 | 73.8 | 3 | 341 |
| 80 | 58.5 | 5.3 | 384.6 | 83.7 | 4 | 353 |
| 90 | 61.3 | 6.4 | 423.1 | 93.7 | 5 | 369 |
| 100 | 64.2 | 6.4 | 423.1 | 103.7 | 5 | 377 |
| 110 | 67.0 | 6.5 | 423.1 | 113.8 | 5 | 417 |
| 120 | 69.6 | 7.1 | 423.1 | 123.8 | 6 | 427 |
| 130 | 72.1 | 7.1 | 423.1 | 133.7 | 7 | 443 |
| 140 | 74.5 | 7.8 | 423.1 | 143.6 | 7 | 453 |
| 150 | 76.7 | 7.8 | 423.1 | 153.2 | 7 | 467 |
| 160 | 78.9 | 8.1 | 423.1 | 162.8 | 8 | 475 |
| 170 | 81.2 | 8.9 | 433.7 | 172.4 | 9 | 495 |
| 180 | 83.3 | 8.9 | 433.7 | 182.0 | 9 | 506 |
| 190 | 85.4 | 9.5 | 510.4 | 191.7 | 9 | 520 |
| 200 | 87.5 | 9.9 | 510.4 | 201.2 | 9 | 532 |
Figure 1.
First-Stage F-Statistics for the Effect of Local area Practice Style on ACE/ARB Use by Local Area Size (10 Patients to 200 Patients)
Figure 2.
Figure 2a: Northeast United States ACE/ARB Area Treatment Ratios Based on Local Areas Defined Using 10 Patients Around 5-Digit ZIP Codes.
Figure 2b: Northeast United States ACE/ARB Area Treatment Ratios Based on Local Areas Defined Using 200 Patients Around 5-Digit ZIP Codes.
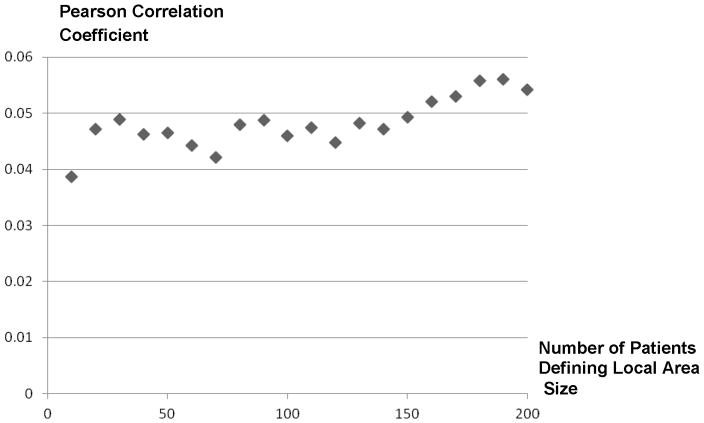
Table 2 contrasts ACE/ARB treatment rates, unadjusted outcome rates, and selected measured covariate averages between patients grouped by ACE/ARB treatment choice, and the 1st and 10th deciles of the ACE/ARB ATR for 10-patient, 100-patient, and 200-patient defined local areas. We also report Cochran-Armitage trend tests for each measured characteristic across the ATR deciles groups for each local area size.61, 62 Of the patients in our sample, 50.5% received an ACE/ARB prescription in the 30-days post AMI discharge. Patients who received an ACE/ARB tended to be younger; had fewer comorbidities prior to AMI admission; had higher-risk AMIs; were more likely to have had a cardiac catheterization during their AMI admission; were less likely to have conditions related to ACE/ARB side effects either prior to or during admission; and were more likely to have used an ACE/ARB prior to their index AMI. Grouping patients by the ACE/ARB ZIP code ATRs revealed substantial ACE/ARB treatment variation but the extent of variation falls with the local area size. For example, ACE/ARB treatment rates varied from 35.0% to 64.0% moving from the 1st to 10th decile with the 10-patient local area size, but varied only from 44.2% to 56.3% using the 200-patient local area size. Compared with grouping patients by ACE/ARB treatment, grouping patients by ZIP code ATRs increased the balance in all measured confounders except patient race. Figure 3 presents the Hausman over-identification statistics for the IV model estimated for each local area size. The Hausman test statistic ranged from 0.47 to 2.21 and all were statistically insignificant except those estimated using the 60 and 100-person sized local areas. The Hausman test statistic appears to trend downward as local area size increases. Figure 4 presents estimated correlations between local area ACE/ARB practice style measures and local area overall life expectancy across local area sizes. A small positive correlation between ACE/ARB practice style and local area life expectancy is observed that trends higher as local area size increases.
Table 2.
Acute Myocardial Infarction Patient Characteristics by ACE/ARB Choice and Local Area Practice Styles Defined Using Different Area Sizes (10, 100, 200)
| Local Area Practice Style Area Size | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE/ARB in 30 days | 10-Person Local Areas | 100-Person Local Areas | 200-Person Local Areas | |||||||||
| No | Yes | p-value (χ2) | 1st decile | 10th decile | Trend Testa | 1st decile | 10th decile | Trend Testa | 1st decile | 10th decile | Trend Testa | |
| Number of Patients | 33,757 | 34,479 | 6,824 | 6,824 | 6,825 | 6,825 | 6,821 | 6,821 | ||||
| Column % | Column % | Column % | Column % | |||||||||
| ACE/ARB Rx%b | 0 | 100 | <0.0001* | 35.0 | 64.0 | <0.0001* | 42.8 | 57.1 | <0.0001* | 44.2 | 56.3 | <0.0001* |
| 1-year Survival % | 81.7 | 87.6 | <0.0001* | 82.8 | 84.7 | 0.0054* | 83.3 | 84.6 | 0.5427 | 83.8 | 84.3 | 0.4997 |
| Age | ||||||||||||
| 66–75 | 35.6 | 41.6 | <0.0001* | 36.9 | 38.5 | 0.0482* | 36.8 | 40.1 | <0.0001* | 36.4 | 38.8 | 0.0038* |
| 76–85 | 39.5 | 39 | 0.2176 | 39.9 | 39.4 | 0.0507 | 40.6 | 37.3 | <0.0001* | 40.5 | 38.4 | <0.0001* |
| 85+ | 24.9 | 19.4 | <0.0001* | 23.2 | 22.1 | 0.9851 | 22.5 | 22.6 | 0.5585 | 23.1 | 22.8 | 0.2538 |
| Race | ||||||||||||
| White | 84.5 | 82.5 | <0.0001* | 84.8 | 82.6 | 0.0163* | 86.2 | 81.8 | <0.0001* | 87.9 | 79.8 | <0.0001* |
| Black | 7.5 | 8.0 | 0.0296* | 7.6 | 9.4 | 0.2996 | 7.6 | 10.1 | <0.0001* | 6.9 | 11.1 | <0.0001* |
| Other | 8.0 | 9.6 | <0.0001* | 7.6 | 8.0 | 0.0299* | 6.2 | 8.1 | <0.0001* | 5.2 | 9.1 | <0.0001* |
| Metroc | 71.2 | 68.7 | <0.0001* | 70.2 | 70.3 | 0.7837 | 70.6 | 71.4 | <0.0001* | 69.2 | 70.2 | <0.0001* |
| Low Per Capita Incomed | 48.3 | 50.8 | <0.0001* | 49.4 | 48.2 | 0.1770 | 49.1 | 49.7 | <0.0001* | 52.1 | 48.0 | 0.0012* |
| Charlson Scoree-- pre-index | ||||||||||||
| 0 | 31.2 | 35.7 | <0.0001* | 31.7 | 32.8 | 0.1944 | 31.7 | 32.8 | 0.5323 | 32.7 | 33.0 | 0.7787 |
| 1 | 22.5 | 24.6 | <0.0001* | 23.3 | 23.1 | 0.5597 | 23.3 | 23.4 | 0.5941 | 23.9 | 23.2 | 0.7940 |
| 2 | 15.2 | 14.0 | <0.0001* | 16.2 | 14.8 | 0.0640 | 15.0 | 13.7 | 0.2998 | 14.8 | 14.8 | 0.5170 |
| 3 | 11.1 | 10.2 | <0.0001* | 11.1 | 10.7 | 0.8890 | 11.1 | 11.4 | 0.4147 | 10.9 | 11.0 | 0.1480 |
| 4+ | 19.9 | 15.5 | <0.0001* | 17.8 | 18.6 | 0.5147 | 18.8 | 18.6 | 0.2869 | 17.6 | 17.9 | 0.9459 |
| Anterior Wall AMIf | 4.9 | 8.2 | <0.0001* | 5.8 | 6.5 | 0.4406 | 5.9 | 6.5 | 0.3104 | 5.7 | 6.6 | 0.4593 |
| Non ST Elevation AMI (NSTEMI)g | 78.7 | 72.8 | <0.0001* | 77.3 | 77.4 | 0.3574 | 77.8 | 76.7 | 0.4354 | 77.6 | 75.9 | 0.2170 |
| ACE/ARB side effect condition -- pre-indexh | 24.1 | 17.4 | <0.0001* | 21.6 | 21.4 | 0.1961 | 21.5 | 21.5 | 0.4023 | 20.6 | 21.2 | 0.9066 |
| ACE/ARB side effect condition – indexh | 34.8 | 25.3 | <0.0001* | 31.9 | 30.3 | 0.0252* | 30.9 | 30.9 | 0.3121 | 30.6 | 30.7 | 0.7472 |
| Cardiac Cath Index | 50.0 | 62.8 | <0.0001* | 55.4 | 55.9 | 0.7898 | 54.8 | 55.2 | 0.3402 | 56.2 | 55.7 | 0.5336 |
| ACE use in 180 days pre-index | 28.2 | 43.1 | <0.0001* | 34.8 | 36.6 | 0.0201* | 34.7 | 38.4 | 0.0005* | 35.2 | 37.1 | 0.0165* |
Cochran-Armitage test of trend in characteristic value across patients grouped into deciles based on local area ACE/ARB practice style measure. For example, the p value Metro tests whether a linear trend in Metro residence exists across the ACE/ARB practice style-based patient groups.
% patients with an ACE/ARB prescription in 30-days post AMI discharge.
Lived in a Metropolitan area according to Rural-Urban Continuum Codes developed by the USDA Economic Research Service (http://www.ers.usda.gov/topics/rural-economy-population/rural-classifications.aspx). 20 patients had unknown metro size.
Mean per capita income in ZIP code below ZIP code mean.
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology, 2000 Dec; 53(12): 1258–67.67
ICD-9 410.0; 410.1
ICD-9 410.7
Patient had either angioedema (995.1); hyperkalemia (276.7); acute renal failure/acute tubular necrosis (584.xx); acute glomerulonephritis (580.xx); of Chronic Kidney Disease (016.00, 016.01, 016.02, 016.03, 016.04, 016.05, 016.06, 095.4, 189.0, 189.9, 223.0, 236.91, 250.40, 250.41, 250.42, 250.43, 271.4, 274.1, 283.11, 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 440.1, 442.1, 572.4, 582.xx, 583.xx, 585.xx, 586.xx, 588.xx, 591, 753.12, 753.13, 753.14, 753.15, 753.16, 753.17, 753.19, 753.20, 753.21, 753.22, 753.23, 753.29, 794.4)
Figure 3.

Over-Identification F-Statistics by Local Area Size (10 Patients to 200 Patients)
Figure 4.
Correlations of Local Area ACE/ARB Area Treatment Ratio and Local Area Overall Life Expectancy by Local Area Size (10 Patients to 200 Patients)
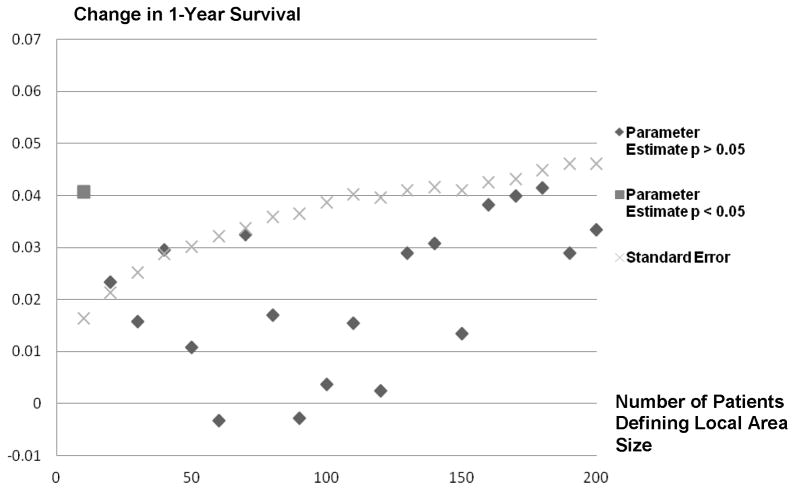
Figure 5 plots the LATE estimates and respective standard errors by local area size. Estimates of LATE ranged from −0.003 to 0.041 (i.e. 41 additional patients per 1000 treated survive for one-year post AMI). Only the IV estimate produced with the 10-person local area (0.041 or 41 patients per additional 1000 treated survive one year) is statistically different from zero. IV estimates trend downward moving from 10-person to 120-person local areas, but trend upward for local areas defined using greater than 120 person.
Figure 5.
Instrumental Variable Parameter Estimates and Standard Errors for the Effect of ACE/ARB Prescribing Post AMI on 1-Year Survival and Standard Error by Local Area Size (10 Patients to 200 Patients)
Discussion
Instrumental variable (IV) methods yield local average treatment effect (LATE) estimates that are representative of the subset of patients whose treatment choices were responsive to the instrument specified in the analysis.13 Methodologists have stressed that LATE estimates are best applied to policy questions that are closely aligned with the treatment variation generated by the instruments specified.63 As such, our LATE estimates of ACE/ARBs on one-year survival post-AMI using local area practice styles would be best used to address policy questions related to whether changes in existing ACE/ARB rates will affect patient survival. For example, what survival benefits will be gained by moving from a 50.5% ACE/ARB treatment rate to a 55% rate, or what benefits, would be lost from reducing the ACE/ARB treatment rate to 45%? As has been argued, estimates of LATE may be more appropriate for answering these questions than estimates from RCTs.14, 19, 64, 65
We found that the area treatment ratio (ATR)-based measures of local practice style describe a statistically significant portion of variation in ACE/ARB use across all local area sizes. All F-statistics across local area sizes were greater than 10 which is considered the threshold of “weak instruments” in the IV literature.23 However, as seen in Figure 1, as the local area size expands, the strength of the relationship between local area practice style measures and individual patient ACE/ARB choice diminishes. A direct effect of this reduction can be seen in the increase in the standard errors of the ACE/ARB treatment effect estimates in Figure 5. If the treatment effect standard error found in the 10-patient local area were maintained across local area sizes, six of the 20 treatment effect estimates would have been statistically significantly different from zero at the .05 level.
We discussed two main sources of bias for LATE estimates using ATR-based measures of local area practice styles in IV estimation. We theorized in Appendix A that using ATR-based instruments will yield estimates of LATE with a positive bias but that this bias will diminish as local area size increases. It is also possible that for certain local area sizes ATR-based measures could be correlated with either average patient characteristics or ecological factors that affect outcome directly. Previous studies have suggested several potential confounding factors, but there are no a priori relationships that would associate these factors with ATR-based measures of local practice styles by local area size. We suggest researchers should look for anomalies in LATE estimates across local area sizes and search for secondary sources of information to assess the validity of estimates. We provide an example of this by estimating correlations between local area ACE/ARB ATR values and local area overall life expectancies. In future research medical charts from the index AMI could be abstracted for a sample of patients in the highest and lowest ACE/ARB ATR areas for several local area sizes to assess whether average patient characteristics such as AMI severity varies with ATR values across local area sizes.
With respect to the effect of ACE/ARBs on one-year survival post-AMI across local area sizes (Figure 5), we found that only the LATE estimate from the 10-person local area size was statistically different from zero and that LATE estimates trended downward as local area size increased from 10 to 120 patients. This trend is consistent with the theory we provide in Appendix A that ATR-based measures of local area practice style will lead to LATE estimates with positive bias but that this bias will diminish as local area size increases. However, this trend clearly does not extend to local area definitions greater than 120 patients as LATE estimates trended upward for local area sizes using from 120 to 200 patients. The U-shaped relationship between LATE estimates and local area size suggests that correlations between local area size and unmeasured confounders exist, but this U-shaped relationship alone is insufficient to pinpoint which local area sizes have the most prominent confounding problem. It is possible that correlations between unmeasured confounding factors and the ATR-based instruments exist in the larger-sized local areas that bias LATE treatment effect estimates in a positive manner. We did find small positive correlations between the ATR values and life expectancy that get stronger as local area size increases that support this conclusion. On the other hand, it is possible that correlations between unmeasured confounding factors and the ATR-based instruments exist in the smaller-sized local areas that bias treatment effects toward zero (independent of the positive bias associated with essential heterogeneity). The over-identification statistic estimates in Figure 3 trend lower with increased local area size which provides some weight to this idea, but secondary data sources are needed to further understand the source of LATE estimate variation across area sizes.
Just over 50% of the AMI patients in our sample filled an ACE/ARB prescription in the 30-days post discharge from their index AMI. If ACE/ARB prescribing is sorted across AMI patients based on expected benefits (essential heterogeneity) we expected that the treatment rate variation identified by local area practice style differences reflects treatment choices for patients whose expected treatment benefits from ACE/ARBs are less definitive. As a result, we expected our estimates of the local average treatment effect (LATE) of ACE/ARBs on one-year patient survival to be lower than the upper limit RCT estimates (50 lives saved per 1000 high-risk patients). Our LATE estimates using various local area sizes are all less than this upper limit. Our LATE estimates are best used to assess whether higher rates of ACE/ARB prescribing post-AMI will increase survival rates. If the IV assumptions hold, our results do not provide clear evidence that higher treatment rates will increase survival rates. While our local practice style-based instrument described a substantial portion of treatment variation across local area sizes and, for the most part, treatment effect estimates were positive, large treatment effect standard errors preclude the rejection of the null hypothesis that no survival benefits are available from higher ACE/ARB prescribing rates. Our results suggest that providers may be correctly sorting ACE/ARB treatments to AMI patients in practice and that increasing the ACE/ARB treatment rate beyond 50% may do little to improve survival rates. However, care should be taken not to generalize these results to all AMI patients especially those patients that receive ACE/ARBs in current practice.
Key Findings.
The use of smaller local areas to measure practice styles as instruments exploits more treatment variation and results in smaller standard errors. However, if treatment effects are heterogeneous, the use of smaller local areas may increase the risk that local practice style measures are dominated by idiosyncratic differences in average treatment effectiveness across areas resulting in treatment effect estimates that are biased toward greater effectiveness.
What this adds to what is known
Local area practice style measures can be useful instruments in instrumental variable analysis, but the use of smaller local area sizes to generate greater treatment variation may result in treatment effect estimates that are biased toward higher effectiveness. Assessment of whether ecological bias can be mitigated by changing local area size requires the use of outside data sources.
What is the implication, what should change now
Researchers should be aware that if treatment effects are heterogeneous across patients, the use of smaller-sized local areas as the basis to measure local practice style as an instrument may result in treatment effect estimates that are biased in favor of treatment.
Appendix A
To assess the properties of our local area practice style measures with regard to local area size we derive a model of local practice style model based on geographic variation in provider treatment effectiveness beliefs. Similar results can be found using other local area factors as the source of practice style differences such as variation in the value of treatment outcomes across areas.66 Let expected patient outcome be described by the following model:
| A1 |
where Yi is the outcome for patient “i”. N designates the size of the geographic area around patient “i” as defined by the shortest driving distance required to locate the N nearest patients with the same clinical condition. TiN is the treatment choice for patient “i” living in the area N-sized. [(β10 + β1S Si) · PN] represents the range of treatment effectiveness beliefs across patients with characteristics Si in living in the N-sized area. Under this specification treatment effectiveness is heterogeneous across patients based on Si. If Si is characterized in a manner so that higher values of Si increase treatment effectiveness β1S will be positive. PN represents average provider treatment effectiveness beliefs in area N-sized. The larger PN the more effective the providers in the area believe treatment is across the range of Si. EN are ecological factors in the N-sized area that effect patient outcomes. Xi represents the measured characteristics for patient “i” that affect the outcome directly, while θiN represents the accumulated unmeasured characteristics of patient “i” living in the N-sized that affect outcome.
Further, describe treatment choice for patient “i” living in the N-sized area as:
| A2 |
where TiN, Xi, PN′ and Si are defined as above. Under essential heterogeneity Si is specified in (A2) because providers are assumed to (1) assess Si for each patient; (2) have knowledge of the effect of Si on treatment effectiveness; and (3) recommend treatment choice based on the value of Si for each patient. Higher Si values increase treatment effectiveness so α2 in (A2) will be positive. If PN is measured so that larger PN represents stronger treatment effectiveness beliefs, α3 will also be positive. Xi has no direct relationship with treatment effectiveness in (A1) so the theoretical justification for specifying Xi in the treatment choice equation is unclear. However, we include Xi in (A2) to remain consistent with general convention. μi equals the accumulated unmeasured characteristics for patient “i” that affect treatment choice but do not affect outcome.
If was able to be measured directly, PN could serve as a valid instrument under the following conditions (1) α3 ≠ 0; (2) Corr(PN, EN) = 0; (3) Corr(PN, Si) = 0; and (4) Corr(PN, θiN) = 0. Under these conditions IV estimation will yield a consistent estimate of the local average treatment effect (LATE) of Ti on Yi − β1PN -- that is specific to the distribution of Si values for the subset of patient whose treatment choices were affected by PN. β1PN will likely represent patients with moderate Si values as patients with very high Si values will likely receive treatment regardless of PN and patients with very low Si values will likely not receive treatment regardless of PN.
In this paper we measure PN using area treatment ratios (ATRs) for the local area around each patient residence ZIP code of size N. TiN, Yi, Xi and are observed by the researcher. We assume Si is not correlated with the underlying PN and is distributed evenly across patients with a mean of μS and variance . However, our ATR approach to measure local treatment preferences for areas of size N solves to:
| A3 |
where T̂i equals the predicted probability of treatment for patient “i” conditional on Xi. S̄N equals the mean of Si in the local area of size N. Local areas, out of chance, with a larger S̄N will have more patients apt to gain from treatment and more patients choosing treatment conditional on Xi. As a result, our ATRN measure will vary with both PN and S̄N. Therefore, when ATRN is used as an instrument when estimating (A2) in the first stage of 2SLS we expect that the ATRN value associated with each local area will be positively correlated with the unmeasured Si values for the patients within each local area. This positive correlation will result in an IV estimate of β1PN that is biased high relative to the true LATE for the subset of patients whose treatment choices were affected by PN. However, S̄N is distributed with mean μS and variance . Therefore, as N increases the variance in S̄N across local areas will diminish and a larger portion of the treatment variation described by ATRN will be generated from variation in PN, and, all else equal, the positive bias associated with estimates of β1PN will diminish.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John M. Brooks, University of Iowa, College of Pharmacy and College of Public Health.
Yuexin Tang, University of Iowa, College of Pharmacy
Cole G. Chapman, University of Iowa, College of Pharmacy
Elizabeth A. Cook, University of Iowa, College of Pharmacy
Elizabeth A. Chrischilles, University of Iowa, College of Public Health.
References
- 1.Sox HC, Goodman SN. The Methods of Comparative Effectiveness Research. Annu Rev Publ Health. 2012;33:425–445. doi: 10.1146/annurev-publhealth-031811-124610. [DOI] [PubMed] [Google Scholar]
- 2.Brooks JM. Supplement 1. Improving Characterization of Study Populations: the Identification Problem. In: Velentgas P, Dreyer NA, editors. Developing a Protocol for Observational Comparative Effectiveness Research (OCER): A User’s Guide. 12-EHC099. Rockville, MD: Agency for Healthcare Research and Quality; 2012. (Prepared by Outcome DEcIDE Center [Quintiles Outcome] under Contract No. HHSA29020050016I TO10) [PubMed] [Google Scholar]
- 3.Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000 Dec;29(6):1102. doi: 10.1093/oxfordjournals.ije.a019909. [DOI] [PubMed] [Google Scholar]
- 4.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 5.McMahon AD. Approaches to combat with confounding by indication in observational studies of intended drug effects. Pharmacoepidemiol Drug Saf. 2003 Oct-Nov;12(7):551–558. doi: 10.1002/pds.883. [DOI] [PubMed] [Google Scholar]
- 6.Zohoori N, Savitz DA. Econometric approaches to epidemiologic data: relating endogeneity and unobserved heterogeneity to confounding. Ann Epidemiol. 1997 May;7(4):251–257. doi: 10.1016/s1047-2797(97)00023-9. [DOI] [PubMed] [Google Scholar]
- 7.Angrist JD, Imbens GW. Two-Stage Least Squares Estimation of Average Causal Effects in Models with Variable Treatment Intensity. Journal of the American Statistical Association. 1995;90(430):431–442. [Google Scholar]
- 8.Angrist JD, Imbens GW, Rubin DB. Identification of Causal Effects Using Instrumental Variables. Journal of the American Statistical Association. 1996;91(434):444–455. [Google Scholar]
- 9.Angrist JD, Krueger AB. Instrumental variables and the search for identification: From supply and demand to natural experiments. Journal of Economic Perspectives. 2001;15:69–85. [Google Scholar]
- 10.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA. 1994 Sep 21;272(11):859–866. [PubMed] [Google Scholar]
- 11.Brooks JM, McClellan M, Wong HS. The marginal benefits of invasive treatments for acute myocardial infarction: does insurance coverage matter? Inquiry. 2000 Spring;37(1):75–90. [PubMed] [Google Scholar]
- 12.Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health Serv Res. 1998 Dec;33(5 Pt 1):1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 13.Imbens GW, Angrist JD. Identification and Estimation of Local Average Treatment Effects. Econometrica. 1994;62(2):467–475. [Google Scholar]
- 14.Harris KM, Remler DK. Who Is the Marginal Patient? Understanding Instrumental Variables Estimates of Treatment Effects. Health Services Research. 1998;33(5):1337–1360. [PMC free article] [PubMed] [Google Scholar]
- 15.McClellan M, McNeil B, Newhouse J. Does More Intensive Treatment of Acute Myocardial Infarction in the Elderly Reduce Mortality? Analysis using instrumental variables. JAMA. 1994;272(11):859–866. [PubMed] [Google Scholar]
- 16.Angrist JD. Two-Stage Least Squares Estimation of Average Causal Effects in Models With Variable Treatment Intensity. Journal of the American Statistical Association. 1995;90(430):431–442. [Google Scholar]
- 17.Angrist JD, Imbens GW, Rubin D. Identification of Causal Effects Using Instrumental Variables. Journal of the American Statistical Association. 1996;91(434):444–472. [Google Scholar]
- 18.McClellan M, McNeil BJ, Newhouse JP. Does More Intensive Treatment of Acute Myocardial-Infarction in the Elderly Reduce Mortality - Analysis Using Instrumental Variables. JAMA-J Am Med Assoc. 1994 Sep 21;272(11):859–866. [PubMed] [Google Scholar]
- 19.Fang G, Brooks JM, Chrischilles EA. Apples and Oranges? Interpretations of Risk Adjustment and Instrumental Variable Estimates of Intended Treatment Effects Using Observational Data. American Journal of Epidemiology. 2012 Jan;175(1):60–65. doi: 10.1093/aje/kwr283. [DOI] [PubMed] [Google Scholar]
- 20.Wong K, Campitelli MA, Stukel TA, Kwong JC. Estimating Influenza Vaccine Effectiveness in Community-Dwelling Elderly Patients Using the Instrumental Variable Analysis Method. Archives of Internal Medicine. 2012 Mar;172(6):484–491. doi: 10.1001/archinternmed.2011.2038. [DOI] [PubMed] [Google Scholar]
- 21.Zeliadt SB, Potosky AL, Penson DF, Etzioni R. Survival benefit associated with adjuvant androgen deprivation therapy combined with radiotherapy for high and low-risk patients with nonmetastatic prostate cancer. International Journal of Radiation Oncology Biol Phys. 2006;66(2):395–402. doi: 10.1016/j.ijrobp.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 22.Lu-Yao GL, Albertson PC, Moore DF, et al. Survival Following Primary Androgen Deprivation Therapy Among Men with Localized Prostate Cancer. Journal of the American Medical Association. 2008;300(2):173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadley J, Polsky D, Mandelblatt JS, et al. An Exploratory Instrumental Variable Analysis of the Outcomes of Localized Breast Cancer Treatments in a Medicare Population. Health Economics. 2003 Mar;12(3):171–186. doi: 10.1002/hec.710. [DOI] [PubMed] [Google Scholar]
- 24.Basu A, Heckman JJ, Navarro-Lozano S, Urzua S. Use of instrumental variables in the presence of heterogeneity and self-selection: An application to treatments of breast cancer patients. Health Economics. 2007 Nov;16(11):1133–1157. doi: 10.1002/hec.1291. [DOI] [PubMed] [Google Scholar]
- 25.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of Observational Studies in the Presence of Treatment Selection Bias: Effects of Invasive Cardiac Management on AMI Survival Using Propensity Score and Instrumental Variable Methods. JAMA. 2007 Jan 17;297(3):278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks JM, Chrischilles EA. Heterogeneity and the interpretation of treatment effect estimates from risk adjustment and instrumental variable methods. Medical Care. 2007 Oct;45(10):S123–S130. doi: 10.1097/MLR.0b013e318070c069. [DOI] [PubMed] [Google Scholar]
- 27.Brooks JM, Chrischilles EA, Scott SD, Chen-Hardee SS. Was breast conserving surgery underutilized for early stage breast cancer? Instrumental variables evidence for stage II patients from Iowa. Health Services Research. 2003 Dec;38(6):1385–1402. doi: 10.1111/j.1475-6773.2003.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang G, Brooks JM, Chrischilles EA. A New Method to Measure Geographic Variation in Prescription Use and Its Implications for Comparative Effectiveness Research. Medical Care. 2010;40:710–717. doi: 10.1097/MLR.0b013e3181e41bb2. [DOI] [PubMed] [Google Scholar]
- 29.Earle CC, Tsai JS, Gelber R, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of Chemotherapy for Advanced Lung Cancer in the Elderly: Instrumental Variable and Propenstiy Analysis. Journal of Clinical Oncology. 2001 Feb 15;19(4):1064–1070. doi: 10.1200/JCO.2001.19.4.1064. [DOI] [PubMed] [Google Scholar]
- 30.Fang G, Brooks JM, Chrischilles EA. Comparison of Instrumental Variable Analysis Using a New Instrument With Risk Adjustment Methods to Reduce Confounding by Indication. American Journal of Epidemiology. 2012 Jun 1;175(11):1142–1151. doi: 10.1093/aje/kwr448. [DOI] [PubMed] [Google Scholar]
- 31.Gray SW, Landrum MB, Lamont EB, McNeil BJ, Jaklitsch MT, Keating NL. Improved Outcomes Associated With Higher Surgery Rates for Older Patients With Early Stage Nonsmall Cell Lung Cancer. Cancer. 2012 Mar 1;118(5):1404–1411. doi: 10.1002/cncr.26363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative Effectiveness of Prostate Cancer Treatments: Evaluating Statistical Adjustments for Confounding in Observational Data. Journal of the National Cancer Institute. 2010 Dec;102(23):1780–1793. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks JM, Chrischilles EA, Landrum MB, et al. Survival Implications Associated with Variation in Mastectomy Rates for Early-Staged Breast Cancer. International Journal of Surgical Oncology. 2012 doi: 10.1155/2012/127854. forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blakely TA, Woodward AJ. Ecological effects in multi-level studies. Journal of Epidemiology and Community Health. 2000 May;54(5):367–374. doi: 10.1136/jech.54.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heckman JJ. Econometric Causality. International Statistical Review. 2008;76(1):1–27. [Google Scholar]
- 36.Brooks JM, Gang F. Interpreting Treatment Effect Estimates with Heterogeneity and Choice: Simulation Model Results. Clinical Therapeutics. 2009;31(4):902–919. doi: 10.1016/j.clinthera.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Angrist JD. Treatment Effect Heterogeneity in Theory and Practice. The Economic Journal. 2004 Mar;114:C52–C83. [Google Scholar]
- 38.Brooks JM, Chrischilles EA. Heterogeneity and the Interpretation of Treatment Effect Estimates from Risk-Adjustment and Instrumental Variable Methods. Medical Care. 2007 Oct;45(10 supplement):S123–S130. doi: 10.1097/MLR.0b013e318070c069. [DOI] [PubMed] [Google Scholar]
- 39.Reeder GS. Angiotensin Converting Enzyme Inhibitors and Receptor Blockers in Acute Myocardial Infarction: Recommendations for Use. In: Basow DS, editor. UpToDate. Waltham, MA: UptoDate; 2012. [Google Scholar]
- 40.Reeder GS. Angiotensin Converting Enzyme Inhibitors and Receptor Blockers in Acute Myocardial Infarction: Clinical trials. In: Basow DS, editor. UpToDate. Waltham, MA: UpToDate; 2012. [Google Scholar]
- 41.Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, Winkelmayer WC. Improvements in Long-Term Mortality After Myocardial Infarction and Increased Use of Cardiovascular Drugs After Discharge. Journal of the American College of Cardiology. 2008;51(13):1247–1254. doi: 10.1016/j.jacc.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 42.Jencks SF, Huff ED, Cuerdon T. Change in the quality of care delivered to Medicare beneficiaries, 1998–1999 to 2000–2001. JAMA-J Am Med Assoc. 2003 Jan;289(3):305–312. doi: 10.1001/jama.289.3.305. [DOI] [PubMed] [Google Scholar]
- 43.Heckman JJ, Urzua S, Vytlacil E. Understanding Instrumental Variables in Models with Essential Heterogeneity. The Review of Economics and Statistics. 2006;88(3):389–432. [Google Scholar]
- 44.Blais C, Hamel D, Rinfret S. Impact of Socioeconomic Deprivation and Area of Residence on Access to Coronary Revascularization and Mortality After a First Acute Myocardial Infarction in Quebec. Can J Cardiol. 2012 Mar-Apr;28(2):169–177. doi: 10.1016/j.cjca.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Freedman VA, Grafova IB, Rogowski J. Neighborhoods and Chronic Disease Onset in Later Life. American Journal of Public Health. 2011 Jan;101(1):79–86. doi: 10.2105/AJPH.2009.178640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao SV, Schulman KA, Curtis LH, Gersh BJ, Jollis JG. Socioeconomic status and outcome following acute myocardial infarction in elderly patients. Archives of Internal Medicine. 2004 May;164(10):1128–1133. doi: 10.1001/archinte.164.10.1128. [DOI] [PubMed] [Google Scholar]
- 47.Molshatzki N, Drory Y, Myers V, et al. Role of Socioeconomic Status Measures in Long-term Mortality Risk Prediction After Myocardial Infarction. Medical Care. 2011 Jul;49(7):673–678. doi: 10.1097/MLR.0b013e318222a508. [DOI] [PubMed] [Google Scholar]
- 48.Gerber Y, Benyamini Y, Goldbourt U, Drory Y Israel Study Grp First Acute M. Neighborhood Socioeconomic Context and Long-Term Survival After Myocardial Infarction. Circulation. 2010 Jan;121(3):375–U354. doi: 10.1161/CIRCULATIONAHA.109.882555. [DOI] [PubMed] [Google Scholar]
- 49.Tonne C, Schwartz J, Mittleman M, Melly S, Suh H, Goldberg R. Long-term survival after acute myocardial infarction is lower in more deprived neighborhoods. Circulation. 2005 Jun;111(23):3063–3070. doi: 10.1161/CIRCULATIONAHA.104.496174. [DOI] [PubMed] [Google Scholar]
- 50.Stjarne MK, Fritzell J, De Leon AP, Hallqvist J, Grp SS. Neighborhood socioeconomic context, individual income and myocardial infarction. Epidemiology. 2006 Jan;17(1):14–23. doi: 10.1097/01.ede.0000187178.51024.a7. [DOI] [PubMed] [Google Scholar]
- 51.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease - A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004 Jun;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 52.Spertus JA, Jones PG, Masoudi FA, Rumsfeld JS, Krumholz HM. Factors Associated With Racial Differences in Myocardial Infarction Outcomes. Annals of Internal Medicine. 2009 Mar 3;150(5):314–324. doi: 10.7326/0003-4819-150-5-200903030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulz AJ, House JS, Israel BA, et al. Relational pathways between socioeconomic position and cardiovascular risk in a multiethnic urban sample: complexities and their implications for improving health in economically disadvantaged populations. Journal of Epidemiology and Community Health. 2008 Jul;62(7):638–646. doi: 10.1136/jech.2007.063222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: Estimating positive predictive value on the basis of review of hospital records. American Heart Journal. 2004 Jul;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Margulis AV, Choudhry NK, Dormuth CR, Schneeweiss S. Variation in initiating secondary prevention after myocardial infarction by hospitals and physicians, 1997 through 2004. Pharmacoepidemiology and Drug Safety. 2011 Oct;20(10):1088–1097. doi: 10.1002/pds.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angrist JD. Estimation of Limited Dependent Variable Models With Dummy Endogenous Regressors: Simple Strategies for Empirical Practice. Journal of Business & Economic Statistics. 2001 Jan;19(1):2–16. [Google Scholar]
- 57.Angrist JD, Pischke J-S. Mostly Harmless Econometrics: An Empiricist’s Companion. Princeton, New Jersey: Princeton University Press; 2009. [Google Scholar]
- 58.Chow G. Tests of Equality between Sets of Coeffiecients in Two Linear Models. Econometrica. 1960;28(3):591–605. [Google Scholar]
- 59.Hausman JA. Specification and Estimation of Simultaneous Equation Models. In: Griliches Z, Intriligator MD, editors. Handbook of Econometrics. Vol. 1. New York: North-Holland Publishing Company; 1983. pp. 392–448. [Google Scholar]
- 60.Murray CJL, Kulkarni SC, Michaud C, et al. Eight Americas: Investigating mortality disparities across races, counties, and race-counties in the United States (vol 3, pg 4, 2005) PLoS Medicine. 2006 Dec;3(12):2465–2465. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cochran WG. Some Methods of Strengthening the Common Chi-Squared Tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 62.Armitage P. Tests for Linear Trends in Proportions and Frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 63.Heckman JJ. Building Bridges Between Structural and Program Approaches to Evaluating Policy. Journal of Economic Literature. 2010 Jun;68(2):356–398. doi: 10.1257/jel.48.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McClellan M, Newhouse JP. Instrumental Variables Analysis Applications in Health Services Research -- A Special Supplement to HSR -- Overview of Supplement Issue. Health Services Research. 2000;35(5):1061–1069. [PMC free article] [PubMed] [Google Scholar]
- 65.Newhouse JP, McClellan M. Econometrics in outcomes research: The use of instrumental variables. Annual Review of Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 66.Brooks JM, Fang G. Interpreting Treatment-Effect Estimates With Heterogeneity and Choice: Simulation Model Results. Clinical Therapeutics. 2009 Apr;31(4):902–919. doi: 10.1016/j.clinthera.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]