Abstract
AIM: To investigate the reasons for the occurrence of the pink-color sign of iodine-unstained lesions.
METHODS: In chromoendoscopy, the pink-color sign of iodine-unstained lesions is recognized as useful for the diagnosis of esophageal squamous cell carcinoma. Patients with superficial esophageal neoplasms treated by endoscopic resection were included in the study. Areas of mucosa with and without the pink-color sign were evaluated histologically. The following histologic features that were possibly associated with the pink-color sign were evaluated. The keratinous layer and basal cell layer were classified as present or absent. Cellular atypia was classified as high grade, moderate grade or low grade, based on nuclear irregularity, mitotic figures, loss of polarity, chromatin pattern and nuclear/cytoplasmic ratio. Vascular change was assessed based on dilatation, tortuosity, caliber change and variability in shape. Vessels with these four findings were classified as positive for vascular change. Endoscopic images of the lesions were captured immediately after iodine staining, 2-3 min after iodine staining and after complete fading of iodine staining. Quantitative analysis of color changes after iodine staining was also performed.
RESULTS: A total of 61 superficial esophageal neoplasms in 54 patients were included in the study. The lesions were located in the cervical esophagus in one case, the upper thoracic esophagus in 10 cases, the mid-thoracic esophagus in 33 cases, and the lower thoracic esophagus in 17 cases. The median diameter of the lesions was 20 mm (range: 2-74 mm). Of the 61 lesions, 28 were classified as pink-color sign positive and 33 as pink-color sign negative. The histologic diagnosis was high-grade intraepithelial neoplasia (HGIN) or cancer invading into the lamina propria in 26 of the 28 pink-color sign positive lesions. There was a significant association between pink-color sign positive epithelium and HGIN or invasive cancer (P = 0.0001). Univariate analyses found that absence of the keratinous layer and cellular atypia were significantly associated with the pink-color sign. After Bonferroni correction, there were no significant associations between the pink-color sign and presence of the basal membrane or vascular change. Multivariate analyses found that only absence of the keratinous layer was independently associated with the pink-color sign (OR = 58.8, 95%CI: 5.5-632). Quantitative analysis was performed on 10 superficial esophageal neoplasms with both pink-color sign positive and negative areas in 10 patients. Pink-color sign positive mucosa had a lower mean color value in the late phase (pinkish color) than in the early phase (yellowish color), and had similar mean color values in the late and final phases. These findings suggest that pink-color positive mucosa underwent color fading from the color of the iodine (yellow) to the color of the mucosa (pink) within 2-3 min after iodine staining. Pink-color sign negative mucosa had similar mean color values in the late and early phases (yellowish color), and had a lower mean color value in the final phase (pinkish color) than in the late phase. These findings suggest that pink-color sign negative mucosa did not undergo color fading during the 2-3 min after iodine staining, and underwent color fading only after spraying of sodium thiosulfate.
CONCLUSION: The pink-color sign was associated with absence of the keratinous layer. This sign may be caused by early fading of iodine staining.
Keywords: Chromoendoscopy, Esophageal cancer, Esophageal squamous neoplasia, Iodine staining, Pink-color sign
Core tip: The pink-color sign of iodine-unstained lesions is useful for the diagnosis of esophageal squamous cell carcinoma. We investigated histologic findings of esophageal neoplasms, and found that absence of the keratinous layer because of neoplastic cell proliferation may be responsible for the pink-color sign. Quantitative analysis of color showed that pink-color sign positive mucosa underwent early color fading from the color of the iodine (yellow) to the color of the mucosa (pink) within 2-3 min. Based on these results, we speculated on the mechanism underlying the pink-color sign. These findings may improve our understanding of the characteristics of esophageal neoplasms.
INTRODUCTION
Esophageal cancer is the sixth most common cause of cancer-related mortality worldwide[1]. The overall survival of patients with esophageal cancer remains poor, regardless of histologic type. However, a favorable prognosis can be expected after treatment with esophagectomy[2,3], chemoradiotherapy[4,5] or endoscopic resection[6-9] if the cancer is detected at an early stage.
Conventional endoscopy has limited usefulness for the treatment of esophageal cancer, because it is not easy to identify early neoplastic changes[10,11]. Chromoendoscopy with iodine staining is reported to be more sensitive for the early diagnosis of esophageal squamous cell carcinoma[12,13], but has low specificity and requires multiple biopsy specimens[10,14]. A dramatic color change after iodine staining, from the initial yellow color to a pink color 2-3 min later, is known as the pink-color sign, and is useful for identifying cancerous lesions[15,16]. This sign has been reported to dramatically improve specificity for esophageal high-grade intraepithelial neoplasia (HGIN) and invasive cancer[15,16]. Choosing adequate biopsy sites is sometimes difficult, especially in patients with scattered-type staining of the esophagus, which is characterized by multiple Lugol-voiding lesions[17]. Some of these iodine-unstained lesions may indicate inflammation or low-grade intraepithelial neoplasia (LGIN), and in such cases a lack of iodine staining is not a good indication for taking a biopsy specimen. Because of its high specificity, the pink-color sign is a good indicator for choosing adequate biopsy sites in patients with scattered-type staining. However, the mechanism underlying the occurrence of the pink-color sign has not been fully investigated. Improved understanding of this mechanism may improve our understanding of the characteristics of the relevant lesions, and increase the likelihood of accurate diagnosis. This study therefore aimed to clarify the histologic changes responsible for the occurrence of the pink-color sign in esophageal squamous neoplasia.
MATERIALS AND METHODS
Endoscopic examination and resection
The current clinical investigation was conducted during routine endoscopic procedures for resection of esophageal squamous lesions. Patients with superficial esophageal neoplasia confirmed by histologic examination were included in the study. Superficial esophageal neoplasia was defined as a lesion limited to the submucosa. Typical endoscopic findings were superficial protruding type, superficial flat type and superficial excavated type. If a lesion had a large broad-based protrusion, crater and stiffened wall, it was diagnosed as advanced cancer. Patients were excluded if they had previously undergone surgery, chemotherapy or radiotherapy for esophageal cancer. The endoscopic procedures were performed using a high-resolution magnifying upper-gastrointestinal endoscope (GIF-Q240Z or GIF-H260Z; Olympus, Tokyo, Japan). The structure enhancement function of the video processor was set at level B8 for narrow-band imaging (NBI). A black soft hood (MB-162 for GIF-Q240Z or MB-46 for GIF-H260Z; Olympus) was mounted on the tip of the endoscope to maintain an adequate distance between the tip of the endoscopic zoom lens and the mucosal surface during observations. The endoscopic procedure was performed under intravenous sedation with midazolam (Dormicam; Yamanouchi Pharma, Tokyo, Japan) and pentazocine (Pentazin; Sankyo Pharmaceuticals, Tokyo, Japan).
The esophageal mucosa was initially examined using white-light imaging or NBI. A catheter was then used to spray 20-40 mL of 0.6%-1.2% iodine solution until the esophageal mucosa was evenly stained, and the subsequent color changes were observed. Immediately after spraying of the iodine solution, the normal mucosa was dark brown, whereas abnormal mucosa suspicious of dysplasia or cancer was yellow. The iodine-unstained (yellow) areas were classified as pink-color sign positive or pink-color sign negative. In pink-color sign positive mucosa, the yellow areas changed to pink within 2-3 min of spraying, and in pink-color sign negative mucosa, these areas remained yellow after 3 min. Representative areas (2-5 mm diameter) were marked with marker dots, and the lesions were resected by endoscopic submucosal dissection or endoscopic mucosal resection. Iodine staining and marking of pink-color sign positive and negative areas were performed immediately before resection. Considering the potential disadvantages of intraoperative endoscopy and iodine staining, patients requiring surgical resection were excluded. To ensure that the study protocol was strictly followed, marking before endoscopic resection and confirmation of marking after endoscopic resection were performed by one of two endoscopists (Ishihara R or Kanzaki H). Procedures that were performed without these two endoscopists in attendance were excluded from the study. Written informed consent was obtained from all patients before endoscopic examination and resection of lesions. Institutional review board approval was granted for a retrospective chart review and analysis of the data.
Histologic evaluation
All specimens were cut into 2-mm slices and embedded in paraffin. Sections were cut from the paraffin blocks and stained with hematoxylin and eosin. The pink-color sign positive and negative lesions marked by the marker dots were examined histologically. The depth of cancer involvement was classified according to the Japanese Classification of Esophageal Carcinoma[18], and intraepithelial neoplasms were classified as LGIN or HGIN, according to the World Health Organization classification[19]. Intraepithelial cancer was included in HGIN.
The following histologic features that were possibly associated with the pink-color sign were evaluated. The keratinous layer (Figure 1) and basal cell layer were classified as present or absent. Cellular atypia was classified as high grade, moderate grade or low grade, based on nuclear irregularity, mitotic figures, loss of polarity, chromatin pattern and nuclear/cytoplasmic ratio. Vascular change was assessed based on dilatation, tortuosity, caliber change and variability in shape. Vessels with these four findings were classified as positive for vascular change[20]. All histologic assessments were performed by the same pathologist (SI), who was blinded to the endoscopic and clinical findings.
Figure 1.
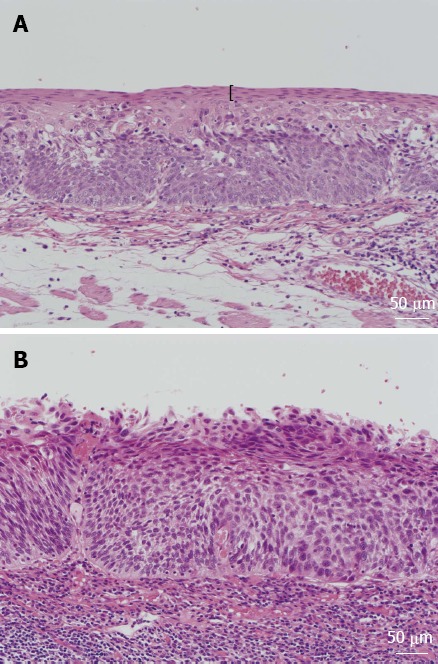
Histologic findings of esophageal lesions. A: Esophageal lesion with keratinous layers, shown by black parentheses; B: Esophageal lesion without keratinous layers.
Quantitative analysis of color
This study included esophageal cancers those have both pink-color sign positive and pink-color sign negative areas. The esophageal mucosa was initially examined with white-light imaging or NBI. A catheter was used to spray 20-40 mL of 1.2% iodine solution until the normal esophageal mucosa was evenly stained, and the subsequent color changes were examined. Sodium thiosulfate solution was then sprayed to relieve symptoms caused by the iodine, which also accelerated the color fading by reduction of the iodine.
The digital images were used to perform quantitative analysis of the color changes after iodine staining. Endoscopic images of the lesions were captured immediately after iodine staining, 2-3 min after iodine staining and after complete fading of iodine staining, taking care to ensure that all images were obtained from a similar direction and a similar distance, to enable accurate analysis (Figure 2). All the images were captured under the instruction of one endoscopist (Ishihara R). The images were stored in bitmap format (.bmp) with a resolution of 640 × 480 pixels. A small region of interest was chosen in both the pink-color sign positive and pink-color sign negative areas. These regions of interest were carefully chosen to be of similar size in each area (Figure 3). The red-green-blue components of each region of interest were calculated using Image J software (National Institutes of Health, Bethesda, MD, United States), and color diagrams were created using the graphing function of Microsoft Excel (Microsoft Corp., Redmond, WA, United States).
Figure 2.
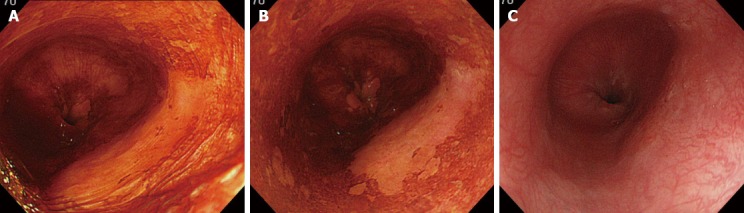
Endoscopic image of type IIc esophageal cancer. A: Endoscopic image of type IIc esophageal cancer immediately after iodine staining; B: Endoscopic image of type IIc esophageal cancer 2 min after iodine staining. The pink-color sign is observed on the right side of the lesion; C: Endoscopic image of type IIc esophageal cancer 3 min after sodium thiosulfate spraying.
Figure 3.
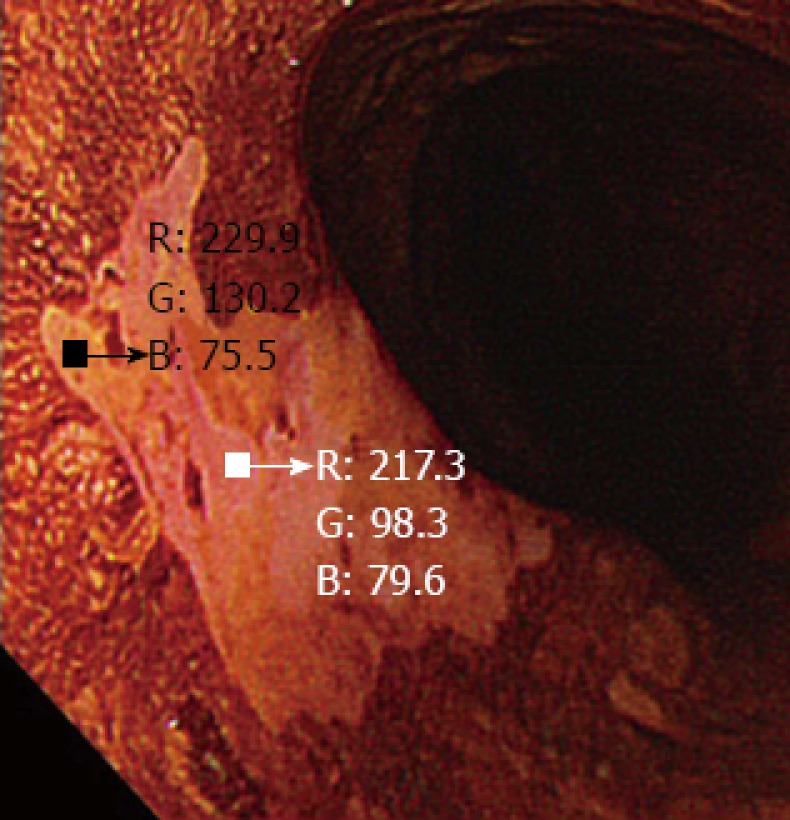
A small region of interest was chosen in both the pink-color sign positive and pink-color sign negative areas. These regions of interest were carefully chosen to be of similar size in each area. The red-green-blue components of each region of interest were calculated using Image J software.
The LU’V’ color system is a uniform color space that was adopted by the Commission Internationale de l’E’ clairage in 1976 and is used to systematically represent the different colors[21,22]. A color system is a model for representing colors in terms of intensity values. Generally, colors are described using color systems with three or four dimensions (red-green-blue or cyan-magenta-yellow-black). The diagram of the LU’V’ system has U’ and V’ coordinates that represent the chromaticity values of each color. This color space is designed to be perceptually uniform, meaning that a given change in value roughly corresponds to the same perceptual difference over any part of the space. Using this system, color can be quantified and evaluated on a two-dimensional plane. However, determining the range of color is challenging because there is no clear border between colors.
Statistical analysis
The relationships between the pink-color sign and each histologic finding were analyzed. If more than one lesion was resected from a patient, each lesion was considered separately for the purposes of statistical analysis. Univariate analyses of the relationships between the pink-color sign and the histologic findings were performed using the χ2 test with Yates’ correction. Factors independently associated with the pink-color sign were identified using multivariate logistic regression analysis. The model fit was assessed using the Hosmer-Lemeshow test. A two-sided P value of < 0.05 was considered statistically significant. A Bonferroni-adjusted P-value was used for multiple comparisons to control for experimental errors due to multiple testing. All analyses were performed using SPSS software version 11.0 (SPSS Inc, Chicago, IL, United States).
RESULTS
Histologic evaluation of pink-color sign positive areas
A total of 97 patients with superficial esophageal cancer were treated by endoscopic resection at the Osaka Medical Center for Cancer and Cardiovascular Diseases from May 31, 2011 to March 1, 2012. Of these, 10 patients were excluded because of previous radiation, 29 were excluded because endoscopic resection was performed without the attendance of the two endoscopists (Ishihara R or Kanzaki H) and 4 were excluded because no images were captured at 2-3 min after iodine staining. A total of 61 superficial esophageal neoplasms in 54 patients were included in the study. The lesions were located in the cervical esophagus in one case, the upper thoracic esophagus in 10 cases, the mid-thoracic esophagus in 33 cases, and the lower thoracic esophagus in 17 cases. The median diameter of the lesions was 20 mm (range: 2-74 mm) (Table 1). Of the 61 lesions, 28 were classified as pink-color sign positive and 33 as pink-color sign negative. The histologic diagnosis was HGIN or cancer invading into the lamina propria in 26 of the 28 pink-color sign positive lesions. Two lesions that were classified as pink-color sign positive were diagnosed as LGIN. One of these lesions had a thin keratinous layer and mild cellular atypia. This lesion showed an obscured pink-color sign, which was classified as positive in this study. In retrospect, it is possible that this lesion should have been classified as pink-color sign negative. The other lesion did not have a keratinous layer and had moderate cellular atypia. However, this lesion showed surface differentiation and was diagnosed as LGIN because obvious cytological abnormalities were confined to the lower half of the squamous epithelium. There was a significant association between pink-color sign positive epithelium and HGIN or invasive cancer (P = 0.0001) (Table 2). There was also a significant association between the presence of a keratinous layer and HGIN or invasive cancer (P = 0.0007).
Table 1.
Characteristics of patients and lesions
| Characteristics | n |
| Gender | |
| Male | 50 |
| Female | 4 |
| Age (yr) | |
| Median (range) | 67 (45-82) |
| Lesion location | |
| Cervical esophagus | 1 |
| Upper thoracic esophagus | 10 |
| Middle thoracic esophagus | 33 |
| Lower thoracic esophagus | 17 |
| Lesion size (mm) | |
| Median (range) | 20 (2-74) |
| Histological diagnosis of the marked area | |
| LGIN | 21 |
| HGIN | 37 |
| LPM | 3 |
LGIN: Low-grade intraepithelial neoplasia; HGIN: High-grade intraepithelial neoplasia; LPM: Cancer invading into the lamina propria.
Table 2.
Associations between histologic diagnosis, keratinous layer and pink-color sign
| HGIN or invasive cancer | LGIN | P value | |
| Pink-color-sign | 0.0001 | ||
| Positive | 26 | 2 | |
| Negative | 14 | 19 | |
| Keratinous layer | 0.0007 | ||
| Present | 19 | 20 | |
| Absent | 21 | 1 |
HGIN: High-grade intraepithelial neoplasia; LGIN: Low-grade intraepithelial neoplasia.
Univariate analyses (Table 3) found significant associations between the pink-color sign and absence of the keratinous layer or cellular atypia. After Bonferroni correction, there were no significant associations between the pink-color sign and presence of the basal membrane or vascular change. Multivariate analyses (Table 4) showed that absence of the keratinous layer was independently associated with the pink-color sign (OR = 58.8, 95%CI: 5.5-632). Hosmer-Lemeshow testing indicated that the model achieved a sufficient goodness-of-fit (P = 0.678).
Table 3.
Associations between pink-color sign and histologic findings (univariate analysis)
| Pink-color-sign positive | Pink-color-sign negative | P value | |
| Keratinous layer | < 0.0001 | ||
| Present | 7 | 32 | |
| Absent | 21 | 1 | |
| Cellular atypia1 | 0.004 | ||
| Mild | 1 | 13 | |
| Moderate | 20 | 19 | |
| Severe | 7 | 1 | |
| Presence of basal membrane | 0.018 | ||
| Yes | 14 | 27 | |
| No | 14 | 6 | |
| Vascular change | 0.070 | ||
| Severe | 20 | 15 | |
| Mild | 8 | 18 | |
Cellular atypia was classified based on nuclear irregularity, mitotic figures, loss of polarity, chromatin pattern and nuclear/cytoplasmic ratio.
Table 4.
Associations between pink-color sign and histologic findings (multivariate analysis)
| OR (95%CI) | P value | |
| Keratinous layer | 0.001 | |
| Present | 1 | |
| Absent | 58.8 (5.5-632) | |
| Cellular atypia1 | 0.580 | |
| Mild | 1 | |
| Moderate | 3.5 (0.3-35.5) | |
| Severe | 3.4 (0.07-165) | |
| Presence of basal membrane | ||
| Yes | 1 | 0.610 |
| No | 1.6 (0.3-9.3) | |
| Vascular change | ||
| Mild | 1 | 0.770 |
| Severe | 1.3 (0.3-6.3) |
Cellular atypia was classified based on nuclear irregularity, mitotic figures, loss of polarity, chromatin pattern and nuclear/cytoplasmic ratio.
Quantitative analysis of the pink-color sign
A total of 1373 patients underwent esophagogastroduodenoscopy at the Osaka Medical Center for Cancer and Cardiovascular Diseases from September 21, 2012 to December 5, 2012. Of these, 29 were diagnosed with esophageal squamous cell carcinoma. Fourteen of these 29 patients were excluded for the following reasons: history of chemoradiotherapy (8 patients), the entire lesion was pink-color sign positive (4 patients), or the pink-color sign was negative (2 patients). Ten of the 15 patients that had lesions with both pink-color sign positive and pink-color sign negative areas were examined under the instruction of the endoscopist (Ishihara R) and were included in the analysis. Endoscopic images of the lesions immediately after iodine staining (early phase), 2-3 min after iodine staining (late phase) and after complete fading of iodine staining (final phase) were analyzed (Figure 4). The mean U’ and V’ values of the pink-color sign positive and negative areas are shown in Figure 5. Pink-color sign positive mucosa had a lower mean V’ value in the late phase (pinkish color) than in the early phase (yellowish color), and had similar mean U’ and V’ values in the late and final phases (Figure 5A). These findings suggest that pink-color positive mucosa underwent color fading from the color of the iodine (yellow) to the color of the mucosa (pink) within 2-3 min after iodine staining. Pink-color sign negative mucosa had similar mean U’ and V’ values in the late and early phases (yellowish color), and had a lower mean V’ value in the final phase (pinkish color) than in the late phase (Figure 5B). These findings suggest that pink-color sign negative mucosa did not undergo color fading during the 2-3 min after iodine staining, and underwent color fading only after spraying of sodium thiosulfate.
Figure 4.
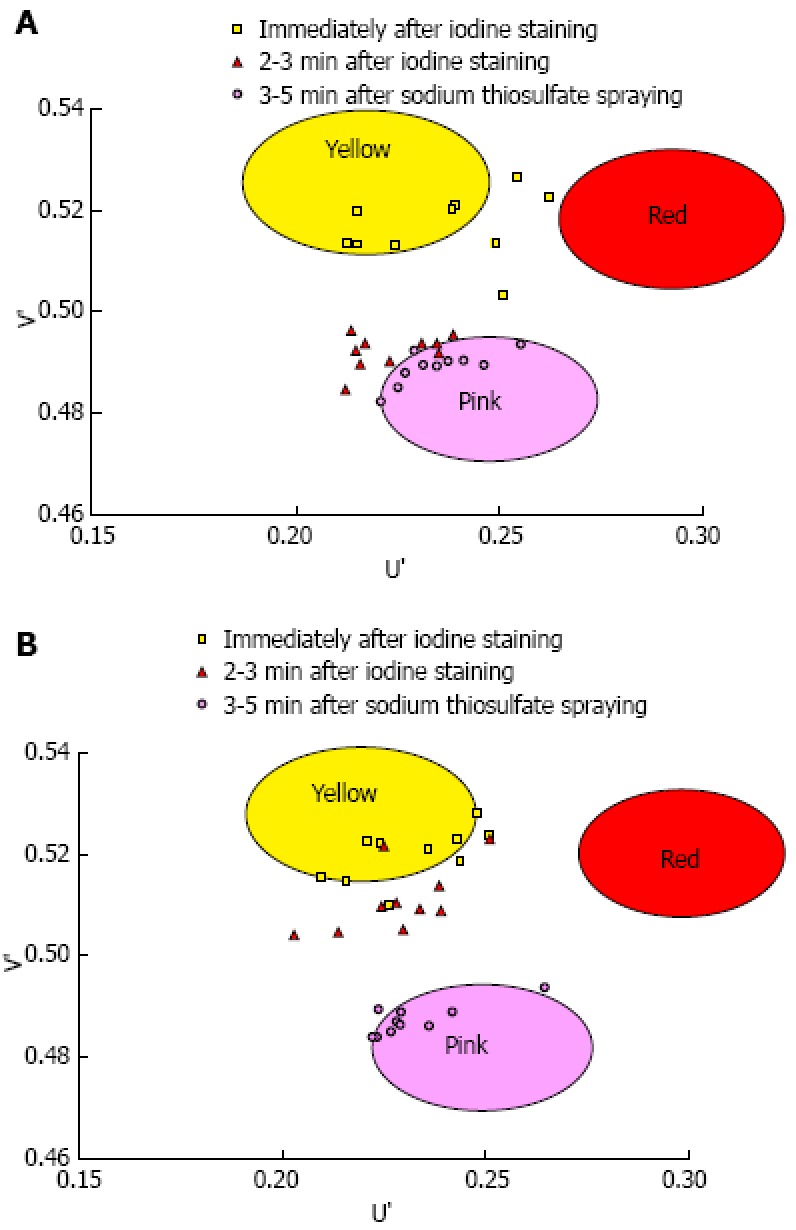
The U’ and V’ values of the pink-color sign positive mucosa and negative mucosa in the early, late and final phases were plotted on a color diagram. A: Pink-color sign positive mucosa; B: Pink-color sign negative mucosa.
Figure 5.
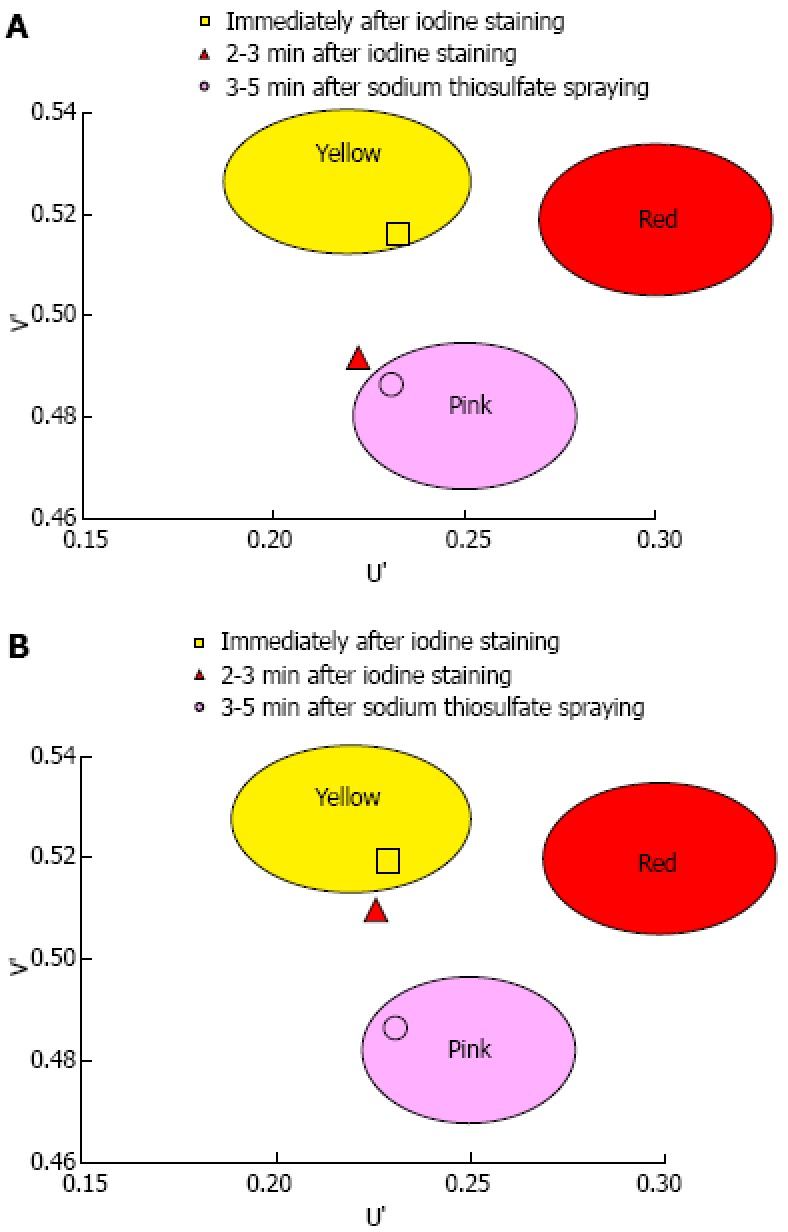
Mean U’ and V’ values of the mucosa in the early, late and final phases were plotted on a color diagram. A: Pink-color sign positive mucosa had a lower mean V’ value in the late phase (pinkish color) than in the early phase (yellowish color), suggesting that the pink-color sign positive mucosa underwent a color change from yellow to pink. The mucosa had similar mean U’ and V’ values in the late and final phases, suggesting that the color of pink-color sign positive mucosa in the late stage was similar to the color of mucosa after complete fading of iodine staining; B: Pink-color sign negative mucosa had similar mean U’ and V’ values in the early and late phases (yellowish color), suggesting that pink-color sign negative mucosa did not change in color during this time period.
DISCUSSION
Analysis of the endoscopic and histologic findings of this study found that absence of the keratinous layer was independently associated with the pink-color sign. Quantitative analysis of color changes found that pink-color sign positive mucosa changed from yellowish to pinkish within 2-3 min after iodine staining, suggesting that the mucosa underwent early color fading from the color of the iodine (yellow) to the color of the mucosa (pink).
This study did not include all patients who met our inclusion criteria, because we wanted to ensure that all procedures were of high quality to obtain accurate results. Marking before endoscopic resection and confirmation of marking after endoscopic resection were performed by one of two endoscopists (Ishihara R or Kanzaki H) to accurately identify the region of interest. All endoscopic images were captured under the instruction of one endoscopist (Ishihara R) to ensure that they were captured under similar conditions. This may have caused some selection bias, but it was felt necessary to limit the number of endoscopists involved for this detailed analysis.
Figure 6 shows our speculated mechanism for the occurrence of the pink-color sign. Locally administered iodine is usually absorbed into the epithelium by passive diffusion[23]. In normal esophageal epithelium, absorbed iodine combines with glycogen in the micro-granules of the prickle cells[24]. The resulting glycogen-iodine complex gives the epithelium a brown color. In the epithelium of neoplastic lesions, the prickle-cell layer is usually replaced by neoplastic cells, and no glycogen-iodine complex is formed. The yellow iodine solution therefore gives the epithelium a yellow color.
Figure 6.
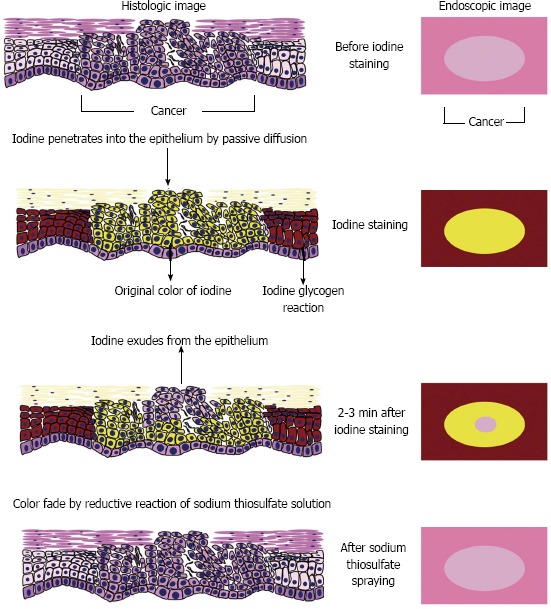
Speculated mechanism of the pink-color sign.
In the epithelium of neoplastic lesions, the yellow color may fade because of reduction of the iodine, absorption of iodine into the bloodstream, or leakage of iodine into the esophageal lumen. Application of sodium thiosulfate solution reduces the adverse effects of iodine staining and accelerates color fading by reduction of the iodine[25]. However, iodine is a strong oxidizing agent[26], and reduction of iodine requires a strong reducing agent. Absorption of iodine by the blood stream may occur in the esophagus[27]. However, iodine is mainly absorbed in the small intestine[28] and the amount of iodine absorbed by the esophagus is not large. Leakage of iodine into the esophageal lumen may therefore be the main cause of color fading, rather than reduction of iodine or absorption of iodine into the bloodstream.
Epithelium plays an important role in regulating the permeability of mucosa, and serves as a barrier between the outside world and the internal milieu of the organism[29,30]. The barrier function of epithelium is determined by its microstructure, and varies among different types of epithelium. The keratin layer of squamous epithelium has a barrier function[29,30], and may play an important role in preventing leakage of iodine from the epithelium. Disruption of the normal epithelial structure, especially of the keratin layer, may increase early leakage of iodine and early color fading. Considering the association between the absence of the keratinous layer and the pink-color sign, this may be the mechanism underlying the occurrence of the sign.
Neoplastic lesions show less staining when exposed to iodine solution than normal mucosa. However, both cancer and LGIN result in iodine-unstained areas. Assessment of iodine staining without assessment of the pink-color sign is therefore not very accurate for the diagnosis of cancer[10,14]. The keratinous layer was found to be absent in areas where most of the epithelium was replaced by neoplastic cells. Most areas that were pink-color sign positive had a proliferation of neoplastic cells in the upper half of the epithelium. The pink-color sign was associated with HGIN or cancer, those are both characterized by abnormal cells in the upper half of the epithelium. Accurate endoscopic diagnosis of esophageal lesions is therefore possible by assessment of the pink-color sign after iodine staining.
Differentiating HGIN or cancer from LGIN is important when deciding on a treatment strategy, because resection of the lesion is required for HGIN and cancer[19]. Histologic diagnosis of biopsy specimens may result in misdiagnosis if the correct area was not biopsied. Areas that are pink-color sign positive should always be biopsied, because this finding is closely associated with HGIN and cancer. Moreover, the keratinous layer is usually absent in areas that are pink-color sign positive, and it may therefore be relatively easy to biopsy neoplastic cells from these areas. However, if further studies confirm that the accuracy of endoscopic diagnosis is similar to that of biopsy diagnosis, cancer could eventually be diagnosed based on the pink-color sign without a need for biopsy.
In conclusion, the pink-color sign was closely associated with absence of the keratinous layer. The pink-color sign may be caused by early leakage of iodine into the esophageal lumen because of impaired barrier function of the epithelium.
COMMENTS
Background
A dramatic color change after iodine staining, from the initial yellow color to a pink color 2-3 min later, is known as the pink-color sign, and is useful for identifying cancerous lesions of the esophagus. This sign has been reported to dramatically improve specificity for esophageal squamous high-grade intraepithelial neoplasia and invasive cancer.
Research frontiers
The mechanism underlying the occurrence of the pink-color sign has not been fully investigated. Improved understanding of this mechanism may improve our understanding of the characteristics of the relevant lesions, and increase the likelihood of accurate diagnosis.
Innovations and breakthroughs
This study clarified the histologic changes responsible for the occurrence of the pink-color sign in esophageal squamous neoplasia as follows. Analysis of the endoscopic and histologic findings of this study found that absence of the keratinous layer was independently associated with the pink-color sign. Quantitative analysis of color changes found that pink-color sign positive mucosa changed from yellowish to pinkish within 2-3 min after iodine staining, suggesting that the mucosa underwent early color fading from the color of the iodine (yellow) to the color of the mucosa (pink). The pink-color sign may be caused by early leakage of iodine into the esophageal lumen because of impaired barrier function of the epithelium.
Applications
The pink-color sign is a good indicator for choosing adequate biopsy sites, because of its high specificity. Improved understanding of this mechanism may improve their understanding of the characteristics of the relevant lesions, and increase the likelihood of accurate diagnosis.
Terminology
Pink-color sign: A dramatic color change after iodine staining, from the initial yellow color to a pink color 2-3 min later. Keratinous layer: The outer layer of the squamous epithelium, which contain a tough, fibrous protein. This layer acts as a protective barrier against outside elements.
Peer review
This study investigated the detailed histologic findings of esophageal neoplasms, and found that absence of the keratinous layer because of neoplastic cell proliferation may be responsible for the pink-color sign. Quantitative analysis of color changes showed that pink-color sign positive mucosa changed from yellowish to pinkish within 2-3 min after iodine staining, suggesting that the mucosa underwent early color fading from the color of the iodine (yellow) to the color of the mucosa (pink). Based on these results, the authors speculated on the mechanism underlying the pink-color sign. These findings may improve understanding of the characteristics of these lesions, and increase the accuracy of diagnosis of esophageal neoplasms.
Footnotes
P- Reviewers Kawakami K, Oka S S- Editor Wen LL L- Editor A E- Editor Ma S
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432–439. [PubMed] [Google Scholar]
- 3.Igaki H, Kato H, Tachimori Y, Daiko H, Fukaya M, Yajima S, Nakanishi Y. Clinicopathologic characteristics and survival of patients with clinical Stage I squamous cell carcinomas of the thoracic esophagus treated with three-field lymph node dissection. Eur J Cardiothorac Surg. 2001;20:1089–1094. doi: 10.1016/s1010-7940(01)01003-x. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto S, Ishihara R, Motoori M, Kawaguchi Y, Uedo N, Takeuchi Y, Higashino K, Yano M, Nakamura S, Iishi H. Comparison between definitive chemoradiotherapy and esophagectomy in patients with clinical stage I esophageal squamous cell carcinoma. Am J Gastroenterol. 2011;106:1048–1054. doi: 10.1038/ajg.2011.42. [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Sato A, Fukuda H, Kagami Y, Udagawa H, Togo A, Ando N, Tanaka O, Shinoda M, Yamana H, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708) Jpn J Clin Oncol. 2009;39:638–643. doi: 10.1093/jjco/hyp069. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara R, Tanaka H, Iishi H, Takeuchi Y, Higashino K, Uedo N, Tatsuta M, Yano M, Ishiguro S. Long-term outcome of esophageal mucosal squamous cell carcinoma without lymphovascular involvement after endoscopic resection. Cancer. 2008;112:2166–2172. doi: 10.1002/cncr.23418. [DOI] [PubMed] [Google Scholar]
- 7.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video) Gastrointest Endosc. 2010;72:255–264, 264.e1-2. doi: 10.1016/j.gie.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Yamashina T, Ishihara R, Nagai K, Matsuura N, Matsui F, Ito T, Fujii M, Yamamoto S, Hanaoka N, Takeuchi Y, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol. 2013;108:544–551. doi: 10.1038/ajg.2013.8. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto CL, Iriya K, Baba ER, Navarro-Rodriguez T, Zerbini MC, Eisig JN, Barbuti R, Chinzon D, Moraes-Filho JP. Lugol’s dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275–282. doi: 10.1111/j.1572-0241.2005.30189.x. [DOI] [PubMed] [Google Scholar]
- 11.Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL, Taylor PR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220–231. [PubMed] [Google Scholar]
- 12.Shiozaki H, Tahara H, Kobayashi K, Yano H, Tamura S, Imamoto H, Yano T, Oku K, Miyata M, Nishiyama K. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer. 1990;66:2068–2071. doi: 10.1002/1097-0142(19901115)66:10<2068::aid-cncr2820661005>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Freitag CP, Barros SG, Kruel CD, Putten AC, Dietz J, Gruber AC, Diehl AS, Meurer L, Breyer HP, Wolff F, et al. Esophageal dysplasias are detected by endoscopy with Lugol in patients at risk for squamous cell carcinoma in southern Brazil. Dis Esophagus. 1999;12:191–195. doi: 10.1046/j.1442-2050.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama A, Ohmori T, Makuuchi H, Maruyama K, Okuyama K, Takahashi H, Yokoyama T, Yoshino K, Hayashida M, Ishii H. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer. 1995;76:928–934. doi: 10.1002/1097-0142(19950915)76:6<928::aid-cncr2820760604>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu Y, Omori T, Yokoyama A, Yoshida T, Hirota J, Ono Y, Yamamoto J, Kato M, Asaka M. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: high-grade intra-epithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol. 2008;23:546–550. doi: 10.1111/j.1440-1746.2007.04990.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara R, Yamada T, Iishi H, Kato M, Yamamoto S, Yamamoto S, Masuda E, Tatsumi K, Takeuchi Y, Higashino K, et al. Quantitative analysis of the color change after iodine staining for diagnosing esophageal high-grade intraepithelial neoplasia and invasive cancer. Gastrointest Endosc. 2009;69:213–218. doi: 10.1016/j.gie.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu Y, Tukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointest Endosc. 2001;54:190–194. doi: 10.1067/mge.2001.116877. [DOI] [PubMed] [Google Scholar]
- 18.Japan Esophageal Society. Japanese Classification of Esophageal Cancer, tenth edition: part I. Esophagus. 2009;6:1–25. doi: 10.1007/s10388-016-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabbert HE, Shimoda T, Hainaut P, Nakamura Y, Field JK, Inoue H. Squamous cell carcinoma of the esophagus. In: Hamilton SR, Aaltonen LA, editors. Pathology and Genetics of the Digestive System: World Health Organization Classification. Lyon: IARC press; 2000. p. 11–19. [Google Scholar]
- 20.Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–295. doi: 10.1016/s0016-5107(03)02532-x. [DOI] [PubMed] [Google Scholar]
- 21.Chung KL, Yang WJ, Yan WM. Efficient edge preserving algorithm for color contrast enhancement with application to color image segmentation. J Vis Commun Image Represent. 2008;19:299–310. [Google Scholar]
- 22.Schanda J. Colorimetry: Understanding the CIE System. New York: John Wiley & Sons Inc; 2007. pp. 61–64. [Google Scholar]
- 23.Dela Cruz F, Brown DH, Leikin JB, Franklin C, Hryhorczuk DO. Iodine absorption after topical administration. West J Med. 1987;146:43–45. [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman S, Barbosa J, Kearns G. Ultrastructural and histochemical localization of glycogen in human normal and hyperkeratotic oral epithelium. Arch Oral Biol. 1971;16:423–434. doi: 10.1016/0003-9969(71)90166-x. [DOI] [PubMed] [Google Scholar]
- 25.Kondo H, Fukuda H, Ono H, Gotoda T, Saito D, Takahiro K, Shirao K, Yamaguchi H, Yoshida S. Sodium thiosulfate solution spray for relief of irritation caused by Lugol’s stain in chromoendoscopy. Gastrointest Endosc. 2001;53:199–202. doi: 10.1067/mge.2001.110730. [DOI] [PubMed] [Google Scholar]
- 26.Knight CA, Stanley WM. THE EFFECT OF SOME CHEMICALS ON PURIFIED INFLUENZA VIRUS. J Exp Med. 1944;79:291–300. doi: 10.1084/jem.79.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vorherr H, Vorherr UF, Mehta P, Ulrich JA, Messer RH. Vaginal absorption of povidone-iodine. JAMA. 1980;244:2628–2629. [PubMed] [Google Scholar]
- 28.Small MD, Bezman A, Longarini AE, Fennell A, Zamcheck N. Absorption of Potassium Iodide from Gastro-Intestinal Tract. Proc Soc Exp Biol Med. 1961;106:450–452. [Google Scholar]
- 29.Powell DW. Barrier function of epithelia. Am J Physiol. 1981;241:G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 30.Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays. 2002;24:789–800. doi: 10.1002/bies.10144. [DOI] [PubMed] [Google Scholar]


