Abstract
AIM: To investigate Krüppel-like factor 8 (KLF8) expression in gastric cancer and its relationship with angiogenesis and prognosis of gastric cancer.
METHODS: One hundred and fifty-four patients with gastric cancer who underwent successful curative resection were retrospectively enrolled in the study. Fifty tumor-adjacent healthy gastric tissues (≥ 5 cm from the tumor margin) obtained during the original resection were randomly selected for comparative analysis. In situ expression of KLF8 and CD34 proteins were examined by immunohistochemistry. The intratumoral microvessel density (MVD) was determined by manually counting the immunostained CD34-positive endothelial cells in three consecutive high-magnification fields (× 200). The relationship between differential KLF8 expression and MVD was assessed using Spearman’s correlation coefficient test. χ2 test was performed to evaluate the effects of differential KLF8 expression on clinicopathologic factors. Kaplan-Meier and multivariate Cox survival analyses were used to assess the prognostic value of differential KLF8 expression in gastric cancer.
RESULTS: Significantly higher levels of KLF8 protein were detected in gastric cancer tissues than in the adjacent non-cancerous tissues (54.5% vs 34.0%, P < 0.05). KLF8 expression was associated with tumor size (P < 0.001), local invasion (P = 0.005), regional lymph node metastasis (P = 0.029), distant metastasis (P = 0.023), and tumor node metastasis (TNM) stage (P = 0.002), as well as the MVD (r = 0.392, P < 0.001). Patients with KLF8 positive expression had poorer overall survival (P < 0.001) and cancer-specific survival (P < 0.001) than those with negative expression. Multivariate analysis demonstrated that KLF8 expression independently affected both overall and cancer-specific survival of gastric cancer patients (P = 0.035 and 0.042, respectively).
CONCLUSION: KLF8 is closely associated with gastric tumor progression, angiogenesis and poor prognosis, suggesting it may represent a novel prognostic biomarker and therapeutic target for gastric cancer.
Keywords: Gastric cancer, Krüppel-like factor 8, Angiogenesis, Prognosis
Core tip: In this study, we found that the expression of Krüppel-like factor 8 (KLF8) was up-regulated in resected gastric cancer tissues. The differential KLF8 expression was significantly associated with enhanced malignant potential, including tumor size, local invasion, regional lymph node metastasis, distant metastasis, and tumor node metastasis stage, as well as the intratumoral microvessel density. Multivariate analysis indicated that KLF8 expression independently affected both overall and cancer-specific survival of gastric cancer patients. Collectively, our findings suggest that KLF8 may be a predictive marker of clinical outcome and represent a novel target for anti-angiogenic therapy of gastric cancer patients.
INTRODUCTION
Gastric cancer is one of the most common malignant tumors worldwide, accounting for the second highest rate of cancer-related death[1]. Despite the decline in gastric cancer mortality has occurred over the past few decades, this disease remains a significant burden in China, with approximately 400000 newly-diagnosed cases being reported each year[2]. Patients with gastric cancer are often asymptomatic or display nonspecific symptoms in the early stages, and by the time symptoms appear the disease has generally reached an advanced stage, when treatment options are limited and the prognosis for long-term survival is poor. Therefore, there remains a critical need for discovery of novel biomarkers that will reflect not only gastric cancer onset but also its progression and risk of mortality; such diagnostic and prognostic indicators may also represent novel therapeutic targets for treating gastric cancer patients at various stages.
Krüppel-like factor 8 (KLF8) was initially identified as a ubiquitously expressed transcriptional repressor[3]. Like other members of the KLF transcription factor family, KLF8 harbors three conserved C2H2 zinc finger DNA-binding domains in its C-terminus, but also has a unique sequence in its N-terminus that is believed to mediate its functional specificity through interactions with other protein binding partners[4,5]. KLF8 activity has been implicated as crucial to a wide range of cellular processes, such as cell cycle progression[6-9], oncogenic transformation[10], epithelial-to-mesenchymal transition, migration and invasion[11,12]. Moreover, overexpression of KLF8 has been found in several human malignant tumors, including those of ovarian, renal and breast origin[13-15]. In vitro studies using gastric cancer cell lines have revealed that lentivirus-mediated knockdown of KLF8 inhibits cell growth and invasion[16,17]. Yet, the clinical significance of KLF8 overexpression in relation to clinicopathologic features of gastric cancer and patient prognosis remains unknown.
Angiogenesis, the formation of new capillary blood vessels, is essential for the growth, progression and metastasis of malignant tumors[18,19]. The microvessel density (MVD) is now widely used to evaluate the degree of tumor angiogenesis and has been proven to be associated with the metastasis and prognosis of a wide range of human tumors[20-22]. One previous study has shown that transfection with KLF8-RNAi lentivirus significantly decreases tumor vessels in a mouse model of liver cancer[11], suggesting that KLF8 may play potentially interesting roles in tumor angiogenesis. However, the angiogenic property of KLF8 protein in primary gastric cancer remains unclear.
In the present study, we detected the expression status of KLF8 by immunohistochemistry in surgically resected gastric cancer, and further explored its potential correlations with clinicopathologic parameters, tumor angiogenesis and prognosis of the patients with gastric cancer.
MATERIALS AND METHODS
Patients and follow-up
Between July 2004 and October 2006, a total of 161 gastric cancer patients underwent curative resection in the Department of General Surgery at Qilu Hospital of Shandong University (Jinan, China). Of these 161 patients, seven cases were lost to follow-up, so a total of 154 patients were eventually enrolled in this study. Retrospective review of the patients’ medical records and direct communication with the patients provided complete diagnosis, treatment and follow-up data for all cases. The resected tumor specimens had been immediately formalin-fixed and paraffin-embedded. Each paraffin block was cut into serial sections at 4 μm intervals for future analysis. In addition, we randomly selected 50 normal gastric tissues (at least 5 cm from the margin of the tumor) as normal controls. The postoperative pathological TNM staging was evaluated based on the 2009 criteria of the International Union Against Cancer (UICC). This study was approved by the Ethics Committee of Qilu Hospital, and written informed consent was obtained from each patient.
None of the patients had received preoperative adjuvant therapy. All patients were followed-up at the outpatient clinic at 3-6 mo intervals, with the follow-up durations ranging from 5-77 mo (median, 45 mo). The follow-up examinations included laboratory testing, physical examination, ultrasound scan, and, if necessary, fibergastroscopy.
Immunohistochemical staining for KLF8 and CD34
Paraffin-embedded tissue sections were baked at 60 °C, dewaxed by soaking in xylene, and rehydrated by passing through an alcohol gradient series. Antigen retrieval was carried out by boiling samples (via microwave) in EDTA (pH 9.0). After cooling to room temperature, the sections were immersed in 3% hydrogen peroxide for 15 min to inhibit endogenous peroxidase activity, followed by blocking in 10% goat serum to reduce nonspecific binding. An overnight incubation at 4 °C was then carried out with anti-KLF8 or anti-CD34 primary antibodies (1:100 in phosphate-buffered saline (PBS); both from Santa Cruz Biotechnology, Santa Cruz, CA), which was followed by incubation with the corresponding horseradish peroxidase-conjugated secondary antibody (Zhongshan Golden Bridge Biotechnology, Beijing, China). The peroxidase reactivity was visualized using 3,3’-diaminobenzidine as substrate. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted with neutral balsam. Negative controls were generated by the same procedure, except with the primary antibody being replaced by PBS alone.
Evaluation of KLF8 expression and MVD
Microscopic evaluation of the immunostained sections was carried out by two pathologists working independently, who were not associated with this study and who were blinded to the patients’ clinicopathologic factors and outcomes. In cases of discrepancy, the slides were re-assessed jointly by both pathologists using a multi-head microscope to establish a consensus result.
KLF8 protein expression was semi-quantitatively assessed by scoring both the immunoreactive staining intensity (0: no staining; 1: weak staining; 2: moderate staining; 3, strong staining) and the proportion of positively-stained cells (0: 0%-9%; 1: 10%-25%; 2: 26%-50%; 3: 51%-75%; 4: > 75%). The overall staining score was then calculated as (percentage score × intensity score), with final scores of 0 indicating negative expression, of 1-5 indicating weakly positive expression, and of ≥ 6 indicating strongly positive expression[11].
MVD was assessed by immunohistochemical staining of CD34, according to the international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumors[23]. The entire sections were scanned at low magnification (× 100) initially to select regions with the highest vascularity (“hot spots”) for focused investigation using high magnification (× 200) to manually count the stained microvessels present in three consecutive fields. A single, countable microvessel was defined as any brown-stained endothelial cell (or cluster) clearly separated from the adjacent microvessels. The average microvessel counts in the three fields under high magnification (× 200) were recorded as the final value of MVD.
Statistical analysis
All statistical analyses were performed using the SPSS software suite, version 17.0 (SPSS, Chicago, IL, United States). χ2 test was used to assess the correlations between KLF8 protein expression and clinicopathologic factors. The relationship between KLF8 protein expression and MVD was determined by Spearman’s correlation coefficient test. Survival curves were calculated by the Kaplan-Meier method, and the significance of intergroup differences in survival was determined by log-rank test. Multivariate survival analysis based on the Cox proportional hazard model was carried out to identify the significant independent prognostic factors. A P value < 0.05 was considered statistically significant.
RESULTS
Correlation of KLF8 expression with clinicopathologic factors
Immunohistochemical analyses of the 154 gastric cancer tissues and 50 adjacent non-cancerous tissues showed KLF8 protein staining of various intensities in both the nuclear and cytoplasmic compartments. Overall, 54.5% (84/154) of the gastric cancer tissues showed KLF8-positivity, including 25.3% which were strongly positive and 29.2% which were weakly positive, and only 34.0% (17/50) of the adjacent non-cancerous tissues showed KLF8-positivity. The difference in KLF8 staining between the gastric cancer and adjacent normal tissues was statistically significant (P < 0.05). Figure 1 shows representative cases with different expression levels of KLF8 protein. Moreover, as shown in Table 1, KLF8-positivity was significantly related to tumor size, local invasion, regional lymph node metastasis, distant metastasis, and TNM stage (all P < 0.05); however, no significant associations were detected for KLF8 expression with patient sex, age, or degree of tumor differentiation (all P > 0.05).
Figure 1.
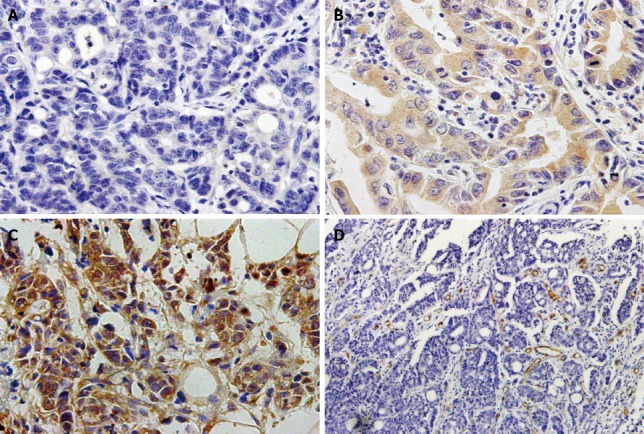
Immunohistochemical staining of Krüppel-like factor 8 and CD34 in gastric cancer specimens. A: Negative expression of Krüppel-like factor 8 (KLF8) in cancer cells; B: Weakly positive expression of KLF8 in cancer cells; C: Strongly positive expression of KLF8 in cancer cells; Magnification: × 400; D: Intratumoral microvessels detected by anti-CD34 antibody (brown) in gastric cancer tissues; Magnification: × 200.
Table 1.
Krüppel-like factor 8 expression and clinicopathologic characteristics in gastric cancer
| Variables | Cases (n) |
KLF8 expression |
P value | |
| Negative | Positive | |||
| Sex | 0.856 | |||
| Male | 122 | 55 | 67 | |
| Female | 32 | 15 | 17 | |
| Age (yr) | 0.136 | |||
| ≤ 60 | 89 | 45 | 44 | |
| > 60 | 65 | 25 | 40 | |
| Tumor (cm) | < 0.001 | |||
| ≤ 5 | 92 | 53 | 39 | |
| > 5 | 62 | 17 | 45 | |
| Tumor differentiation | 0.608 | |||
| Well | 14 | 8 | 6 | |
| Moderate | 43 | 18 | 25 | |
| Poor | 97 | 44 | 53 | |
| Local invasion | 0.005 | |||
| T1-T2 | 31 | 21 | 10 | |
| T3-T4 | 123 | 49 | 74 | |
| Regional lymph node metastasis | 0.029 | |||
| N0 | 45 | 26 | 19 | |
| N1 | 37 | 20 | 17 | |
| N2 | 33 | 9 | 24 | |
| N3 | 39 | 15 | 24 | |
| Distant metastasis | 0.023 | |||
| No | 141 | 68 | 73 | |
| Yes | 13 | 2 | 11 | |
| TNM stage | 0.002 | |||
| I | 20 | 14 | 6 | |
| II | 54 | 30 | 24 | |
| III | 67 | 24 | 43 | |
| IV | 13 | 2 | 11 | |
TNM: Tumor node metastasis; KLF8: Krüppel-like factor 8.
Correlation of KLF8 expression with tumor angiogenesis
As shown in Figure 1D, the intratumoral MVD was determined by CD34 immunohistochemical staining. The MVD values of the 154 cancer specimens ranged from 14 to 62 (mean, 32.0 ± 8.8). When this mean value was applied as a cut-off point, 86 cases (55.8%) were categorized as low MVD (< 32) and 68 cases (44.2%) were categorized as high MVD (≥ 32). KLF8 expression was found to be significantly positively correlated with MVD (r = 0.392, P < 0.001; Table 2).
Table 2.
Correlations between Krüppel-like factor 8 expression and the microvessel density in gastric cancer
| MVD |
KLF8 expression |
P value | r | |
| Negative | Positive | |||
| Low | 54 | 32 | < 0.001 | 0.392 |
| High | 16 | 52 | ||
KLF8: Krüppel-like factor 8; MVD: Microvessel density.
Prognostic significance of KLF8 expression in gastric cancer
Of the 154 patients, 101 (65.6%) cases died during the follow-up period. Among them, the majority (n = 97) died from cancer-related causes, and four cases died from other causes. The 5-year overall and cancer-specific survival rates were 34.4% and 35.3%, respectively.
Kaplan-Meier analysis indicated that KLF8 expression was significantly associated to the survival of gastric cancer patients. The overall 5-year survival rate of patients with KLF8-positive expression was significantly shorter than those with KLF8-negative expression (P < 0.001; Figure 2A). Consistent with this trend, the patients with KLF8-positive expression also had a poorer 5-year cancer-specific survival (P < 0.001; Figure 2B). In addition, we compared the prognostic value of KLF8 strongly positive expression to that of weakly positive expression, and found that there was no significant difference between the two groups for either overall survival (P = 0.116) or cancer-specific survival (P = 0.224). Multivariate analysis using Cox regression indicated that KLF8-positive expression in gastric cancer was a significant independent prognostic factor for both overall survival (95%CI: 1.035-2.539, P = 0.035) and cancer-specific survival (95%CI: 1.017-2.554, P = 0.042) (Table 3).
Figure 2.
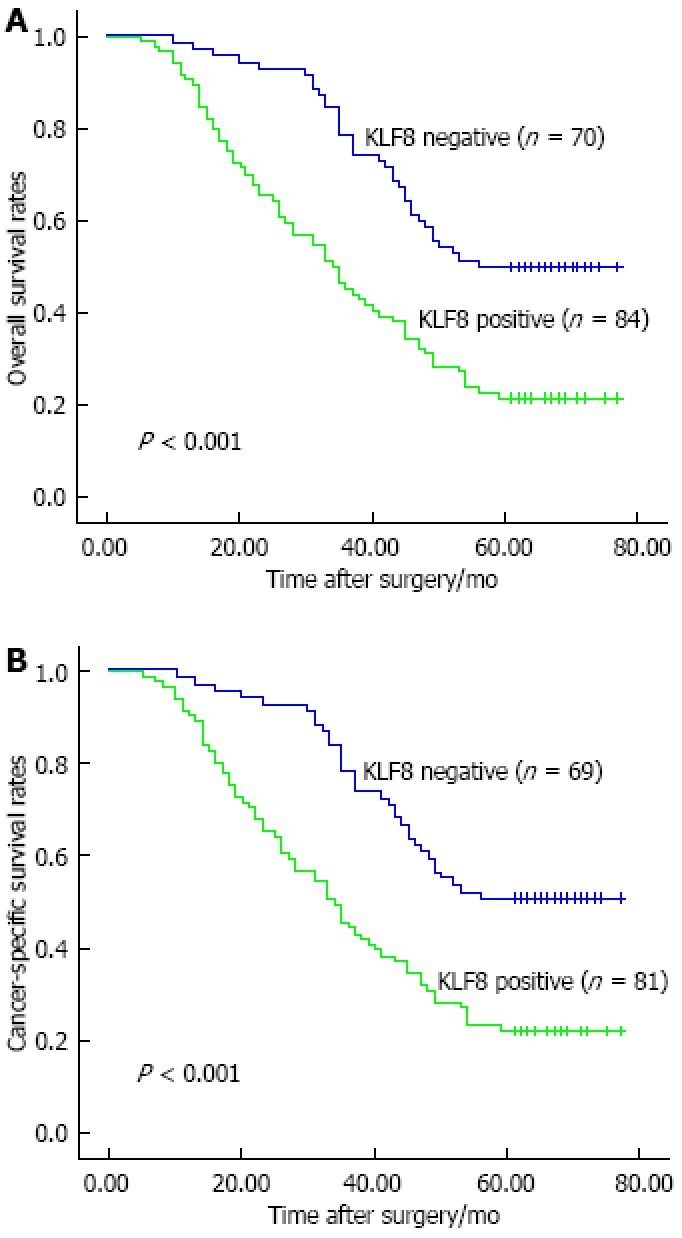
Kaplan-Meier survival curves of overall and cancer-specific survival in gastric cancer patients based on Krüppel-like factor 8 expression. Patients with Krüppel-like factor 8 (KLF8)-positive (weakly and strongly) expression had poorer overall (A) and cancer-specific (B) survival as compared to those with KLF8-negative expression.
Table 3.
Multivariate analysis of prognostic factors in patients with gastric cancer
| Variables |
Multivariate analysis |
|||||
|
Overall survival |
Cancer-specific survival |
|||||
| RR | 95%CI | P value | RR | 95%CI | P value | |
| Sex | 1.132 | 0.678-1.890 | 0.636 | 1.071 | 0.622-1.844 | 0.803 |
| Age | 0.785 | 0.521-1.182 | 0.247 | 0.775 | 0.509-1.180 | 0.235 |
| Tumor size | 1.219 | 0.794-1.872 | 0.365 | 1.220 | 0.790-1.886 | 0.370 |
| Tumor differentiation | 0.841 | 0.591-1.196 | 0.334 | 0.830 | 0.582-1.184 | 0.304 |
| Local invasion | 1.194 | 0.799-1.785 | 0.387 | 1.197 | 0.791-1.811 | 0.395 |
| Regional lymph node metastasis | 1.025 | 0.782-1.343 | 0.858 | 1.029 | 0.783-1.352 | 0.839 |
| Distant metastasis | 6.384 | 1.943-20.975 | 0.002 | 7.091 | 2.104-23.901 | 0.002 |
| TNM stage | 2.401 | 1.182-4.878 | 0.015 | 2.323 | 1.128-4.783 | 0.022 |
| KLF8 expression | 1.621 | 1.035-2.539 | 0.035 | 1.612 | 1.017-2.554 | 0.042 |
| MVD | 2.801 | 1.790-4.381 | 0.000 | 2.782 | 1.751-4.419 | 0.000 |
KLF8: Krüppel-like factor 8; MVD: Microvessel density; TNM: Tumor node metastasis.
DISCUSSION
Surgical resection is the most effective treatment for gastric cancer patients with curative potential, yet the clinical outcomes of these patients are generally poor, largely due to a high ratio of postoperative metastasis or recurrence[24]. In China, the overall 5-year survival rate of gastric cancer patients is only about 40%[25]. Identification of novel biomarkers that can be used as prognostic predictors or therapeutic targets will likely benefit these patients and help to ease the burden of this disease. Aberrant expression of KLF8 has been detected in several types of human malignant tumors and closely associated with oncogenic transformation and tumor progression[10-15]. To the best of our knowledge, however, the study described herein is the first to evaluate the clinicopathologic and prognostic significance of KLF8 in gastric cancer patients.
Our findings confirmed the remarkable up-regulation of KLF8 in gastric cancer tissues and indicated significant associations for KLF8 expression with prognosis-related features, including tumor size, local invasion, regional lymph node metastasis, distant metastasis, and TNM stage. Thus, it is likely that KLF8 plays important roles in gastric cancer progression. Consistent with our results, previous study of renal cell carcinoma has shown that overexpression of KLF8 is strongly associated with larger tumor size and higher clinical stage[14]. However, future studies are still needed to investigate the detailed mechanism of KLF8-mediated gastric cancer progression.
Predicting the clinical outcomes of patients with malignant tumors may provide valuable information for better treatment stratification and personalized therapeutic regimens. Previous study demonstrated that KLF8 overexpression was significantly related with early tumor recurrence and poor prognosis in hepatocellular carcinoma[11]. However, no clinical data has indicated the potential benefit of KLF8 as a biomarker for predicting prognosis of gastric cancer patients. In the current study, multivariate survival analysis based on the Cox proportional hazard model revealed that, among all the factors analyzed, KLF8 expression was a significant independent prognostic factor for both overall and cancer-specific survival of gastric cancer patients following curative resection. These findings clearly demonstrate that aberrant expression of KLF8 may be closely associated with gastric cancer progression in our patient cohort, and suggest its potential as a prognostic biomarker of gastric cancer outcome.
It is well known that tumors are endowed with angiogenic capability, and their growth, invasion, and metastasis are angiogenesis-dependent[26]. The survival rate of patients with highly-vascularized gastric cancer is significantly lower than those with less-vascularized gastric cancer[27]. The findings from the current study indicated a close correlation between KLF8-positivity in tumor specimens and the extent of intratumoral MVD (as determined by CD34 staining), suggesting that KLF8 may facilitate tumor progression via induction of angiogenesis. Therefore, KLF8 may represent a potential novel target of anti-angiogenic therapy for gastric cancer patients. It is important to note, however, that tumor angiogenesis is a complex process involving dynamic interplay of various factors[28], and further studies are needed to elucidate the detailed molecular mechanisms underlying KLF8-mediated tumor angiogenesis before the precise molecular targets of KLF8 are identified and used to develop an optimal treatment for gastric cancer patients.
When interpreting the results presented herein, several inherent limitations to the study design must be considered. First, only gastric cancer patients who underwent curative resection were enrolled in the present study, which restricted the total study population to a relatively low number. Second, the retrospective design provided only previously resected human gastric cancer tissues that were not originally collected with the intent of detecting the prognostic value of KLF8. To address this limitation, multicenter, randomized studies should be conducted to confirm whether KLF8 can be used as an accurate prognostic maker for gastric cancer. In addition, the impact of postoperative adjuvant therapy on prognosis was not evaluated in this study, due to the fact that some gastric cancer patients received different therapeutic regimens (i.e., agents, doses, and cycles of treatment), and not all patients completed the adjuvant therapy because of severe side effects or economic-related restrictions.
In conclusion, this study confirmed the up-regulated expression of KLF8 in gastric cancer tissues and, for the first time, demonstrated its clinicopathologic and prognostic significance. Specifically, positive expression of KLF8 was shown to be significantly correlated with enhanced malignant potential, tumor angiogenesis, and poor prognosis. Furthermore, Cox regression model analysis showed that KLF8-positivity in resected gastric tumors was a meaningful independent prognostic factor for both overall and cancer-specific survival. Collectively, these findings demonstrate that KLF8 may have clinical potential not only as a prognostic marker, by which individuals with risk of poor clinical outcome may be identified, but also as a novel target for anti-angiogenic therapy of gastric cancer patients.
ACKNOWLEDGMENTS
The authors wish to thank Cheng-Jun Zhou (Department of Pathology, The Second Hospital, Shandong University) and Jun-Hui Zhen (Department of Pathology, Qilu Hospital, Shandong University) for their excellent immunostaining evaluation. The authors are also grateful to Shu-Hai Li (Department of Thoracic Surgery, Qilu Hospital, Shandong University) for his expert commentary on the manuscript.
COMMENTS
Background
Gastric cancer remains one of the most common malignancies worldwide, and accounts for the second highest rate of cancer-related death. Despite recent advances in comprehensive therapeutic strategies, the clinical outcome of gastric cancer patients remains poor, mainly due to delayed clinical presentation and a high ratio of postoperative metastasis or recurrence. Therefore, it is necessary to identify novel biomarkers that can be used as prognostic indicators or therapeutic targets for gastric cancer patients.
Research frontiers
Aberrant expression of Krüppel-like factor 8 (KLF8) has been detected in several types of human malignant tumors, including those of ovarian, renal and breast origin. Considerable evidence has indicated that KLF8 expression is closely associated with oncogenic transformation and tumor progression. One previous study showed that lentivirus-mediated interference of KLF8 expression significantly decreased tumor vessels in a mouse model of liver cancer. However, the angiogenic property and prognostic significance of KLF8 in gastric cancer has not been fully elucidated.
Innovations and breakthroughs
In this study, KLF8 expression and microvessel density (MVD) were assessed by immunohistochemical analysis of resected tumors from patients with gastric cancer. Results indicated that the level of KLF8 was significantly higher in gastric cancer tissues than in adjacent non-cancerous tissues. Moreover, the high KLF8 expression was found to be closely associated with prognosis-related features, including tumor size, local invasion, regional lymph node metastasis, distant metastasis, and TNM stage. KLF8-positive expression in tumor specimens was significantly positively correlated with MVD. Patients with KLF8-positive expression had poorer overall and cancer-specific survival than those with KLF8-negative expression. Multivariate analysis demonstrated that the KLF8 expression in tumors independently affected both overall and cancer-specific survival of gastric cancer patients.
Applications
Collectively, the findings from this study demonstrate that KLF8 may have clinical potential not only as a prognostic biomarker, by which individuals with risk of poor clinical outcome may be identified, but also as a novel target for anti-angiogenic therapy of gastric cancer patients.
Terminology
KLF8, which is located on Xp11.21, was initially identified as a ubiquitously expressed transcriptional repressor belonging to the KLF family of transcription factors. Like other members of the KLF family, KLF8 shares three conserved C2H2 zinc finger DNA-binding domains in its C-terminus, but harbors a unique sequence in the N-terminus that is thought to mediate its functional specificity through interactions with other proteins.
Peer review
The authors demonstrated that KLF8 was significantly up-regulated in clinical specimens of gastric cancer, and evaluated its angiogenic property and prognostic significance. The results suggest that KLF8 may represent a novel prognostic biomarker and therapeutic target for gastric cancer.
Footnotes
Supported by The Shandong Province Natural Science Foundation of China, No. ZR2010HZ004; and the National Key Clinical Medical Specialties Foundation
P- Reviewers Baba H, Yi J S- Editor Zhai HH L- Editor A E- Editor Ma S
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Vliet J, Turner J, Crossley M. Human Krüppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 2000;28:1955–1962. doi: 10.1093/nar/28.9.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieker JJ. Krüppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 5.Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Krüppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Mehta TS, Lu H, Wang X, Urvalek AM, Nguyen KH, Monzur F, Hammond JD, Ma JQ, Zhao J. A unique sequence in the N-terminal regulatory region controls the nuclear localization of KLF8 by cooperating with the C-terminal zinc-fingers. Cell Res. 2009;19:1098–1109. doi: 10.1038/cr.2009.64. [DOI] [PubMed] [Google Scholar]
- 7.Urvalek AM, Wang X, Lu H, Zhao J. KLF8 recruits the p300 and PCAF co-activators to its amino terminal activation domain to activate transcription. Cell Cycle. 2010;9:601–611. doi: 10.4161/cc.9.3.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei H, Wang X, Gan B, Urvalek AM, Melkoumian ZK, Guan JL, Zhao J. Sumoylation delimits KLF8 transcriptional activity associated with the cell cycle regulation. J Biol Chem. 2006;281:16664–16671. doi: 10.1074/jbc.M513135200. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell. 2003;11:1503–1515. doi: 10.1016/s1097-2765(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007;26:456–461. doi: 10.1038/sj.onc.1209796. [DOI] [PubMed] [Google Scholar]
- 11.Li JC, Yang XR, Sun HX, Xu Y, Zhou J, Qiu SJ, Ke AW, Cui YH, Wang ZJ, Wang WM, et al. Up-regulation of Krüppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenterology. 2010;139:2146–2157.e12. doi: 10.1053/j.gastro.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Zheng M, Liu G, Xia W, McKeown-Longo PJ, Hung MC, Zhao J. Krüppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res. 2007;67:7184–7193. doi: 10.1158/0008-5472.CAN-06-4729. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Urvalek AM, Liu J, Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem. 2008;283:13934–13942. doi: 10.1074/jbc.M709300200. [DOI] [PubMed] [Google Scholar]
- 14.Fu WJ, Li JC, Wu XY, Yang ZB, Mo ZN, Huang JW, Xia GW, Ding Q, Liu KD, Zhu HG. Small interference RNA targeting Krüppel-like factor 8 inhibits the renal carcinoma 786-0 cells growth in vitro and in vivo. J Cancer Res Clin Oncol. 2010;136:1255–1265. doi: 10.1007/s00432-010-0776-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Lu H, Urvalek AM, Li T, Yu L, Lamar J, DiPersio CM, Feustel PJ, Zhao J. KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene. 2011;30:1901–1911. doi: 10.1038/onc.2010.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Liu N, Xu M, Liu Y, Min J, Pang H, Zhang N, Zhang H, Zhang H. Lentivirus-delivered Krüppel-like factor 8 small interfering RNA inhibits gastric cancer cell growth in vitro and in vivo. Tumour Biol. 2012;33:53–61. doi: 10.1007/s13277-011-0245-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Yang W, Jin W, Wang Y, Tao C, Yu Z. Lentivirus-mediated gene silencing of KLF8 reduced the proliferation and invasion of gastric cancer cells. Mol Biol Rep. 2012;39:9809–9815. doi: 10.1007/s11033-012-1847-x. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J. What is the evidence that tumors are angiogenesis dependent. J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 19.Makrilia N, Lappa T, Xyla V, Nikolaidis I, Syrigos K. The role of angiogenesis in solid tumours: an overview. Eur J Intern Med. 2009;20:663–671. doi: 10.1016/j.ejim.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51:143–158. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Lackner C, Jukic Z, Tsybrovskyy O, Jatzko G, Wette V, Hoefler G, Klimpfinger M, Denk H, Zatloukal K. Prognostic relevance of tumour-associated macrophages and von Willebrand factor-positive microvessels in colorectal cancer. Virchows Arch. 2004;445:160–167. doi: 10.1007/s00428-004-1051-z. [DOI] [PubMed] [Google Scholar]
- 22.Ren J, Liu H, Yan L, Tian S, Li D, Xu Z. Microvessel density and heparanase over-expression in clear cell renal cell cancer: correlations and prognostic significances. World J Surg Oncol. 2011;9:158. doi: 10.1186/1477-7819-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E, Magnani E, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564–1579. doi: 10.1016/s0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 24.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16:260–268. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]
- 26.Ribatti D, Vacca A. The role of microenvironment in tumor angiogenesis. Genes Nutr. 2008;3:29–34. doi: 10.1007/s12263-008-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T. Association of tumour vasculature with tumour progression and overall survival of patients with non-early gastric carcinomas. Br J Cancer. 1997;75:566–571. doi: 10.1038/bjc.1997.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]


