Abstract
AIM: To examine performances regarding prediction of polyp histology using high-definition (HD) i-scan in a group of endoscopists with varying levels of experience.
METHODS: We used a digital library of HD i-scan still images, comprising twin pictures (surface enhancement and tone enhancement), collected at our university hospital. We defined endoscopic features of adenomatous and non-adenomatous polyps, according to the following parameters: color, surface pattern and vascular pattern. We familiarized the participating endoscopists on optical diagnosis of colorectal polyps using a 20-min didactic training session. All endoscopists were asked to evaluate an image set of 50 colorectal polyps with regard to polyp histology. We classified the diagnoses into high confidence (i.e., cases in which the endoscopist could assign a diagnosis with certainty) and low confidence diagnoses (i.e., cases in which the endoscopist preferred to send the polyp for formal histology). Mean sensitivity, specificity and accuracy per endoscopist/image were computed and differences between groups tested using independent-samples t tests. High vs low confidence diagnoses were compared using the paired-samples t test.
RESULTS: Eleven endoscopists without previous experience on optical diagnosis evaluated a total of 550 images (396 adenomatous, 154 non-adenomatous). Mean sensitivity, specificity and accuracy for diagnosing adenomas were 79.3%, 85.7% and 81.1%, respectively. No significant differences were found between gastroenterologists and trainees regarding performances of optical diagnosis (mean accuracy 78.0% vs 82.9%, P = 0.098). Diminutive lesions were predicted with a lower mean accuracy as compared to non-diminutive lesions (74.2% vs 93.1%, P = 0.008). A total of 446 (81.1%) diagnoses were made with high confidence. High confidence diagnoses corresponded to a significantly higher mean accuracy than low confidence diagnoses (84.0% vs 64.3%, P = 0.008). A total of 319 (58.0%) images were evaluated as having excellent quality. Considering excellent quality images in conjunction with high confidence diagnosis, overall accuracy increased to 92.8%.
CONCLUSION: After a single training session, endoscopists with varying levels of experience can already provide optical diagnosis with an accuracy of 84.0%.
Keywords: Colonoscopy, High-definition i-scan, Optical diagnosis, Colorectal polyps, Training
Core tip: Several studies examined the feasibility of optical diagnosis of colorectal polyps using chromoendoscopy, either dye-based or digital techniques, while only a few studies examined the performances using the high-definition (HD) i-scan technology. In addition, experience on prediction of polyp histology using HD i-scan has been reported in an expert setting only, was based on various classification criteria, and paid only a limited attention to the impact of training. In the current study, at our university hospital, we investigated the effect of training on the diagnostic performances of endoscopists with varying levels of experience. We found that, after a short didactic training session, all endoscopists, trainees as well as gastroenterologists, can predict polyp histology with a mean accuracy of 84%.
INTRODUCTION
Image enhanced endoscopy techniques, such as dye-based or digital based chromoendoscopy [i.e., narrow band imaging (NBI), high-definition (HD) i-scan or Fujinon Intelligent Color Enhancement (FICE)] have become largely available in daily practice, yet their additional diagnostic value remains unclear[1,2]. Several studies have demonstrated that expert endoscopists can accurately differentiate adenomatous from non-adenomatous polyps (i.e., optical diagnosis) using such technologies[3-5], thereby enabling an individualized treatment, in which small adenomas are resected without pathologic examination, while small non-adenomatous polyps of the rectosigmoid are left in situ[6,7]. In addition, optical diagnosis might offer an alternative to histologic diagnosis in case polyps cannot be retrieved for histopathology[8]. This practical approach may downsize the pathology costs and reduce the risk of complications, which in turn would increase the efficiency and cost-effectiveness of colonoscopic procedures[6,9].
A large number of studies have focused on the contribution of image enhanced endoscopy techniques in the detection of colorectal polyps[10-14], while information on their role in histologic characterization is still expanding. Feasibility studies on optical diagnosis have been performed using chromoendoscopy[15,16] or NBI[3,14-18], especially in expert setting, while only a few studies examined the feasibility of HD i-scan technology[5,17,19-22], and none of them has been conducted outside an expert setting. Of note, in a study examining diagnostic performances for optical diagnosis of small colorectal polyps using HD i-scan, Chan et al[22] found a 30% difference in accuracy (63% vs 93%) between 2 experienced endoscopists, highlighting the need for training and use of standardized criteria. It is important to understand whether incorporating basic principles of optical diagnosis in the education of practicing endoscopists is sufficient to attain and maintain skills in optical diagnosis[2,7]. At our institution, training on recognition of colorectal lesions, with focus on non-polypoid adenomas, is currently incorporated in the educational curriculum[23]. In the current study, we further extended this training by developing a short training module on optical diagnosis using HD i-scan images. We sought to examine the performances in predicting polyp histology in a group of endoscopists with varying levels of experience. We hypothesized that endoscopists can accurately predict polyp histology after a short training session, irrespective of their prior endoscopy practice experience.
MATERIALS AND METHODS
We conducted a prospective educational study at the Maastricht University Medical Center, the Netherlands. No ethical review was required by the local Institutional Review Board.
Colonoscopy and image collection
We created a library of digital photographs using HD i-scan colonoscopes (Pentax, 90i series, 1.3 × 106 pixels). Twin-pictures [surface enhancement (SE); tone enhancement (TE)] were obtained from consecutive colorectal polyps. Location, size and morphology of colorectal polyps were registered. Colorectal polyps were subdivided according to location into proximal lesions (i.e., located proximal from the rectosigmoid) and distal lesions (i.e., detected in the rectosigmoid), as described previously[18,24]. The size of the polyp was categorized as diminutive (i.e., < 6 mm) or non-diminutive (i.e., ≥ 6 mm). Moreover, colorectal lesions were classified according to morphology into polypoid and non-polypoid (i.e., lesions with a height of less than half of their diameter)[25]. All colonoscopies were performed by one colonoscopist (Sanduleanu S), with previous experience on image enhanced endoscopy techniques[26-28], including the HD i-scan technology. After digital documentation, all colorectal polyps were removed and sent for histopathological assessment. Colorectal polyps were assessed by two experienced gastrointestinal pathologists and classified according to the World Health Organization classification[29]. Tubular adenomas, tubulovillous adenomas, villous adenomas and carcinomas were categorized as adenomatous whereas hyperplastic polyps, other (i.e., inflammatory of lesions) and normal tissue were categorized as polyps with a non-adenomatous histology. Sessile serrated adenomas/polyps were categorized as non-adenomatous polyps, in line with recent insights on the pit pattern of these lesions[30-32].
Training and development of classification table
Pilot phase: We established features associated with adenomatous and non-adenomatous histology, using a different set of 20 colorectal polyps which were examined using both chromoendoscopy and HD i-scan (Figure 1). We built upon previously described, international pit pattern classifications using (digital) chromoendoscopy, namely the Kudo classification and the NBI international colorectal endoscopic classification (NICE classification)[33,34]. We developed a simple classification (i-scan classification for endoscopic diagnosis, ICE-classification), in which the following parameters were separately rated: color, epithelial surface pattern and vascular pattern (Table 1). The quality of images was evaluated by 2 independent examiners and categorized as excellent or good.
Figure 1.
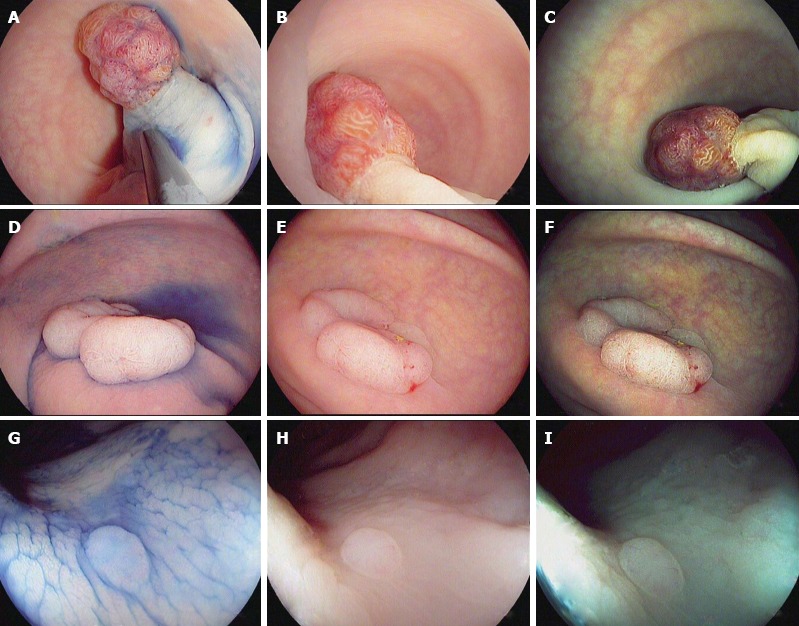
Characterization of colorectal polyps using chromoendoscopy with indigo carmine 0.4% (A, D and G) and high-definition i-scan, surface enhancement (B, E and H)/tone enhancement (C, F and I), the i-scan classification for endoscopic diagnosis. A-C: 20 mm sized pedunculated polyp (Paris Ip). Image enhanced endoscopy shows reddish color, prominent vessels and a type IV pit pattern of the epithelial surface. Histopathology showed a tubulovillous adenoma with low-grade dysplasia; D-F: 40 mm sized non-polypoid (Paris IIa) lesion. Image enhanced endoscopy shows reddish color, dilated, irregular vessels and a type IV pit pattern of the epithelial surface. Histopathology showed a tubulovillous adenoma with high-grade dysplasia; G-I: 3 mm sized non-polypoid (Paris IIa) lesion. Image enhanced endoscopy shows pale color, isolated, lacy vessels and a type II pit pattern. Histopathology showed a hyperplastic polyp.
Table 1.
Classification system for diagnosis of non-adenomatous and adenomatous colorectal polyps using high-definition i-scan (ICE classification)
| Non-adenomatous | Adenomatous | |
| Color | Pale | Reddish |
| Similar to adjacent mucosa | Different from adjacent mucosa | |
| Indiscrete borders | Clearly demarcated | |
| Surface pattern | Round pits of uniform size, no definite pits | Oval, tubular or branched pits |
| Vessel pattern | Isolated, lacy vessels | Dilated, irregular vessels |
Study: For the purpose of this educational study, we assembled an image set of twin pictures (SE; TE) from 50 colorectal polyps to create a test which can be accomplished within 1 h. We developed a training module (Microsoft PowerPoint) including the following information: (1) potential clinical relevance of optical diagnosis; (2) basic principles of the HD i-scan technology; (3) differential criteria of adenomatous vs non-adenomatous polyps using HD i-scan; and (4) clinical examples. Eleven endoscopists, working at our university center, with varying levels of experience, but who did not routinely use HD i-scan prior to this study were invited to participate. All endoscopists received a 20-min didactic training session aiming to familiarize them with the ICE classification of colorectal polyps using HD i-scan. Hereafter, the endoscopists evaluated an image set of 50 colorectal polyps, placed in random order by a computer-generated random number sequence, assessing polyp histology with high- or low confidence levels. High confidence was defined as cases in which the endoscopist could assign a diagnosis with certainty and low confidence in cases in which the endoscopist preferred to send the polyp for formal histopathology.
Statistical analysis
Numbers (percentages) were used to describe categorical variables. Sensitivity, specificity and accuracy in predicting polyp histology were calculated using formal histopathology as gold standard. The positive and negative predictive values (PPVs and NPVs) are highly dependent on the prevalences of adenomatous and non-adenomatous polyps. As our image set may not truly reflect the prevalence of these lesions in the general population, we further calculated the PPVs and NPVs based on our sensitivity and specificity data combined with literature data about the prevalence of adenomatous and non-adenomatous polyps in the general population (i.e., approximately 50% of all colorectal polyps detected during colonoscopy are adenomatous, while 50% non-adenomatous[8,35,36]). A two-step procedure was used to account for the dependency between repeated measurements. First, mean sensitivity, specificity and accuracy per endoscopist were computed and the differences between groups (i.e., gastroenterologists vs trainees) were tested using independent-samples t tests. High vs low confidence diagnoses were compared using the paired-samples t test. For the effect of location, size, morphology and image quality, the data were first summarized per image and differences between groups (i.e., distal vs proximal lesions, diminutive vs non-diminutive lesions, polypoid vs non-polypoid lesions and excellent vs good quality images) were compared using the independent-samples t test. We finally used k statistics to assess the agreement of predicted histology with formal histopathology. We considered a kappa value < 0.20 to indicate poor agreement, 0.21-0.40 slight agreement, 0.41-0.60 moderate agreement, 0.61-0.80 substantial agreement and 0.81-1.00 almost perfect agreement[37]. Two-sided P values ≤ 0.05 were considered statistically significant. Statistical analyses were conducted using the SPSS version 20.0.
RESULTS
A total of eleven endoscopists (4 gastroenterologists and 7 trainees) participated. Median (range) duration of colonoscopic experience was 12 years (range 7-15 years) for the participating gastroenterologists and 3 years (range 0-4 years) for the trainees. A total of 50 twin pictures (Figure 2) were incorporated into the test set, consisting of 14 (28.0%) non-adenomatous and 36 (72.0%) adenomatous polyps. Table 2 depicts the endoscopic and pathologic characteristics of the polyps incorporated into our test set. The majority (65.3%) of colorectal polyps were diminutive in size and 49.0% were located in the distal colon. Moreover, 29 (59.2%) cases had a polypoid and 20 (40.8%) a non-polypoid endoscopic appearance[38]. Image quality was rated as excellent in 29 (58.0%) cases and good in the remaining 21 (42.0%) cases.
Figure 2.
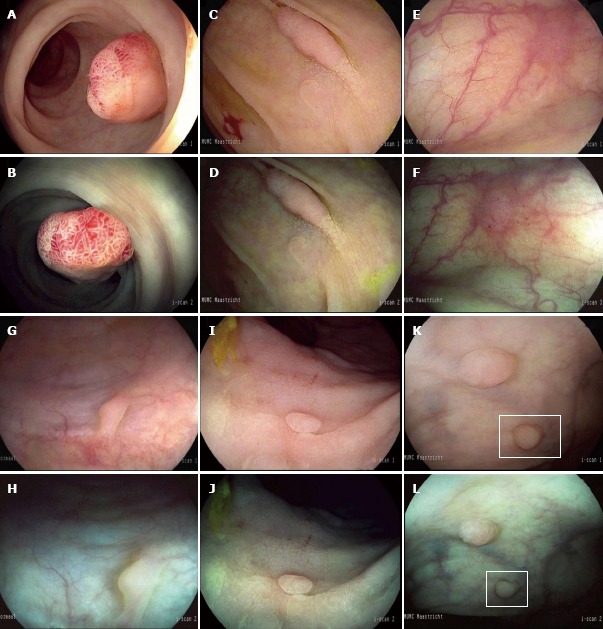
Endoscopic features of non-adenomatous and adenomatous polyps. High-definition (HD) i-scan surface enhancement (SE) (A) and tone enhancement (TE) (B) of a 12 mm pedunculated polyp located in the rectum characterized by a reddish color, prominent vessels and a branch-like (type IV) pit pattern. All endoscopists diagnosed this lesion as adenomatous with high confidence. Histopathology showed a tubulovillous adenoma with low-grade dysplasia. HD i-scan SE (C) and TE (D) of a 20 mm non-polypoid lesion in the ascending colon characterized by a reddish color, irregular vessels and a tubular (type IIIs) pit pattern. Adenomatous histology was predicted by all endoscopists with high confidence. Histopathology showed a tubular adenoma with low-grade dysplasia. HD i-scan SE (E) and TE (F) of a 4 mm non-polypoid lesion located in the proximal colon characterized by a reddish color, surface epithelium different from the surrounding mucosa and indiscrete borders while the vascular pattern could not be clearly observed. Polyp histology was predicted with low confidence by 6 of the 11 endoscopists. Histopathology showed a tubular adenoma with low grade dysplasia. SE (G) and TE (H) of a 3 mm sessile lesion in the sigmoid characterized by a pale color, no vessels and no definite pit pattern. Non-adenomatous histology was predicted with high confidence by 10 of the 11 endoscopists. Histopathology showed normal mucosa. SE (I) and TE (J) of a 3 mm non-polypoid lesion in the rectum characterized by a pale color, no vessels and round pits of uniform size. Non-adenomatous histology was predicted with high confidence by all endoscopists. Histopathology showed a hyperplastic polyp. SE (K) and TE (L) of a 3 mm sessile lesion in the rectum characterized by a pale color and epithelium similar to the adjacent mucosa while the surface pattern and vessel pattern are unclear. Histopathology was predicted with low confidence by 5 out of 11 endoscopists. Histopathology showed a hyperplastic polyp.
Table 2.
Endoscopic and pathologic characteristics of the colorectal polyps incorporated in our test set n (%)
| Characteristic | Polyps |
| Total number of colorectal polyps | 50 |
| Location1 | |
| Proximal colon | 25 (51.0) |
| Distal colon | 24 (49.0) |
| Size1 | |
| Diminutive | 32 (65.3) |
| Non-diminutive | 17 (34.7) |
| Morphology1 | |
| Polypoid | 29 (59.2) |
| Non-polypoid | 20 (40.8) |
| Histopathology | |
| Tubular adenoma | 29 (58.0) |
| Tubulovillous adenoma | 6 (12.0) |
| Carcinoma | 1 (2.0) |
| Hyperplastic polyp | 8 (16.0) |
| Sessile serrated adenoma/polyp | 1 (2.0) |
| Other (i.e., inflammatory) | 2 (4.0) |
| Normal tissue | 3 (6.0) |
| Final histopathology | |
| Non-adenomatous | 14 (28.0) |
| Adenomatous | 36 (72.0) |
| Image quality | |
| Excellent | 29 (58.0) |
| Less than excellent | 21 (42.0) |
Information was missing in 1 case.
Endoscopic features of non-adenomatous and adenomatous polyps
The endoscopic characteristics of non-adenomatous and adenomatous polyps are depicted in Figure 2. The frequencies of endoscopic features predictive of adenomatous and non-adenomatous polyps observed by the eleven endoscopists are shown in Table 3. Reddish color, surface epithelium different from the surrounding mucosa, clear demarcation and oval, tubular or branched pits were frequently seen in adenomatous polyps, whereas thick vessels were observed in only 40.9% of all adenomatous polyps. In contrast, pale color, surface epithelium similar to the adjacent mucosa, indiscrete borders, round pits of uniform size or no definite pits and isolated, lacy vessels were all frequently noticed in non-adenomatous polyps. The surface pattern remained unclear in 9.3% of all adenomatous polyps and 15.6% of all non-adenomatous polyps, whereas a clear vascular pattern could not be observed in 16.2% of the adenomas.
Table 3.
Frequencies of endoscopic features predictive of adenomatous and non-adenomatous polyps
| Adenomatous polyps | Non-adenomatous polyps | |
| Features predictive of adenomas | ||
| Reddish | 62.6% | 2.6% |
| Different adjacent mucosa | 74.7% | 11.7% |
| Clearly demarcated | 72.5% | 33.1% |
| Oval, tubular or branched pits | 71.7% | 11.0% |
| Thick vessels | 40.9% | 8.4% |
| Features predictive of non-adenomas | ||
| Pale | 32.3% | 92.9% |
| Similar adjacent mucosa | 22.5% | 85.7% |
| Indiscrete borders | 26.0% | 65.6% |
| Round pits of uniform size, no definite pits | 18.9% | 73.4% |
| Isolated, lacy vessels | 42.9% | 87.0% |
| Features unclear | ||
| Color | 5.1% | 4.5% |
| Adjacent mucosa | 2.8% | 2.6% |
| Demarcation | 1.5% | 1.3% |
| Surface pattern | 9.3% | 15.6% |
| Vessel pattern | 16.2% | 4.5% |
Diagnostic performances in optical diagnosis using HD i-scan
A total of 550 images were evaluated by the eleven endoscopists. Mean sensitivity, specificity and accuracy for diagnosing adenomas were 79.3%, 85.7% and 81.1%, respectively (Table 4). The corresponding PPV and NPV were 84.7% and 80.5%, respectively.
Table 4.
Diagnostic performances of the eleven endoscopists in predicting polyp histology subdivided according to level of experience, location, size, morphology and image quality
| Sensitivity | Specificity | Accuracy | PPV1 | NPV1 | |
| Overall | 79.3 ± 7.0 | 85.7 ± 9.6 | 81.1 ± 4.7 | 84.7 | 80.5 |
| Experience | |||||
| GE | 76.4 ± 6.6 | 82.1 ± 9.2 | 78.0 ± 3.7 | 81.0 | 77.7 |
| Trainees | 81.0 ± 7.1 | 87.8 ± 9.9 | 82.9 ± 4.5 | 86.9 | 82.2 |
| P value | 0.320 | 0.378 | 0.098 | ||
| Location | |||||
| Proximal | 74.6 ± 30.9 | 80.0 ± 29.7 | 75.6 ± 30.1 | 78.9 | 75.9 |
| Distal | 84.2 ± 29.0 | 88.9 ± 8.8 | 86.0 ± 23.4 | 88.4 | 84.9 |
| P value | 0.353 | 0.547 | 0.187 | ||
| Size | |||||
| Diminutive | 63.2 ± 33.3 | 90.2 ± 7.8 | 74.2 ± 29.1 | 86.6 | 71.0 |
| Non-diminutive | 97.2 ± 7.2 | 27.32 | 93.1 ± 18.3 | 57.2 | 90.7 |
| P value | < 0.001 | 0.008 | |||
| Morphology | |||||
| Polypoid | 81.8 ± 25.1 | 85.1 ± 20.0 | 83.1 ± 23.0 | 84.6 | 82.4 |
| Non-polypoid | 75.4 ± 35.0 | 87.9 ± 13.9 | 77.3 ± 32.8 | 86.2 | 78.1 |
| P value | 0.536 | 0.829 | 0.470 | ||
| Image quality | |||||
| Excellent | 89.3 ± 23.1 | 93.5 ± 6.9 | 90.3 ± 20.3 | 93.2 | 89.7 |
| Good | 63.6 ± 33.3 | 77.9 ± 23.4 | 68.4 ± 30.5 | 74.2 | 68.2 |
| P value | 0.010 | 0.117 | 0.007 |
Data are expressed as mean ± SD.
Based on our sensitivity and specificity data and literature data regarding prevalences of non-adenomatous and adenomatous polyps;
Based on 1 case only, no statistics performed. GE: Gastroenterologist; PPV: Positive predictive value; NPV: Negative predictive value.
Factors influencing the diagnostic performances of the endoscopists
The effects of the level of experience, polyp characteristics and image quality for the prediction of polyp histology on the diagnostic performances of the endoscopists are shown in Table 4. We found no significant difference in mean sensitivity (76.4% vs 81.0%, P = 0.320), mean specificity (82.1% vs 87.8%, P = 0.378) and mean accuracy (78.0% vs 82.9%, P = 0.098) for predicting polyp histology between gastroenterologists and trainees. Of note, mean accuracy for prediction of histology was significantly higher in non-diminutive vs diminutive polyps (93.1% vs 74.2%, P = 0.008). With regard to location, colorectal polyps located in the distal colon were predicted with a higher mean accuracy as compared to colorectal polyps located in the proximal colon, although not statistically significant (86.0% vs 75.6%, P = 0.187). With regard to the morphology, no significant differences were observed between polypoid and non-polypoid lesions (83.1% vs 77.3%, P = 0.470). Of all images, 319 (58.0%) were considered to be of excellent quality. As expected, excellent quality images were predicted with a significantly higher mean accuracy as compared to good quality images (90.3% vs 68.4%, P = 0.007).
High confidence diagnoses
A total of 446 (81.1%) diagnosis could be made with high confidence. High confidence diagnoses corresponded to a significantly higher mean accuracy (84.0% vs 64.3%, P = 0.008) than low confidence diagnoses. A total of 281 (88.1%) excellent quality images were diagnosed with high confidence. High confidence diagnosis in combination with excellent quality images resulted in a significantly higher mean accuracy (92.8% vs 62.3%, P = 0.014) as compared to low confidence diagnosis in combination with high quality images. PPVs and NPVs of high confidence diagnoses were 90.2% and 82.8%, respectively.
Agreement with formal histology
The kappa value, reflecting agreement of the endoscopist prediction with formal histology, ranged from 0.453 to 0.737 among the eleven endoscopists (Table 5). Analysis of high confidence diagnoses only improved the kappa value ranging from 0.519 to 0.821 indicating a moderate to substantial agreement.
Table 5.
Kappa values reflecting agreement of the endoscopist prediction with formal histopathology
| Kappa range | Interpretation1 | |
| All images (n = 50) | ||
| All predictions | 0.453-0.737 | Moderate–substantial agreement |
| High confidence predictions | 0.519-0.821 | Moderate-substantial agreement |
| Excellent quality images (n = 29) | ||
| All predictions | 0.621-0.828 | Substantial-almost perfect agreement |
| High confidence predictions | 0.709-0.900 | Substantial-almost perfect agreement |
Interpretation according to Landis and Koch (reference 37).
A total of 319 (58.0%) images were rated as excellent quality images. Taking into consideration these images only, the kappa values ranged from 0.621 to 0.828. When considering high confidence diagnoses in combination with excellent quality images, the kappa values ranged from 0.709 to 0.900 indicating substantial to almost perfect agreement.
DISCUSSION
The present study shows that after a short didactic training session on optical diagnosis using HD i-scan, endoscopists can already predict colorectal polyp histology, with a mean accuracy of 84.0% for high confidence diagnosis. In addition, we found that specific polyp characteristics might negatively affect the diagnostic performances of endoscopists, in particular a diminutive size.
It is important to systematically evaluate the benefit of new image enhanced endoscopy techniques, as these have become widely available and might enable refinement of diagnosis and a more efficient treatment of colorectal polyps in routine practice[1,2]. Accurate optical diagnosis may allow the endoscopist to leave diminutive non-adenomatous polyps of the rectosigmoid in place and remove and discard diminutive adenomas, thereby reducing the pathology costs and potential risks of complications[6,7]. In addition, these technologies may offer a substitute for histopathology in case colorectal polyps are not retrieved for formal histopathology[8] (i.e., about 8% of all colorectal polyps are histologically uninterpretable in routine practice due to lack of retrieval after polypectomy or damaged material[39]).
Several studies demonstrated optical diagnosis is feasible using chromoendoscopy[15,16] or NBI[3,14-17], while only few studies examined the feasibility using HD i-scan technology[5,17,19-22], and none of them was conducted outside an expert setting. We therefore developed a simple classification system of colorectal polyps using HD i-scan (ICE classification) and evaluated the diagnostic performances for prediction of histology in a group of non-expert endoscopists.
In line with previously reported data[5,17,19-22], the current study shows that endoscopists can predict polyp histology with a mean accuracy of 84.0% (high confidence diagnosis) using HD i-scan still images. Hoffman et al[5,19] reported accuracy rates ranging from 96% to 99% in two single centre studies. All procedures in these studies were conducted by endoscopists with high-level of expertise on image enhanced endoscopy techniques[40], including HD i-scan, and using electronic magnification, which might explain the high accuracy rates observed in these studies[5,19]. The results of our study are consistent with the findings reported in a study by Pigò et al[21] in which an experienced endoscopist predicted the histology of 150 colorectal polyps during colonoscopy with a sensitivity, specificity and accuracy of 95%, 82% and 92%, respectively. Subsequently, still images of these 150 colorectal polyps were evaluated by four other endoscopists after a short teaching session with an overall sensitivity of 88%, specificity of 62% and accuracy of 82%. When evaluating good/excellent quality images only, their overall accuracy improved to 94%, which is in line with our data. A possible explanation for the higher accuracy rates reported by real-time visual assessment compared to still images is perhaps the dynamic observation of lesions (e.g., air insufflation, closer inspection) which may provide additional details. It is therefore plausible that our data underestimate the true diagnostic performances of the endoscopists in routine practice, when polyp histology is predicted by real-time visual assessment.
The present study indicates that a 20 min didactic training session is an effective and efficient way to familiarize endoscopists with the basic principles of optical diagnosis. This is in agreement with existing literature data showing a short and rapid learning curve for accurate evaluation of still images using new image enhanced endoscopy techniques[41,42]. Although several studies showed experienced endoscopists can accurately predict polyp histology[5,14,17], recent data reported lower performances of endoscopists in a community setting[18]. It is possible such lower performances and operator-dependency may reflect different levels of training[6,22], thus emphasizing the need for learning programs comprising both an ex vivo and in vivo phase. By analogy with our previous experience regarding training in detection and management of non-polypoid lesions[23], we suggest the following steps might be considered. First, a short didactic training session might be offered to the endoscopists to familiarize them with the basic principles of optical diagnosis. Second, video training can help to further shape their skills. Finally, individual feedback during colonoscopy by an experienced endoscopists and self-learning (i.e., comparison of optical diagnosis with formal histopathology) might be important to achieve and maintain proficiency in optical diagnosis.
In this study we paid special attention to identify specific factors which may negatively impact prediction of histology, as this may help to establish targets for improvements. We found that colorectal polyps of diminutive size, proximally located or with non-polypoid morphology are more likely predicted with a lower accuracy. In a recent study by our group, proximal neoplasms appeared to be in general more frequently diminutive and have a non-polypoid shape[27]. Taken together these findings emphasize the need for careful inspection of proximal lesions, which might be both more challenging to detect and also to characterize histologically. Optimal bowel preparation, which may be more difficult to achieve in the proximal colon[43] has definitely a key role in reaching these targets.
In this study, we developed a simple classification system (i.e., the ICE classification) of colorectal polyps by means of HD i-scan, applying similar criteria to those described by the NICE classification using NBI[34]. Although several studies now investigated the performance characteristics of optical diagnosis using different image enhanced technologies[4,17,26], the outcomes are difficult to compare due to heterogeneity in the diagnostic criteria used. Implementation of a simple, universal classification system, irrespective of the endoscopic technology used, is essential for comparing the outcomes across studies.
According to the American Society for Gastrointestinal Endoscopy recommendations, a NPV of at least 90% should be attained for a safe implementation of a leave in place approach for diminutive non-adenomatous polyps in routine practice[44]. These benchmarks can be already achieved by expert endoscopists[45], yet data in a non-expert setting are scarce and controversial. In our experience, excellent quality images in conjunction with high confidence diagnosis resulted in a mean accuracy of 92.8%. Studies are currently underway at our institution to assess the performance characteristics of real-time optical diagnosis in a non-expert setting.
Some methodological aspects of this study need further consideration. As a strength, endoscopists with varying levels of experience, and who were previously familiarized on the recognition and management of non-polypoid colorectal neoplasms participated[27,28]. Second, we used a simple classification system (ICE classification), based on previously defined and validated criteria[34]. Third, we examined the influence of polyp characteristics, image quality and use of confidence levels on the diagnostic performances to highlight potential targets for training. As a limitation, we used a selection of images consisting of small and larger colorectal polyps whereas optical diagnosis seems to be applicable for small and diminutive polyps only, given the low rate of advanced histology in these lesions[8]. We assume, however, that simple observation and recognition of the pit-patterns may be the first step when developing educational skills in optical diagnosis. Second, the diagnostic performances of the endoscopists involved in this study did not reach the threshold recommended for a safe implementation of a leave in place approach of non-adenomatous polyps[44]. This was not unexpected, as HD i-scan was only recently introduced at our institution, and hence, the endoscopists might have been still early in their learning curves. Third, the diagnostic performances might have been underestimated as endoscopists predicted polyp histology based on still images instead of real-time visual assessment. Finally, in this study we assigned the sessile serrated adenomas/polyps to the group of non-adenomatous polyps. However, as only 1 out of the 50 colorectal polyps examined turned to be a sessile serrated adenoma/polyp, its re-classification into adenomatous lesion is unlikely to change the results and conclusions of this study.
In conclusion, the present study indicates that optical diagnosis using HD i-scan is feasible for endoscopists with varying levels of experience, with a mean accuracy of 84.0% for high confidence diagnosis. A short training module is an effective tool to familiarize the practicing endoscopists with the basic principles of optical diagnosis, although continuous training and practice may be needed to optimize the skills in optical diagnosis.
ACKNOWLEDGMENTS
We are grateful to all endoscopists, especially Michel Aquarius, José Conchillo, Jeoffrey Haans, Chantal Hoge, Reggy Jaspers, Yolande Keulemans, Ger Koek, Johanna Kruimel, Wout Mares, Tamara Munnecom and Jennifer Wilbrink at the Division of Gastroenterology and Hepatology for their enthusiastic participation in this study.
COMMENTS
Background
Image enhanced endoscopy techniques, such as chromoendoscopy either dye-based or digital [narrow band imaging, high-definition (HD) i-scan or Fujinon Intelligent Color Enhancement] techniques became largely available. Such technologies offer the opportunity to accurately differentiate adenomas from non-adenomatous polyps, allowing their individualized treatment, which in turn may increase the efficiency of colonoscopic procedures.
Research frontiers
Currently, data about the diagnostic performances using HD i-scan are scarce. None of these studies was conducted outside an expert setting, nor applied standardized diagnostic criteria, and none specifically addressed the impact of training.
Innovations and breakthroughs
In this study, authors found that, after a short didactic session, all endoscopists, trainees as well as gastroenterologists, could already predict polyp histology with a mean accuracy of 84%. Authors additionally propose a simple classification of diagnostic criteria of colorectal polyps using HD i-scan (i-scan classification for endoscopic diagnosis), which might represent a practical tool for optical diagnosis in the community setting.
Applications
This study highlights the importance of training in attaining practical skills in optical diagnosis using new image enhanced endoscopy techniques.
Terminology
The HD i-scan technology is a new image enhanced endoscopy technique that allows digital chromoendoscopy via the Pentax EPKi processor.
Peer review
They present the result of examining performances regarding prediction of polyp histology using HD i-scan in a group of endoscopists with varying levels of experience. The authors conclude that endoscopists with varying levels of experience can already provide optical diagnosis with an accuracy of 84.0%. This is a relevant study as optical diagnosis of colorectal polyps might offer an alternative to histologic diagnosis and may downsize the pathology costs and reduce the risk of complications. This manuscript is a well-written paper, however, it may be refined with a minor revision by authors.
Footnotes
P- Reviewer Cha JM S- Editor Gou SX L- Editor A E- Editor Ma S
References
- 1.Vemulapalli KC, Rex DK. Evolving techniques in colonoscopy. Curr Opin Gastroenterol. 2011;27:430–438. doi: 10.1097/MOG.0b013e328349cfc0. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Fennerty MB, Sharma P, Kaltenbach T, Soetikno R. Bringing new endoscopic imaging technology into everyday practice: what is the role of professional GI societies Polyp imaging as a template for moving endoscopic innovation forward to answer key clinical questions. Gastrointest Endosc. 2010;71:142–146. doi: 10.1016/j.gie.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174–1181. doi: 10.1053/j.gastro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 4.dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, Teixeira CR. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol. 2010;22:1364–1371. doi: 10.1097/MEG.0b013e32833a5d63. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827–833. doi: 10.1055/s-0030-1255713. [DOI] [PubMed] [Google Scholar]
- 6.Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10:1171–1178. doi: 10.1016/S1470-2045(09)70329-8. [DOI] [PubMed] [Google Scholar]
- 7.East JE, Saunders BP. Look, remove, and discard: can narrow-band imaging replace histopathology for small colorectal polyps It is time to push the button! Gastrointest Endosc. 2007;66:953–956. doi: 10.1016/j.gie.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 8.East JE, Guenther T, Saunders BP. Novel approaches in colorectal endoscopy: what do we need biopsies for. Pathol Res Pract. 2008;204:459–467. doi: 10.1016/j.prp.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. 2010;8:865–869. doi: 10.1016/j.cgh.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012;1:CD008361. doi: 10.1002/14651858.CD008361.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, Sharma VK. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363–370; quiz 371. doi: 10.1038/ajg.2011.436. [DOI] [PubMed] [Google Scholar]
- 12.Pohl J, Schneider A, Vogell H, Mayer G, Kaiser G, Ell C. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut. 2011;60:485–490. doi: 10.1136/gut.2010.229534. [DOI] [PubMed] [Google Scholar]
- 13.Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G, et al. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73–78. doi: 10.1136/gut.2008.153601. [DOI] [PubMed] [Google Scholar]
- 14.Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, Jonnalagadda SS, Hovis CE, Gaddam S, Wani SB, et al. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc. 2011;74:593–602. doi: 10.1016/j.gie.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 15.Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092–1096. doi: 10.1055/s-2007-966781. [DOI] [PubMed] [Google Scholar]
- 16.Wada Y, Kashida H, Kudo SE, Misawa M, Ikehara N, Hamatani S. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc. 2010;22:192–199. doi: 10.1111/j.1443-1661.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus I-Scan for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc. 2011;74:603–609. doi: 10.1016/j.gie.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 18.Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, Imperiale T, Gunaratnam N. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013;144:81–91. doi: 10.1053/j.gastro.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman A, Kagel C, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis. 2010;42:45–50. doi: 10.1016/j.dld.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Masci E, Mangiavillano B, Crosta C, Fiori G, Trovato C, Viaggi P, Zambelli A, Buffoli F, Staiano T, Manfredi G, et al. Interobserver agreement among endoscopists on evaluation of polypoid colorectal lesions visualized with the Pentax i-Scan technique. Dig Liver Dis. 2013;45:207–210. doi: 10.1016/j.dld.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Pigò F, Bertani H, Manno M, Mirante V, Caruso A, Barbera C, Manta R, Bassotti G, Olivetti G, Conigliaro RL. i-Scan high-definition white light endoscopy and colorectal polyps: prediction of histology, interobserver and intraobserver agreement. Int J Colorectal Dis. 2013;28:399–406. doi: 10.1007/s00384-012-1583-7. [DOI] [PubMed] [Google Scholar]
- 22.Chan JL, Lin L, Feiler M, Wolf AI, Cardona DM, Gellad ZF. Comparative effectiveness of i-SCAN™ and high-definition white light characterizing small colonic polyps. World J Gastroenterol. 2012;18:5905–5911. doi: 10.3748/wjg.v18.i41.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanduleanu S, Rondagh EJ, Masclee AA. Development of expertise in the detection and classification of non-polypoid colorectal neoplasia: Experience-based data at an academic GI unit. Gastrointest Endosc Clin N Am. 2010;20:449–460. doi: 10.1016/j.giec.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Hewett DG, Huffman ME, Rex DK. Leaving distal colorectal hyperplastic polyps in place can be achieved with high accuracy by using narrow-band imaging: an observational study. Gastrointest Endosc. 2012;76:374–380. doi: 10.1016/j.gie.2012.04.446. [DOI] [PubMed] [Google Scholar]
- 25.Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, Matsui S, Friedland S. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 26.Sanduleanu S, Driessen A, Gomez-Garcia E, Hameeteman W, de Bruïne A, Masclee A. In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2010;8:371–378. doi: 10.1016/j.cgh.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, Soetikno RM, Masclee AA, Sanduleanu S. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218–1225. doi: 10.1016/j.gie.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Rondagh EJ, Masclee AA, van der Valk ME, Winkens B, de Bruïne AP, Kaltenbach T, Soetikno RM, Sanduleanu S. Nonpolypoid colorectal neoplasms: gender differences in prevalence and malignant potential. Scand J Gastroenterol. 2012;47:80–88. doi: 10.3109/00365521.2011.638395. [DOI] [PubMed] [Google Scholar]
- 29.Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated (“hyperplastic”) polyposis. In: Bozman FT, Carneiro F, Hruban RH, Theise N, editors. WHO classification of tumours Pathology and genetics Tumours of the digestive system, 4th ed. Berlin: Spinger-Verlag: 2010. [Google Scholar]
- 30.Limketkai BN, Lam-Himlin D, Arnold CA, Arnold MA. The cutting edge of serrated polyps: a practical guide to approaching and managing serrated colon polyps. Gastrointest Endosc. 2013;77:360–375. doi: 10.1016/j.gie.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Kashida H, Ikehara N, Hamatani S, Kudo SE, Kudo M. Endoscopic characteristics of colorectal serrated lesions. Hepatogastroenterology. 2011;58:1163–1167. doi: 10.5754/hge10093. [DOI] [PubMed] [Google Scholar]
- 32.Kimura T, Yamamoto E, Yamano HO, Suzuki H, Kamimae S, Nojima M, Sawada T, Ashida M, Yoshikawa K, Takagi R, et al. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460–469. doi: 10.1038/ajg.2011.457. [DOI] [PubMed] [Google Scholar]
- 33.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 34.Rey JF, Lambert R, Aabakken L, Dekker E, East JE, Kaltenbach T, Kato M, Sharma P, Tanaka S. Proceedings of a preliminary workshop at Gastro 2009--narrow banding imaging in digestive endoscopy: clinical outcome of classification (Omed-Jges Educational Meeting held on 22 November, 2009) Dig Endosc. 2011;23:251–266. doi: 10.1111/j.1443-1661.2010.01083.x. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135:1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai FC, Strum WB. Prevalence of advanced adenomas in small and diminutive colon polyps using direct measurement of size. Dig Dis Sci. 2011;56:2384–2388. doi: 10.1007/s10620-011-1598-x. [DOI] [PubMed] [Google Scholar]
- 37.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 38.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 39.Gschwantler M, Kriwanek S, Langner E, Göritzer B, Schrutka-Kölbl C, Brownstone E, Feichtinger H, Weiss W. High-grade dysplasia and invasive carcinoma in colorectal adenomas: a multivariate analysis of the impact of adenoma and patient characteristics. Eur J Gastroenterol Hepatol. 2002;14:183–188. doi: 10.1097/00042737-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Kiesslich R, Hoffman A, Goetz M, Biesterfeld S, Vieth M, Galle PR, Neurath MF. In vivo diagnosis of collagenous colitis by confocal endomicroscopy. Gut. 2006;55:591–592. doi: 10.1136/gut.2005.084970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010;72:572–576. doi: 10.1016/j.gie.2010.03.1124. [DOI] [PubMed] [Google Scholar]
- 42.Ignjatovic A, Thomas-Gibson S, East JE, Haycock A, Bassett P, Bhandari P, Man R, Suzuki N, Saunders BP. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc. 2011;73:128–133. doi: 10.1016/j.gie.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 43.Belsey J, Crosta C, Epstein O, Fischbach W, Layer P, Parente F, Halphen M. Meta-analysis: the relative efficacy of oral bowel preparations for colonoscopy 1985-2010. Aliment Pharmacol Ther. 2012;35:222–237. doi: 10.1111/j.1365-2036.2011.04927.x. [DOI] [PubMed] [Google Scholar]
- 44.Rex DK, Kahi C, O’Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–422. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 45.McGill SK, Evangelou E, Ioannidis JP, Soetikno RM, Kaltenbach T. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut. 2013:Apr 17; Epub ahead of print. doi: 10.1136/gutjnl-2012-303965. [DOI] [PMC free article] [PubMed] [Google Scholar]


