Abstract
AIM: To investigate the side effects of a zinc sulphate therapy in a cohort of Polish pediatric patients with Wilson’s disease.
METHODS: We retrospectively analyzed a cohort of 53 pediatric patients with Wilson’s disease treated at the Children’s Memorial Health Institute in Warsaw, Poland between the years 1996 and 2011 with zinc sulphate. Patients were diagnosed with Wilson’s disease according to the scoring system of Ferenci, with 49 cases confirmed by mutation analysis. Data about the dosage scheme of zinc sulphate, side effects and efficacy and toxicity of the treatment were collected and recorded in the patient’s medical chart at each visit to the hospital.
RESULTS: Mean age of diagnosis for the entire cohort was 10 years (range, 2.5-17 years). Duration of treatment with zinc sulfate was 83.3 wk (range, 8-344 wk). Side effects, all of gastrointestinal origin, were observed in 21 patients (40% - 9 males and 12 females), irrespective of the duration of therapy. Thirteen out of 21 patients were over the age of 10 years. The most common ATP7B mutation was p.H1069Q. Esophagogastroduodenoscopy, performed in 7 patients (33.3%) suffering from persistent and severe abdominal pain, revealed gastrointestinal ulcerations or erosions with negative Helicobacter pylori tests in all subjects investigated. The above mentioned 7 patients were treated with proton pump inhibitors. Three of those experienced resolution of symptoms, whereas proton-pump inhibitors failed to alleviate symptoms of the remaining four children and conversion of therapy to D-penicillamine was needed.
CONCLUSION: Zinc sulphate appears to cause significant gastrointestinal side effects, which children on therapy for Wilson’s disease should be closely monitored for.
Keywords: Wilson’s disease, Zinc, Abdominal pain, Gastrointestinal ulcer, Therapy
Core tip: The present study demonstrates a considerably higher rate and severity of gastrointestinal adverse effects secondary to zinc sulphate therapy in pediatric patients as previously reported. A total of 40% of our treated patients experienced gastrointestinal symptoms, of which the more severe cases were associated with endoscopically evident gastric ulcerations and erosions. Furthermore it was shown, that proton pump inhibitors were not effective in treating patients with severe zinc associated gastrointestinal side effects, requiring a switch to an alternative treatment regimen.
INTRODUCTION
Wilson’s disease is an autosomal recessive disorder of copper metabolism, which results in impaired copper excretion by the liver. Toxic copper accumulates both in the liver and in the central nervous system. Various therapies are used, which include copper chelators like D-penicillamine and trientine or tetrathiomolybdate, as well as zinc preparations, such as zinc sulphate and zinc acetate. Liver transplantation is used mainly in acute liver failure. In Poland trientine is not registered and the most popular drugs are D-penicillamine (Cuprenil®, Teva Pharmaceuticals Polska) and zinc sulphate (Zincteral®, Teva Pharmaceuticals Polska). Zinc acetate (Wilzin®, Orphan Europe) is registered but not commonly used due to its high costs, which are not covered by the state and often unaffordable for patients.
The most common side effects of zinc therapy described are nausea and abdominal pain. Other complications, secondary to zinc-induced copper deficiency such as hematologic abnormalities, specifically leukopenia (fever, chills, sore throat), neutropenia (oropharyngeal ulcers) and sideroblastic anemia (fatigue, general weakness) are very uncommon but may occur[1-3]. A mild, harmless increase in serum amylase and lipase concentrations without clinical or radiologic evidence of pancreatitis[4] and a 20% reduction of high-density lipoprotein cholesterol in male patients (associated with reduction in total cholesterol) have been described[5]. Zinc may have immunosuppressant effects and reduce leukocyte chemotaxis, however one study found no adverse effects on lymphocyte function with chronic use[6].
Although many potential adverse effects of zinc therapy have been described, zinc sulphate is regarded to be an overall safe and well-tolerated drug. According to Wiggelinkhuizen’s systematic review[7], 12.5% of adult patients with Wilson’s disease suffered from side effects secondary to zinc therapy, all of gastrointestinal origin. There are only few studies focusing on pediatric patients, most of them reporting gastrointestinal adverse effects of zinc sulphate or acetate in children to be uncommon and mild, generally handled by changing dosage scheme or resolving spontaneously[8-12]. However, the analysis of our cohort with 53 pediatric patients with Wilson’s disease led to differing results. Gastrointestinal side effects were detected in a considerably greater proportion of patients and range of severity was clearly wider, with some cases associated with severe and poorly tolerated symptoms and endoscopically evident ulcerations, a finding not been described earlier. Based on this detailed retrospective analysis of Polish children, we raised the concern about the safety of zinc sulphate therapy in pediatric patients with Wilson’s disease.
MATERIALS AND METHODS
The study included 53 patients with hepatic presentation of Wilson’s disease treated at the Children’s Memorial Health Institute in Warsaw, Poland between the years 1996 and 2011. Wilson’s disease was diagnosed according to the scoring system of Ferenci[13,14], with confirmation by mutational analysis in 49 cases. All patients treated with zinc sulphate were selected to the main analysis. Zinc sulphate was used as first choice therapy for 50/53 cases and 3/53 patients were initiated on D-penicillamine and later switched to zinc sulphate as a maintenance therapy (Table 1). Data about dosage scheme of zinc sulphate and adverse effects during therapy were collected and recorded in the patient’s medical chart at each hospital visit. The Local Ethics Committee approved the study. Zinc sulphate was administered at a dose of 135 mg of elemental zinc daily in three divided doses for children with a body weight more than 50 kg. Three patients (males, 14, 15 and 16 years old) received a different zinc sulphate regiment with 180 mg of elemental zinc daily. The dose for younger and smaller children dosage was twice daily 45 mg of elemental zinc. One 9-year-old patient was given a daily dose of 45 mg of elemental zinc. At each visit (for the first year of therapy once every 3 mo, subsequently twice a year) patients were followed for efficacy and toxicity of treatment by measuring liver function tests (alanine transaminase, bilirubin), complete blood count and coagulation parameters, and by physical examination. Adequacy of zinc therapy was monitored with zinc serum levels and with 24-h urinary excretion of copper. Zinc serum levels less than 125 μg/dL and urine copper above 75 μg/d were defined as treatment failure, suggesting non-response or non-compliance. Patients with poor tolerance for zinc sulphate were started on zinc acetate or D-penicillamine.
Table 1.
ATP7B mutations in patients with Wilson’s disease treated with zinc sulphate
| Mutation analysis | n |
| p.H1069Q/p.H1069Q | 22 |
| Diagnosis not confirmed by mutation analysis | 1 |
| p.H1069Q/- | 7 |
| p.H1069Q/p.Q1351X | 2 |
| p.H1069Q/p.A1135fs | 2 |
| p.V845fs/- | 2 |
| p.H1069Q/p.E507fs | 1 |
| p.H1069Q/p.C985Y | 1 |
| p.H1069Q/p.L1325fs | 1 |
| p.H1969Q/p.W779X | 1 |
| p.H1069Q/p.R969Q | 1 |
| p.H1069Q/p.T737I | 1 |
| p.H1069Q/p.P1273L | 1 |
| p.H1069Q/p.V772_I774del | 1 |
| p.H1069Q/p.Arg969Gln | 1 |
| p.H1069Q/p.G1341R | 1 |
| p.A1135fs/p.A1135fs | 1 |
| p.A1135fs/p.R1319X | 1 |
| p.G1158fs/p.G1158fs | 1 |
| p.W779X/p.W779R | 1 |
| p.Q1351X/- | 1 |
| p.A1135fs/- | 1 |
| p.N1270S/- | 1 |
Statistical analysis
The data were collected from patient’s medical charts and analyzed retrospectively. Patients who underwent upper tract endoscopy due to persistent abdominal pain were carefully described. The frequency of findings was presented in numbers and in percentages. Conclusions were based on careful description of findings, as low numbers of patients with presented features did not allow performing statistical analysis.
RESULTS
Characteristics of patients presenting with side effects during treatment with zinc sulphate are illustrated in Table 2. Mean age of diagnosis for our cohort of 53 patients was 10 years (range, 2.5-17 years). Median duration of treatment with zinc sulfate was 83.3 wk (range, 8-344 wk). Side effects secondary to zinc sulphate were observed in 21 children (21/53, 40% of investigated children, 9 males, 12 females; 13/21, 62% aged over 10 years), all symptoms were of gastrointestinal origin: abdominal pain, nausea or vomiting. Adverse effects associated with alternative therapies were noted as well within this group of 21 patients: rash (1 patient) and abdominal pain (1 patient) on D-penicillamine and elevated transaminases with zinc acetate treatment (1 patient).
Table 2.
Characteristic of patients with side effects during zinc therapy
| No. | Sex | Age of onset of symptoms (yr) | Age of diagnosis (yr) | Mutation analysis | Zinc sulphate therapy-dosage scheme (mg) | Treatment history | Cause of conversion to D-penicillamine | Cause of conversion to zinc acetate | Cause of additional intervention | Endoscopic examination | |
| 1 | M | 7 | 8 | p.V845fs | - | 2 × 45 | ZS - P - ZA | Abdominal pain | Rash | No | |
| 2 | F | Asymptomatic, positive family history | 15 | p.V845fs | - | 3 × 45 | ZS - P - ZA | Abdominal pain | Abdominal pain | No | |
| 3 | M | Asymptomatic, positive family history | 6 | p.Q1351X | - | 3 × 45 | ZS - P | Abdominal pain | No | ||
| 4 | M | 7 | 16 | p.W779X | p.W779R | 3 × 45 | ZS - P | Abdominal pain, loss of appetite | No | ||
| 5 | F | 7 | 9 | p.H1069Q | p.H1069Q | 3 × 45 | ZS - P | Vomiting | No | ||
| 6 | M | Lack of data | 12 | p.H1069Q | p.P1273L | 3 × 45 | ZS + PPI | Abdominal pain, symptoms of GERD | No | ||
| 7 | M | 11 | 12 | - | - | 3 × 45 | ZS - ADS | Abdominal pain | No | ||
| 8 | F | 11 | 11 | p.N1270S | - | 3 × 45 | ZS - P | Nausea | No | ||
| 9 | M | 12 | 13.5 | p.G1158fs | p.G1158fs | 5 × 45 | ZS - P | Nausea, vomiting | No | ||
| 10 | F | 13 | 14 | p.H1069Q | p.Q1351X | 3 × 45 | ZS - ZA | Nausea | No | ||
| 11 | F | Lack of data | 12 | p.H1069Q | p.T737I | 3 × 45 | ZS - P | Nausea | No | ||
| 12 | M | 8 | 13 | p.H1069Q | p.V772_I774del | 3 × 45 | P - ZS - ADS | Nausea | No | ||
| 13 | F | 7 | 7 | p.H1069Q | p.G1341R | 2 × 45 | ZS - P | Abdominal pain | No | ||
| 14 | M | 7 | 7 | p.A1135fs | - | 2 × 45 | ZS - ZA - P | Elevated transaminases | Abdominal pain | No | |
| 15 | F | Asymptomatic, positive family history | 5 | p.H1069Q | p.H1069Q | 2 × 45 | ZS + PPI - P | Abdominal pain | Yes | ||
| 16 | F | 8 | 8 | p.H1069Q | p.H1069Q | 2 × 45 | ZS +PPI - P | Abdominal pain | Yes | ||
| 17 | F | 14 | 14 | - | - | 3 × 45 | ZS + PPI | Abdominal pain | Yes | ||
| 18 | F | 12 | 12 | - | - | 3 × 45 | ZS +PPI - P | Abdominal pain | Yes | ||
| 19 | M | Asymptomatic, positive family history | Lack of data | - | - | 2 × 45 | ZS +PPI - P | Abdominal pain | Yes | ||
| 20 | F | 10 | 10 | p.H1069Q | - | 3 × 45 | ZS + PPI | Abdominal pain | Yes | ||
| 21 | F | 9 | 9 | - | - | 1 × 45 | ZS + PPI | Abdominal pain | Yes | ||
ZS: Zinc sulphate therapy; P: D-penicillamine therapy; ZA: Zinc acetate therapy; PPI: Proton pump inhibitor therapy; ADS: Alternative dosage scheme; GERD: Gastroesophageal reflux disease; M: male; F: Female.
Esophagogastroduodenoscopy (EGD) was performed in 7 children (7/21, 33%, 6 females, 1 male) experiencing persistent and severe abdominal pain. Gastritis with ulcerations or erosion was evident in all cases. There was no history of nonsteroidal antiinflammatory drugs use in any of the cases. Histopathology of biopsies showed mild to moderate lymphocytic infiltrations. All subjects investigated tested negative for Helicobacter pylori (H. pylori). Tests were performed during endoscopy by urea test (Table 3 and Figure 1). Three of these seven patients experienced resolution of symptoms on proton pump inhibitors (PPIs) (3 mo therapy: 1 patient; 6-wk therapy: 1 patient; unknown therapy duration due to lack of data: 1 patient). Adverse effects while on PPIs or recurrence of symptoms with discontinuation of PPIs were not observed. Effectiveness of therapy was confirmed by follow up EGD in one case.
Table 3.
Characteristic of patients with persistent abdominal pain
| No. | Abdominal pain before zinc sulphate therapy | Duration of zinc sulphate therapy before abdominal pain occurred (wk) | EGD before intervention | Intervention | Abdominal pain after intervention | EDG after intervention | Additional intervention | Abdominal pain after additional intervention | EGD after additional intervention |
| 1 | Yes | Lack of data | Gastritis with mucosal ulceration | PPI | Yes | Ulcer scar | No | - | - |
| 2 | No | 96 | Gastritis with mucosal ulceration | PPI | No | No | No | - | - |
| 3 | No | 28 | Gastritis with mucosal ulceration | PPI | Yes | Gastritis with mucosal ulceration | Conversion to D-penicillamine | No | No |
| 4 | No | 144 | Gastritis with mucosal ulceration | PPI | Yes | Gastritis | Conversion to D-penicillamine | No | No |
| 5 | No | 12 | Gastritis with mucosal erosion | PPI | No | No | No | - | - |
| 6 | No | 96 | Gastritis with mucosal ulceration | PPI | Yes | No | Conversion to D-penicillamine | No | Normal findings |
| 7 | No | 96 | Gastritis with mucosal erosion | PPI | Yes | No | Conversion to D-penicillamine | No | Normal findings |
EGD: Esophagogastroduodenoscopy; PPI: Proton pump inhibitor.
Figure 1.
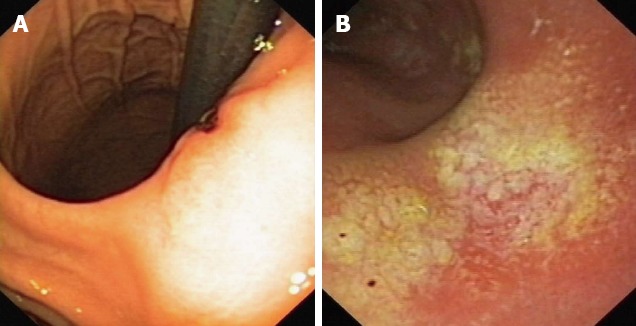
Endoscopic image. A: Endoscopic image of patient 6. Deep ulcer on the minor curvature of the stomach; B: Endoscopic image of patient 1. Flat mucosal ulcerations of the greater curvature of the stomach, about 1 cm in diameter covered with a flat fibrous coating.
Treatment with PPIs failed to alleviate symptoms of the remaining four patients, requiring a switch to D-penicillamine. Follow up EGDs were performed on two of these four patients.
One of the remaining 14 patients presented with GERD symptoms and improved clinically on PPIs, an EGD was not performed.
Three other patients experienced amelioration of symptoms following a conversion to zinc acetate. Two of the latter mentioned three cases had initially been converted to D-penicillamine. However side effects, such as rash in one case and abdominal pain in the other, required a second switch to zinc acetate. With eight patients, amelioration of clinical symptoms was achieved after converting therapy to D-penicillamine. One of those eight cases, although remission of gastrointestinal symptoms had been attained after switching from zinc sulphate to acetate, elevated transaminases necessitated another switch to D-penicillamine. Two other patients improved on the alternative zinc sulphate dosage scheme.
Table 4 illustrates the distrubution of mutations between the two groups: patients with and without side effects during zinc sulphate therapy. However conclusions regarding susceptibility towards adverse effects cannot be drawn from this data.
Table 4.
Characteristic of mutation analysis in two groups of patients with Wilson’s disease: with and without side effects during zinc sulphate therapy
| Mutation analysis |
No. of patients during zinc sulphate therapy |
|
| Without side effects | With side effects | |
| Homozygous for p.H1069Q mutation | 16 | 3 |
| Compound heterozygous for p.H1069Q mutation | 8 | 5 |
| Carrier for p.H1069Q mutation | 4 | 1 |
| Carrier for a mutation other than p.H1069Q | 0 | 5 |
| Mutation other than p.H1069Q on two alleles | 2 | 2 |
| Lack of data | 2 | 5 |
DISCUSSION
The first line therapy for asymptomatic patients and for those with mild hepatic disease is zinc sulphate. It is also being used as a maintenance therapy for patients, whose hepatic symptoms improved under D-penicillamine[15,16]. Another indication for zinc sulphate is the presentation of neurological symptoms[16,17]. The guidelines of the American Association for the Study of Liver Diseases recommend a daily dose for older children and adults of 150 mg in three divided doses[18,19]. However, compliance with the three times per day dosage may be problematic, and it has to be taken at least twice daily to be effective[15,18,19]. For smaller children, with a body weight under 50 kg, the dose is 75 mg/d in three divided doses[18-20]. The dose for children under 5 years of age is not well defined. It is worth noting that taking zinc with food interferes with its absorption[21] and therefore with the effectiveness of treatment. Adequacy of treatment with zinc sulphate can be estimated by measuring serum zinc levels or 24-h urinary zinc excretion. Other goals of treatment monitoring are to ensure patients compliance with therapy, and identify adverse side effects. Patients should be monitored at least twice a year. More frequent observation is needed during the initial phase of treatment, for patients experiencing worsening of symptoms or side effects and for those suspected of noncompliance with therapy[18,19]. Brewer et al[20] advise to test zinc serum and urine copper levels during therapy. A zinc serum level less than 125 μg/dL generally indicates poor compliance. According to the authors the best monitoring tool may be following urine copper levels, with maximum levels around 50 μg/d. Roberts et al[18,19] recommend that urinary copper excretion should not exceed 75 μg/d on a stable treatment. Many studies report sustained elevation of alanine aminotransferase during zinc therapy, which may indicate poor compliance and which may results in fewer and milder side effects. Our patients mostly adhered to zinc therapy but many of them presented sporadically with increased copper excretion in the urine indicating treatment failure.
There are only few studies focusing on side effects of zinc therapy in pediatric patients with Wilson’s disease, and those available studying only small numbers of patients. Abdel Ghaffar et al[11] described nine Egyptian children with Wilson’s disease on a zinc sulphate monotherapy and reported vomiting and epigastric pain, which improved when zinc was taken with a small amount of protein. Most studies however analyzed effectiveness and safety of zinc acetate, which is known to be better tolerated than zinc sulphate. Brewer et al[20] treated 34 children with zinc acetate, with the only side effect being mild gastric irritation in four patients. These were handled by taking the first dose mid-morning, rather than on arising. In cases more difficult to treat, zinc was taken with a small protein meal. Only mild nausea was described by Mizuochi et al[10] in three of four children treated with zinc acetate, while Brewer et al[20] did not observe any adverse effects secondary to zinc acetate in eleven pediatric patients.
More data is available about zinc associated adverse effects in adults. Clinical data of 117 Wilson’s disease patients were analyzed retrospectively by Bruha et al[22]. Side effects associated with zinc sulphate were mild with only 5 patients requiring a switch to zinc acetate (four cases due to gastrointestinal intolerance, one patient due to eosinophilia). No adverse events were noted for zinc acetate therapy.
In a systematic study of Japanese patients with Wilson’s disease zinc acetate was shown to be safe and efficacious. Although more than 50% (20 patients) of their patients experienced adverse effects, including gastrointestinal symptoms and decreased blood iron levels, reported events were mild and patients were able to be continued on the zinc treatment[23].
The present study demonstrates side effects secondary to zinc sulphate therapy in 40% of treated patients, considerably higher compared to previous reports[6,7,11,24]. Complaints were of gastrointestinal origin: epigastric pain, vomiting, nausea or loss of appetite. Seven of these 21 symptomatic children were diagnosed with gastric ulcerations or erosions with negative H. pylori tests and were started on proton pump inhibitors. While three of those patients were relieved of their symptoms, PPIs failed to be effective for the other four, requiring a conversion of their therapy from zinc sulphate to D-penicillamine (Table 2). Most studies report gastrointestinal adverse effects of zinc sulphate or acetate in children to be mild and generally handled by changing the dosage scheme or even resolving spontaneously[10,11]. This maneuver proved to be effective in only two of our patients. One child with abdominal pain experienced relief of symptoms when zinc was taken with a small protein meal given early in the morning and in one case first dose of zinc was taken 30 min after the meal. Still, there are very few reports on side effects of zinc therapy in children. Adult patients with Wilson’s disease experienced mild side effects secondary to zinc sulphate and treatment was converted from zinc sulphate to acetate or gluconate[9,22], in selected cases a switch to D-penicillamine was necessary[24]. Treatment with zinc acetate resulted in only mild complaints and patients were able to continue therapy without any modifications. In his systematic review of zinc sulphate therapy, Wiggelinkhuizen describes that 12.5% (28/224) of patients suffered from side effects, all of gastrointestinal origin and in only two cases discontinuation of therapy was required[7]. However, in the present study, therapy had to be converted from zinc sulphate to D-penicillamine in twelve patients and to zinc acetate in three other probands. One child (Table 2) was converted to D-penicillamine after a failed attempt to treat with zinc acetate and two patients were switched to zinc acetate after unsuccessful D-penicillamine therapy.
We described the distribution of genetic mutations between the two groups of patients, those who experienced side effects and the ones that remained asymptomatic throughout the therapy (Table 4). However, due to the small number of patients, conclusions regarding susceptibility towards adverse effects cannot be drawn from this data.
EGD in children is indicated, only in the presence of severe symptoms and in younger children endotracheal intubation is required to perform an endoscopy. Therefore, there are very little data available on endoscopic findings especially in younger children. We decided to perform endoscopy only in those cases where side effects were expected to cause a withdrawal of treatment. To our knowledge this is the first study to document gastric ulcerations as a significant complication of zinc treatment in Wilson’s disease patients. In the present study we recorded severe side effects secondary to zinc sulphate in seven out of 21 patients and EDG performed in all cases revealed gastritis with mucosal ulceration or erosion. H. pylori test was negative in all subjects investigated. Histopathology of biopsies was unspecific, showing mild to moderate lymphocytic infiltrations. A case report from 1978 by Moore[25], about a 15 year old girl who had been taking zinc sulphate for acne, was the first to describe hemorrhagic gastric erosions associated with zinc sulphate therapy. The ulcerative effect of zinc sulphate was thought to be secondary to the corrosive zinc chloride, which is most likely formed by the action of gastric hydrochloric acid on zinc sulphate[26]. This could explain its isolated effect on the gastric mucosa, leaving the duodenal mucosa intact.
Taking into account the fact that peptic ulcer disease is uncommon in children with an estimated prevalence of 1 in 3000 hospital admissions[27], the high frequency of peptic ulcer disease observed in our study was disquieting. Epidemiological studies show a constant increase in incidence and prevalence of peptic ulcer disease in children[28]. Symptoms in children with suspected peptic ulcer disease commonly include pain associated with food intake, vomiting, bleeding, and a positive family history, and are crucial factors for the diagnosis of peptic ulcer disease in childhood. Poorly localized abdominal pain of a dull character is the most common symptom, but may be localized to the epigastric or periumbilical area in some cases. Unequivocal epigastric pain is relatively uncommon in children and should always prompt further investigation[27]. Esophagogastroduodenoscopy is the diagnostic procedure of choice for children with suspected peptic ulcer. Non-H. pylori ulcer disease can be treated effectively with acid-suppression (proton pump inhibitors, histamine 2 receptor inhibitors)[27]. Our data however demonstrate a failure of PPIs in severe cases of zinc sulphate related abdominal symptoms. Four of our seven patients with gastrointestinal ulcerations or erosions needed to be switched to D-penicillamine due to uncontrollable symptoms despite a treatment with PPIs.
Zinc sulphate is still a commonly used drug to treat Wilson’s disease, especially in patients who otherwise cannot afford therapy. Therefore, it is very important to assess its efficacy and tolerability. Gastrointestinal side effects especially in children are not well studied. They may cause incompliance, which seems to be a major reason for treatment failure[29,30]. To address this problem we performed endoscopy in selected symptomatic patients and identified gastric ulcer disease as the underlying cause of symptoms in 7/21 patients.
The limitation of our study is the retrospective character of data analysis and the relatively small size of the patient group. However, this rare disease limits the options to perform randomized prospective trials.
Adverse reactions such as abdominal pain, nausea and even gastritis are common in children with Wilson’s disease treated with zinc sulphate. They may occur at different stages of therapy. The high frequency of gastritis in patients with chronic abdominal pain indicates the need to perform gastroscopy in selected patients. Discontinuation of zinc sulphate is often inevitable and conversion to D-penicillamine or zinc acetate may be a safer option for these patients. Zinc sulphate appears to cause significant side effects, which should be seriously considered during monitoring the therapy of Wilson’s disease.
COMMENTS
Background
Wilson’s disease represents a metabolic disorder caused by excessive accumulation of copper in tissues. Penicillamine and zinc compounds are used in therapy. Due to costs and availability zinc sulphate is commonly used for treating Wilson’s disease in Poland. It is regarded safe and well tolerated, but there are little data available on side effects, especially in children.
Research frontiers
The most common side effect of zinc therapy described is nausea and abdominal pain. Other uncommon complications are: hematologic abnormalities, specifically leucopenia, neutropenia and sideroblastic anemia, a mild increase in serum amylase and lipase concentrations without clinical or radiologic evidence of pancreatitis, 20% reduction of high-density lipoprotein cholesterol in male patients (but with reduction in total cholesterol) or reduction leukocyte chemotaxis. According to the results of Wiggelinkhuizen’s systematic review in adult patients with Wilson’s disease 12.5% of them suffered from side effects of zinc therapy, all of gastrointestinal origin. There are only few studies focusing on pediatric patients but most of them report gastrointestinal adverse effects of zinc sulphate or acetate in children also to be uncommon and mild, generally handled by changing dosage scheme or resolved spontaneously.
Innovations and breakthroughs
The present study demonstrates side effects secondary to zinc sulphate therapy in 40% of treated patients, which is higher compared to previous reports. The common complaints were of gastrointestinal origin: epigastric pain, vomiting, nausea or loss of appetite. The clinical observation led to conclusions that some side effects seemed to be poorly tolerated by children and endoscopic investigations revealed ulcerations which had not been described earlier. Apart from that authors have some evidence derived from this analysis that proton pump inhibitors may not be effective in zinc sulphate treated children experiencing persistent abdominal symptoms and modification of treatment is necessary.
Applications
The study results suggest that zinc sulphate can cause significant side effects as gastritis or gastric ulcer which should be seriously considered during monitoring the therapy of Wilson’s disease in children. The high frequency of gastritis in patients with chronic abdominal pain indicates the need to perform gastroscopy in selected patients. Discontinuation of zinc sulphate is often inevitable and conversion to penicillamine or zinc acetate may be a safer option for these patients.
Peer review
The manuscript is tackling an important issue in the treatment and management of Wilson’s disease patients. It has excellent introduction and very good discussion, and is well written.
Footnotes
P- Reviewers Bruha R, Usta J S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Fosmire GJ. Zinc toxicity. Am J Clin Nutr. 1990;51:225–227. doi: 10.1093/ajcn/51.2.225. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman HN, Phyliky RL, Fleming CR. Zinc-induced copper deficiency. Gastroenterology. 1988;94:508–512. doi: 10.1016/0016-5085(88)90445-3. [DOI] [PubMed] [Google Scholar]
- 3.Broun ER, Greist A, Tricot G, Hoffman R. Excessive zinc ingestion. A reversible cause of sideroblastic anemia and bone marrow depression. JAMA. 1990;264:1441–1443. doi: 10.1001/jama.264.11.1441. [DOI] [PubMed] [Google Scholar]
- 4.Yuzbasiyan-Gurkan V, Brewer GJ, Abrams GD, Main B, Giacherio D. Treatment of Wilson’s disease with zinc. V. Changes in serum levels of lipase, amylase, and alkaline phosphatase in patients with Wilson’s disease. J Lab Clin Med. 1989;114:520–526. [PubMed] [Google Scholar]
- 5.Brewer GJ, Yuzbasiyan-Gurkan V, Johnson V. Treatment of Wilson’s disease with zinc. IX: Response of serum lipids. J Lab Clin Med. 1991;118:466–470. [PubMed] [Google Scholar]
- 6.Brewer GJ, Johnson V, Kaplan J. Treatment of Wilson’s disease with zinc: XIV. Studies of the effect of zinc on lymphocyte function. J Lab Clin Med. 1997;129:649–652. doi: 10.1016/s0022-2143(97)90200-6. [DOI] [PubMed] [Google Scholar]
- 7.Wiggelinkhuizen M, Tilanus ME, Bollen CW, Houwen RH. Systematic review: clinical efficacy of chelator agents and zinc in the initial treatment of Wilson disease. Aliment Pharmacol Ther. 2009;29:947–958. doi: 10.1111/j.1365-2036.2009.03959.x. [DOI] [PubMed] [Google Scholar]
- 8.Lapere D, Gottrand F, Debray D, Bridoux-Henno L, Lachaux A, Morali A, Lamireau T. Efficacy and tolerance of zinc in the treatment of Wilson disease. Proceedings of European Society for Paediatric Gastroenterology, Hepatology, and Nutrition Annual Meeting; 2010 Jun 9-12; Istanbul, Turkey. J Pediatr Gastroenterol Nutr. 2010;50(Suppl 2):E154–E155. [Google Scholar]
- 9.Linn FH, Houwen RH, van Hattum J, van der Kleij S, van Erpecum KJ. Long-term exclusive zinc monotherapy in symptomatic Wilson disease: experience in 17 patients. Hepatology. 2009;50:1442–1452. doi: 10.1002/hep.23182. [DOI] [PubMed] [Google Scholar]
- 10.Mizuochi T, Kimura A, Shimizu N, Nishiura H, Matsushita M, Yoshino M. Zinc monotherapy from time of diagnosis for young pediatric patients with presymptomatic Wilson disease. J Pediatr Gastroenterol Nutr. 2011;53:365–367. doi: 10.1097/MPG.0b013e31821d5abe. [DOI] [PubMed] [Google Scholar]
- 11.Abdel Ghaffar TY, Elsayed SM, Elnaghy S, Shadeed A, Elsobky ES, Schmidt H. Phenotypic and genetic characterization of a cohort of pediatric Wilson disease patients. BMC Pediatr. 2011;11:56. doi: 10.1186/1471-2431-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LC, Wang JD, Tsai CR, Cheng SB, Lin CC. Clinical features and therapeutic response in Taiwanese children with Wilson’s disease: 12 years of experience in a single center. Pediatr Neonatol. 2010;51:124–129. doi: 10.1016/S1875-9572(10)60022-8. [DOI] [PubMed] [Google Scholar]
- 13.8th International Conference on Wilson Disease and Menkes Disease. Leipzig, Germany, April 16-18, 2001. Abstracts. Z Gastroenterol. 2001;39:245–260. [PubMed] [Google Scholar]
- 14.Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, Schilsky M, Cox D, Berr F. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23:139–142. doi: 10.1034/j.1600-0676.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 15.Brewer GJ, Dick RD, Johnson VD, Brunberg JA, Kluin KJ, Fink JK. Treatment of Wilson’s disease with zinc: XV long-term follow-up studies. J Lab Clin Med. 1998;132:264–278. doi: 10.1016/s0022-2143(98)90039-7. [DOI] [PubMed] [Google Scholar]
- 16.Medici V, Rossaro L, Sturniolo GC. Wilson disease--a practical approach to diagnosis, treatment and follow-up. Dig Liver Dis. 2007;39:601–609. doi: 10.1016/j.dld.2006.12.095. [DOI] [PubMed] [Google Scholar]
- 17.Medici V, Trevisan CP, D’Incà R, Barollo M, Zancan L, Fagiuoli S, Martines D, Irato P, Sturniolo GC. Diagnosis and management of Wilson’s disease: results of a single center experience. J Clin Gastroenterol. 2006;40:936–941. doi: 10.1097/01.mcg.0000225670.91722.59. [DOI] [PubMed] [Google Scholar]
- 18.Roberts EA, Schilsky ML. A practice guideline on Wilson disease. Hepatology. 2003;37:1475–1492. doi: 10.1053/jhep.2003.50252. [DOI] [PubMed] [Google Scholar]
- 19.Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 20.Brewer GJ, Dick RD, Johnson VD, Fink JK, Kluin KJ, Daniels S. Treatment of Wilson’s disease with zinc XVI: treatment during the pediatric years. J Lab Clin Med. 2001;137:191–198. doi: 10.1067/mlc.2001.113037. [DOI] [PubMed] [Google Scholar]
- 21.Pecoud A, Dozel F, Schelling JL. The effect of foodstuffs on the absorption of zinc sulfate. Clin Pharmacol Ther. 1975;17:469–474. doi: 10.1002/cpt1975174469. [DOI] [PubMed] [Google Scholar]
- 22.Bruha R, Marecek Z, Pospisilova L, Nevsimalova S, Vitek L, Martasek P, Nevoral J, Petrtyl J, Urbanek P, Jiraskova A, et al. Long-term follow-up of Wilson disease: natural history, treatment, mutations analysis and phenotypic correlation. Liver Int. 2011;31:83–91. doi: 10.1111/j.1478-3231.2010.02354.x. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu N, Fujiwara J, Ohnishi S, Sato M, Kodama H, Kohsaka T, Inui A, Fujisawa T, Tamai H, Ida S, et al. Effects of long-term zinc treatment in Japanese patients with Wilson disease: efficacy, stability, and copper metabolism. Transl Res. 2010;156:350–357. doi: 10.1016/j.trsl.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Czlonkowska A, Gajda J, Rodo M. Effects of long-term treatment in Wilson’s disease with D-penicillamine and zinc sulphate. J Neurol. 1996;243:269–273. doi: 10.1007/BF00868525. [DOI] [PubMed] [Google Scholar]
- 25.Moore R. Bleeding gastric erosion after oral zinc sulphate. Br Med J. 1978;1:754. doi: 10.1136/bmj.1.6115.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molokhia MM, Portnoy B. Bleeding gastric erosion after oral zinc sulphate. Br Med J. 1978;1:1145. doi: 10.1136/bmj.1.6120.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulllivan PB. Peptic ulcer disease in children. Paediatrics and Child Health. 2010;10:462–464. [Google Scholar]
- 28.Sultz HA, Schlesinger ER, Feldman JG, Mosher WE. The epidemiology of peptic ulcer in childhood. Am J Public Health Nations Health. 1970;60:492–498. doi: 10.2105/ajph.60.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masełbas W, Chabik G, Członkowska A. Persistence with treatment in patients with Wilson disease. Neurol Neurochir Pol. 2010;44:260–263. doi: 10.1016/s0028-3843(14)60040-2. [DOI] [PubMed] [Google Scholar]
- 30.Walshe JM. Cause of death in Wilson disease. Mov Disord. 2007;22:2216–2220. doi: 10.1002/mds.21693. [DOI] [PubMed] [Google Scholar]


