Abstract
AIM: To investigate the prevalence and risk factors of polypoid lesions of the gallbladder (PLGs) in petrochemical employees in Ningbo, Zhejiang Province, China.
METHODS: All active and retired employees aged 20-90 years (n = 11098) of a refinery and chemical plant in eastern China were requested to participate in a health survey. The participants were subjected to interview, physical examination, laboratory assessments and ultrasonography. All the participants were invited to have a physical examination after a face-to-face interview. Fasting blood samples were obtained from the antecubital vein, and the samples were used for the analysis of biochemical values. Abdominal ultrasonography was conducted.
RESULTS: A total of 10461 (7331 men and 3130 women) current and former petrochemical employees attended for screening. The overall prevalence of post-cholecystectomy, gallstones and PLGs was 0.9%, 5.2% and 7.4%, respectively. Compared with the increased prevalence of either gallstones or post-cholecystectomy in older persons, PLGs were more common in the middle-aged, peaking in those aged 40-59 years. Excluding the patients with gallstones, gallstones mixed with PLGs, or those who had undergone cholecystectomy, in the remaining 9828 participants, the prevalence of PLGs in men (8.9%) was significantly higher than that in women (5.5%, P < 0.001). The analyzed risk factors with increased OR for the development of PLGs were male gender (OR = 1.799, P < 0.001), age ≥ 30 years (OR = 2.699, P < 0.001) and hepatitis B surface antigen (HBsAg) positivity (OR = 1.374, P = 0.006).
CONCLUSION: PLGs are not rare among Chinese petrochemical employees. Male gender, HBsAg positivity, and middle age are risk factors for developing PLGs.
Keywords: Prevalence, Risk factors, Polypoid lesions, Gallbladder, Chinese
Core tip: Polypoid lesions of the gallbladder (PLGs) are commonly encountered in clinical practice. This study investigated the prevalence and risk factors of PLGs in a cohort of petrochemical employees in the city of Ningbo, Zhejiang Province, China. It was demonstrated that 7.4% of the petrol-chemical employees studied had PLGs; and male, hepatitis B surface antigen positive and middle-aged employees, especially those aged between 30 and 59 years, had higher risks for developing PLGs.
INTRODUCTION
The widespread application and improved image quality of abdominal ultrasonography in modern clinical practice have led to an increase in the detection of abnormalities of the biliary tree, including polypoid lesions of the gallbladder (PLGs)[1,2]. The significance of PLGs is still poorly understood. In general, PLGs represent a heterogeneous group of changes in the gallbladder wall and include entities such as cholesterol polyps, inflammatory polyps, adenomas, leiomyomas and lipomas[3]. As a result, clinicians are ever more frequently confronted with the question of how to proceed in cases of incidentally discovered PLGs[3]; and patients with PLGs usually require repeated ultrasonography and follow-up[4], enduring a certain degree of anxiety.
The prevalence of PLGs is reported in the range 0.3%-9.5%, depending on the population studied and on the study design. Prevalence in Western studies falls in the range 1.0%-6.1%[5,6], which is lower than that reported in Southeast Asian populations, mainly in Japan, South Korea and Taiwan[7-10]. To date, only a few studies have investigated the prevalence of and risk factors for PLGs in the mainland of China. Yang et al[11] have reported that the prevalence of PLGs in Shanghai was 4.2%. Xu et al[12] have reported that the prevalence in Beijing was 6.9%. However, these two studies were retrospective analyses. To understand better the prevalence of and risk factors for PLGs in Chinese petrochemical employees in Zhejiang Province, a prospectively observational study of 10461 stable participants was conducted in 2009, which is expected to provide a comprehensive update to the results of the previous studies. This cohort of employees will be followed up in the next 5 years. All participants will be invited for annual reassessment, including similar questions, laboratory measurement, ultrasonography, and collection of biological material at baseline. In addition, the progression of PLGs will be observed prospectively.
MATERIALS AND METHODS
Study population
The study of PLGs was carried out as part of the petrochemical employees annual health check-up survey[13]. In 2009, a descriptive cross-sectional study was performed in a stable population of all employees of a refinery and chemical plant in the city of Ningbo, Zhejiang Province, in the eastern region of China. Chinese law requires state-owned companies to provide annual health check-ups for all employees. The study population included all regular employees with at least 1 mo service at the Zhenhai Refinery and Chemical Plant of the SINO-Petrol Chemical Company, in northeastern Ningbo between January 1 and December 31, 2009.
Invitation to participate was by letter and non-responders received one reminder. A research nurse interviewed subjects at Zhenhai Lianhua Hospital, the former Workers Hospital of the Zhenhai Refinery and Chemical Plant.
The present study was conducted in accordance with the Declaration of Helsinki, as amended in Scotland (2000). The protocol and statement of informed consent were approved by the Ethics Committee of Zhenhai Lianhua Hospital, Ningbo. Written informed consent was obtained from each participant.
Interview and physical examination
An appointment was made for each participant at the check-up center of Zhenhai Lianhua Hospital, where a face-to-face interview was conducted by a trained nurse to complete a two-page self-assessment questionnaire that included a gallbladder-related question: “Have you ever had gallbladder surgery”
All individuals were invited to have a physical examination after the face-to-face interview. They were required to fast overnight. Body measurements were performed by a trained medical professional using a standardized protocol. Body weight and standing height were measured in light indoor clothing without shoes. Weight was measured to the nearest 0.1 kg using a calibrated hospital spring scale. Height and waist circumference (WC) were measured in centimeters. WC was measured between the lowest rib and the iliac crest, horizontally through the narrowest part of the torso. Body mass index (BMI) was then calculated as weight in kilograms divided by height in meters squared. Individuals were grouped into two categories based on BMI (< 25 and ≥ 25 kg/m2) and WC (< 90 and ≥ 90 cm for men, and < 80 and ≥ 80 cm for women)[14]. Blood pressure was measured using an automated sphygmomanometer with the subject in a sitting position. Systolic blood pressure and diastolic blood pressure were measured at the first and fifth Korotkoff phases, respectively.
Laboratory tests
Fasting blood samples were obtained from an antecubital vein, and the samples were used for the analysis of biochemical values. The values included triglyceride, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, uric acid, and fasting plasma glucose (FPG). All values were measured by an Olympus AU640 auto-analyzer (Olympus, Kobe, Japan) using standard methods. Hepatitis B surface antigen (HBsAg) was detected with a second-generation enzyme-linked immunosorbent assay (VITROS ECi Immunodiagnostic System; Ortho Clinical Diagnostics, Inc., Johnson and Johnson, Raritan, NJ, United States). The reagents used were offered by the same company. The tests were undertaken strictly according to the instructions of the manufacturers.
Ultrasonography
All participants were asked to come to a morning examination after an overnight fast of ≥ 8 h. Abdominal ultrasonography was conducted using a scanner equipped with a 2.0-5.0-MHz transducer (Voluson 730, GE Healthcare, Pittsburgh, PA, United States). The ultrasongraphers were unaware of the participants’ clinical and laboratory characteristics. Gallstones were present if the gallbladder contained echoes that moved with gravity, except when the stones were large, a septum existed in the gallbladder, or there was an enclosed infundibulum. PLGs were diagnosed as immobile echoes protruding from inside the gallbladder wall into the lumen. The ultrasonic characteristics of PLGs included hyperechoic structures without acoustic shadow, unequivocal visualization in two planes (longitudinal and in cross-section), no change in position of the wall change secondary to change in subjects’ position. In patients with multiple polyps, the size of the largest polyp was measured. Subjects with both gallstones and PLGs were classified into the gallstones group.
Statistical analysis
Data on quantitative characteristics were expressed as mean ± SD. Data on qualitative characteristics were expressed as percentage values or absolute numbers as indicated. The data analysis was performed with SPSS (version 11.5, SPSS Software, Chicago, IL, United States). For continuous variables, non-parametric tests (two-tailed Mann-Whitney U test) were used. Group differences between the numbers of subjects were analyzed using χ2 test (normal data) and analysis of variance (continuous data). A multiple stepwise regression analysis (backward: Wald; cutoff for entry: 0.05, for removal: 0.10) was performed in order to evaluate independent relationships between sex, age and PLG. P < 0.05 was considered statistically significant.
RESULTS
Population studied
All of the 11098 active and retired employees were invited to participate in the study. There were 7760 (69.9%) men and 3338 (30.1%) women. We excluded 637 subjects for not attending the health check program. In all, 7331 (94.5%) of the invited men and 3130 (93.8%) of the women participated in the study. Figure 1 displays the recruitment flow chart. Demographic data on the 10461 attending individuals are shown in Table 1.
Figure 1.
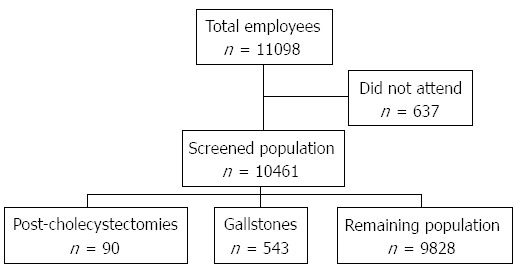
Recruitment flow chart.
Table 1.
Population characteristics (n = 10461) n (%)
| Characteristics | Male (n = 7331) | Female (n = 3130) |
| Age, mean ± SD (range) (yr) | 46.0 ± 13.2 (21-87) | 48.6 ± 12.4 (20-90) |
| Age distribution (yr) | ||
| 20-29 | 551 (7.5) | 113 (3.6) |
| 30-39 | 2221 (30.3) | 736 (23.5) |
| 40-49 | 1903 (26.0) | 896 (28.6) |
| 50-59 | 1506 (20.5) | 776 (24.8) |
| 60-69 | 621 (8.5) | 374 (11.9) |
| ≥ 70 | 529 (7.2) | 235 (7.5) |
| Smoking | 2878 (39.3) | 20 (0.6) |
| Alcohol consumption | 1910 (26.1) | 39 (1.2) |
| Concomitant condition | ||
| Hypertension | 3459 (47.2) | 1093 (34.9) |
| Diabetes mellitus | 368 (5.0) | 162 (5.2) |
Prevalence of post-cholecystectomy, gallstones and PLGs
There were 90 (0.9%) patients who had undergone cholecystectomy. The overall prevalence of PLGs was 7.4% (777/10461), which was higher than that for gallstones, which was 5.2% (543/10461, P < 0.001). Nineteen subjects with both gallstone and PLG were classified into the group of gallstones. The prevalence was further stratified by age among post-cholecystectomy, gallstones and PLGs (Figure 2A). Compared with the increased prevalence of either gallstones or post-cholecystectomy in older persons, the PLGs were more common in the middle-aged, peaking in those aged 40-59 years. The difference between men and women was stratified with age (Table 2). The prevalence of gallstones and post-cholecystectomy in men was lower than that in women in all age groups. In contrast, the prevalence of PLGs in men was higher than that in women in all age groups. Overall, men had a significantly higher prevalence of PLGs compared to women (8.5% vs 5.0%, P < 0.001). In patients with PLGs, the proportion of large polypoid lesions (≥ 10 mm) was small (14/777, 1.8%; 11 male, 3 female). This equated to a general population prevalence of larger PLGs of 0.1%. None of these 14 patients underwent cholecystectomy.
Figure 2.
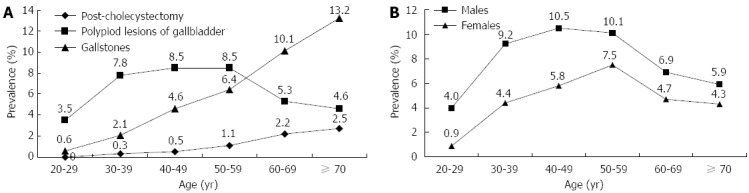
Prevalence of gallbladder diseases in different age groups and polypoid lesions of the gallbladder stratified by sex in Chinese petrochemical employees. A: Prevalence of post-cholecystectomy and gallstones increased with age, whereas polypoid lesions of the gallbladder (PLGs) were more common in the middle-aged people, peaking in those aged 40-59 years; B: Prevalence of PLGs stratified by sex in different age groups in the remaining 9828 Chinese petrochemical employees. Prevalence of PLGs in men was higher than in women in all age groups.
Table 2.
Prevalence, age distribution and gender of patients with post-cholecystectomy, gallstones and polypoid lesions of the gallbladder in 10461 examinees n (%)
| Age group (yr) |
Examinees |
Post-cholecystectomy |
Gallstones |
PLGs |
|||||||
| Male | Female | Male | Female | P value | Male | Female | P value | Male | Female | P value | |
| 20-29 | 551 | 113 | 0 (0.0) | 0 (0.0) | - | 4 (0.7) | 0 (0.0) | 1.000 | 22 (4.0) | 1 (0.9) | 0.153 |
| 30-39 | 2221 | 736 | 4 (0.2) | 6 (0.8) | 0.019 | 40 (1.8) | 22 (3.0) | 0.051 | 201 (9.0) | 31 (4.2) | < 0.001 |
| 40-49 | 1903 | 896 | 9 (0.5) | 4 (0.4) | 0.322 | 79 (4.2) | 50 (5.6) | 0.093 | 190 (10.0) | 49 (5.5) | < 0.001 |
| 50-59 | 1506 | 776 | 6 (0.4) | 18 (2.3) | < 0.001 | 94 (6.2) | 53 (6.8) | 0.588 | 142 (9.4) | 53 (6.8) | 0.035 |
| 60-69 | 621 | 374 | 11 (1.8) | 11 (2.9) | 0.223 | 58 (9.3) | 42 (11.2) | 0.337 | 38 (6.1) | 15 (4.0) | 0.151 |
| ≥ 70 | 529 | 235 | 6 (1.1) | 15 (6.4) | < 0.001 | 68 (12.9) | 33 (14.0) | 0.545 | 27 (5.1) | 8 (3.4) | 0.300 |
| Total | 7331 | 3130 | 36 (0.5) | 54 (1.7) | < 0.001 | 343 (4.7) | 200 (6.4) | < 0.001 | 620 (8.5) | 157 (5.0) | < 0.001 |
PLG: Polypoid lesions of the gallbladders.
Characteristics of subjects according to PLG status
After excluding 543 patients with gallstones and 90 who underwent cholecystectomy, the remaining 9828 participants (93.9% of the total attending population) were further analyzed. Characteristics were compared between the group with PLGs and a control group. Controls were confirmed not to have gallstones, post-cholecystectomy or PLGs in our screening program. The control group consisted of 9051 subjects, including 6332 men and 2719 women (Table 3). Of the remaining 9828 subjects, the overall prevalence of PLGs was 7.9% (777/9828); 8.9% (620/6952) in men and 5.5% (157/2876) in women.
Table 3.
Characteristics of the 9828 remaining subjects with and without polypoid lesions of the gallbladders
| Variables | With PLG (n = 777) | Without PLG (n = 9051) | P value |
| Age (yr), ≥ 30/< 30 | 754/23 | 8414/637 | < 0.001 |
| Sex, male/female | 620/157 | 6332/2719 | < 0.001 |
| Smoking | 245 (32.7) | 2487 (27.5) | 0.002 |
| HBsAg positive | 96 (12.4) | 842 (9.3) | 0.005 |
| Alcohol consumption | 166 (21.4) | 1686 (18.4) | 0.061 |
| History of hypertension | 140 (18.0) | 1615 (17.8) | 0.903 |
| History of diabetes | 27 (3.5) | 428 (4.7) | 0.110 |
| Body mass index (kg/m2) | 23.2 ± 2.8 | 23.0 ± 3.0 | 0.384 |
| Waist circumference (cm) | 80.3 ± 8.4 | 80.0 ± 9.2 | 0.472 |
| Systolic blood pressure (mmHg) | 122.0 ± 14.8 | 122.2 ± 15.4 | 0.655 |
| Diastolic blood pressure (mmHg) | 77.7 ± 10.0 | 77.7 ± 9.9 | 0.992 |
| Total cholesterol (mmol/L) | 5.2 ± 1.0 | 5.3 ± 1.0 | 0.128 |
| LDL cholesterol (mmol/L) | 3.1 ± 0.8 | 3.1 ± 0.8 | 0.475 |
| HDL cholesterol (mmol/L) | 1.4 ± 0.4 | 1.5 ± 0.4 | 0.119 |
| Triglycerides (mmol/L) | 1.4 ± 1.0 | 1.5 ± 1.1 | 0.490 |
| Fasting blood glucose (mmol/L) | 4.8 ± 0.8 | 4.8 ± 0.8 | 0.773 |
| Uric acid (μmol/L) | 339.0 ± 77.1 | 336.3 ± 84.5 | 0.398 |
Data are expressed as absolute numbers (percentage) or mean ± SD. HBsAg: Hepatitis B surface antigen; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; PLG: Polypoid lesions of the gallbladders.
The differences in the prevalence of PLGs in the remaining population between men and women were investigated for every age decade (Figure 2B). The prevalence of PLGs in men was significantly higher in women (P < 0.001). In men, the prevalence was highest among those in their 40s (10.5%), followed by those in their 50s (10.1%), 30s (9.2%), 60s (6.9%), 70s or above (4.9%), and 20s (4.0%). In women, the prevalence was highest among those in their 50s (7.5%), followed by those in their 40s (5.8%), 60s (4.7%), 30s (4.4%), 70s or above (4.3%), and 20s (0.9%). In men, the overall difference in the age-dependent change in prevalence was found to be statistically significant (χ2 = 31.994, P < 0.001). In women, the overall difference in the age-dependent change in prevalence was also significant (χ2 = 13.074, P = 0.023). The highest prevalence (10.5%) of PLGs was found in male participants aged 40-49 years.
Logistic regression analysis of relevant risk factors for PLGs
The factors significantly associated with PLGs were male sex (P < 0.001), age ≥ 30 years (P < 0.001), HBsAg positivity (P = 0.005) and cigarette smoking (P = 0.002) (Table 3). In order to identify the risk factors, we performed multivariate logistic regression analysis (backward stepping) (Table 4.) There was no significant difference between the PLG-positive and -negative groups for cigarette smoking (OR = 1.020, 95%CI: 0.854-1.220, P = 0.823). Male gender (OR = 1.799, 95%CI: 1.472-2.199, P < 0.001), age ≥ 30 years (OR = 2.699, 95%CI: 1.762-4.133, P < 0.001) and HBsAg positivity (OR = 1.374, 95%CI: 1.097-1.721, P = 0.006) were still positively correlated with PLGs. However, the risk of PLGs was 2.938 times higher in middle-aged (30-59 years) subjects than in young persons, and old (≥ 60 years) people had a 2.009 times higher risk of PLGs than young people.
Table 4.
Multivariate logistic regression analysis for polypoid lesions of the gallbladders
| Variables | B1 | OR | 95%CI | P value |
| Sex (male) | 0.587 | 1.799 | 1.472-2.199 | < 0.001 |
| Age (yr) | ||||
| ≥ 30 | 0.993 | 2.699 | 1.762-4.133 | < 0.001 |
| 30-59 | 1.078 | 2.938 | 1.914-4.511 | < 0.001 |
| ≥ 60 | 0.697 | 2.009 | 1.243-3.245 | 0.004 |
| HBsAg (+) | 0.318 | 1.374 | 1.097-1.721 | 0.006 |
1Regression coefficient. The dependent variable was the presence or absence of polypoid lesions of the gallbladders. The covariates include sex, age ≥ 30 years (divided into age 30-59 years and ≥ 60 years), body mass index ≥ 25.0 kg/m2, systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg, fasting blood glucose ≥ 5.6 mmol/L, triglyceride level ≥ 1.7 mmol/L, high-density lipoprotein cholesterol level ≥ 1.03 mmol/L in men or ≥ 1.29 mmol/L in women, and waist circumference ≥ 90 cm in men or ≥ 80 cm in women. HBsAg: Hepatitis B surface antigen.
DISCUSSION
This large population-based survey of current and former petrochemical employees provides a unique opportunity to study the prevalence of gallbladder polyps and the distribution by age and sex in the eastern coastal region of China.
In this stable population, nearly 14% of men and 13% of women aged 20-90 years had gallbladder disorders, including post-cholecystectomy, gallstones and PLGs. Cholecystectomy had been performed in 0.5% of men and 1.7% of women. Gallstones were diagnosed in 4.7% of men and 6.4% of women. PLGs were diagnosed in 8.5% of men and 5.0% of women.
The prevalence of post-cholecystectomy (0.9%) was so low that only 90 participants underwent cholecystectomy. The prevalence of gallstones in this study (5.2%) is similar to that in previous sonographic screening studies conducted in Japan, Taiwan and Korea, where gallstones were identified in 3.6%-11.0% of the population[15-17]. The current study demonstrated a higher prevalence of gallstones in women than in men in all age groups and showed that prevalence increased with age, which is in agreement with previous studies[10].
The prevalence of PLGs was higher than that of gallstones in participants aged < 60 years, and lower than that of gallstones in participants aged ≥ 60 years in this study. These findings are comparable with other studies that have indicated that PLGs were prevalent in middle-aged patients, whereas prevalence of gallstones and cholecystectomy increased with age[18,19]. The true reason for diminishing the prevalence of PLGs with age cannot be clarified in our study. The reported risks for gallstones, such as age, large body size[20,21] and metabolic disorders[22] were not risk factors for development of PLGs in the present study. This difference in risk factors may have been due to the different pathogenesis of these common gallbladder diseases.
PLGs are not rare in Taiwan or in the mainland of China[10-12]. The present study indicated that petrochemical employees in Ningbo had a similar prevalence (7.4%) of PLGs, although a little lower than that in Taiwan (9.5%)[10], but higher than that in Shanghai (4.2%)[11] and Beijing (6.9%)[12]. After excluding 633 patients with gallstones or those who underwent cholecystectomy, in the remaining 9828 participants, the overall prevalence of PLGs was 7.9%; 8.9% in men and 5.5% in women, which was higher than that in Denmark (4.3%)[5], Japan (5.2% and 5.6%)[7,8] and Germany (6.1%)[23]. These differences in the prevalence of PLGs among the Chinese, Japanese, Danish and German people deserve further consideration.
The present study demonstrated that men had a higher prevalence of PLGs than women at all ages. The prevalence of PLGs was highest in middle-aged men in their 40s and 50s. In women, the prevalence of PLGs was highest in those in their 50s. These results were in accordance with a Japanese study[7]. In contrast, the prevalence of post-cholecystectomy or gallstones was higher in women than in men at all ages. However, this study did not identify the mechanisms mediating this sex-related risk.
Hepatitis B virus infection is endemic in China and the HBsAg-positive rate in the general population is approximately 10.0%[24]. The current study found that compared with the PLG-negative group, the PLG-positive group had a significantly higher incidence of HBsAg positivity. Logistic regression analysis showed that HBsAg positivity is a risk factor for PLGs. These results are in agreement with those of some previous studies in the Chinese population[10-12,25], but contrary to others[17,26]. The inconsistent findings may be related to the number of cases, sex ratio, ethnic difference and other factors. The pathophysiology of the association between PLGs and HBsAg positivity deserves further study.
The associations between PLG and sex, age, BMI, WC, blood pressure, serum uric acid, serum lipid level, history of hypertension, history of diabetes, cigarette smoking, and alcohol consumption were all tested independently. Male sex, age ≥ 30 years and cigarette smoking were statistically significant for the PLG group using the χ2 test. Other variables were not significant by univariate analysis. In the multivariate analysis, male sex and age ≥ 30 years were still positively correlated with PLGs. This agreed with previous studies in which male sex was a major risk factor for PLGs. However, cigarette smoking was no longer associated with PLGs, which was in contrast with some previous studies. Okamoto et al[27] have reported that cigarette smoking is inversely related to PLGs in Japanese men. Significant effects of other demographic variables such as BMI, blood pressure, alcohol consumption, and history of hypertension and diabetes on the prevalence of PLGs were not identified in the present study. Similarly, there was no relationship between PLGs and biochemical parameters such as FPG, lipid profile and uric acid.
The present study had some strengths and limitations. First, the employees were a stable population. The demographic characteristics of this group may not be completely generalizable, for example, there were substantially more men than in the general population, but that may be more representative than many other studies in the literature. Second, pathological data were not available for 777 subjects with PLGs, although a proportion (14/777, 1.8%) of the large-sized polyps are likely to develop neoplasms[4,28]. In general, polypoid lesions > 10 mm are an important predictor for malignancy. For small PLGs < 10 mm, it has been shown that most of these lesions are benign and remain so for several years[29,30]. Nevertheless, it is not possible in a screening study to establish the histopathological nature of polypoid lesions. Therefore, a longitudinal study with the same cohort is now underway to observe the progression of PLGs, especially for the 14 cases of larger PLGs.
In conclusion, this study demonstrated that 7.4% of the petrochemical employees had PLGs. Male sex and HBsAg positivity were associated with prevalence of PLGs; and age ≥ 30 years, especially 30-59 years, was positively associated with PLGs. Further research on the prevalence of and risk factors for PLGs is therefore necessary in the general population in the mainland of China.
COMMENTS
Background
The widespread application and improved image quality of abdominal ultrasonography in modern clinical practice have led to an increase in the detection of biliary tree abnormalities, including polypoid lesions of the gallbladder (PLGs). The reported prevalence of PLGs is 0.3%-9.5%, depending on the population studied and on the study design. To date, only a few studies have investigated the prevalence and risk factors for PLGs in China. This study investigated the prevalence of and risk factors for PLGs in a cohort of petrochemical employees in the city of Ningbo, China.
Research frontiers
This large population-based survey provided a unique opportunity to study the prevalence of gallbladder polyps and the distribution by age and sex in the eastern coastal region of China. This study demonstrated that men had a higher prevalence of PLGs than women at all ages. The prevalence of PLGs was highest in middle-aged men in their 40s and 50s. The demographic characteristics of this group may not be completely generalizable, for example, there were substantially more men than in the general population, but that may be more representative than many other studies in the literature.
Innovations and breakthroughs
The prevalence and risk factors of gallbladder polyps from 10461 subjects in eastern China were investigated. The results suggest that these populations do not have a low prevalence of PLGs, and middle-aged men or both sexes positive for hepatitis B surface antigen (HBsAg) have a high risk. These results provide an important reference for the prevention and treatment of gallbladder polyps.
Applications
To understand better the prevalence of and risk factors for PLGs in petrochemical employees in Zhejiang Province, China, a prospective observational study of 10461 stable participants was conducted in 2009, which is expected to provide a comprehensive update to the results of previous studies. The results provide an important evidence for the prevention and treatment of gallbladder polyps.
Terminology
PLGs represent a heterogeneous group of changes in the gallbladder wall and include entities such as cholesterol polyps, inflammatory polyps, adenomas, leiomyomas and lipomas.
Peer review
In this paper, the prevalence and risk factors of gallbladder polyps from 10461 subjects in eastern China were investigated. The results suggest that these populations do not have a low prevalence of PLGs, and middle-aged men or people of both sexes positive for HBsAg have a high risk. These results provide an important reference for the prevention and treatment of gallbladder polyps. Nevertheless, further clarification or revision in some places of the paper are needed.
Footnotes
Supported by Ningbo Social Development and Technology Support Plan Project of China, No. 2011C50021; Ningbo Natural Science Foundation of China, No. 2012A610187; Clinical Research Foundation of Zhejiang Medical Association, No. 2010ZYC-B07, and Zhenhai Social Development and Technology Support Plan Project of Ningbo, No. 2011A2062
P- Reviewer Qiao T S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Andrén-Sandberg A. Diagnosis and management of gallbladder polyps. N Am J Med Sci. 2012;4:203–211. doi: 10.4103/1947-2714.95897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheon YK, Cho WY, Lee TH, Cho YD, Moon JH, Lee JS, Shim CS. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J Gastroenterol. 2009;15:2361–2366. doi: 10.3748/wjg.15.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers RP, Shaffer EA, Beck PL. Gallbladder polyps: epidemiology, natural history and management. Can J Gastroenterol. 2002;16:187–194. doi: 10.1155/2002/787598. [DOI] [PubMed] [Google Scholar]
- 4.Csendes A, Burgos AM, Csendes P, Smok G, Rojas J. Late follow-up of polypoid lesions of the gallbladder smaller than 10 mm. Ann Surg. 2001;234:657–660. doi: 10.1097/00000658-200111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kratzer W, Haenle MM, Voegtle A, Mason RA, Akinli AS, Hirschbuehl K, Schuler A, Kaechele V. Ultrasonographically detected gallbladder polyps: a reason for concern A seven-year follow-up study. BMC Gastroenterol. 2008;8:41. doi: 10.1186/1471-230X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldouri AQ, Malik HZ, Waytt J, Khan S, Ranganathan K, Kummaraganti S, Hamilton W, Dexter S, Menon K, Lodge JP, et al. The risk of gallbladder cancer from polyps in a large multiethnic series. Eur J Surg Oncol. 2009;35:48–51. doi: 10.1016/j.ejso.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Segawa K, Arisawa T, Niwa Y, Suzuki T, Tsukamoto Y, Goto H, Hamajima E, Shimodaira M, Ohmiya N. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992;87:630–633. [PubMed] [Google Scholar]
- 8.Shinchi K, Kono S, Honjo S, Imanishi K, Hirohata T. Epidemiology of gallbladder polyps: an ultrasonographic study of male self-defense officials in Japan. Scand J Gastroenterol. 1994;29:7–10. doi: 10.3109/00365529409090429. [DOI] [PubMed] [Google Scholar]
- 9.Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH, Yu SJ, Kang HY, Lee JY, Park MJ. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps. Gut Liver. 2008;2:88–94. doi: 10.5009/gnl.2008.2.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin WR, Lin DY, Tai DI, Hsieh SY, Lin CY, Sheen IS, Chiu CT. Prevalence of and risk factors for gallbladder polyps detected by ultrasonography among healthy Chinese: analysis of 34 669 cases. J Gastroenterol Hepatol. 2008;23:965–969. doi: 10.1111/j.1440-1746.2007.05071.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang HL, Kong L, Hou LL, Shen HF, Wang Y, Gu XG, Qin JM, Yin PH, Li Q. Analysis of risk factors for polypoid lesions of gallbladder among health examinees. World J Gastroenterol. 2012;18:3015–3019. doi: 10.3748/wjg.v18.i23.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Tao LY, Wu Q, Gao F, Zhang FL, Yuan L, He XD. Prevalences of and risk factors for biliary stones and gallbladder polyps in a large Chinese population. HPB (Oxford) 2012;14:373–381. doi: 10.1111/j.1477-2574.2012.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao YS, Liu ZM, Chen CX, Zhu ZW, Hong ZL. Ningbo thyroid dysfunction prevalence study: a cross-sectional survey in an employees-cohort. Zhonghua Yixve Zazhi (Engl) 2010;123:1673–1678. [PubMed] [Google Scholar]
- 14.Zhou BF. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases--report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. Biomed Environ Sci. 2002;15:245–252. [PubMed] [Google Scholar]
- 15.Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K. Prevalence of gallstone disease in relation to smoking, alcohol use, obesity, and glucose tolerance: a study of self-defense officials in Japan. Am J Epidemiol. 1992;136:787–794. doi: 10.1093/aje/136.7.787. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Huang MH, Yang JC, Nien CK, Etheredge GD, Yang CC, Yeh YH, Wu HS, Chou DA, Yueh SK. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol. 2006;21:1737–1743. doi: 10.1111/j.1440-1746.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 17.Lim SH, Kim DH, Park MJ, Kim YS, Kim CH, Yim JY, Cho KR, Kim SS, Choi SH, Kim N, et al. Is Metabolic Syndrome One of the Risk Factors for Gallbladder Polyps Found by Ultrasonography during Health Screening. Gut Liver. 2007;1:138–144. doi: 10.5009/gnl.2007.1.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century. Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 19.Festi D, Dormi A, Capodicasa S, Staniscia T, Attili AF, Loria P, Pazzi P, Mazzella G, Sama C, Roda E, et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project) World J Gastroenterol. 2008;14:5282–5289. doi: 10.3748/wjg.14.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panpimanmas S, Manmee C. Risk factors for gallstone disease in a Thai population. J Epidemiol. 2009;19:116–121. doi: 10.2188/jea.JE20080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou L, Shu XO, Gao YT, Ji BT, Weiss JM, Yang G, Li HL, Blair A, Zheng W, Chow WH. Anthropometric measurements, physical activity, and the risk of symptomatic gallstone disease in Chinese women. Ann Epidemiol. 2009;19:344–350. doi: 10.1016/j.annepidem.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Tang H, Jiang S, Zeng L, Chen EQ, Zhou TY, Wang YJ. Gender and metabolic differences of gallstone diseases. World J Gastroenterol. 2009;15:1886–1891. doi: 10.3748/wjg.15.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kratzer W, Schmid A, Akinli AS, Thiel R, Mason RA, Schuler A, Haenle MM. [Gallbladder polyps: prevalence and risk factors] Ultraschall Med. 2011;32 Suppl 1:S68–S73. doi: 10.1055/s-0029-1245265. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhang H, Elizabeth A, Liu XQ. Epidemiology of hepatitis B and associated liver diseases in china. Zhongguo Yixve Kexve Zazhi. 2013;27:243–248. doi: 10.1016/s1001-9294(13)60009-7. [DOI] [PubMed] [Google Scholar]
- 25.Lai SW, Lai HC, Liu CS, Liao KF, Lin T, Lin CC. The prevalence of gallbladder polyps is higher in HBsAg positive population. Acta Gastroenterol Belg. 2010;73:294–295. [PubMed] [Google Scholar]
- 26.Chen CY, Lu CL, Chang FY, Lee SD. Risk factors for gallbladder polyps in the Chinese population. Am J Gastroenterol. 1997;92:2066–2068. [PubMed] [Google Scholar]
- 27.Okamoto M, Yamagata Z, Takeda Y, Yoda Y, Kobayashi K, Fujino MA. The relationship between gallbladder disease and smoking and drinking habits in middle-aged Japanese. J Gastroenterol. 2002;37:455–462. doi: 10.1007/s005350200066. [DOI] [PubMed] [Google Scholar]
- 28.Park JY, Hong SP, Kim YJ, Kim HJ, Kim HM, Cho JH, Park SW, Song SY, Chung JB, Bang S. Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol. 2009;24:219–222. doi: 10.1111/j.1440-1746.2008.05689.x. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, Hann LE, D’Angelica M, Allen P, Fong Y, Dematteo RP, Klimstra DS, Blumgart LH, Jarnagin WR. Polypoid lesions of the gallbladder: diagnosis and followup. J Am Coll Surg. 2009;208:570–575. doi: 10.1016/j.jamcollsurg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Colecchia A, Larocca A, Scaioli E, Bacchi-Reggiani ML, Di Biase AR, Azzaroli F, Gualandi R, Simoni P, Vestito A, Festi D. Natural history of small gallbladder polyps is benign: evidence from a clinical and pathogenetic study. Am J Gastroenterol. 2009;104:624–629. doi: 10.1038/ajg.2008.99. [DOI] [PubMed] [Google Scholar]


