Abstract
We report on a 24-year-old male patient with history of bloody diarrhea, abdominal pain and vomiting. Endoscopy revealed massive ulcerative discontinuous proctosigmoiditis with deep, sharply demarcated epithelial denudations and enterotoxigenic methicillin-resistant Staphylococcus aureus (MRSA) was detected in mucosal biopsies. After treatment with linezolide and steroids, a significant amelioration of colitis was detected and testing for MRSA became negative. In face of the case presented here, we suggest that in patients with refractory inflammatory bowel disease (IBD), microbiological assessment should be performed to detect a possible Staphylococcus aureus infection in order to initiate an antimicrobial treatment in addition to IBD-specific treatment.
Keywords: Inflammatory bowel disease, Crohn’s disease, Infectious colitis, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus
Core tip: The case presented here displays the complex situation of Crohn’s disease aggravated by an intestinal bacterial infection, which is a commonly observed clinical scenario. However, the presence of enterotoxigenic methicillin-resistant Staphylococcus aureus (MRSA) in colonic mucosal biopsies is a very rare finding. Nevertheless, in face of the increasing prevalence of MRSA infections, clinicians should be aware of unusual opportunistic infections demanding a sophisticated antimicrobial screening and treatment to be combined with inflammatory bowel disease - specific medical therapy.
INTRODUCTION
Patients with inflammatory bowel disease (IBD) are hospitalized more frequently compared to the general population. In addition, regular use of antibiotics and immunomodulating drugs further increase the patients risk to acquire antimicrobial resistant organisms. Recently, Nguyen et al[1]. demonstrated a 1.4-fold increased prevalence of methicillin-resistant Staphylococcus aureus (MRSA) colonisation of hospitalized IBD patients as compared to general medical patients. This was associated with a seven-fold relative increase in in-hospital mortality. However, the clinical impact of intestinal MRSA infection on the course of IBD still remains unclear.
CASE REPORT
We report on a 24-year-old male Caucasian pig farmer who was transferred to our hospital with history of bloody diarrhea, abdominal cramping and vomiting for a 3-mo period. The preliminary diagnosis in the referring hospital was Crohn’s disease (CD). However, the patient had not received neither immunosuppressive treatment nor steroid medication when he was admitted to our department. Physical examination provided no evidence of tenderness or a pathological abdominal mass. There were no signs of suspicious peripheral lymph nodes. Laboratory findings showed elevated values of inflammatory parameters, including elevated CRP (5 mg/dL) and leukocyte count (13.000/μL) as well as marked anemia (hemoglobin, 7.6 g/dL). Blood cultures for bacteria and polymerase chain reaction (PCR) results for cytomegalovirus (CMV), adenovirus, epstein-barr virus, herpes simplex virus type (HSV)-1, HSV-2 and varicella zoster virus were negative. Ultrasonographic examination of the abdomen revealed a thickened wall of the terminal ileum. On our ward, the patient collapsed due to anemia caused by rectal bleeding requiring blood transfusion. Esophago-gastro-duodenoscopy detected gastritis and a duodenal ulcer without evidence for Helicobacter pylori growth. Histological examination was without any signs of specific inflammation. To evaluate the severity of colonic inflammation, sigmoidoscopy was performed, revealing massive ulcerative proctosigmoiditis in a discontinuous pattern with deep, sharply delineated epithelial denudations (Figure 1A). Biopsies showed a massive infiltrate of inflammatory cells in the mucosa as well as in the submucosa resembling acute Crohn’s colitis, however, granuloma formation was absent. CMV antigen and RNA was undetectable in mucosal biopsies. Furthermore, stool examination was negative for Clostridium difficile, Salmonella spp., Shigella spp., Campylobacter spp. and Yersinia enterocolitica as well as for helminth eggs and protozoan parasites, including Giardia lamblia. Therefore, the patient initially did not receive antimicrobial treatment. Magnetic resonance imaging of the abdomen showed inflammatory changes, predominantly in the area of the descending colon and the left colonic flexure (Figure 1B). Finally, microbiological assessment of four mucosal biopsies indicated growth of MRSA in all biopsies obtained. The MRSA was found to belong to spa-type t003. The strain was tested PCR-positive for sed encoding the staphylococcal enterotoxin D. Other pyrogenic toxin superantigen genes (tst, sea, seb, sec and see) as well as the exfoliative (epidermolytic) toxin encoding genes (eta and etb) and the genes encoding Panton-Valentine leukocidin (PVL) were tested negative applying sets of multiplex PCRs as previously described[2,3]. Remarkably, MRSA was found solely in perianal skin swabs, but not in swabs obtained from nostrils, scalp, axilla, and groin. We decided to commence antibiotic treatment with linezolid (600 mg iv bid). Additionally, steroid therapy with 100 mg prednisolone daily iv was continued. Furthermore, a decolonization therapy for MRSA was performed. Within days, the patient’s clinical condition improved. After 10 d, a further sigmoidoscopy was performed revealing near-total mucosal healing of colitis. Histologically, moderate inflammatory infiltrations were found to be remaining. Diagnostic follow-up was conducted one month later. Meanwhile, the clinical symptoms had improved significantly. Abdominal pain and diarrhea were no longer present. Blood test results for blood count and CRP were normal. Esophago-gastro-duodenoscopy provided neither macroscopical nor histological evidence of inflammation. The duodenal ulcer was no longer detectable. Complete ileo-colonoscopy was performed displaying discrete pancolitis with mucosal friability and reduced vascular pattern. In the distal colon, multiple pseudopolypoid lesions were detectable, along with fibrin-coated ulcers as a correlate of inflammatory changes. The histological examination of colonic biopsies was again indicative of discrete discontinuous colitis resembling findings typical of CD. Microbiologic testing of mucosal specimens and all skin swabs (including perianal) was now negative for MRSA. Again, no sign of CMV infection was found. Consecutively, the steroid medication was tapered.
Figure 1.
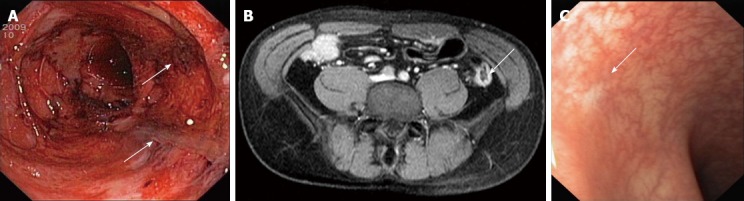
Massive colonic inflammation was detected by endoscopy and magnetic resonance imaging and ameliorated after antibiotic treatment. A: Sigmoidoscopy of the distal colon: Massive ulcerative proctosigmoiditis in a discontinuous pattern with deep and sharply delineated epithelial denudations (arrows) could be detected; B: Magnetic resonance imaging of the abdomen: contrast enhancement of the colonic wall in the area of the descending colon indicated inflammatory changes (arrow); C: Flexible sigmoidoscopy after ten days of antibiotic therapy with linezolid: Macroscopically, a near-total mucosal healing of colitis was detectable (arrow).
Six weeks later, follow-up flexible sigmoidoscopy was performed. No acute inflammatory changes were detectable (Figure 1C). Histologically, mild signs of acute and chronic inflammation with interspersed crypt abscesses were found. At this time, the patient was on 5 mg prednisolone daily and had no complaints. Repeat MR enteroclysis was without signs of small intestinal IBD. Endoscopic follow-up 3 mo later again was showing complete mucosal healing. Histology was indicative of changes in the mucosa as well as a small hyperplasic polyp of the rectosigmoid colon. No signs of an acute flare-up occurred in a follow-up of further 12 mo. In addition, MRSA rescreening by nasal swabs applying polymerase chain reaction was performed and yielded negative results.
DISCUSSION
Staphylococcus aureus (S. aureus) is a leading cause of human bacterial infections worldwide aggravated by the continuing threat of multi-resistant strains as represented by the different clonal lineages of MRSA. It is estimated that 30% of healthy individuals are colonised with commensal S. aureus in their anterior nares that were found to represent the major source and an independent risk factor for subsequent nosocomial infections[4]. Inadequate antibiotic prescribing and poor adherence to infection control guidelines are the two main reasons for the development and spread of MRSA[5]. While the percentage of MRSA among S. aureus clinical isolates is believed to be 35%-70% in the United States[6], pan-European surveillance data on bloodstream infections showed marked variability in the proportion of MRSA ranging from less than 1% to more than 50%[7].
In the context of IBD, the presence of opportunistic infections and infections is of special interest, since infections were found to trigger an acute disease flare[8]. Moreover, the occurrence of opportunistic infections in patients with IBD has become a key safety issue especially with widespread use of immunosuppressive and immunomodulatory drugs[9]. Recently, it was shown that the use of any anti-inflammatory drugs such as corticosteroids, thiopurines, and anti-tumor necrosis factor alpha agents (e.g., infliximab) is associated significantly with an increased risk of opportunistic infection in IBD patients[10]. In addition, the number of immunosuppressive agents combined appears to determine the individual risk of opportunistic infections[10].
Since the course of IBD is often chronically relapsing, IBD patients are frequently hospitalized and, thus, are at higher risk of hospital-associated infections[1]. Dysregulated barrier function of the intestinal surface epithelial lining is believed to represent a key factor for mucosal bacterial invasion. In active IBD, a disrupted epithelial barrier could therefore predispose for colonic S. aureus infections. In literature, only few reports on the possible correlation between IBD and MRSA infection are reported. One report published by Ishiyama et al[11] described an MRSA-associated diarrhea with positive stool cultures in patients after colorectal surgery. Recently, a first systematic analysis regarding this topic was performed by Nguyen et al[1]. It could be demonstrated that hospitalized IBD patients are at increased risk for MRSA infection as compared to non IBD-gastrointestinal and general medicine patients. Moreover, Nguyen et al[1] showed that the presence of MRSA is associated with seven-fold increased in-hospital mortality in the cohort of IBD patients. Further recognized risk factors for MRSA infection include bowel surgery, parenteral nutrition, and long hospitalization.
In our patient, the severe onset of the first acute flare of CD is striking and unusual. Endoscopically and histologically, massive inflammatory changes were present which resulted in relevant GI bleeding with anemia and hypotension. Furthermore, it is remarkable that microbiological testing for MRSA was completely negative except in specimens from colonic mucosa and perianal skin swabs. Lu et al[12] showed that S. aureus enterotoxins B might be associated with acute inflammatory response in mice. The detection of the enterotoxin D encoding gene in the patient’s MRSA isolate makes a toxin-associated trigger of colitis plausible. It is likely that the presence of MRSA in our patient was no simple coincidence but a factor further worsening the disease course. This hypothesis is substantiated by the observation that the treatment with MRSA active antibiotic was accompanied by a rapid amelioration of colitis with mucosal healing.
Although the patient is working as a pig farmer, the MRSA subtype found in this patient belongs to a typical epidemic hospital-acquired MRSA clone and does not belong to the known livestock-associated clonal lineages (CC011/ST398). The relationship to the pig farming seems not to be epidemiologically relevant in this case and an earlier contact to healthcare seems more plausible as a source of colonization. In IBD patients, the risk of severe disease course may be increased. Yet, future studies are needed to define the putative connection between S. aureus and the course of IBD, the influence of the production of staphylococcal enterotoxins on IBD course, and if co-infection with (enterotoxigenic) S. aureus may be a predictor for severe disease course in IBD patients.
In case of refractory courses of IBD, microbiological assessment should be considered to rule out opportunistic S.aureus infection in order to initiate an antimicrobial treatment in addition to the IBD-related treatment.
ACKNOWLEDGMENTS
We like to thank Dr. Akiko Möller-Horigome for translation of Japanese secondary literature. D. Bettenworth was supported by a research fellowship from the Faculty of Medicine, Westfälische Wilhelms-Universität Münster.
Footnotes
P- Reviewer Ip M S- Editor Zhai HH L- Editor A E- Editor Ma S
References
- 1.Nguyen GC, Patel H, Chong RY. Increased prevalence of and associated mortality with methicillin-resistant Staphylococcus aureus among hospitalized IBD patients. Am J Gastroenterol. 2010;105:371–377. doi: 10.1038/ajg.2009.581. [DOI] [PubMed] [Google Scholar]
- 2.Becker K, Roth R, Peters G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol. 1998;36:2548–2553. doi: 10.1128/jcm.36.9.2548-2553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Eiff C, Friedrich AW, Peters G, Becker K. Prevalence of genes encoding for members of the staphylococcal leukotoxin family among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 2004;49:157–162. doi: 10.1016/j.diagmicrobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 4.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 5.Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64 Suppl 1:i3–10. doi: 10.1093/jac/dkp256. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, Leblebicioglu H, Abu Khader I, Miranda Novales MG, Berba R, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010;38:95–104.e2. doi: 10.1016/j.ajic.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Köck R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, et al. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill. 2010;15:19688. doi: 10.2807/ese.15.41.19688-en. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Graff LA, Bernstein CN. Do NSAIDs, antibiotics, infections, or stress trigger flares in IBD. Am J Gastroenterol. 2009;104:1298–1313; quiz 1314. doi: 10.1038/ajg.2009.15. [DOI] [PubMed] [Google Scholar]
- 9.Viget N, Vernier-Massouille G, Salmon-Ceron D, Yazdanpanah Y, Colombel JF. Opportunistic infections in patients with inflammatory bowel disease: prevention and diagnosis. Gut. 2008;57:549–558. doi: 10.1136/gut.2006.114660. [DOI] [PubMed] [Google Scholar]
- 10.Toruner M, Loftus EV, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Ishiyama M, Watanabe C, Utsunomiya K. [Colitis caused by MRSA] Nihon Naika Gakkai Zasshi. 1992;81:1629–1634. [PubMed] [Google Scholar]
- 12.Lu J, Wang A, Ansari S, Hershberg RM, McKay DM. Colonic bacterial superantigens evoke an inflammatory response and exaggerate disease in mice recovering from colitis. Gastroenterology. 2003;125:1785–1795. doi: 10.1053/j.gastro.2003.09.020. [DOI] [PubMed] [Google Scholar]


