Abstract
The mucosal surfaces are constantly exposed to incoming pathogens which can cause infections that result in severe morbidity and/or mortality. Studies have reported that mucosal immunity is important for providing protection against these pathogens and that mucosal vaccination is effective in preventing local infections. For many years, the sublingual mucosa has been targeted to deliver immunotherapy to treat allergic hypersensitivities. However, the potential of vaccine delivery via sublingual mucosal has received little attention until recently. Recent studies exploring such potential have documented the safety and effectiveness of sublingual immunization, demonstrating the ability of sublingual immunization to induce both systemic and mucosal immune responses against a variety of antigens, including soluble proteins, inter particulate antigens, and live-attenuated viruses.
This review will summarize the recent findings that address the promising potential of sublingual immunization in proving protection against various mucosal pathogens.
Keywords: Mucosal immune responses, Lung immunity, Parenteral immunization, Sublingual (s.l.) immunization, Vaccine
INTRODUCTION
Mucosal surfaces form boundaries with the exterior environment, functioning as the main gateway for pathogens entering the host via respiratory, gastrointestinal, and/or genital tracts. Hence, properly functioning mucosal immunity is important for protection against these invading pathogens (1). An ideal vaccine against an infectious pathogen should prime the host for induction of pathogen-specific memory immune responses at the appropriate mucosal compartments, thereby, preventing the entry and/or replication of the invading pathogen at the site of infection. Previous studies have established that mucosal vaccination can efficiently stimulate the local mucosal immunity and the broadly functional systemic immunity and suggested that mucosal vaccine delivery may be a proficient method to induce pathogen-specific secretory antibody responses as well as cytotoxic T lymphocyte (CTL) responses at the target mucosal tissues (2-8). Accordingly, these studies also have reported that mucosal vaccination is highly effective in conferring protection against various mucosal pathogens. Nevertheless, most vaccines that are currently being used are administered via parenteral routes. Although parenteral vaccines are very effective in eliciting general systemic immunes responses, they are inefficient stimulators of mucosal immunity (9). For example, delivering influenza vaccines via parenteral route relies on the systemic induction of IgG antibodies for protection. However, studies have reported that influenza vaccination efficacy is closely correlated to the immune responses induced within the respiratory mucosa, and parenteral vaccines being used presently have been shown to be inefficient in stimulating immune responses at the respiratory mucosa (10). In recent years, a number of studies have explored the potential of sublingual immunization in eliciting desired immune responses against various potential vaccine components which includes soluble protein antigens, virus-like particles, and inactivated or live-attenuated viruses (3-8,11,12). These studies have successfully demonstrated the safety and efficacy of sublingual immunization in inducing antigen-specific systemic and mucosal immune responses and protection against pathogen challenges. This review provides an overview of previous studies that have described a promising prospect for sublingual immunization in vaccine delivery.
SUBLINGUAL MUCOSA: A VIABLE ROUTE FOR PROTEIN ANTIGEN DELIVERY TO ELICIT ANTIGEN-SPECIFIC IMMUNE RESPONSES
Currently, the sublingual route is widely being used to deliver sublingual immunotherapy (SLIT) for treatment of type 1 (allergic) hypersensitivity, as repeated sublingual administration of an allergen has shown to induce allergen-specific immune responses that mediate skewing of allergic Th2 responses to Treg induction and/or Th1 activation and result in reduction of allergic symptoms following subsequent exposure (13,14). For example, a study conducted by Kildsgaard et al. reported that SLIT treatment in allergen sensitized mice increased T cell proliferation as wells as the levels of allergen-specific IgAs in bronchoalveolar lavage (BAL) and nasal lavage (NAL) with no detectable allergen-specific IgEs (15). Another study conducted by Brimnes et al. reported that SLIT treatment in allergen-sensitized mice reduces the allergic symptoms, eosinophilia, and allergen-specific NAL and serum IgE levels upon allergen challenge (16). More importantly, sublingual antigen administration has been shown to be safe as there is no reported cases of anaphylactic shock in clinical studies performed on children (17,18), although a few mild to moderate local adverse effects were observed in one phase I study evaluating the toxicity of grass pollen tablets (19).
Based on these observations, researchers at our institution evaluated the use of sublingual route for vaccine delivery. First, in a study conducted by Cuburu et al., a group of mice received different concentrations (10, 50, 200µg) of OVA (used as a model soluble protein antigen) together with cholera toxin (CT) as mucosal adjuvant (2). Following immunization, OVA-specific systemic and mucosal antibody levels as well as cytokine and cytotoxic T lymphocyte (CTL) responses were examined. The results from this study showed that sublingual OVA-immunization induced both systemic and mucosal antibody responses specific to the administered antigen with the magnitude of antibody responses generated by sublingual immunization being comparable to that observed after intranasal immunization and significantly greater than that elicited by orogastric immunization. The authors also described that sublingual delivery of OVA promoted proliferation of OVA-specific naïve CD4+ T cells with mixed Th1 and Th2 cytokine profiles and significant induction of OVA-specific CTL responses in both local and systemic manners.
Moreover, on a follow-up study, the same group investigated the efficacy of sublingual administration of non-replicating antigen in establishing antigen-specific immunity in the genital mucosa and in conferring protection against human papillomavirus virus (HPV) (3). The study demonstrated that sublingual immunization of mice with OVA with CT adjuvant induced OVA-specific IgAs and IgGs in sera and in cervicovaginal secretions and that the induction of OVA-specific antibodies in the cervicovaginal secretions was associated with CCL28-mediate migration of antibody secreting cells (ASCs) to the genital mucosa. Interestingly, while sublingual, intranasal, and intravaginal immunization evoked comparable genital ASC responses, intragastric immunization failed to generate significant antibody responses in the genital mucosa. Further, sublingual immunization with OVA induced OVA-specific CTLs in the genital mucosa, although the induction of CTL response required the presence of CT adjuvant. Another aspect of this study was to determine whether delivery of vaccine antigen via sublingual route could actually confer protection against live virus challenge. Accordingly, the authors administered sublingual delivery of human papillomavirus virus-like particles (HPVLPs) with or without CT adjuvant and observed that HPVLP-immunization conferred protection against genital challenge with human papillomavirus pseudovirions. On a similar note, a study conducted by Hervouet et al. demonstrated that sublingual immunization of mice with HIV-1 gp41 and a reverse transcriptase polypeptide using cholera toxin B subunit (CTB) as an adjuvant induced gp41-specific IgA antibodies and ASCs, as well as reverse transcriptase-specific CTL responses in the genital mucosa (11). Taken together, these studies recognized the sublingual mucosa as a potential route of vaccine delivery, which promotes the induction broadly distributed humoral and cell-mediated immune response in systemic lymphoid tissues as well as various mucosal compartments, to offer protection against pathogens that possess tropism for mucosal epithelia.
However, results from a recent human study investigating the effectiveness of sublingual immunization with the licensed quadravalent Human Papilloma Virus (HPV) vaccine Gardasil® (Sanofi Pasteur), which contains L1-based virus-like particles (VLPs) representing four HPV types, were less encouraging (20). In this study, eighteen healthy adult female volunteers were immunized three times with Gardasil® at 0, 4 and 16 weeks via either sublingual or intramuscular route. The results demonstrate that intramuscular delivery elicited HPV-specific, pseudovirus-neutralizing serum and cervicovaginal IgGs and primed for circulating ACS responses. However, sublingual delivery generated 38-fold lower serum and 2-fold lower cervicovaginal IgGs levels than intramuscular delivery while inducing neutralizing antibody response in only 3 out of 12 subjects. Moreover, neither route substantially increased HPV-specific mucosal IgAs, indicating the challenge that lie ahead to optimize the immune responses following sublingual immunization of HPV vaccines in humans.
SUBLINGUAL DELIVERY OF INFLUENZA VIRUS VACCINES
Adoption of sublingual vaccine delivery for the establishment protection against respiratory pathogens has been explored in several studies (Table I) (3-8,11,12). In a study published by Song et al., mice sublingually immunized with formalin-inactivated or live influenza A/PR/8 virus (H1N1) were protected against a lethal influenza virus challenge (12). The authors concluded that the observed protection was mediated by the induction of influenza virus-specific IgGs and IgAs in the serum and respiratory mucosa, respectively, which limits the virus entry and replication in the respiratory mucosa. In addition, sublingual delivery of formalin-inactivated A/PR/8 virus in the presence of mucosal adjuvant CTA-LTB induced systemic expansion of IFN-γ-secreting CD4+ and CD8+ T cells and virus-specific cytotoxic T lymphocyte responses. Although the authors did not expound upon the mechanism by which virus-specific CTL response was primed following immunization with a non-replicating, inactivated virus particle, a specialized microenvironment within the proximal draining lymph node for the sublingual mucosa and/or cross-presentation of vaccine antigen(s) within the antigen presenting cells (APCs) may have promoted the induction of influenza virus-specific CTLs response observed in this study (10,12). Moreover, this study demonstrated that a single sublingual administration of live A/PR/8 virus did not cause pathology and established broad-range protection against H1N1 and H3N2 influenza virus subtypes without the risk of potential passage of vaccine virus to the olfactory bulb.
Table I.
Effect of sublingual route against various viral pathogens
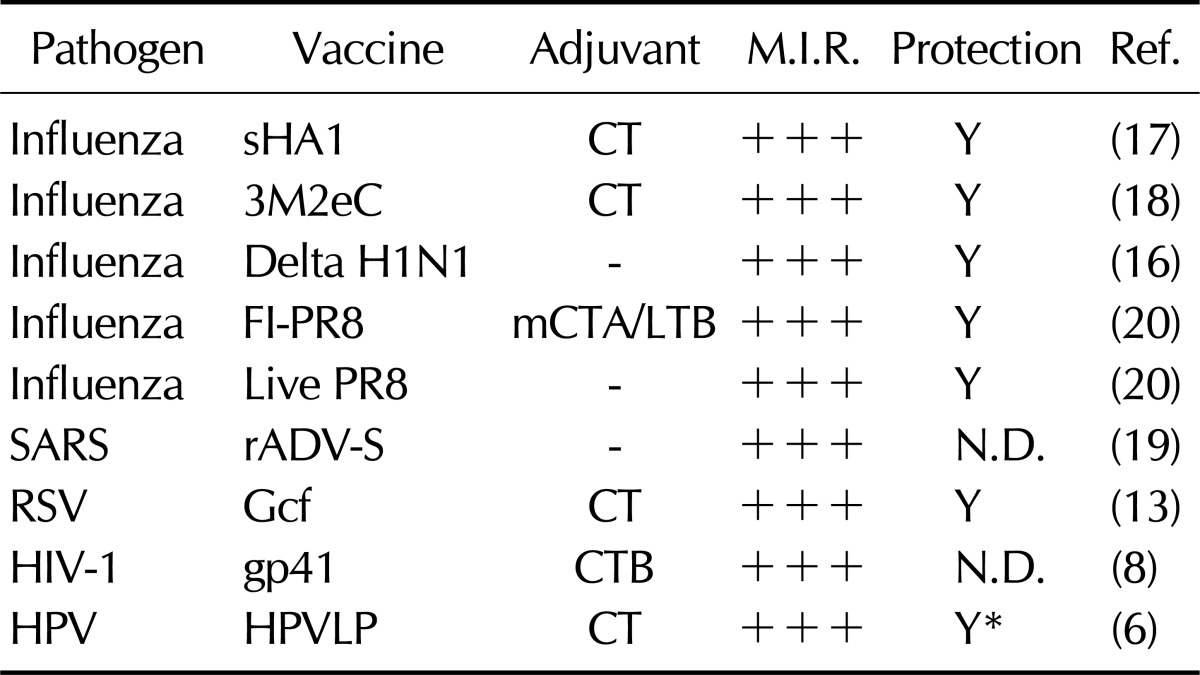
M.I.R., mucosal immune responses; +++, comparable to immune response generated by intranasal or systemic immunization N.D., not determined. *protection against HPV16 pseudovirus challenge.
A recent study by Park et al. also reported that sublingual administration of live-attenuated influenza virus lacking the nonstructural protein 1 (NS1) was safe and effective in inducing protection against homo- and hetero-subtypic influenza virus challenges (5). In this study, mice were given sublingual administration of NS1-deleted recombinant H1N1 or H5N1 influenza virus (designated DeltaNS1 H1N1 or DeltaNS1 H5N1, respectively), and subsequent development of protective immunity generated by the sublingual delivery of live-attenuated influenza virus vaccines was evaluated. The results were promising as sublingually administered live-attenuated influenza virus vaccine offered cross-subtypic protection with the protective efficacy comparable to that induced by intranasal immunization with the same vaccine.
Furthermore, studies from our laboratory also evaluate the protective efficacy generated by sublingual delivery of influenza virus subunit vaccines. In one study, a recombinant influenza virus M2 protein-based subunit vaccine containing three tandem copies of the M2e (3M2eC) was expressed in Escherichia coli, and the protective efficacy of parenteral and sublingual immunizations was compared (7). We observed that parental immunization induced robust M2e-specific antibody responses in the serum but failed to provide complete protection against a lethal challenge with influenza virus. Meanwhile, sublingual immunization with 3M2eC resulted in significant decrease in M2e-specific serum antibody levels compared to parental vaccination. However, sublingual immunization conferred superior protection against influenza virus challenge, and substantial levels of M2e-specific mucosal antibodies were detected in saliva, nasal wash, and BAL of the sublingually immunized mice. This study successfully demonstrated that sublingual delivery of 3M2eC improves the protective efficacy of the subunit vaccine compared to the parenteral delivery and such improvement of protective efficacy can be attributed to the effective induction of antigen-specific antibody responses in the airway mucosa.
In another study published from our laboratory, a recombinant influenza virus hemagglutinin protein 1 (sHA1), derived from 2009 pandemic H1N1 influenza virus, was expressed in E. coli and was administered to mice via sublingual route in combination with CT adjuvant (6). Sublingual sHA1 immunization induced neutralizing antibody responses in the serum and in the respiratory mucosa and provided complete protection against a lethal challenge with pandemic H1N1 influenza A/CA/04/09 virus. Furthermore, the protective efficacy induced by sublingual immunization was comparable to that induced by parenteral immunization. Collectively, growing body of evidence suggests that sublingual delivery of inactivated, live-attenuated, or recombinant protein influenza virus vaccines safely and effectively confer protection against influenza virus infection and offers an alternative strategy to parenteral vaccination for delivering influenza virus vaccines.
SUBLINGUAL DELIVERY OF RSV AND SARS VIRUS VACCINE CANDIDATES
The sublingual mucosa may also be a promising vaccine delivery route for other respiratory pathogens including respiratory syncytial virus (RSV) and severe acute respiratory syndrome (SARS) virus. A recent study published by Kim et al. evaluated a bacterially-expressed subunit vaccine, designated Gcf, which encompasses the central conserved region within the RSV G glycoprotein (4). The authors reported that sublingual immunization of mice with Gcf elicited strong serum IgG and mucosal IgA responses and effectively reduced the lung virus titer following RSV challenge in the absence of Th2-biased cytokine responses or a pronounced pulmonary eosinophilia. This study importantly demonstrated that sublingual delivery of RSV G-based subunit vaccine can prevent RSV infection without priming for any vaccine-induced disease enhancement, affirming sublingual immunization a viable delivery option for RSV subunit vaccines. Furthermore, a study conducted in our laboratory examined sublingual delivery of recombinant adenovirus vector expressing SARS spike (S) protein (rADV-S) in generating SARS virus-specific immune responses (8). In this report, sublingual administration of rADV-S in mice induced SARS virus-specific neutralizing antibody response in the serum and secretory IgA response in the respiratory mucosa. The observed antibody responses were similar in magnitude to those induced following intranasal administration of rADV-S. In addition, sublingual immunization significantly increased the frequency of SARS virus S protein-specific, IFN-γ-secreting CD8+ T cell to the lungs compared to intramuscular immunization. Importantly, unlike intranasal administration, sublingual immunization of rADV-S posed minimum risk for potential retrograde passage of the vaccine component to the CNS as shown by the absence of adenoviral DNA in the olfactory bulb. Overall, these findings provide evidential support for the adoption of sublingual vaccination strategy in administration of vaccines for various respiratory pathogens.
CONCLUSION
Many questions regarding sublingual immunization still remain to be addressed, including the use of proper adjuvant and the optimization of vaccine formulation to further enhance the vaccine efficacy. However, the studies described in this review demonstrate promising aspects of sublingual immunization. In these studies, sublingual immunization has been shown to be safe and highly effective in generating robust immune responses against the administered antigen. Moreover, it has been shown to confer protective immunity by simultaneously eliciting systemic IgG and mucosal IgA antibodies as well as CTL responses in the peripheral lymphoid organs and mucosal tissues. These studies also suggest that sublingual immunization could be a better alternative to the traditional parental route of vaccine delivery against both genital and respiratory pathogens (3,4,6-8,11). Taken together, the findings described in this review provide a foundation for further evaluation of this novel but promising route of vaccine delivery.
ACKNOWLEDGEMENTS
This work was supported by the Regional Technology Innovation Program of the Ministry of Knowledge Economy (MKE) and TBP grant from KRIBB (KGM3110912). The International Vaccine Institute is supported in part by grants from the governments of the Republic of Korea, Kuwait, and Swedish International Development Cooperation Agency (SIDA).
Abbreviations
- s.l.
sublingual
- CTL
cytotoxic T lymphocyte
- SLIT
sublingual immunotherapy
- BAL
bronchoalveolar lavage
- NAL
nasal lavage
- CT
cholera toxin
- HPV
human papillomavirus virus
- ASC
antibody secreting cell
- HPVLP
human papillomavirus virus-like particles
- CTB
cholera toxin B subunit
- VLP
virus-like particle
- APC
antigen presenting cell
- NS1
nonstructural protein 1
- sHA1
hemagglutinin protein 1
- RSV
respiratory syncytial virus
- SARS
severe acute respiratory syndrome
Footnotes
The authors have no financial conflict of interest.
References
- 1.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 2.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, Hofman P, Holmgren J, Anjuere F, Czerkinsky C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25:8598–8610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 3.Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, Holmgren J, Stadler K, Schiller JT, Anjuére F, Czerkinsky C. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J Immunol. 2009;183:7851–7859. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim S, Joo DH, Lee JB, Shim BS, Cheon IS, Jang JE, Song HH, Kim KH, Song MK, Chang J. Dual role of respiratory syncytial virus glycoprotein fragment as a mucosal immunogen and chemotactic adjuvant. PLoS One. 2012;7:e32226. doi: 10.1371/journal.pone.0032226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HJ, Ferko B, Byun YH, Song JH, Han GY, Roethl E, Egorov A, Muster T, Seong B, Kweon MN, Song M, Czerkinsky C, Nguyen HH. Sublingual immunization with a live attenuated influenza a virus lacking the nonstructural protein 1 induces broad protective immunity in mice. PLoS One. 2012;7:e39921. doi: 10.1371/journal.pone.0039921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shim BS, Choi JA, Song HH, Park SM, Cheon IS, Jang JE, Woo SJ, Cho CH, Song MS, Kim H, Song KJ, Lee JM, Kim SW, Song DS, Choi YK, Kim JO, Nguyen HH, Kim DW, Bahk YY, Yun CH, Song MK. Sublingual administration of bacteria-expressed influenza virus hemagglutinin 1 (HA1) induces protection against infection with 2009 pandemic H1N1 influenza virus. J Microbiol. 2013;51:130–135. doi: 10.1007/s12275-013-2399-z. [DOI] [PubMed] [Google Scholar]
- 7.Shim BS, Choi YK, Yun CH, Lee EG, Jeon YS, Park SM, Cheon IS, Joo DH, Cho CH, Song MS, Seo SU, Byun YH, Park HJ, Poo H, Seong BL, Kim JO, Nguyen HH, Stadler K, Kim DW, Hong KJ, Czerkinsky C, Song MK. Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza. PLoS One. 2011;6:e27953. doi: 10.1371/journal.pone.0027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim BS, Stadler K, Nguyen HH, Yun CH, Kim DW, Chang J, Czerkinsky C, Song MK. Sublingual immunization with recombinant adenovirus encoding SARS-CoV spike protein induces systemic and mucosal immunity without redirection of the virus to the brain. Virol J. 2012;9:215. doi: 10.1186/1743-422X-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505–515. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 10.Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003;13:293–310. doi: 10.1002/rmv.398. [DOI] [PubMed] [Google Scholar]
- 11.Hervouet C, Luci C, Cuburu N, Cremel M, Bekri S, Vimeux L, Marañon C, Czerkinsky C, Hosmalin A, Anjuère F. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine. 2010;28:5582–5590. doi: 10.1016/j.vaccine.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008;105:1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viswanathan RK, Busse WW. Allergen immunotherapy in allergic respiratory diseases: from mechanisms to meta-analyses. Chest. 2012;141:1303–1314. doi: 10.1378/chest.11-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calderón MA, Casale TB, Togias A, Bousquet J, Durham SR, Demoly P. Allergen-specific immunotherapy for respiratory allergies: from meta-analysis to registration and beyond. J Allergy Clin Immunol. 2011;127:30–38. doi: 10.1016/j.jaci.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Kildsgaard J, Brimnes J, Jacobi H, Lund K. Sublingual immunotherapy in sensitized mice. Ann Allergy Asthma Immunol. 2007;98:366–372. doi: 10.1016/S1081-1206(10)60884-8. [DOI] [PubMed] [Google Scholar]
- 16.Brimnes J, Kildsgaard J, Jacobi H, Lund K. Sublingual immunotherapy reduces allergic symptoms in a mouse model of rhinitis. Clin Exp Allergy. 2007;37:488–497. doi: 10.1111/j.1365-2222.2006.02624.x. [DOI] [PubMed] [Google Scholar]
- 17.Agostinis F, Tellarini L, Canonica GW, Falagiani P, Passalacqua G. Safety of sublingual immunotherapy with a monomeric allergoid in very young children. Allergy. 2005;60:133. doi: 10.1111/j.1398-9995.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 18.Olaguíbel JM, Alvarez Puebla MJ. Efficacy of sublingual allergen vaccination for respiratory allergy in children. Conclusions from one meta-analysis. J Investig Allergol Clin Immunol. 2005;15:9–16. [PubMed] [Google Scholar]
- 19.Larsen TH, Poulsen LK, Melac M, Combebias A, Andre C, Malling HJ. Malling. 2006. Safety and tolerability of grass pollen tablets in sublingual immunotherapy--a phase-1 study. Allergy. 2006;61:1173–1176. doi: 10.1111/j.1398-9995.2006.01203.x. [DOI] [PubMed] [Google Scholar]
- 20.Huo Z, Bissett SL, Giemza R, Beddows S, Oeser C, Lewis DJ. Systemic and mucosal immune responses to sublingual or intramuscular human papilloma virus antigens in healthy female volunteers. PLoS One. 2012;7:e33736. doi: 10.1371/journal.pone.0033736. [DOI] [PMC free article] [PubMed] [Google Scholar]


