Abstract
To assess efficacy and safety of lornoxicam as analgesic after surgery on head and neck in comparison to tramadol. Forty five patients undergoing operations on head and neck were recruited and randomly assigned to two parallel groups—lornoxicam and tramadol, both given intramuscular on the first post-operative day followed by oral tablets for the consecutive 4 days. Treatment was given single blind. 10 cm visual analog scale (VAS) pain score and wound tenderness assessed by a 3-point ordinal scale were the primary efficacy parameters. Use of rescue medication and percentage of subjects having at least 50 % pain relief by 48 h were also compared as secondary parameters. The groups were comparable at baseline regarding age, sex and VAS score. There was steady decline in VAS pain score from baseline to study end in both the groups, indicating good analgesic efficacy with either drug. Between groups comparisons of VAS score showed no significant difference at any time point. Between groups comparisons of wound tenderness also showed no significant difference. Five patients on lornoxicam and one patient on tramadol experienced at least 50 % pain relief at 48 hours compared to baseline while five patients from the lornoxicam group and eight from the tramadol group required rescue medicine. The tolerability of lornoxicam appeared to be significantly superior to tramadol, with less number of patients experiencing adverse drug reactions. Lornoxicam is safe, effective and comparable to tramadol for relieving postoperative pain after operations on head and neck.
Keywords: Lornoxicam, Tramadol, Post-operative pain, VAS score
Introduction
Pain in the post-operative period is an inevitable consequence of any surgery and relief from pain forms an essential component of post-operative patient care. Pain sensation after operation results from surgical trauma that might occur during manipulation of tissues. Selection of analgesics should be carefully considered in order to suit the patient’s needs. Effective and early pain relief helps in early mobilization and better patient compliance. Drugs used to relieve post-operative pain should be effective as well as safe with minimum adverse effects.
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used for treatment of mild to moderate post-operative pain. Lornoxicam (an oxicam derivative) is a non-steroidal analgesic with relatively rapid onset of action than other oxicam derivatives [1]. It is approved for use in musculoskeletal and joint pain disorders such as osteoarthritis and rheumatoid arthritis; it is also used in the treatment of other painful conditions including postoperative pain. [2, 3]. Lornoxicam is available in formulations suitable for oral as well as parenteral administration.
Tramadol is a centrally acting analgesic used for treatment of post-operative pain [4, 5]. It is synthetic codeine analog with two distinct synergistic mechanisms of action: weak μ-opioid receptor agonism and an inhibition of monoamine neurotransmitter (norepinephrine and serotonin) reuptake. It is not known to cause serious cardiovascular or respiratory depression [6].
Although widely used as analgesics, comparative data on efficacy and safety of these two drugs in post-operative pain relief is sparse [7]. The present study thus aims to assess the comparative efficacy and safety of tramadol and lornoxicam as analgesics after head and neck surgery, when administered by both intramuscular and oral route. All the recruited patients were operated under general anaesthesia.
Materials and Methods
Screening of patients and recruitment were carried out at the ENT (ear–nose–throat) Outpatient Department of a tertiary care hospital in West Bengal during the period March 2011 to December 2011. Altogether 51 patients of either sex, in the age group 20–60 years of age, undergoing operations of head or neck like, partial or total thyroidectomy, thyroglossal cyst, branchial fistula, dentigerous cyst, pre auricular sinus, parapharyngeal tumor etc. were selected for the study.
Patients known to be hypersensitive to any of the study drugs were excluded from the study. Those with history of bronchial asthma, hypertension, peptic ulcer disease or seizures were also excluded. Patients receiving NSAIDs, sedative-hypnotic or psychotropic drugs, MAO inhibitors or having participated in any other clinical trial within the past 1 month were not included.
Ethical Considerations
The study was conducted in accordance with the principles of the Declaration of Helsinki for Biomedical Research Involving Human Subjects. Also, every effort was made to adhere to the Indian Good Clinical Practice and ICMR guidelines. All trial subjects had to give written consent to participate in the trial. The study protocol, case record form and the patient informed consent form received clearance from the Institutional Ethics Committee.
Sample Size
This was determined on the basis of visual analog scale (VAS) pain score as the principal efficacy parameter. For the study to have 90 % power to detect a difference of 3 in VAS score between the treatment groups with significance level of p < 0.05, the number of subjects required was calculated to be 19 in each group. A within group standard deviation of 7.5 was obtained through a pilot study in 10 subjects in the VAS pain score. Assuming a 10 % drop-out rate, 45 subjects were recruited.
Study Design
A total number of 51 patients were screened for the study. Out of these, 6 were excluded as they refused to give written consent. The subjects were randomized into two groups: lornoxicam (n = 22) and tramadol (n = 23).The study was designed as a unicentric, prospective, single blind, randomized, controlled trial with two parallel treatment arms.
For the individual patient, the treatment duration was for 5 days in the post-operative period. Group A patients received intramuscular Lornoxicam 16 mg twice on the first post-operative day followed by 8 mg orally twice daily after food for the consecutive 4 days. Group B patients received intramuscular Tramadol 50 mg twice on the first post-operative day followed by 50 mg orally twice daily after food for the consecutive 4 days.
A base-line assessment (V0) of the patient was done as soon as the subject regained consciousness postoperatively. First follow-up (V1) was done 24 h after the patient received first dose of medication, second (V2) after 48 h, third (V3) after 72 h, fourth and the final assessment (V4) on the 4th post-operative day (after 96 h).
Study Drugs and Concomitant Medication
Injectable preparations and oral tablets of lornoxicam and tramadol were dispensed to the subjects according to randomization. The identity of injections/tablets were not revealed to the subjects as the study was single-blind. The medications were administered by the investigators or the nursing staff at the scheduled time points.
Compliance was assessed at each follow-up and at the end of the study. Compliance was judged satisfactory if a particular subject missed no more than 20 % of the scheduled doses.
The enrolled patients were not allowed to use any non-permitted medication known to interact or potentially alter the response to the study drugs.
At screening a thorough medical history and clinical examination of the potential subjects was undertaken to assess their suitability for participation in the study. Written informed consent was obtained. Body weight, resting pulse rate, respiratory rate and BP were recorded. Non permitted medicines were withdrawn. Blood was sampled for the following laboratory tests: complete blood count, blood glucose, urea, creatinine, ALT, AST, Alkaline phosphatase.
After the baseline assessment, the first dose of study medication was dispensed to the subjects, following the randomization list provided all inclusion and exclusion criteria were satisfied.
Assessment of Efficacy
Patient’s pain perception was noted as the VAS score in cm [8, 9]. Baseline wound tenderness was also recorded by pressing on the wound over a thin antiseptic dressing.
At each subsequent assessment, clinical examination was done and patient’s pain perception was noted as the VAS score in cm. Wound tenderness was also assessed.
The primary endpoints for assessment were the VAS pain score and wound tenderness. The VAS scoring was based on a 10 cm line and the patient was asked to indicate the spot on the line which he/she feels correlates with the pain he/she is experiencing (scored as ‘10’ = worst pain imaginable and ‘0’ = no pain). Wound tenderness was graded on a three-point ordinal scale as 0 = no tenderness, 1 = tender, and 2 = highly tender.
Use of rescue medication and percentage of subjects having at least 50 % pain relief by 48 h were the secondary end points. Paracetamol (500 mg) tablets were used for rescue medication in case of inadequate pain relief. Number of patients receiving rescue medicines were assessed between the groups and used as a surrogate marker of analgesic efficacy.
The physician’s clinician’s global impression (CGI) of efficacy and safety was graded on a four-point scale as poor, satisfactory, good and excellent. The patient’s assessment regarding efficacy and acceptability of treatment was similarly recorded on a 4-point scale as poor, satisfactory, good and excellent.
Compliance was noted and treatment-emergent adverse events were looked for, if any. The final end-of- study visit was done on the 4th post-operative day following the same procedures. Blood tests were repeated on the 6th post-operative day.
Safety Monitoring
Any adverse event reported spontaneously by the subject or noted by the clinician during the follow-up or end-of-trial visits were recorded. In case of an adverse event, a subject could be withdrawn if further continuation was considered harmful for him/her. Any adverse event was to be considered serious if it was fatal, life threatening, disabling, incapacitating or if it prolonged hospitalization of the subject.
Statistical Analysis
Non parametric data was compared by Kruskal–Wallis ANOVA, with p < 0.05 as the cut-off level for significance, which was followed by post hoc Dunn’s test. Comparisons were one-tailed. Data analysis was carried out in Graph Pad instat 3.
Results
Of the 45 patients recruited, 22 received lornoxicam and 23 received tramadol according to randomization. There were two dropouts in the lornoxicam group and five in the tramadol group. There were 38 patients to be analyzed as per protocol at the end of the study. The groups were comparable at baseline regarding age, sex and VAS score at entry.
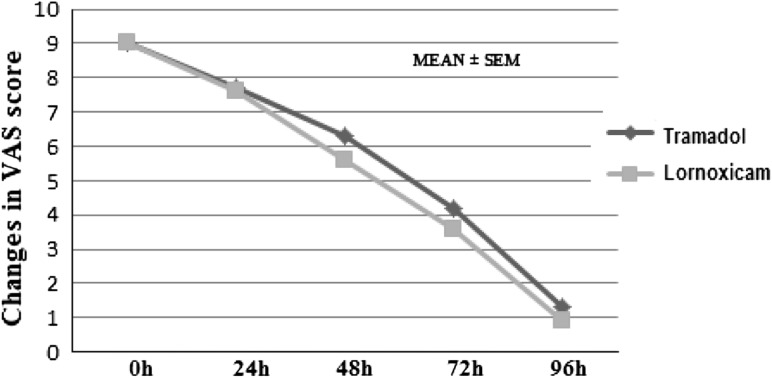
The serial changes in VAS pain score in the two study groups is shown in Table 1. Within group comparisons of VAS pain score was done by Kruskal–Wallis ANOVA followed by post hoc Dunn’s test. There was steady decline in pain intensity from baseline to end of study indicating good analgesic efficacy in both groups. Between groups comparison of VAS score by Kruskal–Wallis ANOVA showed no significant difference at any time point (p > 0.05).
Table 1.
VAS pain score in the two groups (Mean ± SEM)
| Lornoxicam (n = 20) | Tramadol (n = 18) | p value | |
|---|---|---|---|
| Baseline | 9.0 ± 0.14 | 9.0 ± 0.13 | ns |
| 1st follow-up | 7.6 ± 0.14 | 7.7 ± 0.11 | ns |
| 2nd follow-up | 5.6 ± 0.14 | 6.3 ± 0.11 | ns |
| 3rd follow-up | 3.6 ± 0.14 | 4.2 ± 0.15 | ns |
| Study end | 0.9 ± 0.17 | 1.3 ± 0.23 | ns |
p value is from Kruskal–Wallis ANOVA
ns not significant
VAS scoring between the groups in the post- operative period when compared is shown in Fig. 1.
Fig. 1.
Comparison of VAS pain score between lornoxicam and tramadol treated groups at different time intervals
Table 2 shows the distribution of wound tenderness in the two groups. Between groups comparisons of wound tenderness by Kruskal–Wallis ANOVA also showed no significant difference either at baseline or at study end (p > 0.05).
Table 2.
Distribution of wound tenderness in the two groups
| Baseline | V1 | V2 | V3 | V4 | Study end | |
|---|---|---|---|---|---|---|
| Lornoxicam (n = 20) | ||||||
| Non-tender | 0 | 0 | 0 | 0 | 0 | 2 (10 %) |
| Tender | 0 | 1 (5 %) | 11 (55 %) | 12 (60 %) | 15 (75 %) | 18 (90 %) |
| Highly tender | 20 (100 %) | 19 (95 %) | 9 (45 %) | 8 (40 %) | 5 (25 %) | 0 |
| Tramadol (n = 18) | ||||||
| Non-tender | 0 | 0 | 0 | 0 | 0 | 2 (11 %) |
| Tender | 0 | 2 (11 %) | 5 (27 %) | 7 (38 %) | 11 (61 %) | 16 (88 %) |
| Highly tender | 18 (100 %) | 16 (88 %) | 13 (72 %) | 11 (61 %) | 7 (38 %) | 0 |
Difference between groups was not statistically significant at any time point by Kruskal–Wallis ANOVA test
Five patients on lornoxicam and one patient on tramadol experienced at least 50 % pain relief at 48 h compared to baseline. Five patients from the lornoxicam group and eight from the tramadol group required paracetamol 500 mg tablets as rescue medicine. However there was no significant difference noted between the groups in terms of secondary efficacy parameters.
Thus, both the drugs were effective in providing relief in post-operative pain and there was no statistically detectable difference of efficacy in between them.
Commonly occurring adverse events were nausea and abdominal discomfort (Table 3). A total of two subjects in lornoxicam group and five in tramadol group experienced adverse events for which they were excluded from the study and maintained on paracetamol tablets for the rest of their stay in hospital. Vital signs and laboratory parameters at the study end did not show any significant deviations from baseline values in either group.
Table 3.
Distribution of adverse events in the study groups
| Adverse events | Lornoxicam (n = 20) | Tramadol (n = 18) | p value |
|---|---|---|---|
| Nausea | 1 | 1 | ns |
| Vomiting | None | 1 | ns |
| Epigastric pain | 1 | 1 | ns |
| Dizziness | None | None | ns |
| Constipation | None | 2 | ns |
| Drowsiness | None | None | ns |
All values represent number of patients. p value is from Fisher’s Exact Probability test
ns not significant (p > 0.05)
The tolerability of lornoxicam appeared to be significantly superior to tramadol, with less number of patients experiencing adverse drug reactions.
Between groups comparison of CGI of efficacy and safety as assessed by the physician at the end of the study did not show any significant difference (p > 0.05). Also no significant difference could be detected between the groups in patient’s assessment of efficacy and acceptability of treatment (p > 0.05).
Discussion
Pain as defined by the International Association for the Study of Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Early and effective relief from pain in post-operative period is of increasing importance in order to improve patient comfort and to restore patient’s daily function as early as possible. Choice of analgesics in the post-operative period should be guided by an individual’s needs. Treatment of pain in patients after operations on head and neck with a nonopiate analgesic allows close monitoring of the patient’s neurologic and mental conditions and thus NSAIDs are preferred in these situations [10].
NSAIDs do not induce respiratory depression or sedation which allows less intensive post-operative monitoring. However the risk of gastrointestinal disturbances, impaired coagulation, and reduced renal function may limit the use of NSAIDs in some patients [11]. Lornoxicam, an NSAID, combines the high therapeutic potency of oxicams with an improved gastrointestinal toxicity profile as compared to other drugs of this class. This is probably due to the short half-life of lornoxicam (3–5 h) as compared to the other oxicams [12]. It is eliminated following biotransformation to 5-hydroxy-lornoxicam, which does not undergo enterohepatic recirculation. Conjugated metabolites are excreted in urine and faeces with a half-life of about 11 h [13].
There are clinical trials that document the efficacy of lornoxicam as a potent analgesic with excellent antiinflammatory properties in a range of painful and/or inflammatory conditions, including postoperative pain and low back pain [14, 15]. Lornoxicam has also been shown to be effective as pre-emptive analgesic in patients undergoing abdominal operations [16].
Tramadol is an opioid analgesic used for analgesia in post-operative pain. Although nausea, vomiting, constipation, drowsiness, confusion are some common adverse effects with tramadol, it produces fewer typical opioid adverse effects such as respiratory depression and withdrawal symptoms [17].
In our study, there was no significant difference in VAS pain score when compared between the groups at any time point. But within group analysis of VAS pain score in both the groups showed significant reduction in pain score from baseline to end of study visit.
One interesting finding was that there was higher percentage of patients achieving 50 % pain relief at 48 h in lornoxicam group that may be attributed to its early onset of action. This is of advantage with use of lornoxicam as early pain relief results in better patient compliance. Again there was increased use of rescue medication in tramadol group than lornoxicam group. But the trends in these secondary efficacy parameters were not statistically significant.
In this study, the number of patients who dropped out due to adverse effects was less in the lornoxicam group than those in the tramadol group. This is at par with studies which has proved lornoxicam to be safe in both short-term and long-term management of pain [18, 19]. The study subjects showed satisfactory compliance in either group. The limitation of our study is that the subjects were not followed up after discontinuation of the drugs for any delayed adverse events or ‘carry over’ effect.
In terms of average cost of therapy, though lornoxicam scores over tramadol marginally this would hardly be translated into significant cost effectiveness as the duration of such therapy was very short (5 days).
In conclusion, lornoxicam administered by intramuscular route, followed by oral route is well tolerated and comparable to tramadol for relieving postoperative pain after operations on head and neck. The need of rescue medication for breakthrough pain may be less with the former. Lornoxicam may thus be safely used as a short-term analgesic for relieving pain after operations on head and neck.
References
- 1.Burke A, Smyth E, Fitzgerald GA (2006). Analgesic-antipyretic agents; pharmacotherapy of gout. In: Brunton LL, Lazo JS, Parker KL (eds) Goodman & Gillman’s the pharmacological basis of therapeutics, 3rd edn. McGraw –Hill, pp 701
- 2.Sener M, Yilmazer C, Bozdogan N, Ozer C, Donmez A, Arslan G. Efficacy of lornoxicam for acute postoperative pain relief after septoplasty: a comparison with diclofenac, ketoprofen and dipyrone. J Clin Anaesth. 2008;20(2):103–108. doi: 10.1016/j.jclinane.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Frizziero L, Focherini MC, Valentini M, Reta M, Rocchi P. Long term study on the efficacy and safety of lornoxicam in rheumatoid arthritis. Minerva Med. 2002;93(4):315–320. [PubMed] [Google Scholar]
- 4.Ali M, Khan FA. Comparison of analgesic effect of tramadol alone and a combination of tramadol and paracetamol in day-care laparoscopic surgery. Eur J Anaesthesiol. 2009;26(6):475–479. doi: 10.1097/EJA.0b013e328324b747. [DOI] [PubMed] [Google Scholar]
- 5.Scott LJ, Perry CM. Tramadol: a review of its use in postoperative pain. Drugs. 2000;60:139–176. doi: 10.2165/00003495-200060010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Vickers MD, O’ Flaherty D, Szekely SM, Read M, Yoshizumi J. Tramadol: pain relief by an opioid without depression of respiration. J Assoc Anaesth Great Br Irel. 2007;47:291–296. doi: 10.1111/j.1365-2044.1992.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 7.Staunstrup H, Ovesen J, Larsen UT, Elbaek K, Larsen U, Kroner K. Efficacy and tolerability of lornoxicam versus tramadol in postoperative pain. J Clin Pharmacol. 1999;39(8):834–841. doi: 10.1177/00912709922008362. [DOI] [PubMed] [Google Scholar]
- 8.Fischer S, Troidl H, MacLean AA, Koehler L, Paul A. Prospective double-blind randomized study of a new regimen of pre-emptive analgesia for inguinal hernia repair: evaluation of postoperative pain score. Eur J Surg. 2000;166(7):545–551. doi: 10.1080/110241500750008619. [DOI] [PubMed] [Google Scholar]
- 9.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–207. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil Z, Smith DB, Marouani N, Khafif A, Fliss DM. Treatment of pain after head and neck surgeries: control of acute pain after head and neck oncological surgeries. Otolaryngology–Head Neck Surg. 2006;135:182–188. doi: 10.1016/j.otohns.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Analgesics Anti-inflammatory Drugs and Antipyretics (2009) In : Sweetman SC (ed) Martindale the complete drug reference, 36th edn. Pharmaceutical Press, London pp 4
- 12.Radhofer-Welte S, Rabasseda X. Lornoxicam, a new potent NSAID with an improved tolerability profile. Drugs Today. 2000;36(1):55. doi: 10.1358/dot.2000.36.1.566627. [DOI] [PubMed] [Google Scholar]
- 13.Skjodt NM, Davies NM. Clinical pharmacokinetics of lornoxicam: a short half-life oxicam. Clin Pharmacokinet. 1998;34(6):421–428. doi: 10.2165/00003088-199834060-00001. [DOI] [PubMed] [Google Scholar]
- 14.Yakhno N, Guekht A, Skoromets A, et al. Analgesic efficacy and safety of lornoxicam quick-release formulation compared With diclofenac potassium. Clin Drug Investig. 2006;26(5):267–277. doi: 10.2165/00044011-200626050-00004. [DOI] [PubMed] [Google Scholar]
- 15.Narinder R, Karsten K, Marija SM, Charlotte H, Rudolf L. Safety of lornoxicam in the treatment of postoperative pain: a post-marketing study of analgesic regimens containing lornoxicam compared with standard analgesic treatment in 3752 day—case surgery patients. Clin Drug Investig. 2010;30(10):687–697. doi: 10.2165/11538860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Karaman S, Gunusen I, Uyar M, Firat V. The effect of pre-operative lornoxicam and ketoprofen application on the morphine consumption of post-operative patient-controlled analgesia. J Int Med Res. 2006;34(2):168–175. doi: 10.1177/147323000603400206. [DOI] [PubMed] [Google Scholar]
- 17.Analgesics Anti-inflammatory Drugs and Antipyretics (2009). In: Sweetman SC (ed) Martindale the complete drug reference, 36th edn. Pharmaceutical Press, London, pp 131
- 18.Herrmann WA, Geertsen MS. Efficacy and safety of lornoxicam compared with placebo and diclofenac in acute sciatica/lumbo-sciatica: an analysis from a randomised, double-blind, multicentre, parallel-group study. Int J Clin Pract. 2009;63(11):1613–1621. doi: 10.1111/j.1742-1241.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 19.Norholt SE, sindet-Pedersen S, Larsen U, et al. Pain control after dental surgery: a double-blind, randomised trial of lornoxicam versus morphine. Pain. 1996;67:335–343. doi: 10.1016/0304-3959(96)03126-0. [DOI] [PubMed] [Google Scholar]