Abstract
Nasopharyngeal carcinoma (NPC) accounts for less than 1 % of all paediatric cancers. Advances in imaging, radiation delivery, chemotherapy and improvement in multidisciplinary care have resulted in drastic changes in the outcome of this disease. Since NPC is extremely rare in paediatric population and there is paucity of data in Indian patients, we conducted the present study to analyze the outcome of paediatric NPC. Eighteen patients aged 18 years or less, who had pathologically proven diagnosis of NPC and had received treatment at our institute from 2003 to 2010 was included for analysis. These patients were treated with radiotherapy and chemotherapy. Event free survival, local and systemic relapse and progression were analysed. These outcomes were correlated with age, radiation dose and the stage of disease. Majority of patients presented with advanced stage and poor histological grade (94.4 % had WHO Type III disease). The median follow up was 38 months (range 3–91 months). The estimated event free survival at a follow up of 5 years was 64.28 %. Distant metastasis at presentation and lower radiation dose were predictive of poor outcome. Paediatric NPC usually presents in an advanced stage. Our results are comparable to studies which have used only chemoradiotherapy, are far inferior to studies which have used chemoradiotherapy and adjuvant immunotherapy. Larger prospective studies are required to define the role of adjuvant immunotherapy, the optimum dose and schedule of chemoradiotherapy and role of new agents like cetuximab in Indian paediatric patients.
Keywords: Nasopharyngeal carcinoma, Chemotherapy, Event free survival, Radiotherapy
Introduction
Nasopharyngeal carcinoma (NPC) is a common cancer of head and neck region in adults. It is endemic in South East Asian countries. Paediatric NPC is different from its adult counterpart. The occurrence of this tumor in children is very rare; only about 3 % of all NPC occur in patients younger than 18 years [1]. Paediatric NPC is biologically more aggressive (WHO grade III) and most often presents as an advanced locoregional disease. Treatment of paediatric NPC has seen a huge wave of change: initially in the early 1990 irradiation alone was considered curative [2], combination of chemotherapy and radiotherapy became the standard of care in the late 1990 and since the dawn of the new millennium chemo-radiotherapy followed by adjuvant immunotherapy using beta interferon has become the benchmark treatment [3, 4].
Since NPC is extremely rare in paediatric population and there is paucity of data in Indian patients, we conducted the present retrospective study to analyze the demographic profile, clinical features and outcome of paediatric NPC treated with chemotherapy and radiotherapy.
Materials and Methods
One thousand one hundred and twenty five patients were diagnosed to have NPC during 2003–2010 at our institute. Amongst these patients we identified 18 patients aged 18 years or less and were included in this study. Patients who received treatment with palliative intent which included three patients with metastatic disease and one with locally advanced disease having poor performance status were reviewed for demographic profile but were excluded from survival analysis. The case records of these 18 patients were analysed in detail for demographic profile, clinical features, imaging, treatment and the outcome.
The diagnosis was established on the basis of histopathological examination and immunohistochemistry (IHC). IHC was done to differentiate NPC from other common tumors like lymphoma and rhabdomyosarcoma. Local extent of disease was evaluated by clinical and radiographic studies (CT or MRI). Metastatic work up included bone scan, bone marrow examination, chest X-ray and abdominal ultrasound.
The patients were staged according to the TNM staging system and the WHO criteria were used for grading. Chemotherapy was given in the neoadjuvant or adjuvant setting. The regimen consisted 3 cycles of cisplatin 100 mg/m2 on day 1 and 5-fluorouracil 1,000 mg/m2 per day from day 2 to 5 day every 21 days. Patients with metastatic disease received three cycles of chemotherapy upfront, followed by radiotherapy. Patients with non metastatic disease received radiotherapy followed by three cycles of adjuvant chemotherapy. (Tables 1, 2).
Table 1.
Stage distribution of the patients with paediatric nasopharyngeal carcinoma
| Presentation | Number of cases (%) |
|---|---|
| Age | |
| <14 | 6 (33.3) |
| >14 | 12 (66.7) |
| Sex | |
| Male | 14 (77.8) |
| Female | 4 (22.2) |
| Clinical features | |
| Neck swelling | 15 (83.3) |
| Nasal obstruction | 11 (61.1) |
| Epistaxis | 6 (33.3) |
| Headache | 5 (27.7) |
| Eye symptoms | 3 (16.6) |
| Ear symptoms | 2 (11.1) |
| Dysphagia | 1 (5.6) |
| Anti TB drug prior diagnosis | 4 (22.2) |
| WHO type | |
| I | 0 |
| II | 1 (5.6) |
| III | 17 (94.4) |
| Site of metastasis | |
| Bone | 3 |
| Liver | 1 |
| Lung | 1 |
| Bone marrow | 1 |
| Treatment | |
| Induction chemo | 6 |
| Adjuvant chemo | 8 |
| Palliative treatment | 4 |
Table 2.
Distribution of children with nasopharyngeal carcinoma by stage
| Stage | % | |
|---|---|---|
| Stage I | 0 | 0 |
| Stage II | 5.5 | 1 |
| Stage III | 22.2 | 4 |
| Stage IV | 72.3 | 13 |
Radiotherapy was given using conventional technique every 5 days a week. The patients were followed up every 3 monthly for 3 years and 6 monthly thereafter. Progression was defined as appearance of new lesion or increase in size of the primary lesion. Relapse and progression were considered as events. The outcome was correlated with age (less than or more than 14 years), radiation dose (less than or more than 66 Gy) and the stage of disease. The event free survival (EFS) was evaluated using the Kaplan–Meier curve (SPSS 19—SPSS Inc., USA).
Results
This retrospective study included 18 paediatric NPC patients treated at our institution, which formed 1.6 % of all nasopharyngeal cancers. The characteristics of patients are described in table I and II. The median age at presentation was 15.3 years (range 11–18 years).The male: female ratio was 3.5:1. The most common presenting symptom was neck swelling followed by nasal obstruction and epistaxis. 4 patients were wrongly diagnosed as cervical tuberculosis and had received ATT prior to admission at our centre.
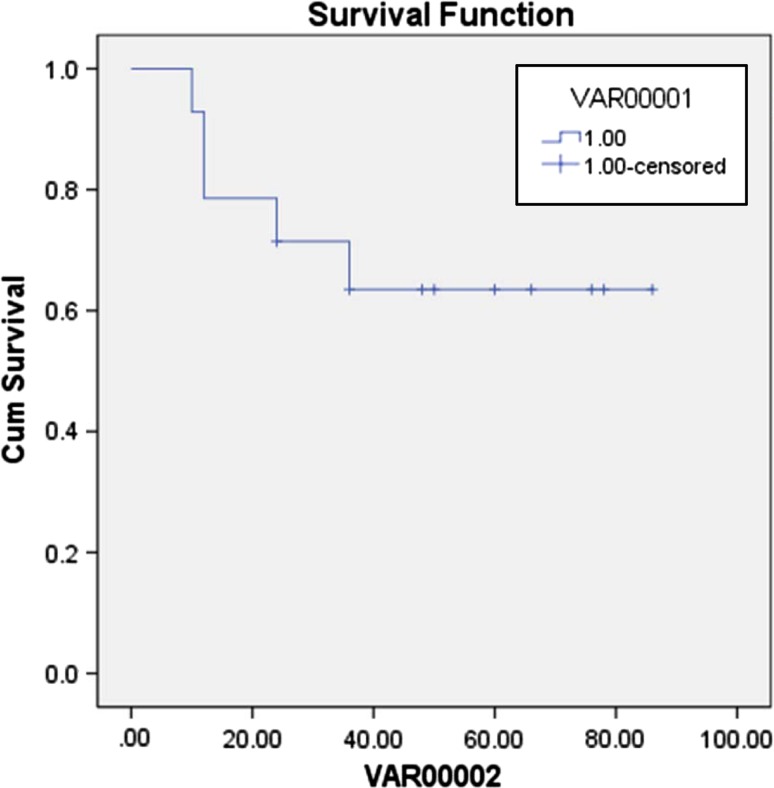
Most of the patients (72.2 %) had stage IV disease at presentation. 6 patients (33 %) presented with metastasis. Bone was the commonest site of metastasis. All except one patient had WHO Type III histology. Six patients received induction chemotherapy followed by radiotherapy and others received radiotherapy followed by adjuvant chemotherapy. The mean dose of radiation given was 70.5 Gy (46–86 Gy) administered as 1.8–2 Gy daily fraction 5 days a week. Mucositis and myelosuppression were the most common complications, observed in 12 patients each. Nephrotoxicity was observed in one patient. The estimated event free survival (EFS) at a follow up of 5 years was 64.28 % (Fig. 1). A total of 5 events (progression of disease in three and relapse in two patients) occurred in as many patients. Of the 2 patients having a relapse, one had local recurrence and one had distant skeletal metastases.
Fig. 1.
Event free survival
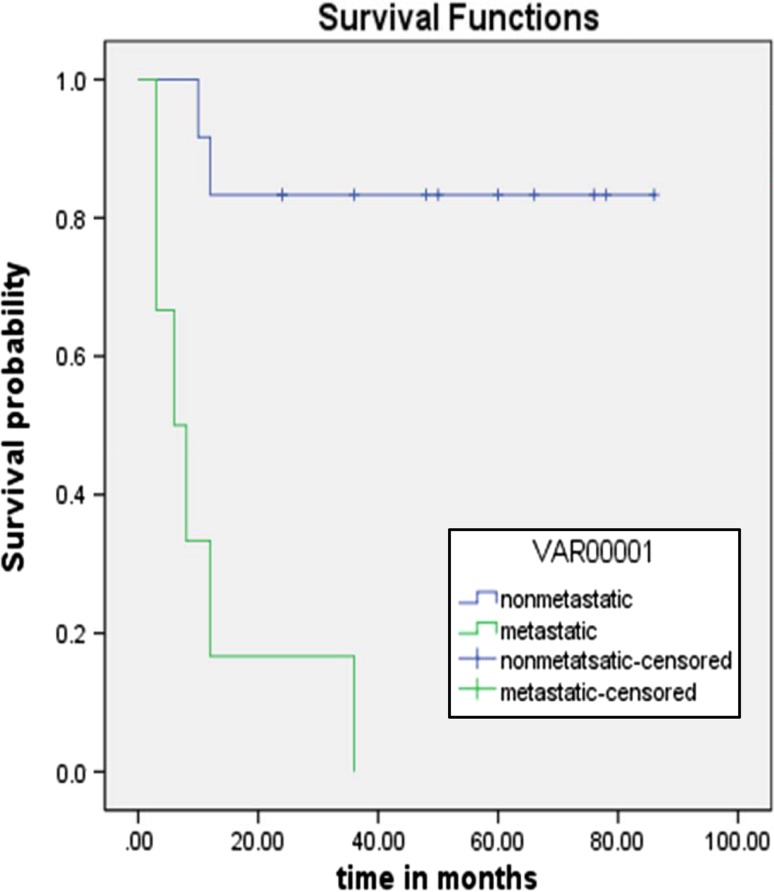
Metastatic disease at presentation was a significant poor prognostic marker (p < 0.001). (Fig. 2) Age did not have significant impact on outcome (p = 0.9). Higher radiation dose also significantly correlated with better outcome (p = 0.007), only one out of 5 patients who had received a radiation dose of <66 Gy were in clinical and radiologic remission at their last follow, while eight out of nine with higher RT dose were in remission.
Fig. 2.
Outcome of disease (metastatic vs. non metastatic)
Discussion
At our institute, paediatric NPC accounted for 1.6 % of all NPC. The most common histological variant was the undifferentiated carcinoma (WHO Type III). The incidence of distant metastasis at presentation, which was 33 % in our series, is similar to world series [5]. All recurrences/relapse were noted within 3 year of diagnosis, this was also comparable to the global data [6].
In a study conducted by paediatric oncology group, which used induction chemotherapy followed by radiotherapy the 4 year EFS was 77 %. [7] Ozyar et al. [8] had reported EFS of 68 % at 5 years in a Rare Cancer Network study involving 8 different countries. In our study the EFS was 62 %, which is shown in figure. In our study metastatic disease had significantly poor prognostic outcome (p < 0.001). This finding was similar to other studies [9–12]. Significant survival advantage was seen in patients receiving higher total radiotherapy dose (>66 Gy), which was similar to other studies [13, 14]. Higher dose of radiotherapy was also associated with increased treatment related toxicity. The common acute toxicities were mucositis, xerostomia and myelosuppression.
The major limitations of our study are the small number of patients, a short follow up, lack of use of concomitant chemo radiotherapy, lack of use of intensity modulated radiotherapy (IMRT) and immunotherapy with interferon-beta after chemotherapy and radiotherapy. Improved outcome and reduced toxicity could have been achieved by use of IMRT which provides superior target coverage and spares the normal tissue [15]. Immunotherapy as per NPC-91-GPOH protocol (Society of Paediatric Oncology and Haematology) which is considered as the standard of care for NPC and achieves very high survival rates, was not used in our study due to financial constraints. We used induction or adjuvant chemotherapy in our study, present studies have shown that concomitant use of chemotherapy with radiotherapy improves overall survival and reduces recurrence [16].
Conclusion
Paediatric NPC usually presents in an advanced stage. A larger prospective studies are required to define the role of adjuvant immunotherapy, optimum dose and schedule of chemotherapy and radiotherapy in pediatric patients and their late toxicities. The role of new agents like gemcitabine, cetuximab in this age group in relapse setting needs to be explored.
References
- 1.Spano JP, Busson P, Atlan D, et al. Nasopharyngeal carcinomas: an update. Eur J Cancer. 2003;39:2121–2135. doi: 10.1016/S0959-8049(03)00367-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee AW, Law SC, Foo W, Poon YF, Chan DK, O SK, Tung SY, Cheung FK, Thaw M, Ho JH. Nasopharyngeal carcinoma: local control by megavoltage irradiation. Br J Radiol. 1993;66(786):528–536. doi: 10.1259/0007-1285-66-786-528. [DOI] [PubMed] [Google Scholar]
- 3.Wolff HA, Rodel RMW, Gunawan B, Overbeck T, Herrmann MKA, Hennies S, et al. Nasopharyngeal carcinoma in adults: treatment results after long-term follow-up with special reference to adjuvant interferonbeta in undifferentiated carcinomas. J Cancer Res Clin Oncol. 2010;136:89–97. doi: 10.1007/s00432-009-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens R, Granzen B, Lassay L, Bucsky P, Hundgen M, Stetter G, Heimann G, Weiss C, Hess CF, Gademann G. Treatment of nasopharyngeal carcinoma in children and adolescents: definitive results of a multicenter study (NPC-91-GPOH) Cancer. 2005;104:1083–1089. doi: 10.1002/cncr.21258. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad A, Stefani S. Distant metastases of nasopharyngeal carcinoma: a study of 256 male patients. J Surg Oncol. 1986;33(3):194–197. doi: 10.1002/jso.2930330310. [DOI] [PubMed] [Google Scholar]
- 6.Li J-X, Lu T-X, Ying H, Fei H. Clinical characteristics of recurrent nasopharyngeal carcinoma in high-incidence area. Sci World J. 2012;2012:8. doi: 10.1100/2012/719754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Galindo C, Wofford M, Castleberry RP, et al. Preradiation chemotherapy with methotrexate, cisplatin, 5-fluorouracil, and leucovarin for paediatric nasopharyngeal carcinoma. Cancer. 2005;103:850–857. doi: 10.1002/cncr.20823. [DOI] [PubMed] [Google Scholar]
- 8.Ozyar E, Selek U, Laskar S, et al. Treatment results of 165 paediatric patients with non-metastatic nasopharyngeal carcinoma: a rare cancer network study. Radiother Oncol. 2006;81:39–46. doi: 10.1016/j.radonc.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Vokes EE, Liebowitz DN, Weichselbaum RR. Nasopharyngeal carcinoma. Lancet. 1997;350:1087–1091. doi: 10.1016/S0140-6736(97)07269-3. [DOI] [PubMed] [Google Scholar]
- 10.Ayan I, Altun M. Nasopharyngeal carcinoma in children: retrospective review of 50 patients. Int J Radiat Oncol Biol Phys. 1996;35:485–492. doi: 10.1016/S0360-3016(96)80010-1. [DOI] [PubMed] [Google Scholar]
- 11.Cvitkovic E, Bachouchi M, Armand JP. Nasopharyngeal carcinoma: biology, natural history and therapeutic implications. Hematol Oncol Clin North Am. 1991;5:821–823. [PubMed] [Google Scholar]
- 12.Fandi A, Bachouchi M, Azli N, et al. Long term disease free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol. 2000;18:1324–1330. doi: 10.1200/JCO.2000.18.6.1324. [DOI] [PubMed] [Google Scholar]
- 13.Ingersoll L, Woo SY, Donaldson S, Giesler J, Maor MH, Goffinet D, Cangir A, Goepfert H, Oswald MJ, Peters LJ. Nasopharyngeal carcinoma in the young: a combined M.D. Anderson and Stanford experience. Int J Radiat Oncol Biol Phys. 1990;19(4):881–887. doi: 10.1016/0360-3016(90)90008-8. [DOI] [PubMed] [Google Scholar]
- 14.Laskar S, Vimal S, Muckaden MA, Laskar S, Bhalla V, Banavali S, Kurkure P, Nair CN, Dinshaw KA. Nasopharyngeal carcinoma in children: ten years experience at the tata memorial hospital, Mumbai. Int. J of Radiat Oncol Biol Phys. 2004;58(1):189–195. doi: 10.1016/S0360-3016(03)00773-9. [DOI] [PubMed] [Google Scholar]
- 15.Laskar S, Bahl G, Muckaden MA, et al. Nasopharyngeal carcinoma in children: comparison of conventional and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(3):728–736. doi: 10.1016/j.ijrobp.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Langendijk JA, Leemans ChR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol. 2004;22(22):4604–4612. doi: 10.1200/JCO.2004.10.074. [DOI] [PubMed] [Google Scholar]