Abstract
Our work is motivated by the observation that rare, broadly neutralizing antibodies (NAbs), 4E10 and 2F5, associate with HIV-1 lipids as part of a required first step in neutralization before binding to membrane-proximal antigens. Subsequently, induction of these types of NAbs may be limited by immunologic tolerance due to autoreactivity with host cell membranes. Despite the significance of this lipid reactivity there is little experimental evidence detailing NAb-membrane interactions. Simple and efficient screening assays are needed to select antibodies that have similar lipid reactivity as known NAbs. To this end we have developed a surface plasmon resonance (SPR) spectroscopy based assay that monitors antibody binding to thiol self-assembled monolayers (SAMs) that replicate salient lipid surface chemistries and NAb binding to lipid surfaces. Specifically, we probed the relative importance of charge and hydrophobicity on antibody-surface interactions. We found that NAb binding to hydrophobic thiol surfaces was significantly greater than that of control monoclonal antibodies (mAbs). Furthermore, we confirmed the importance of charge-mediated antibody surface interactions, originally suggested by results from mAb interactions with conventional lipid vesicle/bilayer surfaces. Our approach, using self-assembled thiol monolayers that replicate the binding behavior of NAbs on lipid surfaces, thus provides an efficient and useful tool to screen interactions of mAbs and lipid-reactive NAbs.
Keywords: HIV-1, Neutralizing antibody, Thiol model surface, Surface plasmon resonance
1. Introduction
The inability to generate broadly neutralizing antibodies (NAbs) is a major obstacle to the development of a successful HIV-1 vaccine (Burton et al., 2004). One promising vaccine target is the membrane-proximal external region (MPER) of viral envelope (Env) protein, gp41. The gp41 MPER is a highly conserved region across diverse HIV-1 strains, which when bound by NAbs, effectively prevents viral fusion with the host cell membrane. Nevertheless, MPER is poorly immunogenic due to its transient structure as it is only transiently exposed during viral fusion during a prehairpin extended intermediate state between the prefusion and postfusion conformations (Alam et al., 2007). Moreover, MPER displays poor sterics, limiting accessibility for immune recognition. These limitations, in part, contribute to the rare production of membrane-proximal NAbs during natural HIV-1 infection. Two such NAbs with great strain breadth and neutralizing ability are 2F5 and 4E10. Although their neutralizing mechanism represents a promising framework for the design of new HIV-1 vaccine candidates, the details of this mechanism are only poorly understood.
Recent research has shown that 2F5 and 4E10 bind the virion via a cooperative two phase mechanism in which the NAbs first interact nonspecifically with the viral lipid membrane and then specifically with the target MPER antigen (Alam et al., 2007; Sun et al., 2008). One explanation for this two-stage interaction is that NAb-membrane interactions likely direct high NAb concentrations toward the viral surface, where the NAb's ability to diffuse within the viral membrane could better position it to encounter its sparse MPER antigen. This unique lipid reactivity may help explain the rarity of 4E10 and 2F5, because induction of these types of NAbs may be limited by immunologic tolerance due to autoreactivity with host cell membranes. Antibody-lipid reactivity may also explain why simple peptide immunogens that mimic neutralizing epitopes on HIV-1 Env gp41 do not elicit NAbs in vivo (Bures et al., 2000). This information suggests that peptide sequence is not the sole determinant of neutralizing ability, and this seems to be particularly true for NAbs that target membrane-proximal antigens of gp41 (Ofek et al., 2004). Thus, it appears a successful HIV-1 vaccine may depend on the proper design of immunogens that account for NAb reactivity with conserved Env proteins and the proximal lipid membrane.
Currently, there is large research activity aimed at eliciting 2F5/4E10-like antibodies that maintain their rare neutralizing breadth and efficacy. However, addressing immune tolerance questions and understanding 2F5/4E10's neutralizing mechanism are major challenges to induce these types of membrane-proximal NAbs and surprisingly, little is known about the underlying NAb-membrane binding mechanism. Accordingly, these NAbs have been shown to be polyspecific, having cross-reactivity with autoantigens such as centromere B and negatively charged phospholipids, such as cardiolipin (Haynes et al., 2005; Sanchez-Martinez et al., 2006). It is also postulated that the complementary determining region (CDR)-3 loop of membrane reactive antibodies, particularly 2F5/4E10, may be structurally poised to react with the proximal lipid membrane. The CDR3 loop has been shown to be an exposed region, away from the NAbs binding paratope, and may act to anchor the NAb into the hydrophobic membrane bilayer. Taken together, this structural information and autoreactive properties provide motivation to develop efficient screening methods for membrane reactive monoclonal antibodies (mAbs) and to test the role of membrane charge and hydrophobicity in HIV-1 antibody binding.
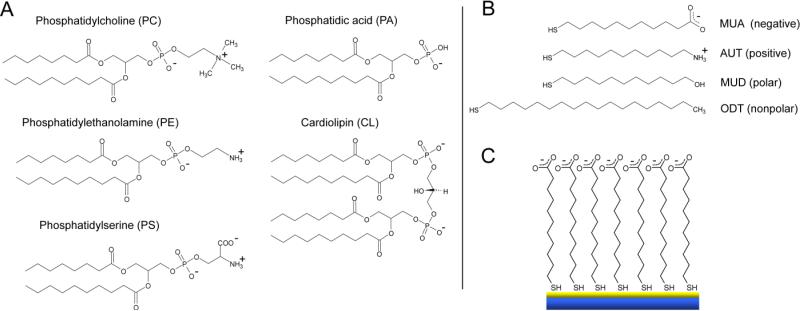
In this work, we present a simple screening platform that chemically mimics viral and host membrane lipids and that replicates NAb membrane interactions. We employ thiol self-assembled monolayers (SAMs) that exhibit a range of chemical functionalities (i.e., variations in hydrophobicity and surface charge) presented on common membrane phospholipids of both the native host CD4+ cells and the HIV-1 virion (Fig. 1). These thiol SAMs (Fig. 1B) present polar (11-mercapto-1-undecanol (MUD)), nonpolar (1-octadecanethiol (ODT)), positively charged (11-amino-1-undecanethiol (AUT)), and negatively charged (11-mercaptoundecanoic acid (MUA)) end-functionality, serving as simple chemical mimics for corresponding lipid head-groups (Fig. 1A). Antibody screening was performed by anchoring the thiol SAMs on custom surface plasmon resonance (SPR) sensor chips (depicted in Fig. 1C).
Fig. 1.
Model thiol SPR system. A. Typical membrane phospholipids, except for cardiolipin. Cardiolipin is most often found in the mitochondria of cells, however, NAbs have been shown to bind the lamellar and reversed hexagonal phases of cardiolipin. For simplicity, the acyl chain for all lipids is shown saturated with an abbreviated chain length. B. ω-substituted alkane thiols with different chemical functionality used to mimic membrane phospholipids. At pH 7.4 thiol monolayers were negatively charged (MUA), positively charged (AUT), polar (MUD), and nonpolar (ODT). C. Schematic of a model 11-mercaptoundecanoic acid (MUA) surface on a gold coated SPR sensor chip.
** Fig. 1 intended for color reproduction on the Web and in print
It is an important and difficult challenge to develop membrane reactive NAbs that selectively target viral lipids and avoid native host cells. Used with surface plasmon resonance (SPR) and other biophysical diagnostic tools, our thiol SAM surfaces may provide insight into required surface chemical functionality that could be mimicked on non-human immunogens. Immunogens comprised with non-human components, such as plant lipids, can potentially present chemical groups that will elicit antibodies with similar 2F5/4E10-membrance reactivity, but yet be different enough in structure to avoid autoreactivity with host cell membranes.
There are many biosensors that effectively probe antibody-membrane interactions. These sensors make use of calorimetry, acoustic (e.g., quartz crystal microbalance), and near-field optical (e.g., surface plasmon resonance (SPR)) assays (see Cooper, 2004 for a review). SPR is a powerful sensing assay in that it can provide information on the specificity, kinetics, and affinity of antibody interactions in a label-free environment. This avoids expensive reagents such as fluorescently labeled compounds and secondary antibodies used in standard ELISAs. Recently, significant progress has been made in developing lipid bilayer systems to be used in biosensor assays, including SPR. These model systems include supported lipid monolayers, tethered lipid bilayers (Wiltschi et al., 2006), pore-spanning bilayers (Im et al., 2010), and polymer supported lipid bilayers, which have all been engineered to screen protein interactions. However, preparation of these planar lipid systems is not always straightforward. For example, the morphology of model membranes formed on surfaces such as the commonly used SPR BIAcore L1 chip, can vary between intact vesicles, a planar lipid bilayer, and even the exposed underlying polymer surface (Cooper, 2004). This variety arises from the complex interplay of the variables that determine whether vesicles rupture to form the desired planar lipid bilayer. Important variables include the temperature, pH, ionic strength and buffer composition, size of the lipid vesicles, lipid transition temperatures, degree of acyl chain saturation, and the presence of cholesterol.
The thiol monolayers prepared here offer a simple alternative to planar lipid bilayers for screening antibody interactions. Thiol surfaces remain stable for months when stored in thiol solution and are economical to produce with high reliability in a large range of screening conditions. Furthermore, the chemical versatility and the well-characterized nature of thiol monolayers also allow distinct chemical groups to be isolated during antibody screening. Our screening platform thus provides an efficient and useful tool not only to study a required step in the mechanism of HIV-1 neutralization, it can also be used to study antibody autoreactivity, especially for newly generated antibodies.
2. Materials and methods
2.1 Antibodies
Human mAb A32 against HIV-1 Env gp120 was the generous gift of James Robinson, Tulane University, New Orleans, LA and was purified as previously described (Scearce and Eisenbarth, 1983). Anti-HIV-1 gp41 (anti-membrane proximal) NAbs 4E10 and 2F5 were purchased from Polymun, Inc., Vienna, Austria. Anti-cardiolipin mAbs IS4 and IS1 were provided by Pojen Chen, University of California, Los Angeles and were derived from an APS patient. Hybridomas were generated as previously described (Zhu et al., 1999). Mouse mAb 13H11 was produced from splenocytes from a mouse immunized with HIV-1 Env oligomer CON-S (Liao et al., 2006), as described (Scearce and Eisenbarth, 1983). P1 was purified using Protein A / Protein G immunoaffinity. All mAbs were purified by affinity chromatography on anti-immunoglobulin columns (Alam et al., 2007).
2.2 Lipid preparation
Palmitoyl oleoyl phosphatidyl serine (POPS) in chloroform (Avanti Polar Lipids) was brought to room temperature for one hour, dried under nitrogen for five minutes, and then dried under vacuum for three hours. The lipid film was reconstituted in 37 °C PBS w/o Ca2+ and Mg2+, pH 7.4 (Gibco Invitrogen, Grand Island, NY), vortexed, sonicated, and extruded 11 times through first a 0.4 μm filter (Whatman, Florham Park, NJ), and then through a 0.1 μm filter (Whatman) (MacDonald et al., 1991). The concentrated lipid solution was then diluted to 0.1 mg/ml in PBS w/o Ca2+ and Mg2+ and vortexed immediately before use. Lipid solutions were used within eight hours after extrusion.
2.3 Model surface preparation
Glass cover slips (VWR) were first cleaned for 30 minutes using a “Piranha” solution (1:3 H2SO4: H2O2) and then rinsed copiously with deionized water and dried under nitrogen. Finally, 5 nm chromium and 45 nm gold were evaporated onto their surface. The coated glass cover slips were sonicated in ethanol before being incubated in 1 mM solutions of mercaptoundecanoic acid (Sigma), mercaptoundecane thiol (Sigma), aminoundecanethiol (Dojindo), or octadecanethiol (Sigma) for at least 12 hours, and up to one month. Before each experiment, slides were sonicated in thiol solution, rinsed with ethanol, and dried with nitrogen. They were then immediately mounted into BIAcore cassettes (BIAcore Inc.), and placed in a BIAcore 3000 or 1000 instrument (BIAcore Inc., Uppsala, Sweden) for SPR measurements.
2.4 Surface plasmon resonance
SPR measurements were performed on a BIAcore 3000 or 1000 instrument. BIAevaluation 3.0 software (BIAcore Inc., Uppsala, Sweden) was used to evaluate the data assuming a bivalent analyte model. For model surfaces, binding of proteins was monitored in real-time at 25°C with a continuous flow of PBS, pH 7.4 (Gibco Invitrogen, Grand Island, NY) at 5 μl/min. For lipid surfaces, POPS liposomes were incubated on a BIAcore SPR L1 chip. A blank in-line reference surface was used to determine non-specific or bulk responses. Bound protein was removed from the liposome sensor surfaces following each cycle of mAb binding by octyl β-D glucopyranoside, and 5 s injections each of 5 mM HCl then 5 mM NaOH.
3. Results & Discussion
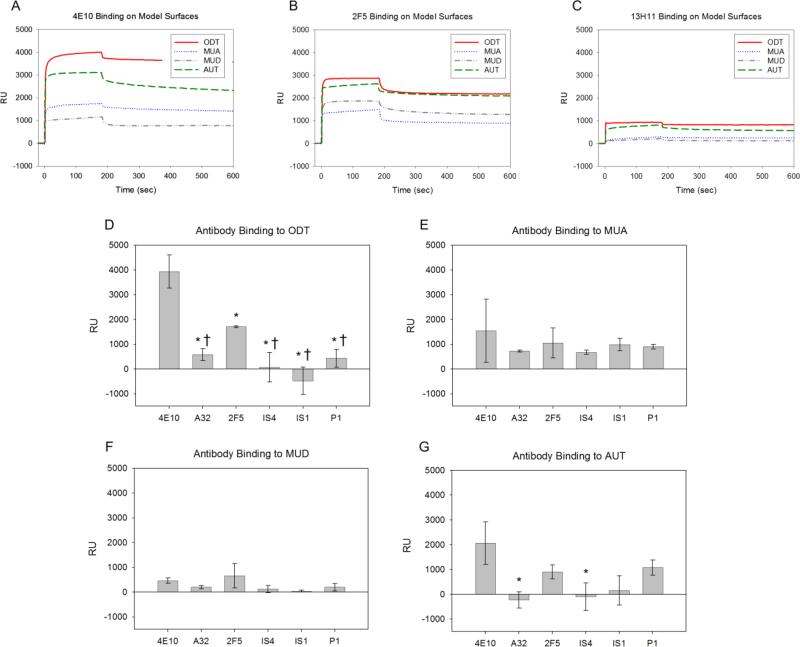
Using SPR spectroscopy, we investigated the binding behavior of a panel of neutralizing (4E10, 2F5), lipid reactive (4E10, 2F5, IS4, IS1, P1), and non lipid reactive nor neutralizing (A32, 13H11) antibodies (Table 1). Antibodies were tested on four model surfaces: negatively charged MUA (contact angle: 33±3°), polar MUD (contact angle: 21±3°), positively charged AUT (contact angle: 60±4°), and nonpolar ODT (contact angle: 104±3°) (Fig. 2).
Table 1.
Panel of screened antibodies and their properties.
| Antibody | Description | Lipid Reactivity | Neutralizing Ability |
|---|---|---|---|
| 4E10 | Human anti-cardiolipin IgG, binds gp41 in MPER | CL, PS, PE, PC, SM (Haynes et al., 2005a) | Broad, potent |
| A32 | Human IgG binds gp120 | None | None |
| 2F5 | Human anti-cardiolipin IgG, binds gp41 in MPER | CL (Haynes et al., 2005a) | Broad, potent |
| 13H11 | Murine mAb binds gp41 in overlapping region of MPER with 2F5. Blocks 2F5 | None | None, control |
| IS4 | Human anti-phospholipid syndrome IgG, | CL (Giles et at., 2005) | None |
| IS1 | Human anti-phospholipid antibody | CL, POPS (Zhu et al., 1999) | Unpublished |
| P1 | Human anti-cardiolipin IgG | CL (personal communication, S.M. Alam) | Unpublished |
Fig. 2.
Antibody-thiol SPR binding response. A-C. Representative SPR response curves for 4E10, 2F5, and 13H11 (non-lipid reactive control) to model surfaces. D-G. Mean RU value from each antibody grouped for each of the four thiols used. Mean values of at least three independent experiments are shown. * Signifies statistically significant (p<0.05) when compared to 4E10. † Signifies statistically significant (p<0.05) when compared to 2F5. The apparent negative amount of bound antibody is due to RU levels below 13H11 background, which was attributed to nonspecific interactions and subtracted from all RU values.
** Fig. 2 intended for color reproduction on the Web and in print
3.1 Antibody screening on thiol model surfaces
Representative SPR curves, in which response units (RU) are plotted as a function of time, are shown in Figures 2A-C. The low levels of 13H11 binding on all surfaces, seen in representative SPR curves (Fig. 2C), was attributed to nonspecific interactions. Although 13H11 shares an epitope on viral Env protein gp41 with 2F5, it is not lipid reactive. Thus, the binding response of 13H11 was subtracted as background from all experiments. The average amount of all antibodies which remained bound following one injection cycle is shown for each of the four model surfaces (Fig. 2D-G). These data demonstrate that our simple thiol SAM surfaces are able to differentiate between NAb binding and binding of other mAbs.
Both 2F5 and 4E10 bound at highest levels to hydrophobic ODT surfaces and had significantly higher binding compared to all other antibodies tested (Figs. 2A-B, and Figs. 2D-G). These results are supported by structural information which shows that the CDR3 region on NAbs 4E10 (EGTTGWGWLGKPIGAFAH) (Zwick et al., 2001) and 2F5 (RRGPTTLFGVPIARGPVNAMDV) (Conley et al., 1994) contain unusually large numbers of hydrophobic and membrane reactive residues (underlined). 4E10's CDR3 also contains zwitterionic tryptophan residues that not only interact with polar head groups but can also embed into the hydrophobic bilayer (Giles et al., 2005). 2F5 bound significantly less on hydrophobic ODT than 4E10, but binding was still significantly elevated relative to all other antibodies. This result is in line with 2F5's smaller lipid reactivity compared to that of 4E10 (Haynes et al., 2005). The relative binding levels of 2F5 and 4E10 on ODT were also similar to binding levels on cardiolipin seen in previous ELISA studies (roughly 1:2) (Haynes et al., 2005).
The SPR screening of NAb 4E10 showed strongest binding on hydrophobic ODT surfaces (Fig. 2D) followed by positively charged AUT (Fig. 2G), and then negatively charged MUA (Fig. 2E). This suggests that if 4E10 is able to penetrate into the membrane bilayer, hydrophobic interactions may dominate its membrane interactions. Furthermore, these results indicate that charge interactions likely contribute to lipid binding as well. 4E10's high affinity to anionic cardiolipin demonstrates this potential charge effect (Haynes et al., 2005).
Human mAb A32 had weak binding to all thiol surfaces, with no particular preference for any one model surface. This agrees with the notion that A32 binds a site far from the lipid membrane on viral Env protein gp120, and is not known to be lipid reactive. The lipid reactive CDR3 in IS4 contains positively charged arginine residues (Giles et al., 2005), and during IS4 screening, the highest binding response occurred on negatively charged MUA thiols (Fig. 2D-G). Furthermore, mAb IS1 is known to interact with cardiolipin and POPS, which are both negatively charged (S.M. Alam, unpublished). This apparent preference for negatively charged lipids is recapitulated by IS1 binding most strongly to negative MUA thiols. mAb P1 interacts with negatively charged cardiolipin, which is also recapitulated by comparable levels of binding to negatively charged thiols. These antibody-binding observations are consistent with a charge-based interaction mechanism.
These results demonstrate that simple, well-characterized self-assembled thiol monolayers on gold present a surface chemistry that is capable to distinguish between broadly neutralizing and control antibodies as well as replicate charge-based interactions between antibodies and lipids.
3.2 Antibody binding on lipid versus thiol model surfaces
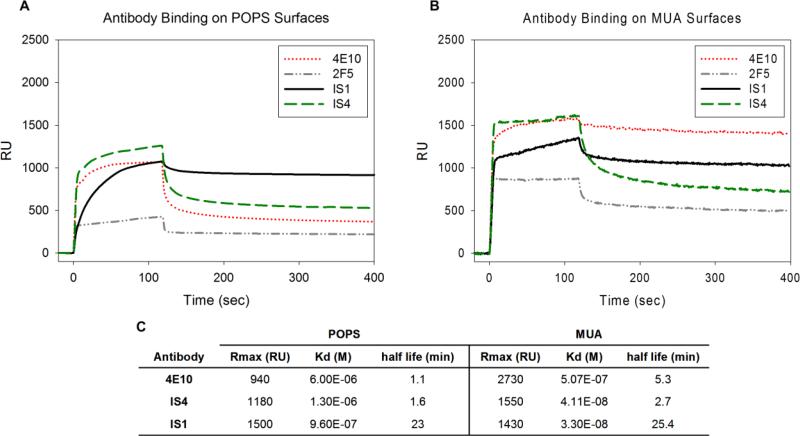
To further investigate the binding behavior of our antibodies, we prepared lipid surfaces using POPS liposomes on a commercially available L1 chip (BIAcore, Sweden). Notably the topography of these lipid surfaces is not clearly defined, possibly presenting a vesicular surface and/or planar lipid bilayer (Cooper, 2004). We monitored binding of 4E10, 2F5, IS4, and IS1 to this more physiologically relevant POPS lipid surface (Fig. 3A) to compare with the binding behavior on the model MUA thiol surface (Fig. 3B). All kinetic data (Fig. 3C) was fitted using a bivalent analyte model to incorporate the bivalent nature of antibody binding. 2F5 binding to POPS was below baseline behavior (similar to A32 and 13H11 binding), preventing the accurate calculation of kinetic data.
Fig. 3.
4E10, 2F5, IS1, and IS4 binding on standard lipid surfaces versus thiol model surfaces. A. Representative SPR response curves for antibodies on negative POPS. B. Representative SPR response curves for antibodies on negative MUA. C. Kinetic properties of antibodies binding on lipid (POPS) and thiol (MUA) model surfaces. Analysis for 2F5 was not performed due to below background binding of 2F5 on POPS.
The MUA surface packing density is approximately three times greater than that of POPS surfaces due to the tightly packed thiol groups (Dubois et al., 1992; Hauser et al., 1981). We thus expect that densely packed thiol surfaces also exhibit a higher surface charge density compared to that of the lipid surfaces. Specifically, a charge-based interaction is likely for mAbs IS1 and IS4, which have a positively charged membrane-reactive region, and could thus explain why MUA surfaces had overall slightly higher antibody binding compared with the lipid surfaces. Importantly, however, the binding order of IS1, IS4, and 2F5 was consistent between the thiol and lipid surfaces, with IS1 binding the highest followed by IS4 and 2F5, respectively. Furthermore, the half-life of IS1 and IS4 correlated well between the POPS and MUA surface. These results are consistent with known antibody properties. For example, IS1 and IS4 bind strongly to negatively charged cardiolipin. Additionally, 2F5 is known to interact only weakly with POPS (Haynes et al., 2005), and bound at lowest levels on both POPS and MUA surfaces. On MUA, 4E10 bound at relatively higher levels than IS1. While 4E10 and IS1 reached an equivalent average steady state association on POPS, 4E10 exhibited much larger variability on the MUA surface (Fig. 2E). Taken together, these observations suggest that the interaction characteristics of NAbs and mAbs with both the thiol and lipid surfaces are largely conserved.
4. Conclusions
The thiol SAM model surfaces used here admittedly lack many characteristics of the complex lipid bilayer including, membrane proteins, lipid heterogeneity, and membrane mobility. Nevertheless, by simply mimicking lipid chemistry, these thiol SAMs allowed us to isolate and distinguish chemical groups that could potentially contribute to specific antibody-lipid interactions. Our results showed that only 2F5 and 4E10 bound strongly to hydrophobic thiols. This observation correlates with findings that suggest that 2F5 and 4E10 embed into the hydrophobic membrane core. This NAb-lipid interaction could then facilitate NAb's ability to diffuse within the viral membrane, positioning the NAb to more likely encounter its sparse MPER antigen. We note, however, that the NAb's high affinity for hydrophobic ODT could be attributed to denaturation of the NAb on the SAM surface which may not occur at the membrane interface. Regardless, this information translates to vaccine design by suggesting that immunogens designed to elicit 2F5/4E10-like antibodies may require an accessible hydrophobic component available for B-cell receptor recognition.
The hydrophobic ODT surface clearly distinguished between neutralizing and non-neutralizing antibodies, both lipid reactive and non-lipid reactive, and thus is promising as a proficient screening platform for broadly neutralizing mAbs that are designed to replicate 2F5 and 4E10's neutralizing breadth and efficacy. If these antibodies have similar binding interaction to ODT thiol surfaces as 2F5/4E10, then they may also possess the same broadly neutralizing ability as 4E10/2F5. In order to determine if specific chemical groups have cooperative effects, the complexity of the model system can be increased by creating mixed monolayer systems or by introducing different end-functionalized thiols. Peptides specific for 4E10 or 2F5 could also be incorporated into the model surface system via covalent attachment.
In conclusion, the surface chemical diversity that can be achieved with thiol SAMs provides a useful and simple platform for screening a wide variety of monoclonal antibodies for 4E10/2F5-like lipid reactivity. These model surfaces also provide a method to infer the importance of different chemical functionalities on NAb-lipid interactions. Such information may contribute to a more complete mechanistic understanding of HIV-1 neutralization as provided by 2F5 and 4E10, and ultimately help guide vaccine design efforts.
Highlights.
Surface plasmon resonance assay that monitors antibody binding to thiol monolayers.
HIV-1 neutralizing antibodies (NAbs) bind to hydrophobic thiols.
Confirmed charge mediated antibody surface interactions.
Suggest NAbs can embed into the hydrophobic lipid membrane core.
Acknowledgements
We thank Dr. J. Robinson (Tulane University) for mAb A32 and Dr. P. Chen (University of California, Los Angeles) for mAbs IS1 and IS4. We also acknowledge Dr. Moses Sekaran for his work with the SPR model surface experiments, and Ms. Alyx Rosen for her work with the SPR POPS experiments. This research was initially supported by the NSF through grant NSF DMR-0239769 CAREER (S.Z.), and the AHA and NIAID (S.M.A.). G.J.H gratefully acknowledges the financial support from The Center for Biomolecular and Tissue Engineering, and the Duke Center for AIDS Research (CFAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178:4424–35. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bures R, Gaitan A, Zhu T, Graziosi C, McGrath KM, Tartaglia J, Caudrelier P, El Habib R, Klein M, Lazzarin A, Stablein DM, Deers M, Corey L, Greenberg ML, Schwartz DH, Montefiori DC. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 2000;16:2019–35. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–6. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- Dubois LH, Nuzzo RG. Synthesis, structure, and properties of model organic-surfaces. Annu Rev Phys Chem. 1992;43:437–63. [Google Scholar]
- Cooper MA. Advances in membrane receptor screening and analysis. J Mol Recognit. 2004;17:286–315. doi: 10.1002/jmr.675. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Kessler JA, 2nd, Boots LJ, Tung JS, Arnold BA, Keller PM, Shaw AR, Emini EA. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc Natl Acad Sci U S A. 1994;91:3348–52. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles I, Lambrianides N, Latchman D, Chen P, Chukwuocha R, Isenberg D, Rahman A. The critical role of arginine residues in the binding of human monoclonal antibodies to cardiolipin. Arthritis Res Ther. 2005;7:R47–56. doi: 10.1186/ar1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H, Pasher I, Pearson RH, Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochem Biophys Acta. 1981;650:21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–8. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Im H, Wittenberg NJ, Lesuffleur A, Lindquist NC, Oh SH. Membrane protein biosensing with plasmonic nanopore arrays and pore-spanning lipid membranes. Chem Sci. 2010;1:688–96. doi: 10.1039/C0SC00365D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho ZT, Ma BJ, Li YY, Decker JM, Nabel GJ, Montefiori DC, Hahn BH, Korber BT, Gao F, Haynes BF. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and CHIV-1 primary viruses. Virology. 2006;353:268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald RC, MacDonald RI, Menco BP, Takeshita K, Subbarao NK, Hu LR. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78:10724–37. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martinez S, Lorizate M, Hermann K, Kunert R, Basanez G, Nieva JL. Specific phospholipid recognition by human immunodeficiency virus type-1 neutralizing anti-gp41 2F5 antibody. FEBS Lett. 2006;580:2395–99. doi: 10.1016/j.febslet.2006.03.067. [DOI] [PubMed] [Google Scholar]
- Scearce RM, Eisenbarth GS. Production of monoclonal antibodies reacting with the cytoplasm and surface of differentiated cells. Methods Enzymol. 1983;103:459–69. doi: 10.1016/s0076-6879(83)03032-3. [DOI] [PubMed] [Google Scholar]
- Sun ZY, Oh KJ, Kim M, Yu J, Brusic V, Song L, Qiao Z, Wang JH, Wagner G, Reinherz EL. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28:52–63. doi: 10.1016/j.immuni.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Wiltschi B, Knoll W, Sinner EK. Binding assays with artificial tethered membranes using surface plasmon resonance. Methods. 2006;39:134–46. doi: 10.1016/j.ymeth.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Zhu M, Olee T, Le DT, Roubey RA, Hahn BH, Woods VL, Jr., Chen PP. Characterization of IgG monoclonal anti-cardiolipin/anti-beta2GP1 antibodies from two patients with antiphospholipid syndrome reveals three species of antibodies. Br J Haematol. 1999;105:102–9. [PubMed] [Google Scholar]
- Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]