Ablation of astrocytic laminin disrupted the interaction between vascular smooth muscle cells and astrocytes, down-regulated contractile protein expression, and weakened vascular integrity in deep brain regions, leading to hemorrhage.
Abstract
Astrocytes express laminin and assemble basement membranes (BMs) at their endfeet, which ensheath the cerebrovasculature. The function of astrocytic laminin in cerebrovascular integrity is unknown. We show that ablation of astrocytic laminin by tissue-specific Cre-mediated recombination disrupted endfeet BMs and led to hemorrhage in deep brain regions of adult mice, resembling human hypertensive hemorrhage. The lack of astrocytic laminin led to impaired function of vascular smooth muscle cells (VSMCs), where astrocytes have a closer association with VSMCs in small arterioles, and was associated with hemorrhagic vessels, which exhibited VSMC fragmentation and vascular wall disassembly. Acute disruption of astrocytic laminin in the striatum of adult mice also impaired VSMC function, indicating that laminin is necessary for VSMC maintenance. In vitro, both astrocytes and astrocytic laminin promoted brain VSMC differentiation. These results show that astrocytes regulate VSMCs and vascular integrity in small vessels of deep brain regions. Therefore, astrocytes may be a possible target for hemorrhagic stroke prevention and therapy.
Introduction
The integrity of blood vessel walls is essential for proper functioning of the circulatory system and for preventing hemorrhage. The vascular walls in the central nervous system (CNS) are composed of an endothelial cell layer, basement membranes (BM), vascular smooth muscle cells (VSMCs) or pericytes, and astrocytic endfeet. The vascular BM is composed of laminin, collagen type IV, nidogen, thrombospondin, and various proteoglycans (Mohan and Spiro, 1986; Yurchenco and Patton, 2009). BM proteins play important roles in proliferation, migration, differentiation, survival, and function of cellular components of the vascular wall (Eble and Niland, 2009; Wagenseil and Mecham, 2009). Mutations in collagen type IV that affect vascular BMs can lead to intracerebral hemorrhage (ICH) in both mice and humans during development (Gould et al., 2005, 2006). Direct damage to the BM under pathological conditions such as ischemic stroke can also cause blood brain barrier (BBB) breakdown and hemorrhage (Wang and Shuaib, 2007; Zhang et al., 2007; Zhao et al., 2007; del Zoppo, 2009; Lo and Rosenberg, 2009).
Laminin, a major component of all BMs, are heterotrimeric molecules composed of α, β, and γ chains (Timpl and Brown, 1994; Miner and Yurchenco, 2004). Two laminin isoforms, laminins-111 and -211, which are produced by astrocytes, are found in the vasculature of the CNS (Jucker et al., 1996; Sixt et al., 2001). In addition, laminins-411 and -511 are expressed in the endothelial BM of most tissues (Frieser et al., 1997; Sorokin et al., 1997; Sixt et al., 2001).
The laminin γ1 chain is an essential subunit of most laminin isoforms (Timpl and Brown, 1994; Hallmann et al., 2005; Durbeej, 2010), and global knockout of laminin γ1 leads to early embryonic lethality (Smyth et al., 1999). To study its function, we generated a laminin γ1 chain conditional knockout mouse line using the Cre/LoxP system (Chen and Strickland, 2003). Using this mouse line, our studies in the nervous system demonstrated the importance of this subunit in the expression and assembly of laminin isoforms and the BM (Chen and Strickland, 2003; Yu et al., 2005, 2007, 2009; Chen et al., 2008).
The expression of astrocytic laminin in CNS blood vessels suggests that it is important for BBB formation and CNS vascular integrity. To explore the specific roles of astrocytic laminin in the cerebrovasculature, we crossed our floxed laminin γ1 mice with a nestin-Cre transgenic mouse line (Tronche et al., 1999), producing a line that does not express laminin in glial cells and neurons (referred to as LNγ1-KO). In this transgenic mouse line, Cre is active in neural precursor cells that give rise to both neurons and glia as early as embryonic day 10.5, but Cre is not expressed in endothelial cells (Graus-Porta et al., 2001). This Cre line has been used to distinguish the role of endothelial (tie2-Cre) and nonendothelial (nestin-Cre, used in this study) β8 integrin in brain vascular development (Proctor et al., 2005).
The LNγ1-KO mice showed disruption of astrocytic laminins in the brain and presented with spontaneous hemorrhagic stroke in adulthood in deep brain regions such as the basal ganglia, which are most affected in human patients. Detailed analysis showed that ablation of astrocytic laminin disrupted VSMC/astrocyte interaction, down-regulated VSMC contractile protein expression, and weakened vascular integrity in deep brain regions, leading to hemorrhage. These results revealed a novel function of astrocytes in regulating VSMC function and vascular integrity in the brain. It is noteworthy that this phenotype is similar to that observed in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL), caused by Notch 3 mutations (Hervé and Chabriat, 2010); these patients have VSMC degeneration often leading to ICH (Viswanathan et al., 2006; Oide et al., 2008).
ICH is a severe form of stroke with high rates of death and long-term disability (Qureshi et al., 2009). Unfortunately, the cellular and molecular mechanisms of how and why hemorrhage occurs are unclear (NINDS ICH Workshop Participants, 2005; Steiner et al., 2011). The spontaneous brain region–specific hemorrhagic phenotype presented by the LNγ1-KO mice suggests an involvement of astrocytic laminin in human hemorrhagic stroke. Therefore, this knockout mouse line may be a useful animal model for studying the mechanisms underlying hemorrhagic stroke. Furthermore, astrocytic laminin may be a novel target in the development of preventive and therapeutic strategies for ICH.
Results
Spontaneous hemorrhagic stroke in LNγ1-KO mice
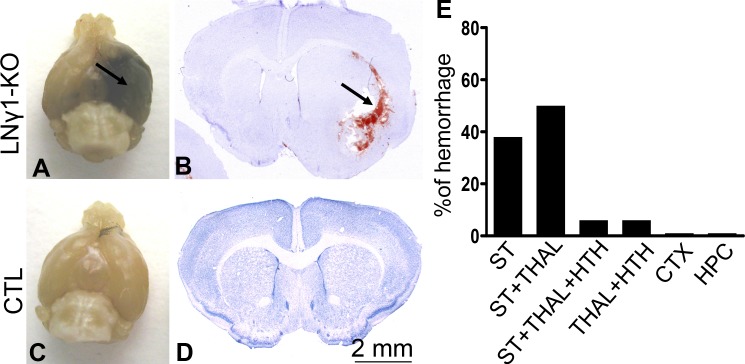
LNγ1-KO mice were born at the expected Mendelian ratio and were smaller than their littermate controls. They began to present with spontaneous ICH in adulthood: 20% of LNγ1-KO mice had ICH by 2–3 months of age, and 60% had ICH by 6 months (Fig. 1 A). These results indicate that the ICH phenotype worsened as LNγ1-KO mice aged. Most hemorrhages occurred in small arterioles in deep brain regions such as the basal ganglia, thalamus, and hypothalamus (Fig. 1 B). Analysis of 16 hemorrhagic LNγ1-KO mice revealed that 94% of hemorrhages were associated with the striatum, with 38% having hemorrhage only in striatum, 50% in both striatum and thalamus, and 6% in striatum, thalamus, and hypothalamus. Hemorrhage was not observed in the cerebral cortex or hippocampus of LNγ1-KO mice (Fig. 1 E). The severity of hemorrhage varied between mice—in the most severe cases, the temporal lobes were filled with blood (Fig. 1 B), but in other cases, the hemorrhage was constrained to the vicinity of blood vessels. Littermate control mice, either homozygous for the floxed allele but lacking nestin-Cre or heterozygous for the floxed laminin γ1 allele and also hemizygous for the Cre transgene, showed no signs of hemorrhage (Fig. 1, C and D).
Figure 1.
LNγ1-KO mice present with ICH in adulthood. Brains of 4-mo-old LNγ1-KO (A) and control littermate (C) mice. The LNγ1-KO mouse brain shows ICH in the temporal lobe (A, arrow), whereas the control mouse is normal. Hematoxylin-stained brain sections of LNγ1-KO (B) and control (D) mice. There is evidence of hemorrhage in the LNγ1-KO (B, arrow) but not in the control mouse brain sections. Brains of LNγ1-KO mice were enlarged near sites of hemorrhage, likely due to edema. Analysis of hemorrhages in different brain regions of LNγ1-KO mice showed that most hemorrhages occurred in striatum and thalamus (E; n = 16 mice). CTX, cortex; HPC, hippocampus; HTH, hypothalamus; ST, striatum; THAL, thalamus. Bar, 2 mm.
Laminin expression changes in the brain blood vessels of LNγ1-KO mice
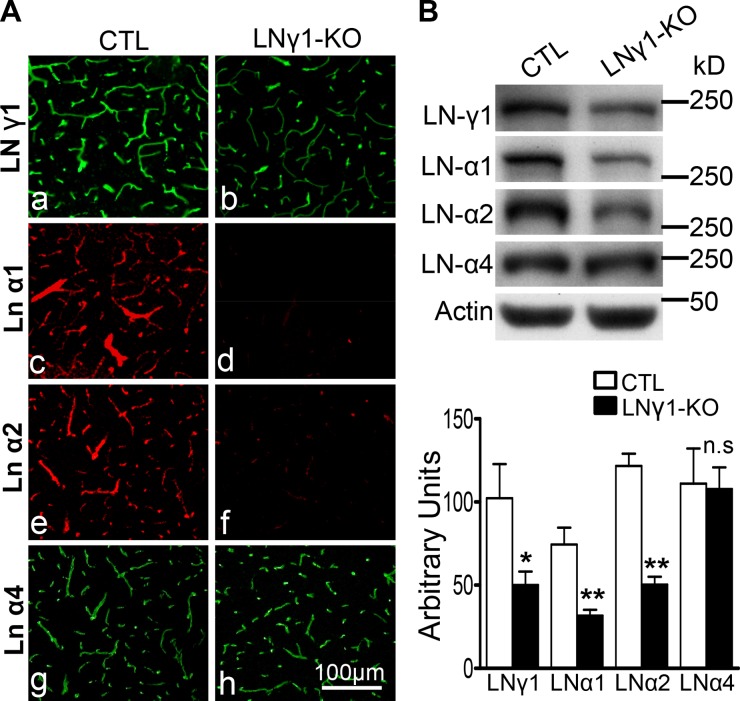
Because LNγ1-KO mice showed a prominent ICH phenotype, we analyzed changes in laminin expression in the brain vasculature by immunohistochemistry and Western blot. Laminin γ1 was expressed in both control and LNγ1-KO brain tissues, but its expression was decreased in LNγ1-KO mice compared with controls (Fig. 2, Ab vs. Aa). Low-level expression of laminin γ1 was expected in the LNγ1-KO mice because nestin-Cre would not be expressed by endothelial cells (Tronche et al., 1999; Proctor et al., 2005) and they would therefore still express laminin γ1.
Figure 2.
Disruption of astrocytic but not endothelial laminins in LNγ1-KO mice. (A) Immunohistochemical analysis showed vascular laminin γ1 is expressed in both control and LNγ1-KO mice (a and b), but its expression level is decreased in LNγ1-KO mice compared with control (a vs. b). Expression levels of laminin α1 and α2 (astrocytic laminins) were dramatically decreased in LNγ1-KO mice (c and e vs. d and f). However, laminin α4 (endothelial) expression remained similar between control and LNγ1-KO mice (g vs. h). Bar, 100 µm. (B) Western blot analysis and quantification of different laminin chain expression levels in the brains of control and LNγ1-KO mice showed lamininγ1, α1, and α2 were significantly decreased in LNγ1-KO mice, but laminin α4 expression was similar between control and KO mice (Student’s t test, n = 7 in each group). n.s: not significant.
Endothelial cells and astrocytes assemble their own BM. Under basal conditions, these two layers of BM are fused together and cannot be distinguished by light microscopy (Sixt et al., 2001). Interestingly, endothelial cells and astrocytes express different laminin α chains (Sixt et al., 2001). We have shown in Schwann cells of the PNS that disruption of laminin γ1 expression results in concurrent lack of expression of the α and β laminin chains (Yu et al., 2005), presumably due to an inability of the cell to assemble the laminin trimers in the absence of γ1, resulting in intracellular retention or degradation of the α and β chains. Based on these observations, we sought to determine whether astrocytic laminin was specifically depleted. We used antibodies targeting astrocyte- and endothelia-specific laminin α chains to assess which α chain was affected. Laminins α1 and α2, which are expressed in astrocytes (Sixt et al., 2001), were dramatically decreased in blood vessels of LNγ1-KO mice (Fig. 2, Ad and Af vs. Ac and Ae). However, levels of laminin α4, which is expressed in endothelial cells (Fig. S1), were similar between KO and control mice (Fig. 2, Ag and Ah). Laminin α2 was deposited at the astrocytic endfeet (stained by S100) ensheathing blood vessels in control mice, but was absent from these structures in LNγ1-KO mice (Fig. S2 A). Western blot analysis and quantification confirmed that expression of laminin γ1, α1, and α2, but not α4, were significantly decreased in LNγ1-KO mice (Fig. 2 B). The decrease in α1 and α2 in LNγ1-KO mice appeared more dramatic by immunohistochemistry than by Western blot, probably due to partial intracellular retention of these chains in the absence of γ1 chain in astrocytes, which can still be detected by Western blot but not by immunohistochemistry. These results show that astrocytic endfeet–associated laminins are specifically disrupted in LNγ1-KO mice.
Because neurons also produce laminin γ1 and nestin-Cre is expressed in neuronal progenitor cells that give rise to neurons and glia (Graus-Porta et al., 2001), neuronal laminin could also contribute to the vascular BM. To investigate whether knocking out neuronal laminin γ1 could affect vascular laminins, we analyzed the expression of different laminin chains (γ1, α2, and α4) in CaMKII-Cre/laminin γ1 conditional KO and littermate control mice (neuron-specific laminin γ1 KO; Chen et al., 2008). There was no change in laminin expression in the vasculature between control and CaMKII-Cre/laminin γ1 KO mice (Fig. S2 B), indicating that the disruption of laminin α1 and α2 expression in LNγ1-KO mice is due to ablation of laminin γ1 in astrocytes and not neurons or endothelial cells. We also analyzed Cre expression patterns in the nestin-Cre mouse line by crossing this Cre line with the ROSA26-enhanced green fluorescent protein (ROSA26-EGFP) reporter transgenic mice (Mao et al., 2001). Our results showed that Cre is active in the parenchyma cells (astrocytes and neurons), but not in VSMCs in the nestin-Cre mouse line during postnatal development (P14; unpublished data) and in adulthood (Fig. S3). These results indicate that the ICH phenotype observed in LNγ1-KO mice is likely due to the disruption of astrocytic laminin.
Brain vascular wall changes in LNγ1-KO mice
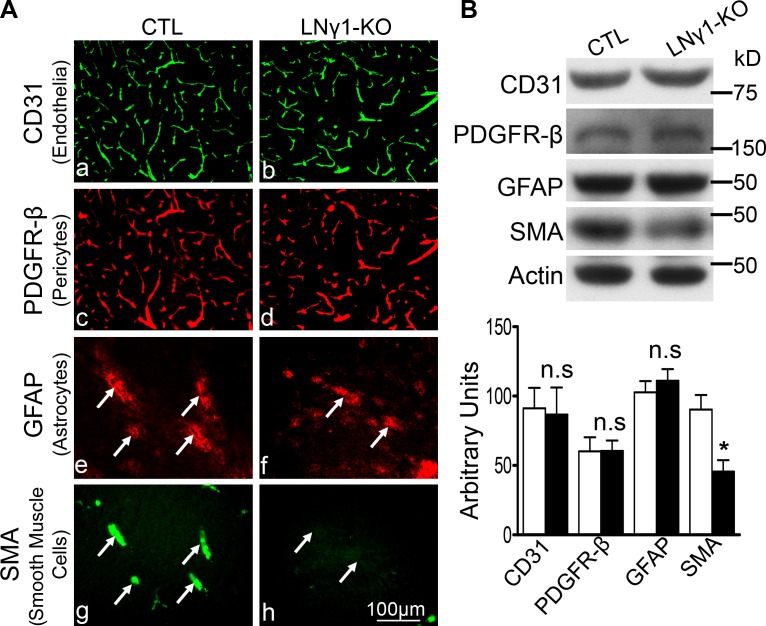
To investigate the pathological changes that may be responsible for hemorrhage in LNγ1-KO mice, we examined structural changes in the vascular walls of LNγ1-KO mouse striatum, the most affected region. We used cell type–specific markers for the cellular components of CNS vasculature: cluster of differentiation 31 (CD31) for endothelial cells; platelet-derived growth factor receptor-β (PDGFR-β) for pericytes; glial fibrillary acidic protein (GFAP) for activated astrocytes; and smooth muscle α-actin (SMA) for VSMCs. CD31 staining showed a similar pattern and intensity in LNγ1-KO and control mice (Fig. 3, Aa and Ab). PDGFR-β staining was also similar between LNγ1-KO and controls as it covered most of the blood vessels in both mouse lines (Fig. 3, Ac and Ad). GFAP expression was similar in both mouse lines in basal ganglia (Fig. 3, Ae and Af), and activated astrocytes associated with large blood vessels (Fig. 3, Ae and Ag). However, there was a dramatic decrease in SMA expression in LNγ1-KO mice compared with controls (Fig. 3, Ag and Ah). GFAP-positive astrocytes were associated with SMA expression in control blood vessels (Fig. 3, Ae and Ag), but GFAP staining was not associated with SMA staining in LNγ1-KO mice (Fig. 3, Af and Ah). Western blot analysis and quantification showed expression levels of CD31, PDGFR-β, and GFAP were not significantly changed in LNγ1-KO mice compared with controls (Fig. 3 B). However, SMA expression was significantly decreased in LNγ1-KO mice compared with controls. These results indicate that VSMCs were affected in LNγ1-KO mice.
Figure 3.
Cerebral vascular wall changes in the striatum of LNγ1-KO mice. (A) Immunohistochemistry analysis revealed expressions of CD31 (endothelial cell marker; Aa and Ab), PDGFR-β (pericyte marker; Ac and Ad), and GFAP (astrocyte marker; Ae and Af) were similar between control and LNγ1-KO mice. However, expression of SMA (smooth muscle cell marker) was decreased in the striata of LNγ1-KO compared with control mice (Ag and Ah). GFAP expression was associated with SMA in the striata of control mice (Ae and Ag, arrows), but minimal SMA expression was observed in the striata of LNγ1-KO mice (Af and Ah). Bar, 100 µm. (B) Western blot analysis and quantification showed CD31, PDGFR-β, and GFAP expressions were not significantly changed in LNγ1-KO mice, but SMA expression was significantly decreased in LNγ1-KO mice compared with control mice (Student’s t test, n = 7 in each group). n.s: not significant.
Vascular smooth muscle cell changes in LNγ1-KO mouse brains
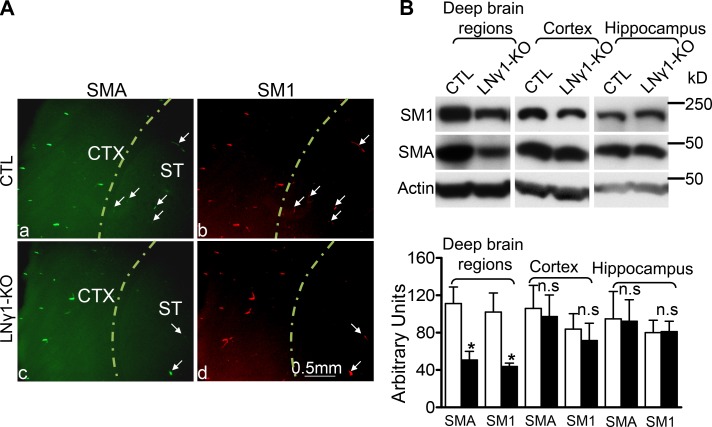
Because SMA was decreased in the blood vessels of LNγ1-KO mouse striatum (Fig. 3) and hemorrhages were mostly observed in the same regions (Fig. 1), we reasoned there might be a correlation between VSMC changes and region-specific hemorrhages. To address this question, we compared SMC contractile protein expression in different cerebrum regions between control and LNγ1-KO mice by immunohistochemistry and Western blot to investigate whether VSMCs were affected in hemorrhagic regions. Immunohistochemistry for SMA and smooth muscle myosin heavy chain 1 (SM1) showed that expression of these two proteins in the cerebral cortex was not affected (Fig. 4 A), but in the striatum their expression was decreased (Fig. 4 A). To quantify regional differential changes of VSMC contractile protein expressions, we performed Western blot analysis. Cerebrums were separated into three different regions: cerebral cortex, hippocampus, and the rest of the cerebrum (deep regions), which include basal ganglia, thalamus, and hypothalamus. Western blot analysis revealed that expression of both SMA and SM1 was significantly decreased in deep cerebral regions of LNγ1-KO mice (Fig. 4 B). However, levels of SMA and SM1 were similar in the cerebral cortex and hippocampus in control and LNγ1-KO mice (Fig. 4 B). These results showed VSMC contractile protein expression was affected in deep brain regions where hemorrhages occurred but not in the cerebral cortex and the hippocampus where no hemorrhage was observed, indicating a possible involvement of VSMC in hemorrhages in LNγ1-KO mice.
Figure 4.
Region-specific VSMC contractile protein expression changes in LNγ1-KO mice. (A) Immunohistochemistry showed that SMA (a and c) and SM1 (b and d) expression in cerebral cortex were similar between control and LNγ1-KO mice, but decreased in striatum of LNγ1-KO mice compared with controls (arrows). (B) Western blot analysis and quantification showed that expression levels of SMA and SM1 were significantly decreased in deep cerebral regions of LNγ1-KO mice compared with controls. SMA and SM1 expression in cerebral cortex and hippocampus of LNγ1-KO mice were not significantly changed compared with the same regions of control mice (Student’s t test, n = 7 in each group). n.s: not significant.
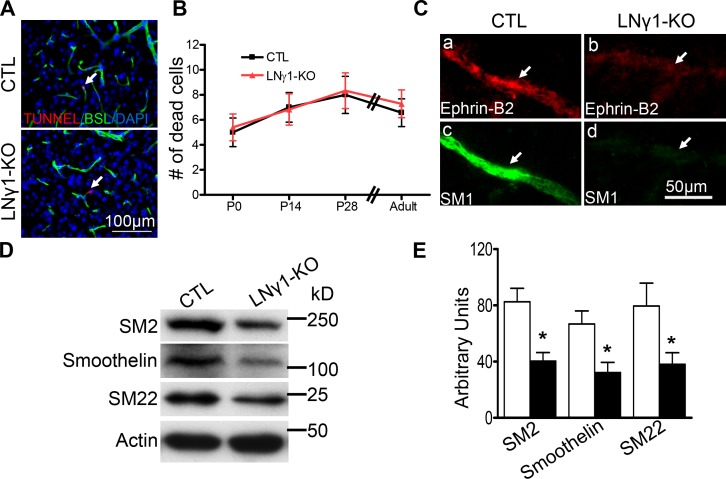
Decreased VSMC marker protein expression in LNγ1-KO mice could be due to SMC death or impaired differentiation. To address this question, we performed TUNEL staining on brain sections from P0, P14, P28, and adult LNγ1-KO and control mice. Our results showed there was no significant difference in blood vessel–associated apoptotic cell death in LNγ1-KO mouse striatum compared with the controls during development stages and adulthood (Fig. 5, A and B). These results showed that decreased VSMC marker protein expression in LNγ1-KO mice was not due to VSMC death.
Figure 5.
Impaired VSMC differentiation but not cell survival in striatum of LNγ1-KO mice. TUNEL staining showed blood vessel–associated apoptotic cell death was not significantly increased in LNγ1-KO mice compared with controls during development (P0, P14, and P28) and in adulthood (A and B; Student’s t test, n = 5–7 in each group at each time point). (C) Impairment of arterial SMC differentiation. Arteries labeled by EphrinB2 (a and b) expressed high level of SM1 in control (c) but very low level in LNγ1-KO (d) mice. Western blot analysis and quantification of VSMC differentiation protein expression in the striatum of control and LNγ1-KO mice. Expression of SM2, Smoothelin, and SM22 were significantly decreased in the striatum of LNγ1-KO mice compared with control mice (E). Bars: (A) 100 µm; (C) 50 µm.
To examine whether there were SMCs in the arteries or arterioles in LNγ1-KO mouse striatum, we used EphrinB2 as a marker for arteries (Wang et al., 1998; Li et al., 2011). Although EphrinB2-positive arteries expressed high levels of SM1 in control mice (Fig. 5, Ca and Cc), EphrinB2-positive arteries expressed very low levels of SM1 in LNγ1-KO mice (Fig. 5, Cb and Cd). EphrinB2 expression was also decreased, indicating VSMCs were likely associated with blood vessels but their differentiation was impaired. Blood vessels with diameters between 8–20 µm were most affected, and large diameter blood vessels (>25 µm) were not affected in LNγ1-KO mouse striatum (not depicted). To further analyze VSMC differentiation in LNγ1-KO mouse striatum, we performed Western blot for additional SMC differentiation proteins. Expression levels of smooth muscle myosin heavy chain 2 (SM2), smoothelin, and smooth muscle 22 α (SM22 α) were all significantly decreased in LNγ1-KO mouse striatum (Fig. 5, D and E). These results further suggest that VSMC differentiation was impaired in the striatum of LNγ1-KO mice.
We then investigated whether decreased SMC contractile protein expression in adult LNγ1-KO mice was due to developmental changes by analyzing SMA and SM1 expression in postnatal stages. Cerebrums from control and LNγ1-KO mice at 4 wk of age were separated into three regions in the same way as the adult brains. Expression levels of SMA and SM1 in these three regions were compared between control and LNγ1-KO mice by Western blot. In deep brain regions both SMA and SM1 showed a significant decrease in LNγ1-KO mice compared with the littermate controls (Fig. S4). However, there were no significant differences in SMA and SM1 expression in cerebral cortex and hippocampus between control and LNγ1-KO mice (Fig. S4). These results suggest that SMC contractile protein expression was already decreased in LNγ1-KO mice during postnatal development, indicating that the decreased expression of VSMC contractile proteins in LNγ1-KO mice was probably due to developmental defects.
Ablation of astrocytic laminin in adult mice disrupted SMC contractile protein expression
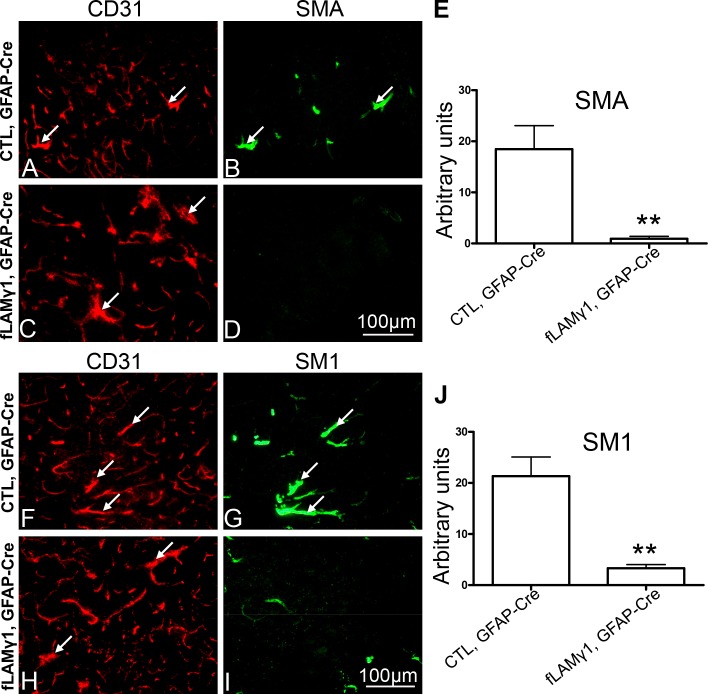
To further analyze whether astrocytic laminin is necessary for the maintenance of VSMC differentiation in adult animals, we disrupted laminin expression in astrocytes of adult mice using adenovirus expressing Cre recombinase under the control of an astrocyte-specific promoter (ad-pGFAP-Cre). We injected ad-pGFAP-Cre into the striata of control and homozygous floxed laminin γ1 allele mice (fLAMγ1). 7 d after injection, the mice were analyzed for adenovirus infection, astrocytic laminin expression, and VSMC differentiation. The ratio of astrocytes infected by adenovirus was 86 ± 8%, and disruption of astrocytic but not endothelial laminin in fLAMγ1mice was confirmed (unpublished data). In contrast to control animals, the fLAMγ1 mice showed significant decreases in SMA and SM1 expression (Fig. 6). The large caliber blood vessels in control mice, shown by CD31 staining, still expressed SMA (Fig. 6, A and B, arrows) and SM1 (Fig. 6, F and G, arrows) after ad-pGFAP-Cre injection. However, these large caliber blood vessels in fLAMγ1 mice had decreases in SMA (Fig. 6, D and E) and SM1 (Fig. 6, I and J) after astrocytic laminin depletion by ad-pGFAP-Cre, and these decreases were statistically significant (Fig. 6, E and J). These results indicate that acute ablation of astrocytic laminin in adult animals can disrupt VSMC contractile protein expression, revealing its importance in the maintenance of brain VSMC differentiation.
Figure 6.
Ablation of astrocytic laminin in adult mice disrupts VSMC contractile protein expression. Adenovirus expressing Cre recombinase under the control of GFAP promoter (ad-pGFAP-Cre) was injected into the striata of control or fLAMγ1 mice. 7 d after ad-pGFAP-Cre injection, both SMA (B and D) and SM1 (G and I) were dramatically decreased in fLAMγ1 mice (D and I) when compared with control (B and G). Blood vessels were visualized by CD31 staining (A, C, F, and H). Large caliber blood vessels express SMA (A and B, arrows) and SM1 (F and G, arrows) in control mice, whereas expression of SMA (C and D, arrows) and SM1 (H and I, arrows) in fLAMγ1 mice was decreased. The decreases in SMA and SM1 in fLAMγ1 mice after ad-pGFAP-Cre injection were statistically significant when compared to control by Student’s t test (E and J). Bar, 100 µm.
Astrocytes and astrocytic laminins promote brain VSMC differentiation in vitro
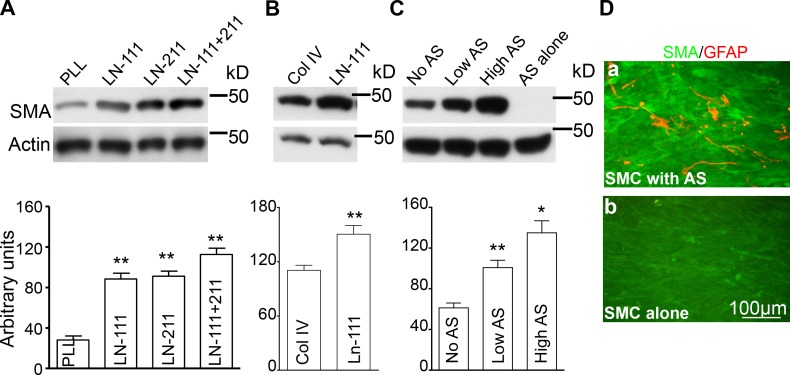
Because disruption of astrocytic laminin impaired brain VSMC (BVSMC) contractile protein expression in vivo, we analyzed whether astrocytic laminins or astrocytes have direct effects on BVSMC differentiation in vitro. Astrocytes express laminins-111 and -211, so we compared SMA expression levels by BVSMCs cultured on plates coated with poly-l-lysine (PLL) or laminin-111 or -211 or a combination of laminins-111 and -211. BVSMCs cultured on laminin-111– or -211–coated plates showed significantly more SMA expression compared with PLL-coated plates (Fig. 7 A). Interestingly, BVSMCs cultured on plates coated with a combination of laminins-111 and -211 showed significantly more SMA expression than BVSMCs cultured on plates coated with laminin-111 or -211 alone (Fig. 7 A). Because astrocytes produce both laminins-111 and -211 and a combination of these laminin isoforms can additively promote BVSMCs differentiation, astrocytes may be well positioned to regulate BVSMCs differentiation.
Figure 7.
Astrocytes and astrocytic laminins promote BVSMC differentiation in vitro. (A) Western blot analysis showed BVSMCs cultured on plates coated with laminin-111 and/or -211 had increased SMA expression compared with cells cultured on PLL-coated plates by ANOVA (n = 18 in each group). (B) BVSMCs cultured on plates coated with laminin-111 had significantly more SMA expression that cells cultured on collagen type IV–coated plates by Student’s t test (n = 18 in each group). (C) BVSMCs co-cultured with astrocytes had significant increases in SMA expression compared with controls and is astrocyte number dependent by ANOVA, whereas astrocytes did not express SMA (n = 18 in each group). (D) Immunocytochemistry showed BVSMCs co-cultured with astrocytes had obvious SMA expression and astrocytes were GFAP positive (a), whereas BVSMCs cultured alone only maintained a basal low level SMA expression and there were no GFAP-positive cells (b). Bar, 100 µm.
We also compared SMA expression levels by culturing BVSMCs on plates coated with laminin and collagen type IV, also a major component of the BM. BVSMCs cultured on laminin expressed significantly more SMA than cells cultured on collagen type IV (Fig. 7 B). These results indicate astrocytic laminin can directly promote BVSMC differentiation, and the effect of laminin on BVSMC differentiation is stronger that other components of the BM such as collagen type IV.
We then analyzed whether astrocytes can directly regulate BVSMC differentiation by co-culturing BVSMCs with astrocytes. BVSMCs were grown to 90% confluence. Different amounts of astrocytes were added into BVSMC culture, and they were co-cultured for 6 d. Compared with the controls, BVSMCs co-cultured with astrocytes showed significantly more SMA expression and was astrocyte number dependent (Fig. 7 C). However, astrocytes by themselves did not express SMA (Fig. 7 C). We also performed SMA and GFAP immunocytochemistry to compare SMA expression in BVSMCs cultured with or without astrocytes. In BVSMC/astrocyte co-culture, SMA expression was elevated and astrocytes were positive for GFAP staining (Fig. 7 D). However, in BVSMCs cultured without astrocytes, SMA expression remained at basal levels and there was no GFAP expression, indicating the purity of the SMC (Fig. 7, Db). These results indicate that astrocytes could directly promote BVSMC differentiation.
Brain region–specific vascular changes in LNγ1-KO mice
Because most hemorrhages were observed in the deep brain regions and VSMCs were impaired in these same regions, we investigated why there was such a regional specificity. One possibility is that the regional heterogeneous expression of Cre recombinase resulted in more complete knockout of laminin in deep brain regions compared with cerebral cortex or hippocampus. Therefore, we compared astrocytic laminin expression between deep brain regions and cerebral cortex or hippocampus in LNγ1-KO mice. However, there was no significant difference (unpublished data). The regional differences could be due to different association between astrocytes and VSMCs. While arteries penetrate through the brain parenchyma, the pia meninges cover the blood vessels and directly contact VSMC in the media layer of arteries. Astrocytic endfeet cover the pia meninges that penetrate the arteries, and as big arteries branch into small arteries and small arteries ramify into arterioles, the pia meninges become thinner. At the capillary level, there are no pia meninges, and astrocytic endfeet directly contact pericytes or endothelial cells. One possible explanation for the effects of disruption of astrocytic laminin on VSMC in the deep brain regions could be that small arteries and arterioles in deep brain regions had closer contact with astrocytes than those arteries in cerebral cortex or hippocampus and were therefore more influenced by astrocytes.
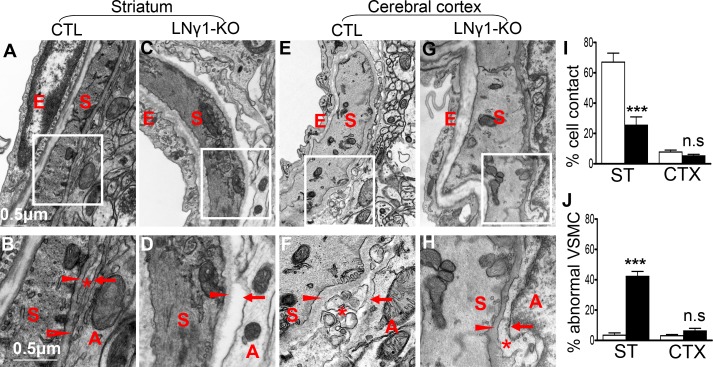
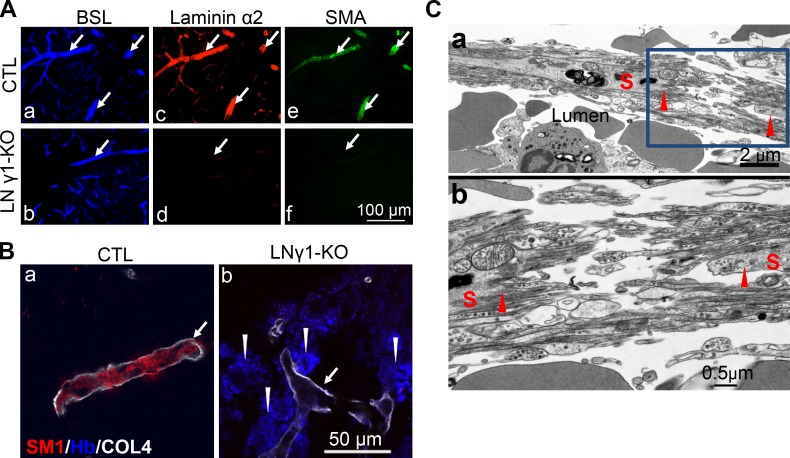
To investigate this possibility, we compared the relationship between astrocytes and VSMCs in small arteries or arterioles in striatum and cerebral cortex by electron microscopy (EM). In small arteries or arterioles in the striatum, there were regions where the BMs of VSMCs and astrocytes were fused together and astrocytes showed close contact with VSMCs (Fig. 8, A and B). However, this close relationship between astrocytes and VSMCs was not observed in small arteries or arterioles in the cerebral cortex (Fig. 8, E and F). In LNγ1-KO mice, ablation of astrocytic laminin γ1 disrupted astrocytic endfeet BM formation and contact between astrocytes and VSMC in small arteries and arterioles in the striatum, but the VSMC BMs were mostly intact (Fig. 8, C and D) due to the absence of Cre expression in this cell type in nestin-Cre mouse line (Fig. S3). However, in small arteries or arterioles in the cerebral cortex of LNγ1-KO mice, VSMCs were covered by pia meninges, located between VSMC and astrocytes, and loss of astrocytic endfeet BM did not affect the contact between VSMCs and pia meninges (Fig. 8, G and H). Interestingly, disruption of astrocyte/VSMC contact in the striatum of LNγ1-KO mice caused abnormal VMSC morphology with less contractile machinery (Fig. 8, C and D). We quantified the percentage of VSMCs (with diameter between 8 and 20 µm) showing direct contact with astrocytes and percentage of VSMCs with abnormal morphology in different brain regions between control and LNγ1-KO mice. There was a decreased percentage of VSMCs showing direct contact with astrocytes in the striatum but not in the cortex in LNγ1-KO mice compared with controls (Fig. 8 I). VSMCs with abnormal morphology (less contractile machinery) were dramatically increased in the striatum, but not in the cortex in LNγ1-KO mice (Fig. 8 J). In control mice, many more VSMCs showed direct contact with astrocytes in striatum than in cortex (Fig. 8 I). These results indicate that astrocytes were more closely associated with VSMCs in small arteries and arterioles in striatum than in the cerebral cortex. Ablation of astrocytic laminin disrupted astrocytic endfeet BM and affected the contact between VSMC and astrocytes in striatum but not in the cerebral cortex. These regional differences may explain why striatum were mostly affected.
Figure 8.
Region-specific vascular changes in LNγ1-KO mice. (A and B) Relationship between astrocytes (A), VSMC (S), and pia meninges (*) in small arterioles in striatum of control mice; there are some regions in which the BM of VSMC and astrocytes are fused together (arrow). (B) Higher magnification of the boxed area in A showed details of the BM of VSMC (arrowhead) and astrocytes (arrow) fused together and astrocyte showed close contact with VSMC (open arrowhead). In some regions the pia meninges (*) lied between VSMC and astrocytes. (C and D) Relationship between VSMC and astrocytes in striatum of LNγ1-KO mice. Astrocytic endfeet detached from the wall of blood vessel. (D) Higher magnification of boxed area in C showed that ablation of astrocytic laminin γ1 disrupted astrocytic endfeet BM formation (arrow) as well as the contact between astrocytes and VSMC (the space between them). However, SMC BMs still exist (arrowhead). (E and F) Relationship between VSMCs, astrocytes, and pia meninges in cerebral cortex in control mice. Pia meninges are found between VSMCs and astrocytes, and there is no direct contact between VSMCs and astrocytes. (F) Higher magnification in boxed area in E showed that the BMs of VSMCs and astrocytes were always separated, and the pia meninges are between them. (G and H) Relationship between VSMCs and astrocytes in cerebral cortex of LNγ1-KO mice. VSMC were covered by pia meninges (*). (H) Higher magnification of the boxed area in G shows that ablation of astrocytic laminin γ1 disrupted astrocytic endfeet BM formation (arrow), but the VSMCs were covered by pia meninges (*) and the BM of VSMCs was intact (arrowhead). (I) Quantification of EM micrographs (n = 7 mice per genotype) revealed that VSMC/astrocytic endfeet direct contact was dramatically decreased in the striatum in LNγ1-KO mice (black bar) compared with controls (white bar). There were dramatically more VSMCs showing direct contact with astrocytic endfeet in striatum than in cerebral cortex in control mice (I). There was no significant difference in percentages of VSMCs showing direct contact with astrocytic endfeet between control and LNγ1-KO cortex (I). There were significantly more VSMCs showing abnormal morphology in the striatum in LNγ1-KO (black bar) than in control mice (white bar), but there was no such difference between control and LNγ1-KO cortex (J). All statistical analyses were by Student’s t test. A, astrocytes; CTX, cortex; E, endothelium; n.s: not significant; S, smooth muscle cell; ST, striatum. Bars in A, C, E, and G are the same and is shown in A; Bars in B, D, F, and H are the same and is shown in B.
Disruption of astrocytic laminin impairs VSMC differentiation and leads to hemorrhagic stroke
We examined the relationship between astrocytic laminin expression, VSMC contractile protein expression, and hemorrhage. In control mice, blood vessels (identified by BSL, fluorescein-bandiraea simplicifolia lectin; Daneman et al., 2010) expressed laminin α2 (Fig. 9, Aa and Ac) and laminin α1 (Fig. S5 C). The expression of astrocytic laminin in large caliber blood vessels correlated with the expression of SMA (Fig. 9, Aa, Ac, and Ae; and Fig. S5, E and G). However, in LNγ1-KO mice, the large caliber blood vessels did not express laminin α2 (Fig. 9, Ad), laminin α1 (Fig. S5 D), or SMA (Fig. 9, Ab, Ad, and Af; and Fig. S5, F and H). Furthermore, analysis of hemorrhagic regions of LNγ1-KO mice showed that hemoglobin, indicating hemorrhage, was deposited outside the blood vessels that had dramatically decreased SM1 expression in the striatum (Fig. 9, Bb), whereas no hemoglobin was detected in control mouse brain (Fig. 9, Ba).
Figure 9.
Disruption of astrocytic laminin impaired VSMC differentiation and led to ICH. (A) Blood vessels were visualized by BSL (a and b). Laminin α2 was expressed in these blood vessels in control (c) but not LNγ1-KO (d) mice. Laminin α2 was coexpressed with SMA in large caliber blood vessels in control mice (e), but this correlation was absent in LNγ1-KO mice (f). (B) In control mouse striatum hemoglobin was not detected around large caliber blood vessels expressing SM1 (arrow). In LNγ1-KO mice, however, hemorrhagic regions (stained by hemoglobin, arrowheads) was associated with large caliber blood vessels expressing little SM1 (arrow, a representative image). (C) A ruptured blood vessel in LNγ1-KO mice (a) was identified, which shows that the wall of the vessel was disassembled and smooth muscle cells were fragmented (arrowheads). (b) Higher magnification of boxed area in panel a showed the details of fragmented smooth muscle cells (arrowhead). COL4, collagen type IV; Hb, hemoglobin. Bars: (A) 100 µm; (B) 50 µm; (Ca) 2 µm; (Cb) 0.5 µm.
Ultrastructural analysis was performed on hemorrhagic blood vessels in the brains of LNγ1-KO mice. Ruptured blood vessels were identified by the presence of blood cells outside the vessels and in the parenchyma (Fig. 9, Ca). EM revealed that the vascular unit was disassembled and smooth muscle cells were fragmented (Fig. 9, Ca and Cb, arrowheads). Together, our results suggest that astrocytic laminin is important for the integrity of blood vessels in deep cerebral regions, and disruption of astrocytic laminin destabilizes the blood vessels and eventually leads to ICH.
Discussion
Astrocytes are the most abundant cell type in the CNS and are closely associated with the vasculature. They play important roles in regulating cerebral vascular development and function, maintaining BBB integrity and homeostasis of the CNS microenvironments, and neurovascular coupling (Abbott et al., 2006; Iadecola and Nedergaard, 2007; Zlokovic, 2008). Astrocytes interact with CNS vasculature through astrocytic endfeet where a BM is assembled. The astrocytic BM is different from the endothelial BM in that it contains different laminin isoforms. Our results showed that ablation of astrocytic laminin disrupted endfeet BM formation and impaired VSMC development in small arteries or arterioles in deep brain regions with decreased VSMC contractile protein expression. In these deep brain regions, astrocytes were more closely associated with VSMCs in small arteries or arterioles. While in the cerebral cortex or hippocampus where astrocytes are not as closely associated with VSMCs as in the deep brain regions, VSMCs were not significantly affected by the loss of astrocytic laminin. We also showed that disruption of astrocytic laminin caused dissociation of the astrocytic endfeet from the vascular wall, therefore destabilizing the blood vessels. These blood vessels were eventually disassembled, which led to ICH. In adult mice, acute ablation of astrocytic laminin in the striatum also disrupted VSMC contractile protein expression, indicating astrocytic laminin was necessary for the maintenance of differentiation of this cell type. In vitro, both astrocytic laminin and astrocytes promoted BVSMC differentiation. Because the regulation of VSMC development and maintenance by astrocytes has not been reported, our studies reveal a novel function of astrocytes in the brain.
Region-specific effects of disruption of astrocytic laminin on VSMC development and vascular integrity
Hemorrhages were only observed in the deep brain regions such as basal ganglia, thalamus, and hypothalamus, but not in cerebral cortex or hippocampus of LNγ1-KO mice. Consistent with the region-specific hemorrhagic phenotype, expression levels of VSMC contractile proteins were only significantly decreased in the deep cerebral regions but not in cerebral cortex or hippocampus, indicating the involvement of VSMC changes in the hemorrhagic phenotype observed. Because the amount of astrocytic laminin was similar in different brain regions of LNγ1-KO mice, the region-specific phenotype was probably not due to more complete disruption of astrocytic laminin in the deep brain regions. Our further analyses revealed that astrocytes were more closely associated with VSMCs in small arterioles of the deep cerebral regions than those in the cerebral cortex or hippocampus. We showed by EM that the BMs of astrocytes and VSMCs were fused together in some areas, and knockout of astrocytic laminin resulted in dissociation of astrocytic endfeet from the vascular wall. However, in the cerebral cortex, astrocytes were less closely associated with VSMCs in small arterioles and the pia meninges almost completely covered the entire outmost surface of VSMCs. This different relationship between astrocytes and VSMCs could be one of the mechanisms responsible for regional differences of hemorrhage in LNγ1-KO mice.
In human hypertensive hemorrhagic stroke patients, the most affected regions are the deep brain regions such as basal ganglia and thalamus (Mayer and Rincon, 2005; Sutherland and Auer, 2006; Qureshi et al., 2009), the same regions that showed hemorrhages in our LNγ1-KO mice. The reasons why these regions are more susceptible to ICH had been unclear. Hemodynamic forces in small arteries or arterioles in these regions probably accounts for one mechanism (Yen et al., 2005; Penn et al., 2011). Our results also reveal that abnormities in astrocytes, such as astrocytic BM changes, lead to hemorrhages in the same brain regions as the human hypertensive hemorrhagic patients, indicating that astrocytic changes may play an important role in the pathogenesis of hemorrhagic stroke in humans.
Astrocytic laminins in BVSMC development and maintenance
Previous in vitro studies show that laminin induces and maintains contractile protein expression in VSMCs (Thyberg and Hultgårdh-Nilsson, 1994; Hultgårdh-Nilsson and Durbeej, 2007). However, the in vivo function of laminin in VSMC development and differentiation had not been investigated. The extracellular environment of VSMCs is rich in laminins. On the interfaces with endothelium, VSMCs interact with endothelial BM which contains laminins-411 and -511(Sixt et al., 2001). On the outmost side, VSMCs contact pia meninges that likely contain laminin-111.
Furthermore, as our current study revealed, in some small arterioles in the deep brain regions, VSMCs may also interact with the astrocytic endfeet BM which contains laminins-111 and -211. Endothelium BM can promote VSMC maturation (Campbell and Campbell, 1986; Scheppke et al., 2012). The BM is composed of various extracellular matrix proteins, all of which may have different effects on VSMC differentiation, and the net effects of BM from each cellular source may differ. It is not clear whether astrocytic endfeet BM exhibits a different role in promoting VSMC maturation compared with endothelial BM. Our in vitro experiments showed that a combination of laminins-111 and -211, which are produced by astrocytes in vivo and are components of the astrocytic endfeet BM, showed stronger effects on BVSMC differentiation (Fig. 7 A) than these two laminin isoforms alone. It will be interesting to compare the effects of endothelial laminin (−411and −511) and astrocytic laminin on BVSMC differentiation. Interestingly, co-culture of BVSMCs with astrocytes also promoted SMC differentiation, which may be due to the laminins or other factors expressed by astrocytes. VSMCs may also produce laminin and assemble BMs (Glukhova et al., 1993). However in nestin-Cre mice, Cre is not expressed in brain VSMC (Fig. S3), and VSMC BMs were mostly intact in LNγ1-KO mice. Because vascular cell death was not significantly changed during development stages and adulthood in LNγ1-KO mice, astrocytic laminin plays a role in VSMC differentiation, whereas laminins from other cell types may be important for cellular viability.
VSMC degeneration is a prominent pathological change in the cerebrovascular wall of hypertensive ICH patients (Qureshi et al., 2009). EM analysis of ruptured arteries in surgically removed brain samples from hypertensive ICH patients showed VSMC atrophy and fragmentation (Takebayashi and Kaneko, 1983), suggesting that one of the major mechanisms underlying hypertensive ICH is VSMC degeneration. However, it remains unclear as to why smooth muscle cells degenerate under hypertensive conditions. In the ruptured arteries of our LNγ1-KO mice, VSMCs also showed fragmentation (Fig. 9, Cb), similar to the ruptured arteries of hypertensive ICH patients. Other studies show the association between astrocytic endfeet swelling, BBB breakdown, and neuronal death in hypertensive rats (Tagami et al., 1990), suggesting an important role of astrocytes in regulating vascular function under hypertensive conditions. In LNγ1-KO mice, even though their blood pressure was normal (unpublished data), ablation of astrocytic laminin disrupted the astrocyte–vasculature interaction in deep brain regions, leading to impairment of VSMC maturation, vascular instability, and ICH. It is also possible that abnormalities in astrocytes may contribute to vascular wall changes in human hypertensive hemorrhagic stroke pathogenesis. Further analysis of samples from human hemorrhagic stroke patients would shed light on whether astrocytic abnormities are involved in hypertensive hemorrhages.
Laminin receptors in mediating laminin function in BVSMC development and vascular integrity
Knockout of either integrin αv or β8 from the neuroepithelium leads to a defective association between cerebral microvessels and the cells of the surrounding brain parenchyma as well as the development of ICH at embryonic and postnatal stages (McCarty et al., 2002, 2005; Proctor et al., 2005). In contrast to our LNγ1-KO mice that present with adult onset and age-dependent ICH, adult integrin knockout mice do not experience ICH (McCarty et al., 2005; Proctor et al., 2005). The differences between LNγ1-KO mice and integrin αv or β8 knockout mice are likely due to distinctive underlying mechanisms. The TGFβ pathway appears to be involved in integrin αv or β8 function in cerebral vascular development (Nguyen et al., 2011), whereas the major laminin receptors expressed in the neurovascular unit in postnatal stages or adulthood are integrin β1 and dystroglycan (del Zoppo and Milner, 2006). However, knockout of integrin β1 or dystroglycan from astrocytes did not show ICH during development or adulthood (Graus-Porta et al., 2001; Moore et al., 2002; Nguyen et al., 2011). Because our results indicate that disruption of astrocytic laminin and VSMC impairment is the likely mechanism responsible for ICH, laminin receptors expressed by VSMCs may play an important role in mediating this effect. Among laminin receptors, integrin α7β1 seems to play an important role in VSMC development and vascular integrity. Knockout of integrin α7 in VSMCs results in reduced VSMCs and cerebral vascularization and cerebrovascular hemorrhage (Flintoff-Dye et al., 2005). Integrin β1 receptors also play important roles in VSMC development and phenotype maintenance as knockout of integrin β1 in VSMCs leads to SMC defects, vascular aneurysms, and neonatal or postnatal lethality (Abraham et al., 2008; Turlo et al., 2012). Dystroglycan, another laminin receptor, may also play an important role in VSMC development and function (Moiseeva, 2001; Hultgårdh-Nilsson and Durbeej, 2007). However, the role of laminin and its receptors in the development and function of VSMCs in cerebrovasculature is not well studied. The effects of astrocytic laminin on VSMC development in small arteries and arterioles in deep brain regions could be mediated by any of the laminin receptors expressed by VSMCs. It would be interesting to determine whether VSMCs in small arteries or arterioles in deep brain regions express different laminin receptors than those in other brain regions. Most of the studies on vascular development and maintenance thus far have focused on the vascular wall components themselves; however, vascular interaction with their environments may also play important roles in their development and function, as we showed here.
VSMCs play important roles in blood vessel integrity and strength. Abnormal changes of VSMCs are implicated not only in hypertensive ICH (Takebayashi and Kaneko, 1983; Pleşea et al., 2005), but also in hereditary cerebral hemorrhage with amyloidosis (Herzig et al., 2004; McCarron and Nicoll, 2004) and atherosclerosis, among others (Orr et al., 2010). Our results reveal a novel pathway that regulates cerebral VSMC differentiation and function. These results may provide the building blocks for developing new preventative and therapeutic strategies in hemorrhagic stroke and transformation in humans.
Materials and methods
Animals
To generate the nestin-Cre/laminin γ1 knockout mice, homozygous floxed laminin γ1 (fLAMγ1) mice on a C57BL6 background were mated with nestin-Cre transgenic mice on a C57BL6 background (Tronche et al., 1999). The fLAMγ1 mice contain two loxP sites inserted into exons 1 and 2 of the laminin γ1 gene so that after Cre-mediated recombination, the allele is nonfunctional (Tronche et al., 1999; Chen and Strickland, 2003). The double heterozygous fLAMγ1/+;nestin-Cre/+ mice were mated with homozygous fLAMγ1 mice to obtain mice that are homozygous for the floxed laminin γ1 allele and also carry the Cre transgene (LNγ1-KO mice). To generate the CaMKII-Cre/laminin γ1 knockout mice, CaMKII-Cre transgenic mice, where Cre is expressed in neurons, were used to obtain mice that are homozygous for the floxed laminin γ1 allele and also carry the CaMKII-Cre transgene (C57BL6 background; Chen et al., 2008). Littermates that were homozygous for the floxed laminin γ1 allele but did not carry the Cre transgenes or heterozygous for the floxed laminin γ1 allele and also carry the Cre transgenes were used as controls in all experiments. To monitor Cre expression patterns in nestin-Cre transgenic mice, ROSA26-EGFP reporter mice were used (C57BL6 background; Mao et al., 2001). Mice that were hemizygous for both the nestin-Cre and ROSA26-EGFP transgenes at P14 and P56 (adult) were used to determine Cre expression patterns in the brain. ROSA26-EGFP hemizygous mice without the nestin-Cre transgene were used as controls. Genomic DNA was analyzed by PCR for genotyping as described previously (Chen and Strickland, 2003).
Stereotaxic adenovirus injection
Adult (8–12 wk of age) control (C57bl6) or fLAMγ1 mice were anesthetized with atropine (0.6 µg/g of body weight) and avertin (0.02 ml/g of body weight) intraperitoneally. After making an incision, a burr hole was drilled at stereotaxic coordinates of −1 mm posterior to bregma and 2.5 mm lateral from midline. Then, 2 µl Ad-pGFAP-Cre (a gift from Arturo Alvarez-Buylla, University of California, San Francisco, San Francisco, CA) was injected into the brain at a depth of 3.5 mm over 5 min. The needle was kept in place for 2 min to prevent reflux. 1 wk after injection, the mice were transcardially perfused and the brains were sectioned for analysis. We conducted all experiments in accordance with the guidelines of the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and with approval from the Animal Care and Use Committee of The Rockefeller University.
Histology and immunohistochemistry
Histology and immunohistochemistry was performed as described previously (Chen et al., 2008). In brief, control and LNγ1-KO mice were anesthetized, the animals perfused, the brains removed, and cryo-sections prepared. For hematoxylin staining, mice were perfused with 4% PFA, and the brains removed and postfixed. Free-floating brain sections were prepared and stained with hematoxylin. Brain hemorrhages were counted in different brain regions of LNγ1-KO mice. For immunostaining, the sections were fixed in 4% PFA in 0.1 M phosphate buffer for 30 min. After washing in PBS, sections were blocked in PBS containing 0.3% Triton X-100 and 5% normal donkey serum. Primary antibodies used were against: laminin α2, S-100, and SMA (Sigma-Aldrich); laminin α1 and γ1 (EMD Millipore); CD31 (BD); SM1 (Kamiya Biomedical Company); laminin α4 (R&D Systems); PDGFR-β (eBioscience); GFAP (Dako); collagen type IV (Abcam); hemoglobin (Santa Cruz Biotechnology, Inc.); EGFP (Molecular Probes); and EphrinB2 (R&D Systems). The sections were incubated at 4°C overnight with primary antibody diluted in PBS containing 0.3% Triton X-100 and 3% normal donkey serum. After rinsing in PBS, sections were incubated with the appropriate secondary antibodies for 1 h. The sections were rinsed in PBS, cover-slipped, and examined using fluorescence microscopy (Axiovert 200; Carl Zeiss).
Terminal deoxynucleotidyl transferase–mediated biotinylated UTP nick end labeling assay (TUNEL) was performed using the In situ Cell Death Detection kit (Roche) according to the manufacturer’s instructions. LNγ1-KO and control mice at P0, P14, P28, and adult were perfused with ice-cold saline, and fresh frozen brain sections were prepared on a cryostat (Leica). Brain sections were fixed in 4% PFA, and permeabilized in 0.1% Triton X-100/0.1% sodium citrate on ice for 2 min and stained with the kit. Blood vessels were visualized with BSL (Vector Laboratories) and nuclei were counterstained with DAPI. Total numbers of blood vessel–associated TUNEL and DAPI double-labeled nuclei were determined and counted in the striatum of LNγ1-KO and control mice (5–7 mice in each group at each time point), and the differences were analyzed by two-tailed Student’s t test.
Western blot analysis
Control and LNγ1-KO mice were perfused with cold PBS (n = 7/group). Brains were collected, and the olfactory bulb and cerebellum were removed and then the right hemisphere of the cerebrum was separated into the cerebral cortex, hippocampus, and the rest of the deep brain regions including basal ganglia, thalamus, and hypothalamus (collectively referred as to deep brain regions). The left hemispheres of the brain were used for brain section preparation and immunohistochemistry. The samples were frozen in dry ice. The tissues were homogenized on ice in 2% SDS, 95 mM NaCl, 25 mM Tris, pH 7.4, 10 mM EDTA, and protease inhibitor cocktail (Roche). After centrifugation, extracts were run on a reducing 8 or 10% SDS-PAGE, blotted to PVDF membrane (EMD Millipore), incubated overnight at 4°C in primary antibody (rat anti-laminin γ1, Thermo Fisher Scientific; rat anti-laminin α1, EMD Millipore; mouse anti-laminin α2, Santa Cruz Biotechnology, Inc.; rabbit anti-laminin α4, Santa Cruz Biotechnology, Inc.; mouse anti-CD31, Abcam; rabbit anti-PDGFR-β, Cell Signaling Technology; mouse anti-GFAP, Sigma-Aldrich; mouse anti-SMA, Sigma-Aldrich; rat anti-SM1, Kamiya Biomedical Company; mouse anti-SM2, Abcam; goat anti-smoothelin, Santa Cruz Biotechnology, Inc.; and rabbit anti-SM22, Gene Tex), and then incubated with HRP-labeled secondary antibody (Jackson ImmunoResearch Laboratory) for 1 h at room temperature. Protein expression was observed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). After stripping, the membranes were reblotted with mouse anti-actin antibody (Sigma-Aldrich). All Western blot results were normalized to actin.
Cell culture
Human brain vascular smooth muscle cells (BVSMCs; Schwartz et al., 1986; Braun et al., 1999) and human astrocytes (van der Laan et al., 1997; Hatton, 2002; Chen and Swanson, 2003), obtained from ScienCell Research Laboratory, were cultured in an incubator with 5% CO2/95% air at 37°C. The second or third passages of the cells were used for experiments. Plates were coated with poly-l-lysine (PLL, 2 µg/cm2; ScienCell Research Laboratory), collagen type IV (4 µg/cm2; Gibco), laminin-111 (Gibco), laminin-211 (4 µg/cm2; a gift from Peter Yurchenco, Robert Wood Johnson Medical School, New Brunswick, NJ), or laminin-111 and -211 (2 µg/cm2 of each). The BVSMCs were plated with medium 231(Gibco) with smooth muscle growth supplement (Gibco) and 5% fetal bovine serum (FBS). Once the cells reached 80–90% confluence, medium was changed to medium 231 with 0.5% FBS and replaced every other day. Human astrocytes were cultured on PLL-coated plates with astrocyte medium containing 2% FBS (ScienCell Research Laboratory). Once they reached 80–90% confluence, astrocytes were collected for co-culture with BVSMCs.
For BVSMC and astrocyte co-culture, BVSMCs were cultured on PLL-coated plates. Once they reached 80–90% confluence, astrocytes (1,000 or 4,000 cells/cm2) were seeded with BVSMCs, and they were co-cultured for 6 d in astrocyte medium. The medium was replaced every other day. In BVSMC sister control cultures, the culture conditions and media were exactly the same as the co-cultures but without astrocytes.
For Western blot analysis, the cells were washed with cold PBS three times and lysed in lysis buffer as described above. In BVSMC and astrocyte co-culture experiments, the total cell numbers of control cultures and BVSMC/astrocyte co-cultures were determined. The percentages of astrocytes in the co-culture were determined by GFAP (a marker of astrocytes) immunocytochemistry (number of GFAP-positive cells versus total number of DAPI). The same percentage of astrocytes was added to the BVSMC control cultures before lysing the cells to ensure the SMC numbers were similar between the two groups for comparison of their differentiation.
Richardson’s staining and EM analysis
Mice were anesthetized and perfused with PBS and 2.5% PFA/2.5% glutaraldehyde in sodium cacodylate buffer. The brains were removed and immersed in the same fixative. Brain sections (100 µm) were cut on a vibratome and collected in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, post-fixed in reduced osmium (1% osmium tetroxide, 1.6% K-ferrocyanide in 0.1% cacodyate buffer), blocked, stained in 1% uranyl acetate, and embedded in resin. Semi-thin sections were cut and stained with Richardson’s staining for proteins. For EM analysis, ultra-thin sections were cut on a microtome (Ultracut E; Reichert-Jung) and post-stained with uranyl acetate and lead. Sections were examined and photographed on a transmission electron microscope (100CXII; JEOL) at 80 KV. For analysis of percentages of VSMCs showing direct contact with astrocytes or with abnormal morphology in different brain regions in LNγ1-KO and control mice (n = 7/group), the analyzer was blind to the genotypes of the samples. The differences between LNγ1-KO and control mice were analyzed by Student’s t test.
Imaging analysis
After immunohistochemistry or immunocytochemistry, brain sections were analyzed with a microscope (Axiovert 200; Carl Zeiss) equipped with Plan-Neofluar (1.25× NA 0.03; 20× NA 0.5, and 40× NA 0.75) objective lenses at room temperature. The imaging medium was air for all the objective lenses used. The AxioCam color camera (Carl Zeiss) and AxioVision software (Carl Zeiss) were used for image collection. Each set of stained sections was processed under identical gain and laser power settings. Each set of obtained images was processed under identical brightness and contrast settings using Adobe Photoshop. Figures were prepared using Microsoft PowerPoint. For quantification of fluorescence staining intensity after ad-GFAP-Cre injection, three brain sections containing the injection sites from three ad-GFAP-Cre–injected control and fLAMγ1 mice were used. The immunostaining signal intensity was analyzed using ImageJ (National Institutes of Health). The Western blot films were digitized, and the signal intensity of the bands was analyzed using ImageJ.
Statistical analysis
Prism 4 (GraphPad Software) was used for all statistical analysis as indicated in the figure legends by either Student’s t test or one-way analysis of variance (ANOVA).
Online supplemental material
Fig. S1 shows that all endothelial cells express laminin α4 in both control and LNγ1-KO mice. Fig. S2 shows disruption of laminin α2 expression at the astrocytic endfeet of LNγ1-KO mice and that neuronal laminin does not contribute to the vascular matrix. Fig. S3 shows Cre expression patterns in nestin-Cre mouse line. Fig. S4 shows region-specific VSMC contractile protein expression changes in LNγ1-KO mice during development. Fig. S5 shows relationship between astrocytic laminin α1 and SMA expression. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201212032/DC1.
Supplementary Material
Acknowledgments
We thank Eleana Sphicas and Kunihiro Uryu in the Electron Microscopy Resource Center at The Rockefeller University for technical support and assistance, Peter Yurchenco at UMDNJ for recombinant laminin-211, Arturo Alvaraz-Buylla at UCSF for Ad-pGFAP-Cre, and GENSAT at The Rockefeller University for ROSA26-EGFP mice. We also thank Anita Ramnarain and all members of the Strickland Laboratory for scientific support and advice.
This work was supported by NIH grant NS050537 and the Louis Herlands Fund.
Footnotes
Abbreviations used in this paper:
- BBB
- blood brain barrier
- BM
- basement membrane
- BVSMC
- brain vascular smooth muscle cell
- CD31
- cluster of differentiation 31
- CNS
- central nervous system
- EGFP
- enhanced green fluorescent protein
- EM
- electron microscopy
- GFAP
- glial fibrillary acidic protein
- ICH
- intracerebral hemorrhage
- LN
- laminin
- LNγ1-KO mice
- laminin γ1 knockout mice
- PDGFR-β
- platelet-derived growth factor receptor-β
- PLL
- poly-l-lysine
- SM1
- smooth muscle myosin heavy chain 1
- SM2
- smooth muscle myosin heavy chain 2
- SM22
- smooth muscle protein 22 α
- SMA
- smooth muscle actin α
- VSMC
- vascular smooth muscle cell
References
- Abbott N.J., Rönnbäck L., Hansson E. 2006. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7:41–53 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- Abraham S., Kogata N., Fässler R., Adams R.H. 2008. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ. Res. 102:562–570 10.1161/CIRCRESAHA.107.167908 [DOI] [PubMed] [Google Scholar]
- Braun M., Pietsch P., Schrör K., Baumann G., Felix S.B. 1999. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc. Res. 41:395–401 10.1016/S0008-6363(98)00302-2 [DOI] [PubMed] [Google Scholar]
- Campbell J.H., Campbell G.R. 1986. Endothelial cell influences on vascular smooth muscle phenotype. Annu. Rev. Physiol. 48:295–306 10.1146/annurev.ph.48.030186.001455 [DOI] [PubMed] [Google Scholar]
- Chen Y., Swanson R.A. 2003. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 23:137–149 10.1097/00004647-200302000-00001 [DOI] [PubMed] [Google Scholar]
- Chen Z.L., Strickland S. 2003. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 163:889–899 10.1083/jcb.200307068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.L., Yu H., Yu W.M., Pawlak R., Strickland S. 2008. Proteolytic fragments of laminin promote excitotoxic neurodegeneration by up-regulation of the KA1 subunit of the kainate receptor. J. Cell Biol. 183:1299–1313 10.1083/jcb.200803107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A.A., Barres B.A. 2010. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 468:562–566 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo G.J. 2009. Relationship of neurovascular elements to neuron injury during ischemia. Cerebrovasc. Dis. 27(Suppl 1):65–76 10.1159/000200442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo G.J., Milner R. 2006. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler. Thromb. Vasc. Biol. 26:1966–1975 10.1161/01.ATV.0000232525.65682.a2 [DOI] [PubMed] [Google Scholar]
- Durbeej M. 2010. Laminins. Cell Tissue Res. 339:259–268 10.1007/s00441-009-0838-2 [DOI] [PubMed] [Google Scholar]
- Eble J.A., Niland S. 2009. The extracellular matrix of blood vessels. Curr. Pharm. Des. 15:1385–1400 10.2174/138161209787846757 [DOI] [PubMed] [Google Scholar]
- Flintoff-Dye N.L., Welser J., Rooney J., Scowen P., Tamowski S., Hatton W., Burkin D.J. 2005. Role for the alpha7beta1 integrin in vascular development and integrity. Dev. Dyn. 234:11–21 10.1002/dvdy.20462 [DOI] [PubMed] [Google Scholar]
- Frieser M., Nöckel H., Pausch F., Röder C., Hahn A., Deutzmann R., Sorokin L.M. 1997. Cloning of the mouse laminin alpha 4 cDNA. Expression in a subset of endothelium. Eur. J. Biochem. 246:727–735 10.1111/j.1432-1033.1997.t01-1-00727.x [DOI] [PubMed] [Google Scholar]
- Glukhova M., Koteliansky V., Fondacci C., Marotte F., Rappaport L. 1993. Laminin variants and integrin laminin receptors in developing and adult human smooth muscle. Dev. Biol. 157:437–447 10.1006/dbio.1993.1147 [DOI] [PubMed] [Google Scholar]
- Gould D.B., Phalan F.C., Breedveld G.J., van Mil S.E., Smith R.S., Schimenti J.C., Aguglia U., van der Knaap M.S., Heutink P., John S.W. 2005. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 308:1167–1171 10.1126/science.1109418 [DOI] [PubMed] [Google Scholar]
- Gould D.B., Phalan F.C., van Mil S.E., Sundberg J.P., Vahedi K., Massin P., Bousser M.G., Heutink P., Miner J.H., Tournier-Lasserve E., John S.W. 2006. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N. Engl. J. Med. 354:1489–1496 10.1056/NEJMoa053727 [DOI] [PubMed] [Google Scholar]
- Graus-Porta D., Blaess S., Senften M., Littlewood-Evans A., Damsky C., Huang Z., Orban P., Klein R., Schittny J.C., Müller U. 2001. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 31:367–379 10.1016/S0896-6273(01)00374-9 [DOI] [PubMed] [Google Scholar]
- Hallmann R., Horn N., Selg M., Wendler O., Pausch F., Sorokin L.M. 2005. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 85:979–1000 10.1152/physrev.00014.2004 [DOI] [PubMed] [Google Scholar]
- Hatton G.I. 2002. Glial-neuronal interactions in the mammalian brain. Adv. Physiol. Educ. 26:225–237 [DOI] [PubMed] [Google Scholar]
- Hervé D., Chabriat H. 2010. Cadasil. J. Geriatr. Psychiatry Neurol. 23:269–276 10.1177/0891988710383570 [DOI] [PubMed] [Google Scholar]
- Herzig M.C., Winkler D.T., Burgermeister P., Pfeifer M., Kohler E., Schmidt S.D., Danner S., Abramowski D., Stürchler-Pierrat C., Bürki K., et al. 2004. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat. Neurosci. 7:954–960 10.1038/nn1302 [DOI] [PubMed] [Google Scholar]
- Hultgårdh-Nilsson A., Durbeej M. 2007. Role of the extracellular matrix and its receptors in smooth muscle cell function: implications in vascular development and disease. Curr. Opin. Lipidol. 18:540–545 10.1097/MOL.0b013e3282ef77e9 [DOI] [PubMed] [Google Scholar]
- Iadecola C., Nedergaard M. 2007. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10:1369–1376 10.1038/nn2003 [DOI] [PubMed] [Google Scholar]
- Jucker M., Tian M., Norton D.D., Sherman C., Kusiak J.W. 1996. Laminin alpha 2 is a component of brain capillary basement membrane: reduced expression in dystrophic dy mice. Neuroscience. 71:1153–1161 10.1016/0306-4522(95)00496-3 [DOI] [PubMed] [Google Scholar]
- Li F., Lan Y., Wang Y., Wang J., Yang G., Meng F., Han H., Meng A., Wang Y., Yang X. 2011. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev. Cell. 20:291–302 10.1016/j.devcel.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Lo E.H., Rosenberg G.A. 2009. The neurovascular unit in health and disease: introduction. Stroke. 40(3, Suppl):S2–S3 10.1161/STROKEAHA.108.534404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Fujiwara Y., Chapdelaine A., Yang H., Orkin S.H. 2001. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 97:324–326 10.1182/blood.V97.1.324 [DOI] [PubMed] [Google Scholar]
- Mayer S.A., Rincon F. 2005. Treatment of intracerebral haemorrhage. Lancet Neurol. 4:662–672 10.1016/S1474-4422(05)70195-2 [DOI] [PubMed] [Google Scholar]
- McCarron M.O., Nicoll J.A. 2004. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol. 3:484–492 10.1016/S1474-4422(04)00825-7 [DOI] [PubMed] [Google Scholar]
- McCarty J.H., Monahan-Earley R.A., Brown L.F., Keller M., Gerhardt H., Rubin K., Shani M., Dvorak H.F., Wolburg H., Bader B.L., et al. 2002. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol. Cell. Biol. 22:7667–7677 10.1128/MCB.22.21.7667-7677.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J.H., Lacy-Hulbert A., Charest A., Bronson R.T., Crowley D., Housman D., Savill J., Roes J., Hynes R.O. 2005. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 132:165–176 10.1242/dev.01551 [DOI] [PubMed] [Google Scholar]
- Miner J.H., Yurchenco P.D. 2004. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 20:255–284 10.1146/annurev.cellbio.20.010403.094555 [DOI] [PubMed] [Google Scholar]
- Mohan P.S., Spiro R.G. 1986. Macromolecular organization of basement membranes. Characterization and comparison of glomerular basement membrane and lens capsule components by immunochemical and lectin affinity procedures. J. Biol. Chem. 261:4328–4336 [PubMed] [Google Scholar]
- Moiseeva E.P. 2001. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc. Res. 52:372–386 10.1016/S0008-6363(01)00399-6 [DOI] [PubMed] [Google Scholar]
- Moore S.A., Saito F., Chen J., Michele D.E., Henry M.D., Messing A., Cohn R.D., Ross-Barta S.E., Westra S., Williamson R.A., et al. 2002. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 418:422–425 10.1038/nature00838 [DOI] [PubMed] [Google Scholar]
- Nguyen H.L., Lee Y.J., Shin J., Lee E., Park S.O., McCarty J.H., Oh S.P. 2011. TGF-β signaling in endothelial cells, but not neuroepithelial cells, is essential for cerebral vascular development. Lab. Invest. 91:1554–1563 10.1038/labinvest.2011.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NINDS ICH Workshop Participants 2005. Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke. 36:e23–e41 10.1161/01.STR.0000155685.77775.4c [DOI] [PubMed] [Google Scholar]
- Oide T., Nakayama H., Yanagawa S., Ito N., Ikeda S., Arima K. 2008. Extensive loss of arterial medial smooth muscle cells and mural extracellular matrix in cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL). Neuropathology. 28:132–142 10.1111/j.1440-1789.2007.00864.x [DOI] [PubMed] [Google Scholar]
- Orr A.W., Hastings N.E., Blackman B.R., Wamhoff B.R. 2010. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J. Vasc. Res. 47:168–180 10.1159/000250095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn D.L., Komotar R.J., Sander Connolly E. 2011. Hemodynamic mechanisms underlying cerebral aneurysm pathogenesis. J. Clin. Neurosci. 18:1435–1438 10.1016/j.jocn.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Pleşea I.E., Cameniţă A., Georgescu C.C., Enache S.D., Zaharia B., Georgescu C.V., Tenovici M. 2005. Study of cerebral vascular structures in hypertensive intracerebral haemorrhage. Rom. J. Morphol. Embryol. 46:249–256 [PubMed] [Google Scholar]
- Proctor J.M., Zang K., Wang D., Wang R., Reichardt L.F. 2005. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J. Neurosci. 25:9940–9948 10.1523/JNEUROSCI.3467-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A.I., Mendelow A.D., Hanley D.F. 2009. Intracerebral haemorrhage. Lancet. 373:1632–1644 10.1016/S0140-6736(09)60371-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppke L., Murphy E.A., Zarpellon A., Hofmann J.J., Merkulova A., Shields D.J., Weis S.M., Byzova T.V., Ruggeri Z.M., Iruela-Arispe M.L., Cheresh D.A. 2012. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood. 119:2149–2158 10.1182/blood-2011-04-348706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S.M., Campbell G.R., Campbell J.H. 1986. Replication of smooth muscle cells in vascular disease. Circ. Res. 58:427–444 10.1161/01.RES.58.4.427 [DOI] [PubMed] [Google Scholar]
- Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O., Sorokin L.M. 2001. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 153:933–946 10.1083/jcb.153.5.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth N., Vatansever H.S., Murray P., Meyer M., Frie C., Paulsson M., Edgar D. 1999. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 144:151–160 10.1083/jcb.144.1.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin L.M., Pausch F., Frieser M., Kröger S., Ohage E., Deutzmann R. 1997. Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev. Biol. 189:285–300 10.1006/dbio.1997.8668 [DOI] [PubMed] [Google Scholar]
- Steiner T., Petersson J., Al-Shahi Salman R., Christensen H., Cordonnier C., Csiba L., Harnof S., Krieger D., Mendelow D., Molina C., et al. ; European Research Network on Intracerebral Haemorrhage 2011. European research priorities for intracerebral haemorrhage. Cerebrovasc. Dis. 32:409–419 10.1159/000330653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland G.R., Auer R.N. 2006. Primary intracerebral hemorrhage. J. Clin. Neurosci. 13:511–517 10.1016/j.jocn.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Tagami M., Nara Y., Kubota A., Fujino H., Yamori Y. 1990. Ultrastructural changes in cerebral pericytes and astrocytes of stroke-prone spontaneously hypertensive rats. Stroke. 21:1064–1071 10.1161/01.STR.21.7.1064 [DOI] [PubMed] [Google Scholar]
- Takebayashi S., Kaneko M. 1983. Electron microscopic studies of ruptured arteries in hypertensive intracerebral hemorrhage. Stroke. 14:28–36 10.1161/01.STR.14.1.28 [DOI] [PubMed] [Google Scholar]
- Thyberg J., Hultgårdh-Nilsson A. 1994. Fibronectin and the basement membrane components laminin and collagen type IV influence the phenotypic properties of subcultured rat aortic smooth muscle cells differently. Cell Tissue Res. 276:263–271 10.1007/BF00306112 [DOI] [PubMed] [Google Scholar]
- Timpl R., Brown J.C. 1994. The laminins. Matrix Biol. 14:275–281 10.1016/0945-053X(94)90192-9 [DOI] [PubMed] [Google Scholar]
- Tronche F., Kellendonk C., Kretz O., Gass P., Anlag K., Orban P.C., Bock R., Klein R., Schütz G. 1999. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23:99–103 10.1038/12703 [DOI] [PubMed] [Google Scholar]
- Turlo K.A., Noel O.D., Vora R., LaRussa M., Fassler R., Hall-Glenn F., Iruela-Arispe M.L. 2012. An essential requirement for β1 integrin in the assembly of extracellular matrix proteins within the vascular wall. Dev. Biol. 365:23–35 10.1016/j.ydbio.2012.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan L.J., De Groot C.J., Elices M.J., Dijkstra C.D. 1997. Extracellular matrix proteins expressed by human adult astrocytes in vivo and in vitro: an astrocyte surface protein containing the CS1 domain contributes to binding of lymphoblasts. J. Neurosci. Res. 50:539–548 [DOI] [PubMed] [Google Scholar]
- Viswanathan A., Guichard J.P., Gschwendtner A., Buffon F., Cumurcuic R., Boutron C., Vicaut E., Holtmannspötter M., Pachai C., Bousser M.G., et al. 2006. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 129:2375–2383 10.1093/brain/awl177 [DOI] [PubMed] [Google Scholar]
- Wagenseil J.E., Mecham R.P. 2009. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 89:957–989 10.1152/physrev.00041.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.X., Shuaib A. 2007. Critical role of microvasculature basal lamina in ischemic brain injury. Prog. Neurobiol. 83:140–148 10.1016/j.pneurobio.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Wang H.U., Chen Z.F., Anderson D.J. 1998. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 93:741–753 10.1016/S0092-8674(00)81436-1 [DOI] [PubMed] [Google Scholar]
- Yen C.P., Lin C.L., Kwan A.L., Lieu A.S., Hwang S.L., Lin C.N., Howng S.L. 2005. Simultaneous multiple hypertensive intracerebral haemorrhages. Acta Neurochir. (Wien). 147:393–399, discussion :399 10.1007/s00701-004-0433-y [DOI] [PubMed] [Google Scholar]
- Yu W.M., Feltri M.L., Wrabetz L., Strickland S., Chen Z.L. 2005. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J. Neurosci. 25:4463–4472 10.1523/JNEUROSCI.5032-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.M., Yu H., Chen Z.L. 2007. Laminins in peripheral nerve development and muscular dystrophy. Mol. Neurobiol. 35:288–297 10.1007/s12035-007-0026-x [DOI] [PubMed] [Google Scholar]
- Yu W.M., Yu H., Chen Z.L., Strickland S. 2009. Disruption of laminin in the peripheral nervous system impedes nonmyelinating Schwann cell development and impairs nociceptive sensory function. Glia. 57:850–859 10.1002/glia.20811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P.D., Patton B.L. 2009. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 15:1277–1294 10.2174/138161209787846766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Winkles J.A., Gongora M.C., Polavarapu R., Michaelson J.S., Hahm K., Burkly L., Friedman M., Li X.J., Yepes M. 2007. TWEAK-Fn14 pathway inhibition protects the integrity of the neurovascular unit during cerebral ischemia. J. Cereb. Blood Flow Metab. 27:534–544 10.1038/sj.jcbfm.9600368 [DOI] [PubMed] [Google Scholar]
- Zhao B.Q., Tejima E., Lo E.H. 2007. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 38(2, Suppl):748–752 10.1161/01.STR.0000253500.32979.d1 [DOI] [PubMed] [Google Scholar]
- Zlokovic B.V. 2008. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 57:178–201 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.