Abstract
In many eukaryotes, cytokinesis requires an actomyosin contractile ring that is crucial for cell constriction and new membrane organization. Two studies in this issue (Onishi et al. 2013. J. Cell Biol. http://dx.doi.org.10.1083/jcb.201302001 and Atkins et al. 2013. J. Cell Biol. http://dx.doi.org.10.1083/jcb.201301090) establish that precise activation and/or inactivation of Rho1 and Cdc42 GTPases is important for the correct order and successful completion of events downstream of actomyosin ring constriction in budding yeast.
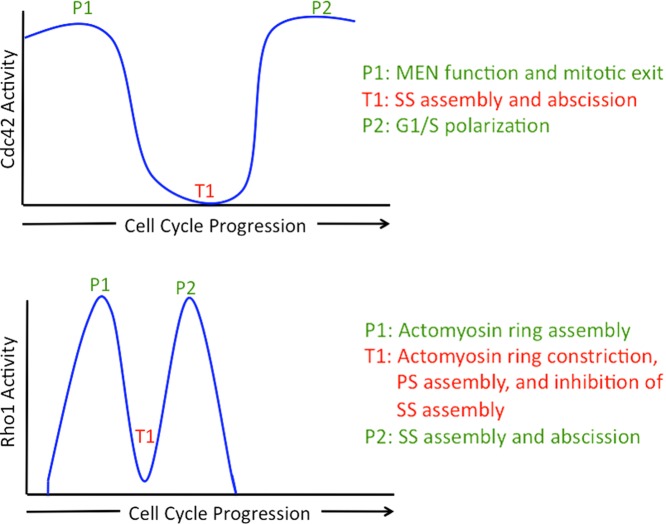
Cytokinesis is the terminal step in the cell cycle through which one cell physically divides into two daughters (Schroeder, 1990; Balasubramanian et al., 2004; Green et al., 2012). In many eukaryotes, ranging from yeast to human, cytokinesis depends on a division apparatus (known by several names, such as actomyosin ring, contractile ring, and cleavage furrow), which is composed of >100 proteins, including filamentous actin, the motor protein myosin II, IQGAP, and F-BAR domain–containing proteins (Oliferenko et al., 2009; Pollard and Wu, 2010). The cytokinetic–actomyosin ring generates constrictive force as well as guides the assembly of new membranes and the cell wall (in yeasts and fungi). Finally, through the process of abscission, the remaining cytoplasmic connections are resolved to liberate the two daughters (Neto et al., 2011; Green et al., 2012). How the later steps of cytokinesis (such as membrane/cell wall assembly and abscission) are coordinated with the earlier steps of cytokinesis (such as actomyosin ring maturation and contraction) remains poorly understood. Two papers in this issue of The Journal of Cell Biology (Atkins et al.; Oishi et al.) significantly clarify the molecular controls that coordinate the terminal steps of cytokinesis. From these studies, a picture emerges of exquisite and previously unappreciated temporal regulation of Rho1/A and Cdc42 family GTPases (Fig. 1) that is important for successful completion of cytokinesis in the budding yeast Saccharomyces cerevisiae.
Figure 1.
The highs and lows of Rho1 and Cdc42 during the cell cycle. Activity profiles of Rho1 and Cdc42 through the budding yeast cell cycle and the proposed functions for activation and inactivation of Rho1 and Cdc42. P1, P2, and T1 refer to peak 1, peak 2, and trough 1, respectively, of the activity of the GTPases described. PS and SS refer to primary septum and secondary septum, respectively. MEN refers to the mitotic exit network signaling module.
The Rho superfamily of GTPases, which comprise Cdc42, Rho, and Rac (Ridley, 1995; Tapon and Hall, 1997), regulate actin cytoskeletal remodeling and function during cell polarization and cytokinesis. In yeast and animal cells, Rho1/A (Rho1 in yeast and RhoA in animals) plays important roles in major aspects of actomyosin ring function (Tolliday et al., 2002; Piekny et al., 2005; Yoshida et al., 2006; Fededa and Gerlich, 2012). In its GTP-bound active form, Rho1/A binds to the actin filament nucleator formin to regulate actin polymerization at the division site. In animals, it also binds to the Rho-associated protein kinase (ROCK) through which it regulates myosin II contractility. Although Rho1/A has a clear role in cytokinesis, whether regulation of Cdc42, another key member of the Rho superfamily, is important for cytokinesis is unknown.
Onishi et al. (2013) further examine the role of Rho1 in cytokinesis in budding yeast. In this organism, the division septum is assembled in two stages, each thought to be indispensable. A primary septum, largely composed of chitin, is first assembled concomitant with actomyosin ring constriction. Subsequently, a secondary septum, largely composed of 1,3-β-glucan, is assembled on both sides of the primary septum (Bi and Park, 2012). Using electron microscopy, Onishi et al. (2013) found that even in wild-type cells, small gaps in the primary septum were masked by additional growth of the secondary septum. Furthermore, they found that the secondary septum, in addition to forming part of the cell wall of the daughter cells, itself might participate in cytokinetic abscission. Because the secondary septum was able to bypass partial loss of the primary septum, Onishi et al. (2013) searched for mechanisms regulating secondary septum assembly through high dosage genetic suppressor analysis with mutants strongly defective in primary septum synthesis. Remarkably, the authors found that up-regulation of Rho1 GTPase or down-regulation of Cdc42 GTPase activities led to secondary septum assembly and viability, even in cells devoid of Chs2, the enzyme involved in primary septum synthesis. These and previous studies (Tolliday et al., 2002; Yoshida et al., 2006) led to two conclusions: (1) Rho1 activation was key to actomyosin ring assembly and (2) Rho1 activation was essential for secondary septum synthesis and abscission. Through the use of temporally regulated expression of a constitutively active version of Rho1, Onishi et al. (2013) found that these two high activity states of Rho1 had to be interrupted by a phase in which Rho1 was maintained in an inactive state. The presence of a trough of Rho1 activity explains why secondary septum assembly occurs only after actomyosin ring constriction and primary septum assembly, despite the localization of Rho1-GTP effector Fks1 (enzyme that synthesizes 1,3-β-glucan in the secondary septum) before actomyosin ring constriction.
How is Rho1 temporarily inactivated, during actomyosin ring constriction and primary septum formation, to facilitate progression of cytokinesis? Onishi et al. (2013) reasoned that the SH3 and transglutaminase (TGc) domain protein Cyk3 (Korinek et al., 2000), a component of the actomyosin ring, might be part of this mechanism because both septa formed simultaneously in cyk3 mutants (possibly as a result of premature Fks1 activation in the absence of a mechanism maintaining Rho1 in its inactive GDP-bound form). Onishi et al. (2013) found that the TGc domain of Cyk3, which lacks enzymatic activity, physically interacted preferentially with GDP-bound Rho1. This biochemical interaction could also be observed in fluorescence-based protein interaction assays, leading them to conclude that the TGc domain of Cyk3 functioned akin to a GDP dissociation inhibitor for Rho1. Thus, it appears that the two peaks and one trough of Rho1 activity are all important for proper cytokinesis.
Although Onishi et al. (2013) found that down-regulation of Cdc42 promoted secondary septum assembly and cytokinesis, their study was focused on Rho1. The complementary study by Atkins et al. (2013) sheds light on how Cdc42 inhibition is regulated and how such an inhibition might regulate cytokinesis. These authors measured the fraction of active GTP-bound Cdc42 during the cell cycle using the Cdc42-GTP reporter CRIB (Burbelo et al., 1995). Interestingly, they found that Cdc42 was active in two peaks: in anaphase and during cell polarization at G1/S. These two phases of peak Cdc42 activity were interrupted by a period of trough during cytokinesis, when Cdc42 was predominantly GDP bound. Because expression of an activated form of Cdc42 was toxic to cells partially defective in actomyosin ring function and cytokinesis, Atkins et al. (2013) concluded that active Cdc42 interfered with cytokinesis.
How is Cdc42 inactivated in a temporally precise manner, and what downstream cytokinetic events depend on Cdc42 inactivation? Through a variety of genetic and biochemical experiments, Atkins et al. (2013) found that the Cdc5 protein kinase (related to Polo kinase in animals) was important for the inactivation of Cdc42 via phosphorylation of Bem2 and Bem3, which are GTPase-activating proteins for Cdc42 (Bi and Park, 2012). Consistently, Atkins et al. (2013) found that bem2 mutants (in which Cdc42 is inappropriately active) were defective in cell separation, suggesting a role for Bem2 (and likely Bem3) in aspects of actomyosin ring function or septum assembly. Through protein localization experiments, Atkins et al. (2013) found that Iqg1 (Epp and Chant, 1997; Osman and Cerione, 1998; Shannon and Li, 1999), a protein essential for actomyosin ring assembly and septum formation, and Inn1 (Sanchez-Diaz et al., 2008; Nishihama et al., 2009), a protein that links the plasma membrane to the actomyosin ring and participates in primary septum assembly, failed to properly localize in bem2 mutants. Conversely, increasing the level of Iqg1 rescued the cytokinesis defect of bem2 mutants, establishing that Iqg1 was a key effector affected by increased Cdc42 activity. Interestingly, Iqg1 localization and the cell separation defect were rectified in double mutants defective in bem2 and the Cdc42 effector kinase Ste20, suggesting that the down-regulation of a known canonical Cdc42 response pathway was key to proper cytokinesis. Thus, it appears that inactivation of Cdc42 is essential for the localization of proteins important for actomyosin ring constriction and secondary septum assembly to the division site.
Where do these studies leave us, and what are the open questions that emerge? An important question that follows is what is the precise temporal correlation between the activities of Rho1 and Cdc42 and is the temporary inactivation of Rho1 and Cdc42 activities necessary to avoid cross talk between these GTPase signaling pathways? Simultaneous analysis of Rho1 and Cdc42 activity and function in the same cell populations should begin to address this question. A second important question is precisely how does inhibition of Cdc42 lead to Iqg1 and Inn1 localization and what are the targets of Rho1 (other than Fks1) that participate in secondary septum formation during its second activity peak? The studies of Onishi et al. (2013) and Atkins et al. (2013) are remarkable in their breadth and depth, in that they have together shed detailed mechanistic insight into the physiological roles of proteins that are evolutionarily highly conserved. Whether similar mechanisms operate in other organisms can now be investigated.
Acknowledgments
We acknowledge Dr. Ramanujam Srinivasan for help with EndNote and Ms. Dhivya Subramaniam for helpful comments on the manuscript.
This work was supported by research funds from Temasek Life Sciences Laboratory, Singapore.
References
- Atkins B.D., Yoshida S., Saito K., Wu C.-F., Lew D.J., Pellman D. 2013. Inhibition of Cdc42 during mitotic exit is required for cytokinesis. J. Cell Biol. 202:231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M.K., Bi E., Glotzer M. 2004. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 14:R806–R818 10.1016/j.cub.2004.09.022 [DOI] [PubMed] [Google Scholar]
- Bi E., Park H.O. 2012. Cell polarization and cytokinesis in budding yeast. Genetics. 191:347–387 10.1534/genetics.111.132886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo P.D., Drechsel D., Hall A. 1995. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 270:29071–29074 10.1074/jbc.270.49.29071 [DOI] [PubMed] [Google Scholar]
- Epp J.A., Chant J. 1997. An IQGAP-related protein controls actin-ring formation and cytokinesis in yeast. Curr. Biol. 7:921–929 10.1016/S0960-9822(06)00411-8 [DOI] [PubMed] [Google Scholar]
- Fededa J.P., Gerlich D.W. 2012. Molecular control of animal cell cytokinesis. Nat. Cell Biol. 14:440–447 10.1038/ncb2482 [DOI] [PubMed] [Google Scholar]
- Green R.A., Paluch E., Oegema K. 2012. Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 28:29–58 10.1146/annurev-cellbio-101011-155718 [DOI] [PubMed] [Google Scholar]
- Korinek W.S., Bi E., Epp J.A., Wang L., Ho J., Chant J. 2000. Cyk3, a novel SH3-domain protein, affects cytokinesis in yeast. Curr. Biol. 10:947–950 10.1016/S0960-9822(00)00626-6 [DOI] [PubMed] [Google Scholar]
- Neto H., Collins L.L., Gould G.W. 2011. Vesicle trafficking and membrane remodelling in cytokinesis. Biochem. J. 437:13–24 10.1042/BJ20110153 [DOI] [PubMed] [Google Scholar]
- Nishihama R., Schreiter J.H., Onishi M., Vallen E.A., Hanna J., Moravcevic K., Lippincott M.F., Han H., Lemmon M.A., Pringle J.R., Bi E. 2009. Role of Inn1 and its interactions with Hof1 and Cyk3 in promoting cleavage furrow and septum formation in S. cerevisiae. J. Cell Biol. 185:995–1012 10.1083/jcb.200903125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliferenko S., Chew T.G., Balasubramanian M.K. 2009. Positioning cytokinesis. Genes Dev. 23:660–674 10.1101/gad.1772009 [DOI] [PubMed] [Google Scholar]
- Onishi M., Ko N., Nishihama R., Pringle J.R. 2013. Distinct roles of Rho1, Cdc42, and Cyk3 in septum formation and abscission during yeast cytokinesis. J. Cell Biol. 202:311–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman M.A., Cerione R.A. 1998. Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton. J. Cell Biol. 142:443–455 10.1083/jcb.142.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekny A., Werner M., Glotzer M. 2005. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 15:651–658 10.1016/j.tcb.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Pollard T.D., Wu J.Q. 2010. Understanding cytokinesis: lessons from fission yeast. Nat. Rev. Mol. Cell Biol. 11:149–155 10.1038/nrm2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J. 1995. Rho-related proteins: actin cytoskeleton and cell cycle. Curr. Opin. Genet. Dev. 5:24–30 10.1016/S0959-437X(95)90049-7 [DOI] [PubMed] [Google Scholar]
- Sanchez-Diaz A., Marchesi V., Murray S., Jones R., Pereira G., Edmondson R., Allen T., Labib K. 2008. Inn1 couples contraction of the actomyosin ring to membrane ingression during cytokinesis in budding yeast. Nat. Cell Biol. 10:395–406 10.1038/ncb1701 [DOI] [PubMed] [Google Scholar]
- Schroeder T.E. 1990. The contractile ring and furrowing in dividing cells. Ann. NY Acad. Sci. 582:78–87 10.1111/j.1749-6632.1990.tb21669.x [DOI] [PubMed] [Google Scholar]
- Shannon K.B., Li R. 1999. The multiple roles of Cyk1p in the assembly and function of the actomyosin ring in budding yeast. Mol. Biol. Cell. 10:283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N., Hall A. 1997. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol. 9:86–92 10.1016/S0955-0674(97)80156-1 [DOI] [PubMed] [Google Scholar]
- Tolliday N., VerPlank L., Li R. 2002. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12:1864–1870 10.1016/S0960-9822(02)01238-1 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kono K., Lowery D.M., Bartolini S., Yaffe M.B., Ohya Y., Pellman D. 2006. Polo-like kinase Cdc5 controls the local activation of Rho1 to promote cytokinesis. Science. 313:108–111 10.1126/science.1126747 [DOI] [PubMed] [Google Scholar]