Abstract
Because glomerular functions are modulated by numerous humoral agents, probably acting through cyclic nucleotides, the effects of some polypeptide hormones and biogenic amines on cyclic AMP (cAMP) and cyclic 3′,5′-guanosine monophosphate (cGMP) were studied in glomeruli isolated from rat renal cortex. Glomeruli and cortical tubules were prepared by a combination of sieving and density-gradient centrifugation. Under basal conditions, the contents of cAMP and cGMP in glomeruli were significantly higher than in tubules and unfractionated renal cortical tissue.
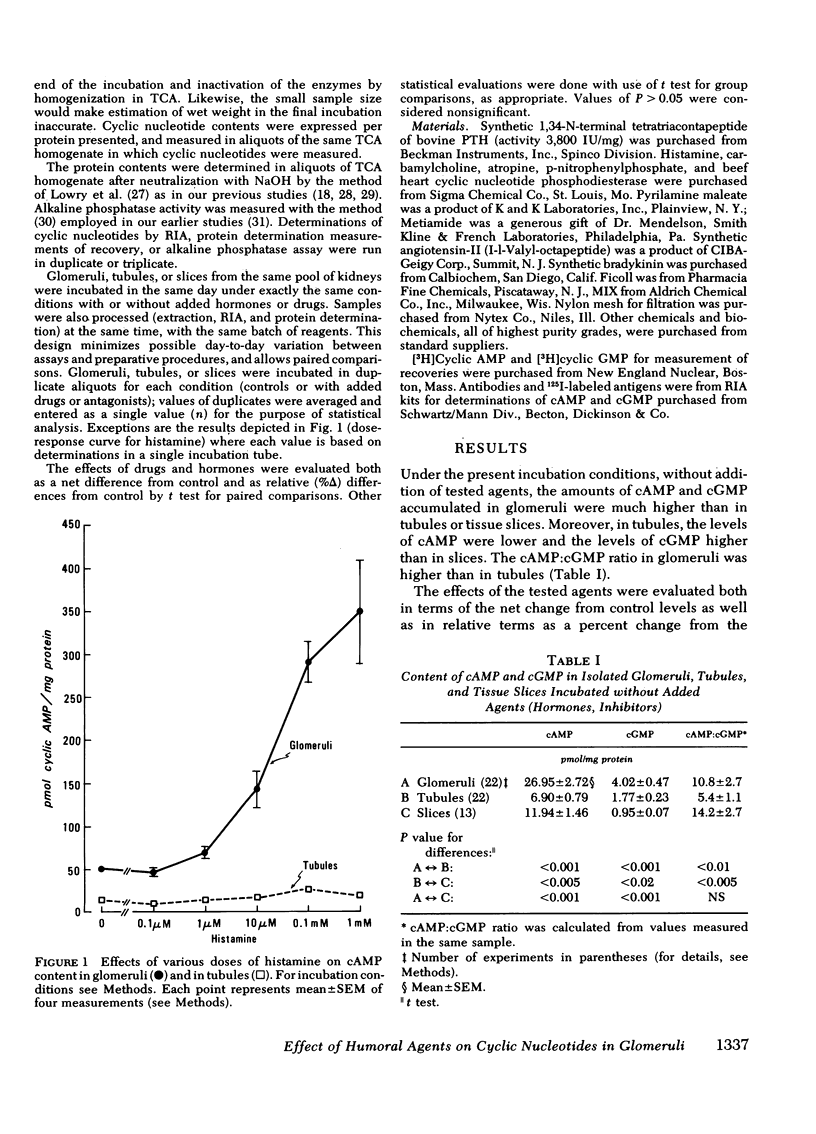
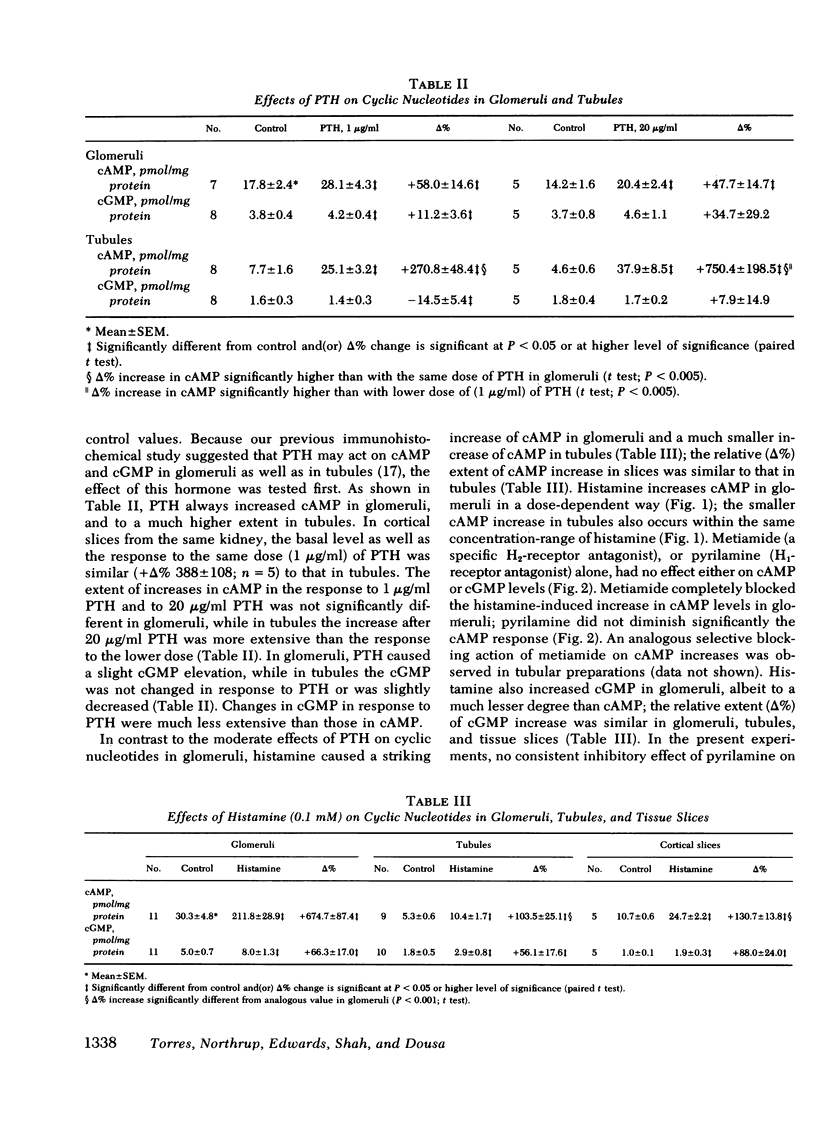
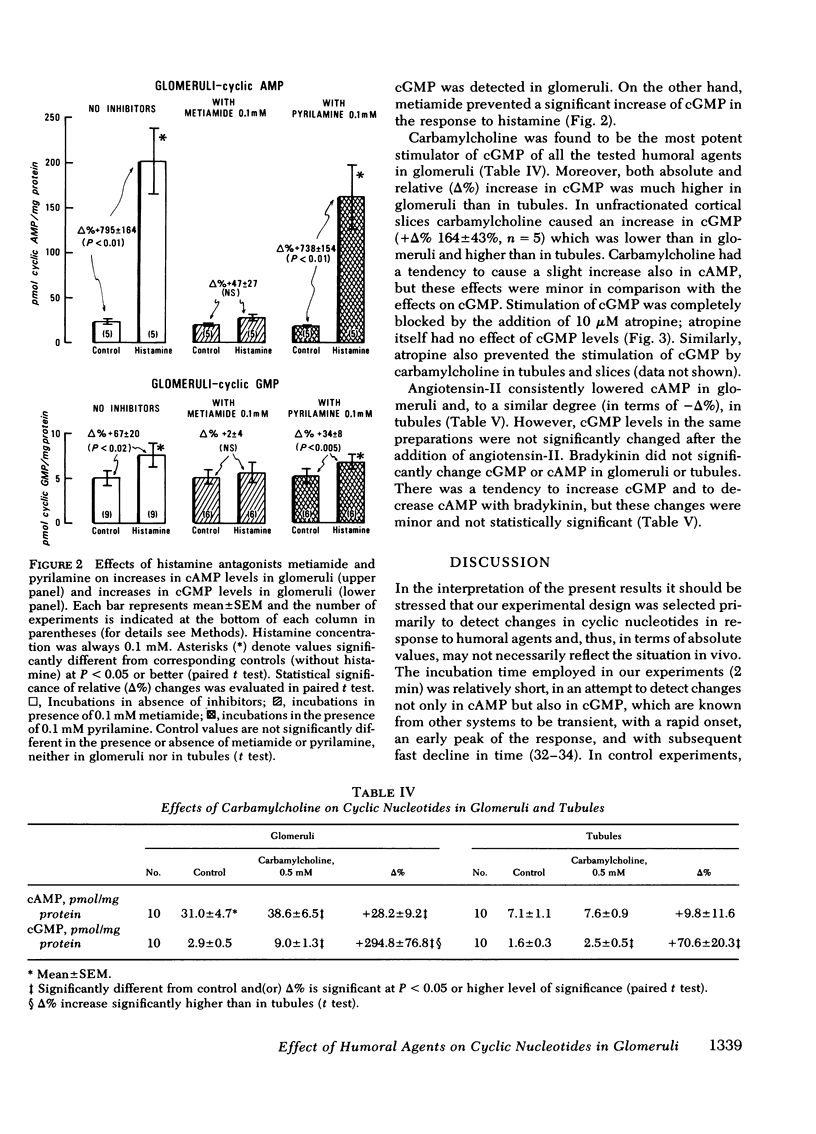
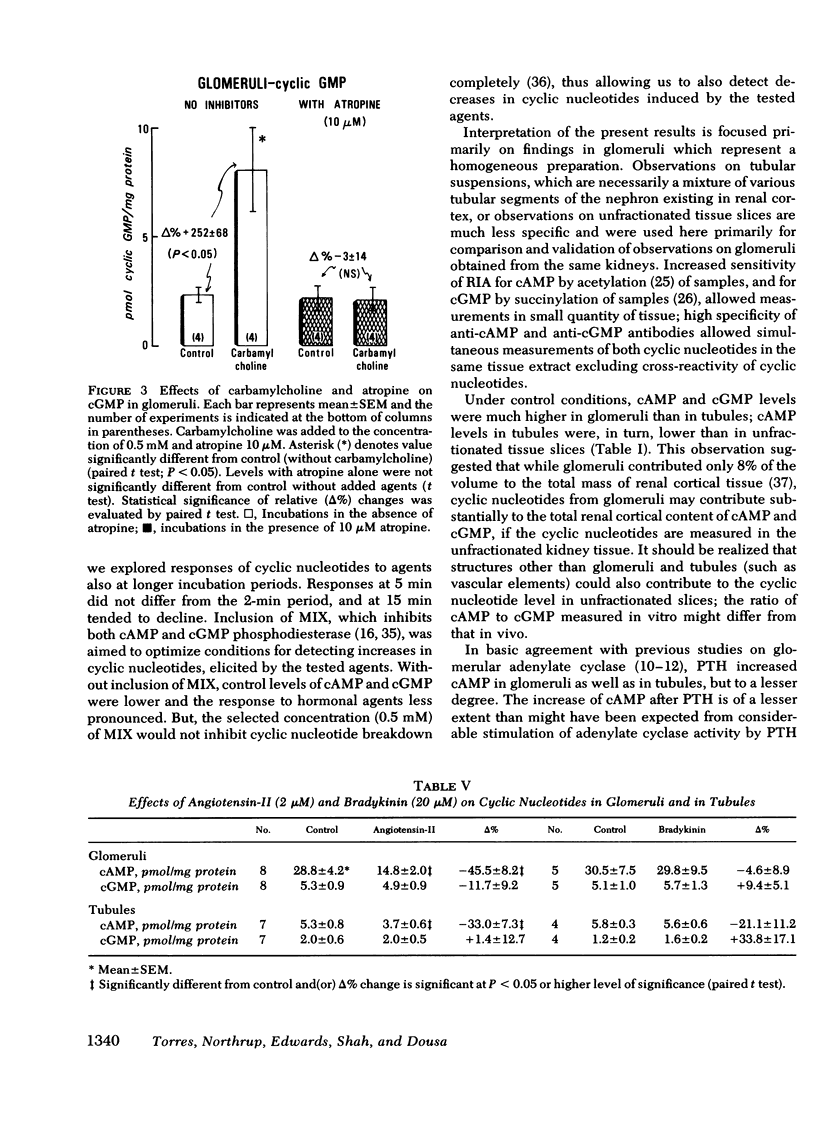
Histamine caused a striking increase in cAMP in glomeruli (+Δ% 675±87) and, to a lesser degree, increased cAMP in tubules (+Δ% 103±25) or in tissue slices. This stimulation was dose-dependent in the range of 1 μM-1 mM histamine. Metiamide (an H2-antagonist), but not pyrilamine (an H1-antagonist) blocked the effect of histamine on cAMP, which indicates that histamine causes its effect via interaction with H2 receptors. Histamine caused less extensive increases in cGMP in both glomeruli and tubules. Carbamylcholine caused a marked increase in cGMP in glomeruli (+Δ 295±7) and a much lower increase in tubules (+Δ% 70±20); these effects were blocked by atropine. Parathyroid hormone (1 μg/ml) increased cAMP and, to a much lesser degree, also cGMP in glomeruli. In tubules, parathyroid hormone caused much more extensive increases in cAMP than in glomeruli; no changes, or rather a small decline in cGMP, was observed. Angiotensin-II (2 μM) markedly lowered cAMP in glomeruli (−Δ% −45±8) and in tubules (−Δ% 33±7) but had no effect on cGMP. Bradykinin (20 μM) did not consistently influence either cAMP or cGMP in glomeruli or tubules.
Present results demonstrate that cAMP and cGMP metabolism in glomeruli are controlled independently by humoral agents known to alter glomerular functions in vivo. Our findings are consistent with the view that histamine and cholinergic agents generated and (or) released locally in glomeruli or in their vicinity may play important roles as mediators of immunopathological injury of glomeruli, and that these effects are mediated by cAMP and (or) cGMP through interaction with H2 receptors and muscarinic receptors. Likewise, our results suggest that the effects of angiotensin-II and parathyroid hormone on glomerular dynamics may be mediated by cyclic nucleotides.
Thus, we surmise that extrarenal as well as intrarenal humoral agents may play an important role in the pathology and physiology of glomeruli through mediation of either cAMP, cGMP, or both.
Full text
PDF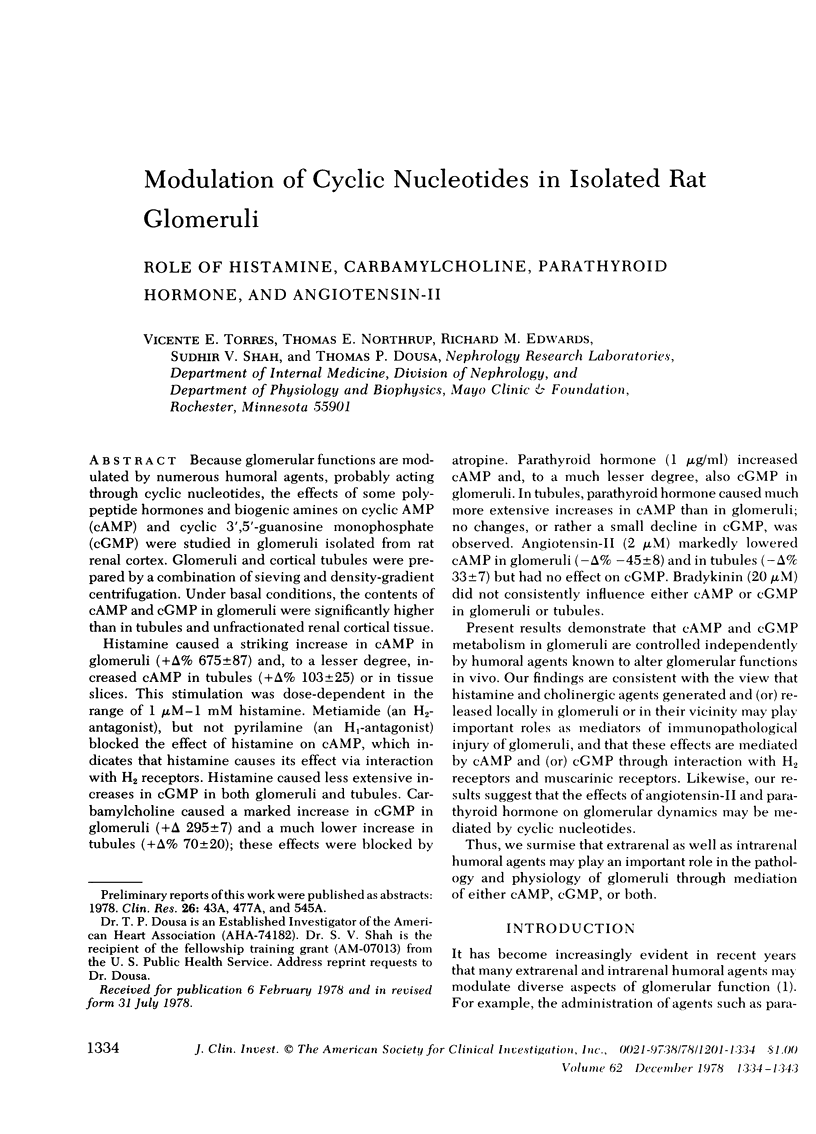





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylis C., Deen W. M., Myers B. D., Brenner B. M. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976 Apr;230(4):1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Konnen K. S., Tucker B. J. Angiotensin II effects upon the glomerular microcirculation and ultrafiltration coefficient of the rat. J Clin Invest. 1976 Feb;57(2):419–434. doi: 10.1172/JCI108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer M. P., Deen W. M., Robertson C. R., Brenner B. M. Mechanism of angiotensin II-induced proteinuria in the rat. Am J Physiol. 1977 Jul;233(1):F13–F21. doi: 10.1152/ajprenal.1977.233.1.F13. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Baylis C., Deen W. M. Transport of molecules across renal glomerular capillaries. Physiol Rev. 1976 Jul;56(3):502–534. doi: 10.1152/physrev.1976.56.3.502. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Orloff J. Effect of temperature and medium K on Na and K fluxes in separated renal tubules. Am J Physiol. 1966 Oct;211(4):1005–1010. doi: 10.1152/ajplegacy.1966.211.4.1005. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Sandler J. A., Manganiello V. C., Vaughan M. Guanosine 3',5'-monophosphate and adenosine 3',5'-monophosphate content of human umbilical artery. J Clin Invest. 1975 May;55(5):1020–1025. doi: 10.1172/JCI108002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. A. Properties of the guanylate cyclase-guanosine 3':5'-monophosphate system of rat renal cortex. Activation of guanylate cyclase and calcium-independent modulation of tissue guanosine 3':5'-monophosphate by sodium azide. J Biol Chem. 1976 Aug 10;251(15):4651–4658. [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D., Ong S. H., Steiner A. L. Immunohistochemical localization of 3':5'-cyclic AMP and 3':5'-cyclic GMP in rat renal cortex: effect of parathyroid hormone. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3569–3573. doi: 10.1073/pnas.74.8.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T. P., Duarte C. G., Knox F. G. Effect of colchicine on urinary phosphate and regulation by parathyroid hormone. Am J Physiol. 1976 Jul;231(1):61–65. doi: 10.1152/ajplegacy.1976.231.1.61. [DOI] [PubMed] [Google Scholar]
- Earp H. S., Smith P., Huang Ong S. H., Steiner A. L. Regulation of hepatic nuclear guanylate cyclase. Proc Natl Acad Sci U S A. 1977 Mar;74(3):946–950. doi: 10.1073/pnas.74.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci. 1976 Mar 1;18(5):529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Hall D. A., Barnes L. D., Dousa T. P. Cyclic AMP in action of antidiuretic hormone: effects of exogenous cyclic AMP and its new analogue. Am J Physiol. 1977 Apr;232(4):F368–F376. doi: 10.1152/ajprenal.1977.232.4.F368. [DOI] [PubMed] [Google Scholar]
- Harbon S., Clauser H. Cyclic adenosine 3',5' monophosphate levels in rat myometrium under the influence of epinephrine, prostaglandins and oxytocin, correlations with uterus motility. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1496–1503. doi: 10.1016/s0006-291x(71)80255-3. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Heald J. I., Hollis T. M. Histidine decarboxylase-mediated histamine synthesis in glomeruli from rat kidneys. Am J Physiol. 1976 May;230(5):1349–1353. doi: 10.1152/ajplegacy.1976.230.5.1349. [DOI] [PubMed] [Google Scholar]
- Helwig J. J., Bollack C., Mandel P., Goridis C. Renal cortex guanylate cyclase. Preferential enrichment in glomerular membranes. Biochim Biophys Acta. 1975 Feb 19;377(2):463–472. doi: 10.1016/0005-2744(75)90326-5. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Ichikawa I., Troy J. L., Brenner B. M. Evidence for a parathyroid hormone-dependent influence of calcium on the glomerular ultrafiltration coefficient. J Clin Invest. 1978 Jan;61(1):32–40. doi: 10.1172/JCI108922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa I., Humes H. D., Dousa T. P., Brenner B. M. Influence of parathyroid hormone on glomerular ultrafiltration in the rat. Am J Physiol. 1978 May;234(5):F393–F401. doi: 10.1152/ajprenal.1978.234.5.F393. [DOI] [PubMed] [Google Scholar]
- Juhlin L. Determination of histamine in small biopsies and histological sections. Acta Physiol Scand. 1967 Sep;71(1):30–36. doi: 10.1111/j.1748-1716.1967.tb03706.x. [DOI] [PubMed] [Google Scholar]
- Kaliner M., Austen K. F. Immunologic release of chemical mediators from human tissues. Annu Rev Pharmacol. 1975;15:177–189. doi: 10.1146/annurev.pa.15.040175.001141. [DOI] [PubMed] [Google Scholar]
- Kapoor C. L., Krishna G. Hormone-induced cyclic guanosine monophosphate secretion from guinea pig pancreatic lobules. Science. 1977 May 27;196(4293):1003–1005. doi: 10.1126/science.193187. [DOI] [PubMed] [Google Scholar]
- Katsuki S., Murad F. Regulation of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol. 1977 Mar;13(2):330–341. [PubMed] [Google Scholar]
- Kim J. K., Frohnert P. P., Hui Y. S., Barnes L. D., Farrow G. M., Dousa T. P. Enzymes of cyclic 3',5'-nucleotide metabolism in human renal cortex and renal adenocarcinoma. Kidney Int. 1977 Sep;12(3):172–183. doi: 10.1038/ki.1977.98. [DOI] [PubMed] [Google Scholar]
- Knox F. G., Preiss J., Kim J. K., Dousa T. P. Mechanism of resistance to the phosphaturic effect of the parathyroid hormone in the hamster. J Clin Invest. 1977 Apr;59(4):675–683. doi: 10.1172/JCI108686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lansing A. I., Belkhode M. L., Lynch W. E., Lieberman I. Enzymes of plasma membranes of liver. J Biol Chem. 1967 Apr 25;242(8):1772–1775. [PubMed] [Google Scholar]
- Murad F., Kimura H. Cyclic nucleotide levels in incubations of guinea pig trachea. Biochim Biophys Acta. 1974 Apr 22;343(2):275–286. doi: 10.1016/0304-4165(74)90092-0. [DOI] [PubMed] [Google Scholar]
- Murad F., Manganiello V., Vaughan M. A simple, sensitive protein-binding assay for guanosine 3':5'-monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):736–739. doi: 10.1073/pnas.68.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgaard J. O. A new method for the isolation of ultrastructurally preserved glomeruli. Kidney Int. 1976 Mar;9(3):278–285. doi: 10.1038/ki.1976.30. [DOI] [PubMed] [Google Scholar]
- Osborne M. J., Droz B., Meyer P., Morel F. Angiotensin II: renal localization in glomerular mesangial cells by autoradiography. Kidney Int. 1975 Oct;8(4):245–254. doi: 10.1038/ki.1975.108. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Sullivan T. J., Wedner H. J. Cyclic AMP and the immune response;. Adv Cyclic Nucleotide Res. 1974;4(0):1–79. [PubMed] [Google Scholar]
- Sraer J. D., Sraer J., Ardaillou R., Mimoune O. Evidence for renal glomerular receptors for angiotensin II. Kidney Int. 1974 Oct;6(4):241–246. doi: 10.1038/ki.1974.105. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stoner J. S., Manganiello V. C., Vaughan M. Guanosine cyclic 3',5'-monophosphate and guanylate cyclase activity in guinea pig lung: effects of acetylcholine and cholinesterase inhibitors. Mol Pharmacol. 1974 Jan;10(1):155–161. [PubMed] [Google Scholar]
- Taylor D. G., Price R. G., Robinson D. The distribution of some hydrolases in glomeruli and tubular fragments prepared from rat kidney by zonal centrifugation. Biochem J. 1971 May;122(5):641–645. doi: 10.1042/bj1220641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres V. E., Hui Y. S., Shah S. V., Northrup T. E., Dousa T. P. Cyclic nucleotide phosphodiesterases in glomeruli of rat renal cortex. Kidney Int. 1978 Nov;14(5):444–451. doi: 10.1038/ki.1978.149. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Craven P. A., DeRubertis F. R., Davis B. B. Differential inhibition of cyclic AMP and cyclic GMP hydrolysis in rat renal cortex. Arch Biochem Biophys. 1977 Jan 30;178(2):598–606. doi: 10.1016/0003-9861(77)90231-4. [DOI] [PubMed] [Google Scholar]



