Abstract
Rationale
Endothelial progenitor cells (EPCs) contribute to the regeneration of endothelium. Aging-associated senescence results in reduced number and function of EPCs, potentially contributing to increased cardiac risk, reduced angiogenic capacity, and impaired cardiac repair effectiveness. The mechanisms underlying EPC senescence are unknown. Increasing evidence supports the role of microRNAs in regulating cellular senescence.
Objective
We aimed to determine whether microRNAs regulated EPC senescence and, if so, what the underlying mechanisms are.
Methods and Results
To map the microRNA/gene expression signatures of EPC senescence, we performed microRNA profiling and microarray analysis in lineage-negative bone marrow cells from young and aged wild-type and apolipoprotein E–deficient mice. We identified 2 microRNAs, microRNA-10A* (miR-10A*), and miR-21, and their common target gene Hmga2 as critical regulators for EPC senescence. Overexpression of miR-10A* and miR-21 in young EPCs suppressed Hmga2 expression, caused EPC senescence, as evidenced by senescence-associated β–galactosidase upregulation, decreased self-renewal potential, increased p16Ink4a/p19Arf expression, and resulted in impaired EPC angiogenesis in vitro and in vivo, resembling EPCs derived from aged mice. In contrast, suppression of miR-10A* and miR-21 in aged EPCs increased Hmga2 expression, rejuvenated EPCs, resulting in decreased senescence-associated β–galactosidase expression, increased self-renewal potential, decreased p16Ink4a/p19Arf expression, and improved EPC angiogenesis in vitro and in vivo. Importantly, these phenotypic changes were rescued by miRNA-resistant Hmga2 cDNA overexpression.
Conclusions
miR-10A* and miR-21 regulate EPC senescence via suppressing Hmga2 expression and modulation of microRNAs may represent a potential therapeutic intervention in improving EPC-mediated angiogenesis and vascular repair.
Keywords: aging, angiogenesis, cellular transplantation, endothelial progenitor cells, high-mobility group A, microRNAs, senescence
Postnatal neovascularization involves endothelial progenitor cells (EPCs) derived from the bone marrow. EPCs play important roles in vascular homeostasis and compensatory vasculogenesis with their plasticity to differentiate into endothelial cells (ECs) and as a source of paracrine pro-angiogenic factors. EPCs are usually defined retrospectively by their ability to differentiate into ECs. Because diverse progenitor cell types have been shown to differentiate into ECs, there is no universally accepted molecular definition for EPCs. Several types of EPCs have been studied: (1) EPCs defined by cell surface markers, including CD34, CD133/CD34, CD133/vascular endothelial growth factor receptor-2 or CD133/CD34/vascular endothelial growth factor receptor-2; (2) early outgrowth EPCs, of myeloid origin, which stimulate angiogenic responses through paracrine secretion of angiogenic factors; and (3) late outgrowth EPCs, of nonhematopoietic origin, possessing the capacity of de novo vessel formation through direct incorporation into the growing vasculature.1 Regardless of what markers/techniques are used to define EPCs, it is conceivable that these cells are enriched in the lineage-negative bone marrow cells (lin− BMCs). Remarkably, multiple studies have shown that the number and angiogenic function of EPCs decline with aging.2,3 Furthermore, cardiovascular risk factors accelerate the numerical exhaustion and senescence of these cells.4,5 Little is known, however, regarding the molecular mechanisms governing these senescent changes.
MicroRNAs (miRNAs) are a class of small noncoding RNAs of ≈22 nucleotides. During miRNA biogenesis, 1 strand of the duplex, the guide strand, is preferentially incorporated by the argonaute proteins into the RNA-induced silencing complex, promoting degradation or inhibiting translation of transcripts with basepair complimentarity.6 In contrast, the partner strand miRNA* accumulates to lower levels than the guide strand and generally is assumed to be degraded. Emerging evidence, however, indicates that miRNA* species can coaccumulate with their guide strand and mediate gene regulation.7 MiRNAs have been implicated in regulating diverse cellular processes, including differentiation, proliferation, and senescence.8 Recent evidence has shown that miRNAs also may play a role in regulating angiogenesis and endothelial functions.9–12 For example, miR-27b, let-7f, miR-17–92 cluster, and miR-126 play a proangiogenic role, whereas miR-221/miR-222, miR-34, and miR-217 act as antiangiogenic miRNAs in ECs.10,11 miR-21 is dysregulated in many cancers.13 An increased expression of miR-21 in tumor cells is associated with a higher proliferation, invasion capacity, and increased angiogenesis.14 Interestingly, miR-21 in ECs exhibits antiangiogenic functions.15 The expression profile and functions of miRNAs in EPCs remain to be defined.
Hmga2 is a member of the high-mobility group A (Hmga) family that encodes a small, chromatin-associated protein that modifies transcription by altering chromatin structure.16 Recent studies have shown that Hmga2 is preferentially expressed in fetal and young adult neural stem cells (NSCs), but not in aged adult NSCs. Importantly, Hmga2 expression decreases with aging of embryonic stem cells, hematopoietic stem cells, and NSCs, and deletion of Hmga2 impairs NSC function.17,18 Furthermore, Hmga2 promotes NSC self-renewal activity by negatively regulating p16Ink4a and p19Arf expression.18–21 Disrupting the repression of Hmga2 enhances colony formation of NIH3T3 cells.21 Downregulation of Hmga2 by let-7, miR-23a, miR-26a, and miR-30a accelerates cellular senescence of human umbilical cord blood–derived multipotent stem cells.22 The putative role of Hmga2 in regulating EPC senescence and the miRNAs involved remain to be elucidated.
In this report, we show that miR-10A* and miR-21 are progressively expressed and Hmga2 expression is gradually decreased in lin− BMCs, cells that are enriched for EPCs, during aging. We provide evidence that miR-10A* and miR-21 regulate Hmga2 expression. Importantly, the miR-10A*/miR-21–Hmga2–P16Ink4A/P19Arf axis controls EPC senescence and angiogenesis.
Methods
Detailed Methods are available in the Online Data Supplement.
Animals
Apolipoprotein E–deficient (apoE−/−) and wild-type (WT) C57BL/6J mice and FVB/N mice were used.
Isolation and Culture of Lin− BMCs
Mouse lin− BMCs were isolated from the WT or apoE−/− C57B/6 mice of different ages by magnetic separation using mouse Lineage Cell Depletion Kit.
RNA Isolation and Quantitative Reverse-Transcription Polymerase Chain Reaction
Detailed Methods are available in the Online Data Supplement material.
miRNA Microarray Hybridization and Data Analysis
Low-molecular-weight RNA was sent to Ocean Ridge Biosciences for analysis.
Hierarchical Clustering of MiRNA Array Data
Data for the 282 mouse miRNA probes were clustered using Cluster 3.0 software.
Gene Expression Microarray Hybridization and Data Analysis
High-molecular-weight RNA fraction from each sample was used for hybridization to the mouse exonic evidence-based oligonucleotide microarrays containing 38 083 70-mer-oligonucleotide probes.
miRNA and mRNA Inverse Correlation Analysis
miRNA gene targets were determined using the online miRNA database. The MicroCosm Targets version 5 was used.
miRNA Transfection
To study the biological effects of miRNAs on target gene expression, we performed transfection of pre-miR mimic or anti-miR inhibitors.
Lentivirus Production and EPC Cell Lines
Lentiviral supernatants were produced using standard procedures.
Self-Renewal Assay and Cell Proliferation
Self-renewal potential was examined by colony formation assay.
Western Blotting Analysis
Cell extracts were separated by SDS-PAGE in reducing and denaturing conditions.
Scratch Wound Migration Assay
The scratch wound migration assay was used to assess the potential effects of miR-10A*, miR-21, and Hmga2 on EC migration and wound healing.
In Vitro Angiogenesis Assay
The in vitro angiogenic activity of lin− BMCs was determined by Matrigel tube formation assay.
In Vivo Angiogenesis Assay
The Matrigel plug assay and hindlimb ischemic mouse model were performed to study the effects of miR-10A*, miR-21, and their anti-miRs on angiogenesis in vivo.
Senescence-Associated β-Galactosidase Staining and P16Ink4a/P19Arf Expression
Lin− BMC senescence was determined by in situ staining for senescence-associated β-galactosidase (SA-β-gal) and by Western blot and fluorescence-activated cell sorter analysis for p16Ink4a/p19Arfexpression.
Site-Directed Mutagenesis
Point mutations in the miR-10A* and miR-21 binding sites within the Hmga2 3′ untranslated region (UTR) were introduced with the QuikChange II XL site-directed mutagenesis kit (Stratagene).
Luciferase Reporter Assay
The effects of miRNAs on their target mRNA expression were examined by luciferase reporter assay in 293 T cells.
Statistical Analysis
Data were expressed as mean±SD. Student t test and analysis of variants were used to assess differences, with P<0.05 considered to be significant.
Results
miR-10A* and miR-21 Are Upregulated in Aged EPCs
To identify miRNAs involved in EPC senescence, we performed miRNA profiling using the Sanger 13 miRNA. Array targeting 686 mouse miRNAs in lin− BMCs from young (3-week-old apoE−/− and WT) and aged (1-year-old apoE−/− and 2.5-year-old WT) mice, 2 extremes of the aging spectrum. After quality-control, background subtraction, transformation, and normalization of probe intensity, 282 miRNAs were found to be present in at least 1 group of lin− BMCs. Fifty-six miRNAs were differentially expressed in young vs aged cells at a false discovery rate of 0.05. Among them, 38 miRNAs had consistent changes in expression patterns in lin− BMCs from young vs aged apoE−/− and WT mice: 14 were elevated and 24 were downregulated in aged mice. Principal component analysis demonstrated that these differentially expressed miRNAs classified mice well by age regardless of the apoE genotype (Figure 1A), suggesting that the aging process impacts the expression of these miRNAs in lin− BMCs, independent of apoE expression. The top 20 differentially expressed miRNAs in young and aged mice with P<0.001 are listed in Online Table II. Two of the most robustly upregulated miRNAs in aged mice were miR-10A* and miR-21. To validate these findings, we performed reverse-transcription polymerase chain reaction (qRT-PCR) for these 2 miRNAs, which revealed fold changes comparable with those found by miRNA array (Figure 1B).
Figure 1. Differential microRNA (miRNA) and target gene expression in young and aged lineage-negative bone marrow cells (lin− BMCs).
Unsupervised hierarchical clustering of differentially expressed miRNAs (A) in lin− BMCs from old apolipoprotein E–deficient (apoE−/ −) (AO) and wild-type (WO) as well as young apoE−/ − (AY) and wild-type (WY) mice. Red indicates higher expression; green denotes lower expression. Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) validation of miR-10A* and miR-21 (B) and their target Hmga2 expression (C) reveals similar reciprocal expression changes in young and aged lin− BMCs between microarray and qRT-PCR analyses. Regulation of Hmga2 by miR-10A* (D) and miR-21 (E) was confirmed by luciferase reporter and mutagenesis assays. human embryonic kidney cell line 293T cells were cotransfected with precursors of miR-10A*, miR-21, or miR-Ctr, renilla luciferase reporter constructs containing WT or 3 mutated 3′ untranslated regions (UTRs) of Hmga2, and a firefly luciferase reporter control vector to normalize the transfection efficiency. Luciferase was measured 48 hours after transfection. The luciferase activities represent the Renilla/firefly luciferase ratios (mean±SD, n≥6).**P<0.01. WT indicates wild-type.
Hmga2 Is Downregulated in Aged EPCs
Using the same lin− BMC samples, we performed genomewide analysis of mRNA expression using mouse exonic evidence-based oligonucleotide microarray containing 38 467 probes for mouse genes and alternative transcripts. Approximately half of the genes targeted by the mouse exonic evidence-based oligonucleotide array were present in at least 1 group of lin− BMCs. Principal component analysis found 1135 genes to be differentially expressed in these young and aged lin− BMCs, irrespective of apoE expression. Of the 1135 genes that passed the permutation analysis, 67% showed an increased expression in aged mice. Genes with greatest increases were those encoding immunoglobulin and inflammation. Genes that showed decreased expression included insulin-like growth factor 2 mRNA binding protein 3, secreted acidic cysteine rich glycoprotein, and procollagen type Iα2. The top 100 differentially expressed mRNAs in young and aged mice with P<0.00001 are listed in Online Table III. Intriguingly, Hmga2, a gene that regulates stem cell senescence, showed the greatest decrease (86-fold) in aged lin− BMCs. This was further confirmed by qRT-PCR (Figure 1C).
miR-10A* and miR-21 Regulate Hmga2 Expression
To identify those genes whose expression might be regulated by miRNA during EPC aging among the differentially expressed genes, we first performed in silico search for candidate mRNA targets for the identified miRNAs using different miRNA target prediction algorithms such as MicroCosm, TargetScan, PicTar, and EMBL. Seventy-eight of the differentially expressed genes were predicted to be the targets of the 38 candidate miRNAs, and their expression levels also correlated inversely with the miRNA expression levels. Of these, 12 genes, including Hmga2, have been implicated in aging, cell senescence, cell differentiation, and proliferation in other tissues. Hmga2 expression was inversely correlated with the levels of its predicted miRNAs, miR-10A*, and miR-21. Furthermore, computational miRNA target analysis by TargetScan algorithm and RNAHybrid23,24 identified 3 and 2 conserved sites in the Hmga2 3′UTR that are complementary to the sequences of miR-10A* and miR-21, respectively, suggesting that Hmga2 may be a shared molecular target for miR-10A* and miR-21 (Online Figure I).
To determine whether miR-10A* and miR-21 directly targeted Hmga2 3′UTR, we constructed luciferase reporter constructs with WT Hmga 2 3′UTR (WT3′UTR) and the 3′UTR with point mutations disrupting the 3 binding sites (M3′UTR1, M3′UTR2) for miR-10A* and the 2 binding sites (M3′UTR) for miR-21. In human embryonic kidney cell line 293T cells, cotransfection of miR-10A* or miR-21 and WT3′UTR repressed luciferase activity, whereas cotransfection of an unrelated miRNA had little effect (miR-199b). Cotransfection of miR-10A* or miR-21 and M3′UTR1/M3′UTR2 and M3′UTR, respectively, abolished the repression of luciferase activity (Figure 1D and 1E). These data indicate that miR-10A* and miR-21 directly interact with Hmga2 3′UTR, repressing Hmga2 expression.
To determine whether miR-10A* and miR-21 could negatively regulate endogenous Hmga2 expression in lin− BMCs, cells from young mice (3 weeks old) with a considerable level of Hmga2 expression were infected with lentivirus encoding miR-10A*, miR-21, a combination of the 2, or a scrambled miRNA control (miR-Ctr). MiR-10A* and miR-21 repressed Hmga2mRNA and protein expression as compared with miR-Ctr. The combined overexpression of miR-10A* and miR-21 had greater repressive effects. We also infected young lin− BMCs with lentivirus coding for WT Hmga2 or mutant Hmga2, where the 3 binding sites for miR-10A* and 2 binding sites for miR-21 in the 3′UTR were mutated in the presence and absence of miR-10A*, miR-21, and miR-Ctr. The repressive effects of miR-10A* and miR-21 on the mutant Hmga2 expression in lin− BMCs were not observed, whereas considerable repression of exogenous WT Hmga2 was detected (Figure 2A–2C). Next, we analyzed lin− BMCs isolated from aged WT mice (2.5 years old) in which miR-10A* and miR-21 levels were high and Hmga2 expression was low. Lentiviral-mediated transduction of anti-miRs for miR-10A* and miR-21 led to a reduction of miR-10A* and miR-21 expression, resulting in an increased Hmga2 expression level (Figure 2D–2F). Collectively, these data indicate that miR-10A* and miR-21 causally regulate Hmga2 expression, particularly in lin− BMCs. To determine whether aging and culture induced a shift in the composition of cell populations that might have accounted for the candidate miRNA and target gene expression changes, we repeated all expression and miRNA and anti-miR transduction experiments in CD34+ EPCs; similar findings were observed in the single EPC phenotype, indicating that the expression changes of miR-10A*, miR-21, and Hmga2, as well as their regulatory relationship, were not attributable to cell compositional changes (Online Figure II).
Figure 2. MicroRNA (miR)-10A* and miR-21 repress endogenous and exogenous Hmga2 expression in lineage-negative bone marrow cells (lin− BMCs).
Young wild-type (WT) lin− BMCs were infected with lentivirus encoding miR-10A*, miR-21, or miR-Ctr alone or in combination with WT Hmga2 3′ untranslated region (UTR) (WT 3′UTR) or mutant Hmga2 3′UTR (M3′UTR). The Hmga2 mRNA and protein expression were detected by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) (A) and Western blotting (B) and quantified by densitometry (C) after 48 hours and 72 hours, respectively. Aged WT lin− BMCs were infected with lentivirus encoding anti-miR-10A*, anti-miR-21, and miR-Ctr. The effects of miR-10A* and miR-21 on endogenous Hmga2 mRNA and protein expression were detected by qRT-PCR (D) and Western blotting (E and F). The blots are representatives of 3 independent experiments. Mutant 10A*-2 is mutant Hmga2 3′UTR disrupting binding for miR-10A* at binding sites 860 and 1548; mutant miR-10A* is mutant Hmga2 3′UTR for miR-10A* at binding sites 860 and 1504. qRT-PCR and densitometry data are presented as means±SD. *P<0.05, **P<0.01 vs corresponding controls (n≥3).
miR-10A* and miR-21 Regulate EPC Senescence and Self-Renewal Potential
Because Hmga2 promotes NSC self-renewal and hematopoietic progenitor cell clonal expansion, and because miR-10A* and miR-21 suppress Hmga2 expression, we reasoned that these 2 miRNAs might impact on the senescence of lin− BMCs via regulating Hmga2 expression. Young lin− BMCs were infected with lentivirus encoding miR-10A*, miR-21, a combination of the 2, or miR-Ctr. As expected, transduction of miR-10A* and miR-21 resulted in Hmga2 repression and increased expression of senescence-associated β–galactosidase (SA-β-gal) and cell cycle inhibitors p16Ink4a/p19Arf. Furthermore, simultaneous transduction of miR-10A* and miR-21 together resulted in more pronounced lin− BMC senescence (Online Figure III). Remarkably, overexpression of miR-10A* and miR-21 reduced the self-renewal potential of young lin− BMCs compared with miR-Ctr (Figure 3A–3D). In contrast, inhibition of miR-10A* or miR-21 by lentivector-mediated overexpression of anti-miR-10A* and anti-miR-21 in aged lin− BMCs upregulated Hmga2 expression, decreased SA-β-gal and p16Ink4a/p19Arf expression, and increased self-renewal potential, compared with miR-Ctr (Figure 3A–3D and Online Figure IV). Moreover, anti-miR-10A* and anti-miR-21 resulted in increased proliferating cell nuclear antigen staining and increased cell numbers by 3-[4,5-dimethylthiazol-zyl]-2,5-dipheny-tetrazolium bromide assay relative to control infected cells (Figure 3E and 3F). These data indicate that miR-10A* and miR-21 affect lin− BMC senescence and self-renewal potential, and this effect might be mediated via Hmga2 and p16Ink4a/p19Arf.
Figure 3. MicroRNA (MiR)-10A* and miR-21 regulate lineage negative bone marrow cell (lin− BMC) senescence, proliferation, and self-renewal through Hmga2 and p16Ink4a/p19Arf.
Young and aged wild-type (WT) lin− BMCs were infected with lentivirus encoding miR-10A* and miR-21 sense sequences or their anti-miRs, respectively. The effects of miR-10A* and miR-21 overexpression and inhibition on the expression of Hmga2 and p16Ink4a/p19Arf (A), cell senescence as evidenced by senescence-associated β-galactosidase (SA-β-gal) staining (B), self-renewal potential as evaluated by the secondary(2°) colony forming ability in methylcellulose-based media (C, D), and cell proliferation as assessed by proliferating cell nuclear antigen (PCNA) staining and confirmed by 3-[4,5-dimethylthiazol-zyl]-2,5-dipheny-tetrazolium bromide (MTT) cell proliferation assay (E, F) are shown.*P<0.05, **P<0.01 (n≥3) relative to miR-Ctr. Western blots are representative of experiments performed at least in triplicate. Densitometry data for A are presented in Online Figure IVA–IVC.
miR-10A* and miR-21 Induce EPC Functional Impairment
Cell migration and vascular tube formation are key functions of EPCs and play important roles in the angiogenic process. Lin− BMCs are enriched for EPCs and can differentiate into spindle-shaped cells, which express EC surface markers (Online Figure V).5 To investigate whether miR-10A* and miR-21 affected lin− BMC functions as a result of senescent changes, we first performed a wound closure assay to evaluate cell migration. Lentivirus-mediated overexpression of miR-10A* or miR-21 reduced the ability of young lin− BMCs to close the scratch to a level equivalent to that of aged cells, whereas control infection did not show any effects (Figure 4A, top panel). Furthermore, inhibition of miR-10A* or miR-21 by anti-miR-10A* and anti-miR-21 resulted in increased ability of aged lin− BMCs to close the scratch to a level comparable with that of young cells (Figure 4A, bottom panel). These effects were further confirmed by the relative migration distances of the infected cells (Figure 4B). Next, we tested whether miR-10A* or miR-21 overexpression affected angiogenesis in vitro; we performed Matrigel tube formation assay. Young lin− BMCs infected with lentiviral vectors coding for miR-10A*, miR-21, or miR-Ctr were loaded onto Matrigel. miR-10A*-infected and miR-21-infected cells showed a significant impairment of capillary tube formation after10 hours of incubation (Figure 4C and 4D, top panel). Conversely, in aged lin− BMCs, inhibition of miR-10A* and miR-21 by overexpressing anti-miR-10A* and anti-miR-21 rescued tube formation in Matrigel assay (Figure 4C and 4D, bottom panel). Importantly, the miR-10A*-induced and miR-21-induced functional changes were associated with reciprocal changes in Hmga2 and p16Ink4a/p19Arf expression (Figure 5A and Online Figure VI). These data indicate that miR-10A*and miR-21 affect lin− BMC functional impairment associated with cell senescence. The data also implicate the role of Hmga2 and p16Ink4a/p19Arf in mediating the effects of miR-10A* and miR-21.
Figure 4. MicroRNA (miR)-10A* and miR-21 expression regulates wound healing and angiogenesis in vitro.
Young wild-type (WT) lineage-negative bone marrow cells (lin− BMCs) were infected with lentivirus encoding miR-Ctr, miR-10A*, or miR-21 (A, top), or aged WT lin− BMCs were infected with lentivirus encoding miR-Ctr, anti-miR-10A*, and anti-miR-21(A, bottom). Wound closure was assessed and quantified by the relative migration distance 24 hours after performing a scratch in confluent monolayer of cells (n=3; B). The effects of miR-10A* and miR-21 in young (C, top) and aged (C, bottom) lin− BMCs to form capillary tubes in Matrigel assay were determined 10 hours after seeding. The images are representatives of at least 3 independent experiments. Relative tube lengths from 5 microscopic fields are shown in (D). **P<0.01 relative to miR-Ctr.
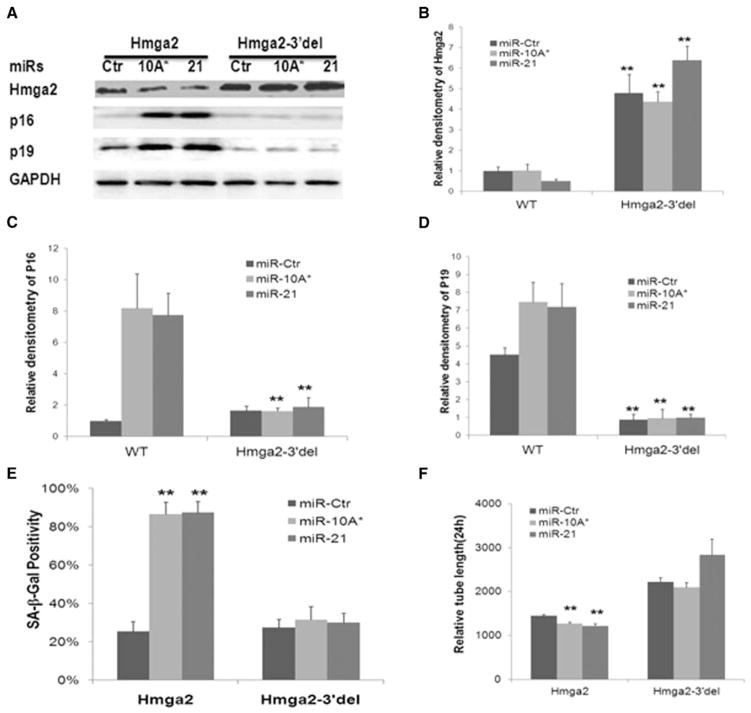
Figure 5. Hmga2 regulates lineage negative bone marrow cell (lin− BMC) senescence, self-renewal potential, proliferation, and vascular tube formation via activation of p16Ink4a/p19Arf.
ShRNA-induced knockdown and wild-type (WT) Hmga2 cDNA or Hmga2 open reading frame (ORF) with 3′ untranslated region (UTR) deletion (Hmga2-3′del)-induced overexpression of Hmga2 result in opposite changes in Hmga2 and p16Ink4a/p19Arf mRNA and protein expression in young and aged lin− BMCs, respectively (A). ShRNA-induced Hmga2 knockdown in young lin− BMCs results in increased senescence-associated β-galactosidase (SA-β-gal) staining (B); decreased self-renewal potential by 2° colony forming units (C); decreased proliferation by proliferating cell nuclear antigen staining and 3-[4,5-dimethylthiazol-zyl]-2,5-dipheny-tetrazolium bromide (MTT) assay (D) and decreased capillary tube formation (E) as compared with shRNA control. Overexpression of Hmga2 by Hmga2-3′del transfection decreases SA-β-gal staining and enhances self-renewal potential (F), increases cell proliferation by MTT assay (G), and improves capillary tube formation (H) in aged lin− BMCs, whereas WT Hmga2 overexpression only transiently improved self-renewal potential and cell proliferation. Data are presented as mean±SD (n=3). *P<0.05, **P<0.01 (n≥3). Western blots are representative of experiments performed at least in triplicate. Densitometry data for western blotting (B) are shown in Online Figure VI.
Hmga2 Mediates the Effects of miR-10A* and miR-21 in lin− BMCs
Because miR-10A* and miR-21 negatively regulate Hmga2 expression and Hmga2 has been shown to regulate the senescence and functions of NSCs and umbilical cord blood-derived mesenchymal stem cells, we asked whether Hmga2 mediated the effects of miR-10A* and miR-21 on lin− BMC senescence, self-renewal potential, and angiogenic functions. Short hairpin RNA (shRNA)-mediated Hmga2 silencing in young lin− BMCs resulted in decreased Hmga2 expression, increased SA-β-gal and p16Ink4a/p19Arf expression, decreased self-renewal potential as determined by secondary and tertiary colony formation, and decreased cell proliferation (Figure 5A–5D), to a degree similar to that of miR-10A* and miR-21 overexpression. These cells also showed decreased tube formation (Figure 5E). We then infected aged lin− BMCs with lentiviral vectors coding for Hmga2 open reading frame (ORF) with 3′UTR deletion (Hmga2-3′del), a form of Hmga2 resistant to miRNA repression, or WT Hmga2. Hmga2-3′del transduction resulted in increased Hmga2 expression, decreased SA-β-gal and p16Ink4a/p19Arf expression, increased self-renewal potential and cell proliferation, and increased vascular tube formation (Figure 5F–5H). These effects were similar to that achieved with miR-10A* and miR-21 repression by their anti-miRs. Although WT Hmga2 overexpression transiently improved self-renewal potential and cell proliferation (Figure 5F–5G), it had no effects on lin− BMC senescence and vascular tube formation after 72 hours incubation, compared with miR-Ctr, suggesting that WT Hmga2 expression might be inhibited by endogenous miR-10A*, miR-21, and other miRNAs (Figure 5F and 5H). Remarkably, when young lin− BMCs were infected with lentiviral vectors encoding miR-10A* and miR-21, together with Hmga2-3′del, or WT Hmga2, overexpression of Hmga2-3′del, but not WT Hmga2, rescued the effects of miR-10A* and miR-21 (Figure 6A–6F). These data establish that Hmga2 works downstream of miR-10A* and miR-21 to affect lin− BMC senescence and functions.
Figure 6. Overexpression of Hmga2 lacking a 3′ untranslated region (UTR) rescues the effects of microRNA (miR)-10A* and miR-21 overexpression.
Coinfection of the lentivirus encoding miR-10A*, miR-21, or miR-Ctr together with Hmga2 open reading frame (ORF) with 3′ UTR deletion (Hmga2-3′del), but not wild-type (WT) Hmga2, in young lineage-negative bone marrow cells (lin− BMCs) results in upregulation of Hmga2 and downregulation of p16Ink4a/p19Arf (A–D). Cotransfection of Hmga2-3′del with miR-10A* or miR-21, but not WT Hmga2, rescues the effects of miR-10A* or miR-21 on senescence-associated β-galactosidase (SA-β-gal) staining (E) and vascular tube formation as shown by relative tube length (F).*P<0.05, **P<0.01 for Hmga2 or Hmga2-3′del-treated groups relative to control. Western blots are representative of experiments performed at least in triplicate. Relative densitometry data (mean±SD) are from 3 independent experiments normalized with GAPDH.
P16Ink4a/p19Arf Mediate the Effects of miR-10A*/miR-21 and Hmga2
We have demonstrated that miR-10A* and miR-21 promote lin− BMC senescence and functional impairment via regulating Hmga2 expression, and that these effects were associated with alterations in p16Ink4a/p19Arf expression. To test whether p16Ink4a and p19Arf mediated the effects of miR-10A*/miR-21 and Hmga2, we infected young lin− BMCs with lentiviral vectors encoding p16Ink4a and p19ArfcDNAs. These cells have low expression levels of endogenous miR-10A*/miR-21 and a considerable amount of Hmga2. The p16Ink4a and p19Arf overexpression increased SA-β-gal expression, decreased self-renewal potential, and decreased vascular tube formation, resembling Hmga2 shRNA-transfected young cells or cells overexpressing miR-10A* and miR-21 (data not shown). We also infected aged lin− BMCs with lentivirus coding for p16Ink4a or p19Arf shRNA. These cells displayed decreased SA-β-gal expression, increased self-renewal potential, and increased vascular tube formation, similar to Hmga2-overexpressing cells or cells in which miR-10A* and miR-21 were repressed (data not shown). Next, we transduced young lin− BMCs with lentiviral vectors coding for miR-10A*, miR-21, or Hmga2 shRNA in the presence and absence of shRNA for p16Ink4a and p19Arf. Remarkably, knockdown of p16Ink4a and p19Arf largely blocked the senescent effects of miR-10A* and miR-21 overexpression or Hmga2 repression (Online Figure VIIA). We then infected aged lin− BMCs with lentiviral vectors encoding anti-miR-10A* and anti-miR-21 and Hmga2-3′del in the presence and absence of p16Ink4a and p19Arf cDNAs. As expected, p16Ink4a and p19Arf overexpression mostly inhibited the effects of miR-10A* and miR-21 repression or Hmga2 overexpression-induced rejuvenation and functional improvement of aged lin− BMCs (Online Figure VIIB). Taken together, these results indicate that p16Ink4a and p19Arf act in tandem with miR-10A*/miR-21 and Hmga2, mediating at least partially their effects on lin− BMC senescence and functions.
miR-10A*/miR-21 and Hmga2 Regulate Angiogenesis In Vivo
To determine the role of miR-10A*/miR-21 and Hmga2 in regulating the angiogenic capability of lin− BMCs in vivo, we first performed Matrigel plug assay. We determined the extent of and the time required for neovascularization of Matrigel plugs containing young vs aged lin− BMCs. As shown in Figure 7A, Matrigel containing aged lin− BMCs showed minimal vascularization, and there was no increase in vascularization in the plugs over a 7- to 14-day period. In contrast, plugs containing young lin− BMCs demonstrated a substantial degree of neovascularization with a maximum increase in the number of vessels and tube length at day 11 after plug injection, as shown by the deep red color. Consistent with our in vitro angiogenesis study, Matrigel containing young lin− BMCs overexpressing miR-10A* and miR-21 or ShRNA-mediated Hmga2 silencing showed a marked reduction in neovascularization, compared with miR-Ctr (Figure 7B). Quantification of angiogenesis revealed that the angiogenic activity of the miR-10A*, miR-21, and ShRNA-mediated Hmga2 silencing overexpressing young lin− BMCs resembled that of aged lin− BMCs. In contrast, plugs containing aged lin− BMCs overexpressing anti-miR-10A* and anti-miR-21 or Hmga2-3′del displayed significantly improved neovascularization, similar to young cells (Figure 7C–7E).
Figure 7. MicroRNA (miR)-10A* and miR-21 regulate angiogenesis in vivo.
Matrigel (250 μL per plug) was mixed with 1.3×106 young or aged lineage negative bone marrow cells (lin− BMCs) transduced with lentivirus coding for miR-10A*, miR-21, and ShRNA-mediated Hmga2 silencing (shRNA-Hmga2), or their anti-miRs, and Hmga2 open reading frame (ORF) with 3′ untranslated region (UTR) deletion (Hmga2-3′del) miR-Ctr, respectively, and was injected subcutaneously into C57BL/6J mice (n=3). Noninfected or control infected lin− BMCs from young and aged mice were also included. After 11 days, Matrigel plugs were removed, and capillary tube formation was detected by confocal microscopy. Representative pictures of indicated groups are shown in (A) and (B) (×100 magnification). Quantitative analysis of tube formation in Matrigel plugs is shown in (C–E). Data represent means±SD.*P<0.05 and**P<0.01 vs control group. The capillaries in green and red are newly formed vessels. Red fluorescence indicates blood vessels stained by DiL, green fluorescence indicates green fluorescent protein (GFP)-positive infected cells, double-labeling indicates capillaries derived from the injection of GFP-positive lentiviral transduced lin− BMCs.
To validate the role of miR-10A*/miR-21 and Hmga2 in regulating lin− BMC-induced angiogenesis in vivo, we injected genetically modified young and aged lin− BMCs intramuscularly in the hindlimbs of mice whose femoral arteries were ligated on 1 side (hindlimb ischemic model) and measured blood flow by serial laser Doppler perfusion imaging analyses and angiogenesis by immunofluorescence staining. Similar to the in vivo Matrigel plug assay, young lin− BMCs overexpressing miR-10A*, miR-21, or ShRNA-mediated Hmga2 silencing showed a marked reduction in the ratio of blood flow (ischemic/nonischemic leg) and in the degree of neovascularization compared with miR-Ctr-transduced cells (Figure 8A–8C and Online Figure VIIIB). In contrast, aged lin− BMCs overexpressing anti-miR10A*, anti-miR-21, or Hmga2-3′del significantly improved blood flow and neovessel formation, whereas lin− BMCs overexpressing WT Hmga2 were not effective (data not shown). Remarkably, the combined modulation of miR-10A* and miR-21 was more effective in improving blood flow at 7 days, but not at 21 days postoperatively. Furthermore, the rate of green fluorescent protein+ lin− BMC incorporation (endothelial differentiation) was increased in mice treated with aged lin− BMCs transduced with anti-miR-10A* and anti-miR-21, whereas the endothelial differentiation rate was decreased in mice receiving young cells overexpressing miR-10A* and miR-21, indicating that the effects of miR-21 and miR-10A* on angiogenesis correlate with their effects on endothelial differentiation. Collectively, these findings support the notion that miR-10A* and miR-21 inhibit the angiogenic capability of lin− BMCs via negatively regulating Hmga2 expression.
Figure 8. MicroRNA (miR)-21 regulates neovascularization in hindlimb ischemia model.
Reperfusion in the ischemic hindlimb (A and B) and neovascularization (C) were improved when young endothelial progenitor cells (EPCs) infected with lentivirus coding for miR-Ctr or aged EPCs transduced with scrambled anti-miRNA control (anti-miR-Ctr), anti-miR-21 or Hmga2-3′ deletion (Hmga2 3′del) were injected, as compared with young EPCs infected with lentivirus coding for miR-21 or shRNA for Hmga2 or aged cells transduced with anti-miR-Ctr. Laser Doppler perfusion imaging was obtained at 0, 7, and 21 days after operation, and recovery of perfusion was assessed. **P<0.01 vs miR-Ctr (n=4 for each group).
Discussion
As humans age, the functions of multiple systems deteriorate, resulting in the development of degenerative disease, such as atherosclerosis. Adult stem cells fuel the renewal of many tissues. Thus, aging problem may lie, at least in part, within the tissue-specific stem cells.25 Several human studies showed that the proliferation, differentiation, and migration capacity of circulating EPCs were reduced in the elderly.26,27 Aged mice have reduced number of EPCs, and these cells have decreased migratory, differentiating, and proliferative activities.5 Bone marrow-derived EPCs from young apoE−/− mice prevent atherosclerotic lesion formation, whereas progenitor cells from aged atherosclerotic apoE−/− mice do not.4 In the current study, we present evidence showing that lin− BMCs undergo senescent changes in association with aging. These aged cells display decreased self-renewal potential and reduced proliferative and migratory activity. We also have demonstrated that age-related lin− BMC senescence is associated with decreased angiogenic capability. Because EPCs play an important role in cardiac repair by promoting angiogenesis and improving blood supply, these findings indicate that lin− BMC senescence may not only promote atherogenesis but also diminish cardiac repair by impairing angiogenesis, thus serving as a double-edged sword affecting the development and prognosis of cardiovascular disease.2–5,28
Increasing evidence supports the role of miRNAs as a common molecular mechanism that underlies cellular senescence in different cell types.11,19,29 For example, miR-34a induces the senescence of culture-derived EPCs, whereas miR-217 modulates EC senescence. Both miRNAs act via inhibiting silent information regulator 1-p53 pathway.10,11 Other miRNAs involved in the senescence of different cell types include miR-29, miR-30, and miR-128a.30,31 To thoroughly characterize the miRNA changes in the senescence of lin− BMCs, cells enriched for EPCs, we utilized a miRNA microarray platform to profile genomewide miRNA expression changes. To effectively identify miRNA candidates and their downstream pathways relevant to lin− BMC senescence, we also analyzed genomewide gene expression profiles. We performed in silico analysis to search for putative mRNA targets for the candidate miRNAs that showed differential expression changes in young vs aged lin− BMCs. We then overlaid the 3 datasets to identify the miRNA candidates whose expression levels correlated inversely with their predicted target genes. Through these robust and stepwise analyses and extensive experimental approaches, we provide evidence showing that miR-10A* and miR-21 serve as novel miRNAs regulating Hmga2 expression in lin− BMCs, where established miRNAs for Hmga2, including the let-7 family, miR-23a, miR-26a, and miR-30a miRNAs, do not show expression changes in association with cell senescence.21,22
Hmga2 is abundantly and ubiquitously expressed and plays a crucial role during embryonic development.32 Hmga2 also is highly expressed in fetal NSCs but its expression declines with age. Hmga2 deficiency reduced NSC frequency and self-renewal by increasing the expression of p16Ink4a/p19Arf, which are cell cycle inhibitors that regulate the pRb and p53 pathways, respectively. Activation of Ink4a/Arf promotes cell cycle arrest that contributes to both organismal aging and tumor suppression. Induction of p16Ink4a contributes to the decline of hematopoietic and NSC function in aged animals.18,22 In the present study, we have demonstrated that the effects of miR-10A* and miR-21 overexpression on lin− BMC senescence are rescued by the overexpression of mutant Hmga2 with 3′UTR deletion, but not WT Hmga2. Remarkably, the overexpression of mutant Hmga2 with 3′UTR deletion, but not WT Hmga2, in aged lin− BMCs rejuvenates the cells, indicating that WT Hmga2 might be subjected to repression by endogenous miR-10A*, miR-21, and perhaps other unidentified miRNAs. Furthermore, p16Ink4a/p19Arf overexpression rescues the effects of Hmga2-induced lin− BMC rejuvenation. These findings demonstrate that Hmga2 and p16Ink4a/p19Arf act downstream of miR-10A* and miR-21, regulating lin− BMC senescence. Importantly, using in vivo Matrigel plug assay and the more relevant hindlimb ischemic model, we show that modification of the miR-10A*/miR-21–Hmga2–p16Ink4a/p19Arf axis improves senescent lin− BMC/EPC-induced angiogenesis, indicating that modulation of this pathway rejuvenates lin− BMCs/EPCs. Remarkably, the combined treatment with miR-10A* and miR-21 antagonists promotes the initiation of angiogenesis but, given enough time, cells treated with each miRNA antagonist alone are able to catch up with the multiple treated cells. Considering that restoration of blood supply in the early stage after myocardial infarct is crucial, these findings may have clinical implications for the justification of multiple miRNA inhibition.
It is important to note that other miR-10A* and miR-21 targets also may have a role in regulating lin− BMC senescence and angiogenesis. For example, it has been recently shown that reactive oxygen species and angiogenic factor RhoB are targets of miR-21.15,33 Based on miRNA TargetScan software analysis, other target genes, including Smad7, VEGFC, SOX2, SOX5, KLF2, PTEN, BCL-2, may be involved in mediating the senescence and antiangiogenic effects of miR-21. Similarly, miR-10A* may act via Rgs13, Bmi-1, Myb, Wnt2, RhoB, Smad7, and CDK1 to exert its effects on cell senescence and angiogenesis. KLF2 was significantly upregulated in aged lin− BMCs and was found to be a key factor in regulating EC differentiation, which will be reported in our follow-up study. None of the other putative target genes showed meaningful changes in expression with aging, and thus were not selected for further study. Given that multiple factors are likely to work together to induce lin− BMC senescence and differentiation, it is conceivable that additional miRNAs and target genes are involved and are awaiting further investigation. The less prominent expression changes of miR-10A* and miR-21 relative to that of Hmga2 in young vs aged lin− BMCs underscores the potential involvement of other miRNAs and perhaps other epigenetic mechanisms in regulating their senescence and rejuvenation.
In summary, our data reveal the existence of a novel pathway that regulates lin− BMC senescence; miR-10A* and mi-21 expression increases with aging, resulting in downregulation of Hmga2, which, in turn, activates p16Ink4a/p19Arf expression, causing decreased self-renewal potential and impaired angiogenic capability. These findings not only may be helpful in developing approaches to rejuvenate lin− BMCs and to enhance angiogenesis for cardiovascular repair, but also may be beneficial in finding new ways to inhibit angiogenesis in the case of cancer treatment.
Supplementary Material
Novelty and Significance.
What Is Known?
Endothelial progenitor cells (EPCs) have been suggested to be essential for the formation of new blood vessel formation and vascular repair. EPC number and angiogenic functions decline as a function of aging.
Treatment with exogenous bone marrow stem/progenitor cells, such as EPCs, can repair injured vessels and induce angiogenesis.
MicroRNAs (miRNAs) can regulate diverse cellular processes, including cellular senescence and angiogenesis.
What New Information Does This Article Contribute?
Aging is associated with functional impairment and numerical exhaustion of EPCs.
In comparison with EPCs from young mice, aged mouse EPCs have elevated levels of miRNAs, miR-10A*, and miR-21, leading to the downregulation of Hmga2 expression, a key regulator of stem and progenitor cell self-renewal and functionality, resulting in p16Ink4a/p19Arf-mediated cellular senescence.
Application of modified EPCs, in which miR-10A* and miR-21 expression is inhibited or Hmga2 is overexpressed, improves new blood vessel formation and blood perfusion in a mouse model of hindlimb ischemia.
EPCs play important roles in maintaining cardiovascular health by promoting vascular regeneration and repair. EPC functionality and number, however, are reduced as a consequence of biological or pathological aging. Therefore, we sought to understand the molecular mechanisms underlying age-associated EPC senescence. Using high-content genomic approaches, we found that the regulatory pathways controlled by miR-10A* and miR-21 are necessary for the maintenance of EPC viability and functionality. We show that overexpression of miR-10A* and miR-21 in EPCs from young animals leads to Hmga2 silencing, which, in turn, promotes the expression of the pro-senescence genes p16Ink4a/p19Arf. The upregulation of p16Ink4a/p19Arf significantly impairs the angiogenic functions of mouse EPCs. In contrast, targeted silencing of miR-10A* and miR-21 in aged EPCs results in upregulation of Hmga2, rejuvenation of EPCs, and improved angiogenic activity. These findings suggest that the miR-10A*/miR-21–Hmga2–p16Ink4a/p19Arf pathway regulates EPC senescence and, therefore, genetic manipulation of this pathway could be a novel therapeutic intervention to improve EPC-mediated angiogenesis and vascular repair.
Acknowledgments
Sources of Funding
This work was supported by the startup funds to C.M. Dong by University of Miami and by a grant from National Institute of Health (AG023073) to P.J. Goldschmidt-Clermont.
Non-standard Abbreviations and Acronyms
- 3′UTR
3′ untranslated region
- EC
endothelial cell
- EPC
endothelial progenitor cell
- GFP
green fluorescent protein
- HEK 293T
human embryonic kidney cell line 293T
- Hmga2-3′del
Hmga2 open reading frame (ORF) with 3′ untranslated region (UTR) deletion
- lin− BMC
linea genegative bone marrow cell
- miR-Ctr
scrambled miRNA control
- miRNA
microRNA
- MTT
3-[4,5-dimethylthiazol-zyl]-2,5-dipheny-tetrazolium bromide
- NSC
neural stem cell
- ORF
open reading frame
- PCNA
proliferating cell nuclear antigen
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SA-β-gal
senescence-associated β–galactosidase
- shRNA
short hairpin RNA
- shRNA-Hmga2
shRNA-mediated Hmga2 silencing
- WT
wild type
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.112.280016.
Disclosures
None.
References
- 1.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 2.Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 3.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:E89–E93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 4.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 5.Zhu S, Liu X, Li Y, Goldschmidt-Clermont PJ, Dong C. Aging in the atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:113–119. doi: 10.1161/01.ATV.0000252035.12881.d0. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JS, Phillips MD, Betel D, Mu P, Ventura A, Siepel AC, Chen KC, Lai EC. Widespread regulatory activity of vertebrate microRNA* species. RNA. 2011;17:312–326. doi: 10.1261/rna.2537911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 9.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 11.Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab. 2010;299:E110–E116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 13.Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Georgoulias V, Lianidou ES. Prognostic value of mature microRNA-21 and microR-NA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR. Clin Chem. 2008;54:1696–1704. doi: 10.1373/clinchem.2007.101741. [DOI] [PubMed] [Google Scholar]
- 14.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1a expression. PLoS ONE. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabatel C, Malvaux L, Bovy N, Deroanne C, Lambert V, Gonzalez ML, Colige A, Rakic JM, Noël A, Martial JA, Struman I. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS ONE. 2011;6:e16979. doi: 10.1371/journal.pone.0016979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- 17.Li O, Vasudevan D, Davey CA, Dröge P. High-level expression of architectural factor HMGA2 and its association with nucleosomes in human embryonic stem cells. Genesis. 2006;44:523–529. doi: 10.1002/dvg.20242. [DOI] [PubMed] [Google Scholar]
- 18.Nishino J, Kim I, Chada K, Morrison SJ. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 2008;135:227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grillari J, Grillari-Voglauer R. Novel modulators of senescence, aging, and longevity: Small non-coding RNAs enter the stage. Exp Gerontol. 2010;45:302–311. doi: 10.1016/j.exger.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 21.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Jung JW, Park SB, Roh K, Lee SY, Kim JH, Kang SK, Kang KS. Histone deacetylase regulates high mobility group A2-targeting microR-NAs in human cord blood-derived multipotent stem cell aging. Cell Mol Life Sci. 2011;68:325–336. doi: 10.1007/s00018-010-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res. 2008;18:404–411. doi: 10.1101/gr.6587008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krüger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34:W451–W454. doi: 10.1093/nar/gkl243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 26.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. DNA. [DOI] [PubMed] [Google Scholar]
- 28.Zeng H, Li L, Chen JX. Overexpression of angiopoietin-1 increases CD133+/c-kit+ cells and reduces myocardial apoptosis in db/db mouse infarcted hearts. PLoS ONE. 2012;7:e35905. doi: 10.1371/journal.pone.0035905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mudhasani R, Zhu Z, Hutvagner G, Eischen CM, Lyle S, Hall LL, Lawrence JB, Imbalzano AN, Jones SN. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. J Cell Biol. 2008;181:1055–1063. doi: 10.1083/jcb.200802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez I, Cazalla D, Almstead LL, Steitz JA, DiMaio D. miR-29 and miR-30 regulate B-Myb expression during cellular senescence. Proc Natl Acad Sci USA. 2011;108:522–527. doi: 10.1073/pnas.1017346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkataraman S, Alimova I, Fan R, Harris P, Foreman N, Vibhakar R. MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS ONE. 2010;5:e10748. doi: 10.1371/journal.pone.0010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monzen K, Ito Y, Naito AT, Kasai H, Hiroi Y, Hayashi D, Shiojima I, Yamazaki T, Miyazono K, Asashima M, Nagai R, Komuro I. A crucial role of a high mobility group protein HMGA2 in cardiogenesis. Nat Cell Biol. 2008;10:567–574. doi: 10.1038/ncb1719. [DOI] [PubMed] [Google Scholar]
- 33.Fleissner F, Jazbutyte V, Fiedler J, Gupta SK, Yin X, Xu Q, Galuppo P, Kneitz S, Mayr M, Ertl G, Bauersachs J, Thum T. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ Res. 2010;107:138–143. doi: 10.1161/CIRCRESAHA.110.216770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.