Abstract
Cynomolgus monkeys, Macaca fascicularis, fed cholesterol-containing saturated-fat diets develop increased levels of high molecular weight plasma low density lipoproteins (LDL), associated with accelerated atherosclerosis. To study the composition and structure of these abnormal particles, LDL from monkeys, fed atherogenic and control diets, were characterized chemically and examined by differential scanning calorimetry and low-angle X-ray scattering. LDL from animals on the experimental diet showed an increase in molecular weight (4.0 to 7.0 × 106, experimental diet compared with 3.0 to 3.7 × 106, control diet) associated with a large increase in cholesterol ester content and concomitant smaller increases in protein, phospholipid, and free cholesterol. There was a strong positive correlation between molecular weight and the number of saturated and monounsaturated cholesterol esters in the particle. In contrast, particle content of polyunsaturated cholesterol esters remained constant despite large changes in total particle cholesterol esters.
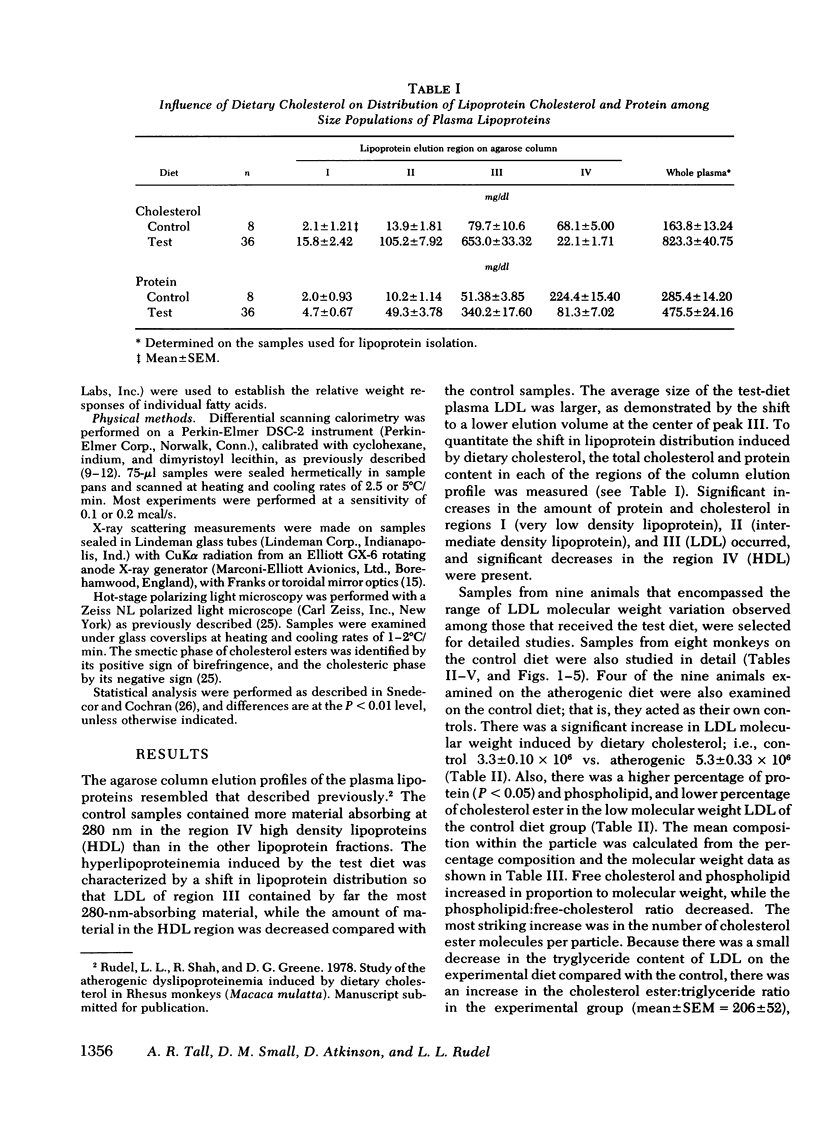
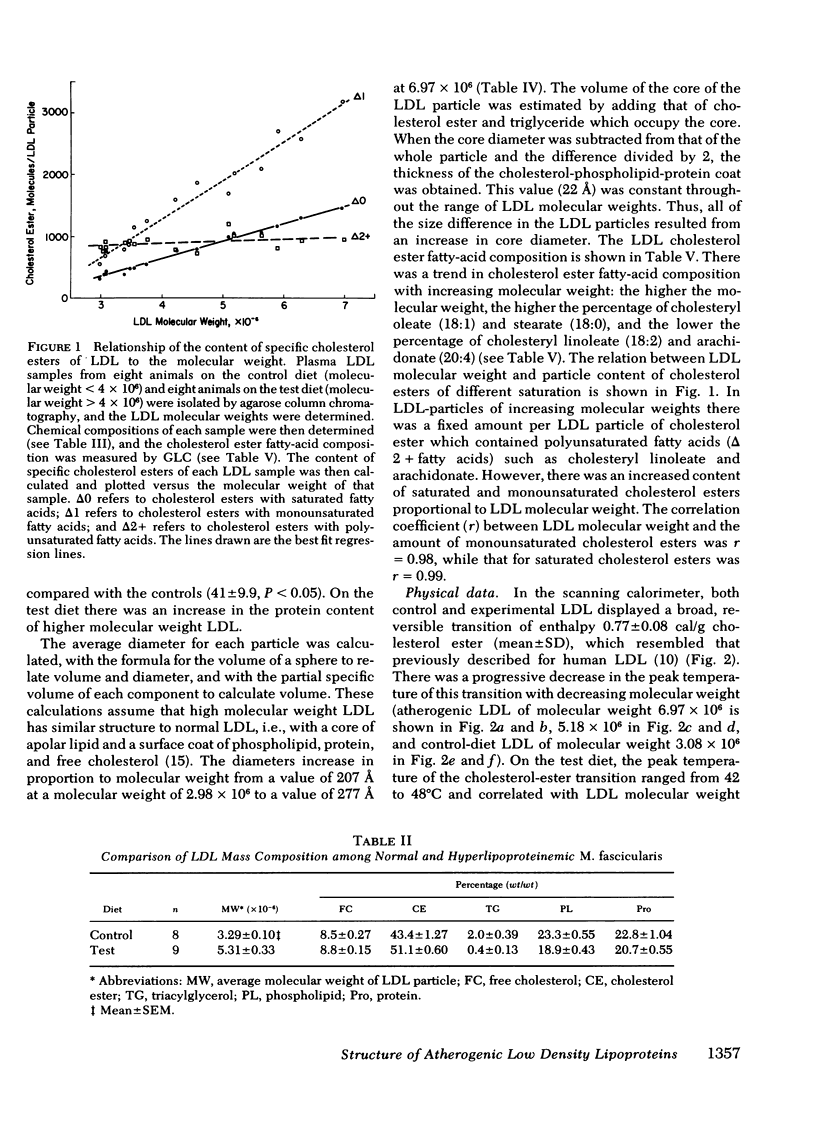
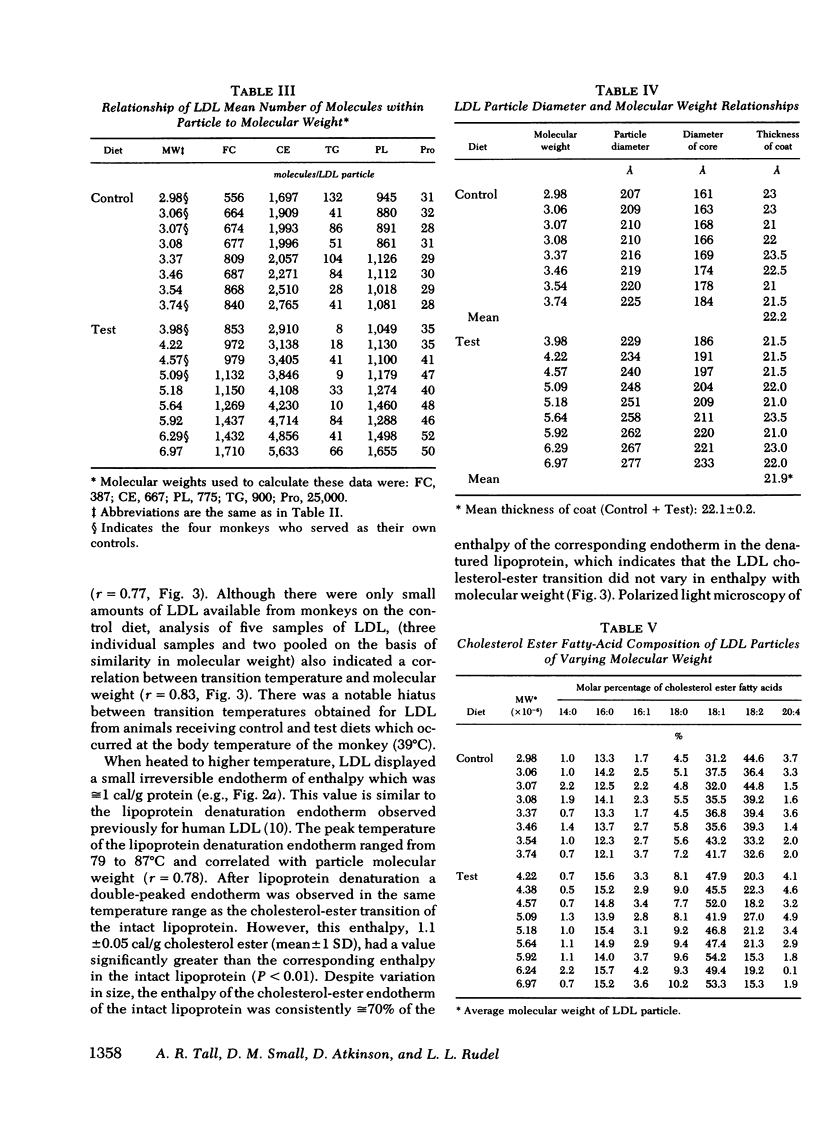
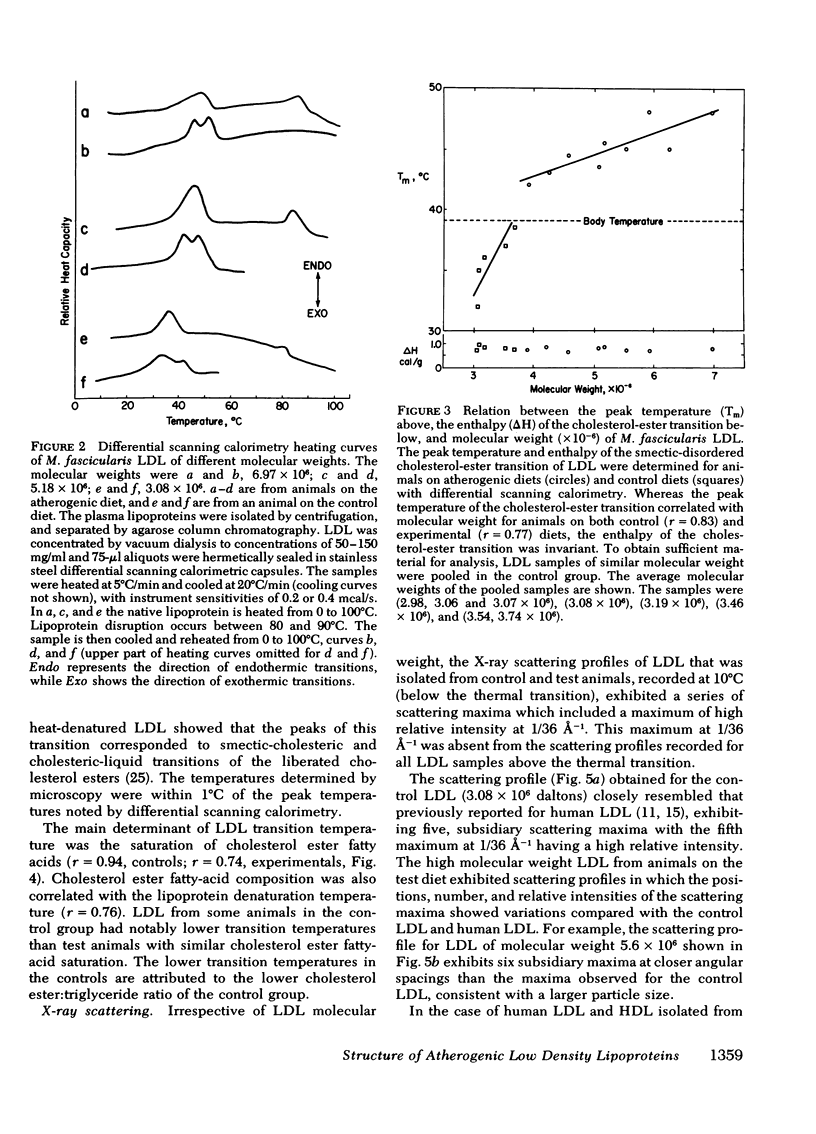
When examined by calorimetry and X-ray scattering, LDL from monkeys on both diets diplayed a reversible transition of cholesterol esters from an ordered smeticlike (layered) structure to a more disordered state. For all animals on the experimental diet, the peak temperature of the cholesterol-ester transition (42-48°C) was above body temperature (39°C), but below body temperature on the control diet (34-38.5°C). In the experimental group, the transition temperature was correlated with the LDL molecular weight. However, after thermal disruption of LDL, liquid-crystalline transitions of LDL cholesterol esters were observed in the same temperature range as in the intact lipoprotein, which shows that changes in particle size had little effect on the cholesterol-ester transition temperature. Rather, the transition temperature was determined by the degree of saturation of the LDL cholesterol ester fatty acids and the LDL cholesterol ester: triglyceride ratio, both of which correlated with increased LDL molecular weight.
The existence of smectic-like cholesterol ester in LDL at body temperature was clearly a discriminating feature between monkeys on control and experimental diets. Diet-induced changes in the lipid composition of precursor lipoproteins of LDL appeared to lead to the existence of smectic-like cholesterol ester in LDL above body temperature. The altered composition and structure of the core lipids of high molecular weight LDL probably account, in part, for the previously documented correlation between increased LDL molecular weight and atherosclerosis in this species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D., Deckelbaum R. J., Small D. M., Shipley G. G. Structure of human plasma low-density lipoproteins: molecular organization of the central core. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1042–1046. doi: 10.1073/pnas.74.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson D., Tall A. R., Small D. M., Mahley R. W. Structural organization of the lipoprotein HDLc from atherosclerotic swine. Structural features relating the particle surface and core. Biochemistry. 1978 Sep 19;17(19):3930–3933. doi: 10.1021/bi00612a007. [DOI] [PubMed] [Google Scholar]
- Bates S. R., Wissler R. W. Effect of hyperlipemic serum on cholesterol accumulation in monkey aortic medial cells. Biochim Biophys Acta. 1976 Oct 21;450(1):78–88. doi: 10.1016/0005-2760(76)90300-3. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. J., Shipley G. G., Small D. M. Structure and interactions of lipids in human plasma low density lipoproteins. J Biol Chem. 1977 Jan 25;252(2):744–754. [PubMed] [Google Scholar]
- Deckelbaum R. J., Tall A. R., Small D. M. Interaction of cholesterol ester and triglyceride in human plasma very low density lipoprotein. J Lipid Res. 1977 Mar;18(2):164–168. [PubMed] [Google Scholar]
- Fless G. M., Wissler R. W., Scanu A. M. Study of abnormal plasma low-density lipoprotein in rhesus monkeys with diet-induced hyperlipidemia. Biochemistry. 1976 Dec 28;15(26):5799–5805. doi: 10.1021/bi00671a017. [DOI] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- KARMEN A., WHYTE M., GOODMAN D. S. FATTY ACID ESTERIFICATION AND CHYLOMICRON FORMATION DURING FAT ABSORPTION. 1. TRIGLYCERIDES AND CHOLESTEROL ESTERS. J Lipid Res. 1963 Jul;4:312–321. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. A., Morris M. D. The effect of cholesterol feeding on primate serum lipoproteins. I. Low density lipoprotein characterization from rhesus monkeys with high serum cholesterol. Biochem Med. 1976 Oct;16(2):116–126. doi: 10.1016/0006-2944(76)90014-4. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. Atherogenic hyperlipoproteinemia induced by cholesterol feeding the Patas monkey. Biochemistry. 1976 Jul 13;15(14):2979–2985. doi: 10.1021/bi00659a007. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T., Brewer H. B., Jr Characterization of the plasma lipoproteins and apoproteins of the Erythrocebus patas monkey. Biochemistry. 1976 May 4;15(9):1928–1933. doi: 10.1021/bi00654a021. [DOI] [PubMed] [Google Scholar]
- Nelson C. A., Lee J. A., Brewster M., Morris M. D. Flotation equilibrium of serum low density lipoproteins. Anal Biochem. 1974 May;59(1):69–74. doi: 10.1016/0003-2697(74)90010-4. [DOI] [PubMed] [Google Scholar]
- Peters T. J., De Duve C. Lysosomes of the arterial wall. II. Subcellular fractionation of aortic cells from rabbits with experimantal atheroma. Exp Mol Pathol. 1974 Apr;20(2):228–256. doi: 10.1016/0014-4800(74)90057-4. [DOI] [PubMed] [Google Scholar]
- Ross A. C., Zilversmit D. B. Chylomicron remnant cholesteryl esters as the major constituent of very low density lipoproteins in plasma of cholesterol-fed rabbits. J Lipid Res. 1977 Mar;18(2):169–181. [PubMed] [Google Scholar]
- Rudel L. L., Greene D. G., Shah R. Separation and characterization of plasma lipoproteins of rhesus monkeys (Macaca mulatta). J Lipid Res. 1977 Nov;18(6):734–744. [PubMed] [Google Scholar]
- Rudel L. L., Lee J. A., Morris M. D., Felts J. M. Characterization of plasma lipoproteins separated and purified by agarose-column chromatography. Biochem J. 1974 Apr;139(1):89–95. doi: 10.1042/bj1390089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Lofland H. B. Circulating lipoproteins in nonhuman primates. Primates Med. 1976;9:224–266. [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D., Felts J. M. The transport of exogenous cholesterol in the rabbit. I. Role of cholesterol ester of lymph chylomicra and lymph very low density lipoproteins in absorption. J Clin Invest. 1972 Oct;51(10):2686–2692. doi: 10.1172/JCI107087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel L. L., Pitts L. L., 2nd, Nelson C. A. Characterization of plasma low density lipoproteins on nonhuman primates fed dietary cholesterol. J Lipid Res. 1977 Mar;18(2):211–222. [PubMed] [Google Scholar]
- Sears B., Deckelbaum R. J., Janiak M. J., Shipley G. G., Small D. M. Temperature-dependent 13C nuclear magnetic resonance studies of human serum low density lipoproteins. Biochemistry. 1976 Sep 21;15(19):4151–4157. doi: 10.1021/bi00664a003. [DOI] [PubMed] [Google Scholar]
- Slack J., Mills G. L. Anomalous low density lipoproteins in familial hyperbetalipoproteinaemia. Clin Chim Acta. 1970 Jul;29(1):15–25. doi: 10.1016/0009-8981(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Small D. M. Cellular mechanisms for lipid deposition in atherosclerosis (second of two parts). N Engl J Med. 1977 Oct 27;297(17):924–929. doi: 10.1056/NEJM197710272971710. [DOI] [PubMed] [Google Scholar]
- St Clair R. W., Smith B. P., Wood L. L. Stimulation of cholesterol esterification in rhesus monkey arterial smooth muscle cells. Circ Res. 1977 Feb;40(2):166–173. doi: 10.1161/01.res.40.2.166. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Atkinson D., Small D. M., Mahley R. W. Characterization of the lipoproteins of atherosclerotic swine. J Biol Chem. 1977 Oct 25;252(20):7288–7293. [PubMed] [Google Scholar]
- Tall A. R., Deckelbaum R. J., Small D. M., Shipley G. G. Thermal behavior of human plasma high density lipoprotein. Biochim Biophys Acta. 1977 Apr 26;487(1):145–133. doi: 10.1016/0005-2760(77)90051-0. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Shipley G. G., Small D. M. Conformational and thermodynamic properties of apo A-1 of human plasma high density lipoproteins. J Biol Chem. 1976 Jun 25;251(12):3749–3755. [PubMed] [Google Scholar]
- Tall A. R., Small D. M., Deckelbaum R. J., Shipley G. G. Structure and thermodynamic properties of high density lipoprotein recombinants. J Biol Chem. 1977 Jul 10;252(13):4701–4711. [PubMed] [Google Scholar]



