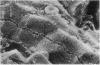
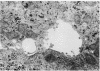
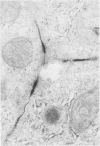
Abstract
Transepithelial movement of water and solute occurs both through the cell membrane as well as across the intercellular junctional complex (paracellular shunt pathways). Permeability of paracellular shunt pathways is increase by transmucosal osmotic gradients, and in certain epithelia these changes are associated with bullous-like deformations (blisters) of the zonula occludens and localization of lanthanum within junctional complexes. Although bile acids increase biliary secretion by osmotic forces, the source of this water movement into bile is not known. In the present studies we examined whether a choleretic infusion of sodium dehydrocholic acid (DHC) or its taurine conjugate, taurodehydrocholate, altered the solute permeability characteristics and morphologic appearance of the junctional complexes of rat hepatocytes. Animals were continuously infused for 1 hr with 1% albumin--0.9% NaCl alone or 120 mumol of DHC and bile flow and biliary clearance of [14C]sucrose, an indirect marker of biliary permeability were measured. The number of intercellular blisters adjacent to the bile canaliculus were counted in an unbiased manner from photographs obtained with scanning electron microscopy. Bile flow and the biliary sucrose clearance remained unchanged in control animals whereas DHC infusions resulted in a progressive increase in the biliary clearance of [14C]sucrose during the 60 min of infusion even though the choleretic response to DHC was stable during the final 30 min of infusion. DHC infusions produced surface invaginations, or blisters, (0.1--0.7 micrometer in diameter) which were located immediately adjacent to the hemi-bile canaliculus and occurred with a frequency of 1.62 +/- 0.08 per hepatocyte surface, which was fivefold greater than observed in controls. In separate groups of animals 5 mM ionic lanthanum chloride was perfused intraportally after taurodehydrocholate infusions, and the number of junctional complexes that contained the electron dense marker were quantitated by transmission electron microscopy. Localization of lanthanum in the junctional complexes of fasted control animals was not observed, whereas approximately equal to 50% of the zonula occludens in DHC-infused animals contained lanthanum which was also occasionally identified within the lumen of the bile canaliculus. These results indicate that infusions of DHC cause blisters adjacent to the junctional complex of rat hepatocytes in association with changes in solute conductivity of the zonula occludens to cations such as ionic lanthanum chloride, and presumably to larger solutes such as sucrose. Qualitatively similar morphologic findings were also observed during the infusion of sodium taurocholate at physiologic rate (40 mumol/h). These studies suggest that the paracellular shunt pathway in the liver is an important site for bile acid-induced water and solute movement into bile.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockman D. E. Route of flow and micropathology resulting from retrograde intrabiliary injection of India ink and ferritin in experimental animals. A combined light- and electron-microscopic study. Gastroenterology. 1974 Aug;67(2):324–332. [PubMed] [Google Scholar]
- Boyer J. L., Bloomer J. R. Canalicular bile secretion in man. Studies utilizing the biliary clearance of (14C)mannitol. J Clin Invest. 1974 Oct;54(4):773–781. doi: 10.1172/JCI107817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel M., Sahar A., Erlij D. The movement of lanthanum across diffusion barriers in the choroid plexus of the cat. Brain Res. 1974 Feb 15;67(1):178–184. doi: 10.1016/0006-8993(74)90311-4. [DOI] [PubMed] [Google Scholar]
- Chalcroft J. P., Bullivant S. An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J Cell Biol. 1970 Oct;47(1):49–60. doi: 10.1083/jcb.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., DiBona D. Pathways for movement of ions and water across toad urinary bladder. II. Site and mode of action of vasopressin. J Membr Biol. 1974;19(3):195–220. doi: 10.1007/BF01869978. [DOI] [PubMed] [Google Scholar]
- Claude P., Goodenough D. A. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol. 1973 Aug;58(2):390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagno J., Grisham J. W. Scanning electron microscopy of extrahepatic biliary obstruction. Arch Pathol. 1974 Jun;97(6):348–351. [PubMed] [Google Scholar]
- Creemers J., Jacques P. J. Endocytic uptake and vesicular transport of injected horseradish peroxidase in the vacuolar apparatus of rat liver cells. Exp Cell Res. 1971 Jul;67(1):188–203. doi: 10.1016/0014-4827(71)90634-3. [DOI] [PubMed] [Google Scholar]
- DiBona D. R., Civan M. M. Pathways for movement of ions and water across toad urinary bladder. I. Anatomic site of transepithelial shunt pathways. J Membr Biol. 1973;12(2):101–128. doi: 10.1007/BF01869994. [DOI] [PubMed] [Google Scholar]
- DiBona D. R. Passive intercellular pathway in amphibian epithelia. Nat New Biol. 1972 Aug 9;238(84):179–181. doi: 10.1038/newbio238179a0. [DOI] [PubMed] [Google Scholar]
- Erlij D., Martínez-Palomo A. Opening of tight junctions in frog skin by hypertonic urea solutions. J Membr Biol. 1972;9(3):229–240. [PubMed] [Google Scholar]
- Erlinger S., Dhumeaux D. Mechanisms and control of secretion of bile water and electrolytes. Gastroenterology. 1974 Feb;66(2):281–304. [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWCETT D. W. Observations on the cytology and electron microscopy of hepatic cells. J Natl Cancer Inst. 1955 Apr;15(5 Suppl):1475–1503. [PubMed] [Google Scholar]
- Fahimi H. D. Perfusion and immersion fixation of rat liver with glutaraldehyde. Lab Invest. 1967 May;16(5):736–750. [PubMed] [Google Scholar]
- Forker E. L. Hepatocellular uptake of inulin, sucrose, and mannitol in rats. Am J Physiol. 1970 Dec;219(6):1568–1573. doi: 10.1152/ajplegacy.1970.219.6.1568. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Mechanisms of hepatic bile formation. Annu Rev Physiol. 1977;39:323–347. doi: 10.1146/annurev.ph.39.030177.001543. [DOI] [PubMed] [Google Scholar]
- Forker E. L. The effect of estrogen on bile formation in the rat. J Clin Invest. 1969 Apr;48(4):654–663. doi: 10.1172/JCI106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend D. S., Gilula N. B. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972 Jun;53(3):758–776. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Glasinović J. C., Dumont M., Duval M., Erlinger S. Hepatocellular uptake of bile acids in the dog: Evidence for a common carrier-mediated transport system. An indicator dilution study. Gastroenterology. 1975 Oct;69(4):973–981. [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPTON J. C. Electron microscopic study of extrahepatic biliary obstruction in the mouse. Lab Invest. 1961 May-Jun;10:502–513. [PubMed] [Google Scholar]
- Hardison W. G. Metabolism of sodium dehydrocholate by the rat liver: its effect on micelle formation in bile. J Lab Clin Med. 1971 May;77(5):811–820. [PubMed] [Google Scholar]
- Javitt N. B. Hepatic bile formation. (Second of two parts). N Engl J Med. 1976 Dec 30;295(27):1511–1516. doi: 10.1056/NEJM197612302952705. [DOI] [PubMed] [Google Scholar]
- Layden T. J., Boyer J. L. Taurolithocholate-induced cholestasis: taurocholate but not dehydrocholate, reverses cholestasis and bile canalicular membrane injury. Gastroenterology. 1977 Jul;73(1):120–128. [PubMed] [Google Scholar]
- Layden T. J., Schwarz, Boyer J. L. Scanning electron microscopy of the rat liver. Studies of the effect of taurolithocholate and other models of cholestasis. Gastroenterology. 1975 Sep;69(3):724–738. [PubMed] [Google Scholar]
- Ma M. H., Laird W. A., Scott H. Cytopempsis of horseradish peroxidase in the hepatocyte. J Histochem Cytochem. 1974 Mar;22(3):160–169. doi: 10.1177/22.3.160. [DOI] [PubMed] [Google Scholar]
- Machen T. E., Erlij D., Wooding F. B. Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J Cell Biol. 1972 Aug;54(2):302–312. doi: 10.1083/jcb.54.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Palomo A., Erlij D., Bracho H. Localization of permeability barriers in the frog skin epithelium. J Cell Biol. 1971 Aug;50(2):277–287. doi: 10.1083/jcb.50.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Palomo A., Erlij D. Structure of tight junctions in epithelia with different permeability. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4487–4491. doi: 10.1073/pnas.72.11.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter A., Orci L., Rouiller C. A study on the permeability barriers between Disse's space and the bile canaliculus. J Ultrastruct Res. 1969 Oct;11:1–71. [PubMed] [Google Scholar]
- Motta P., Fumagalli G. Structure of rat bile canaliculi as revealed by scanning electron microscopy. Anat Rec. 1975 Aug;182(4):499–513. doi: 10.1002/ar.1091820408. [DOI] [PubMed] [Google Scholar]
- Nemchausky B. A., Layden T. J., Boyer J. L. Effects of chronic choleretic infusions of bile acids on the membrane of the bile canaliculus. A biochemical and morphologic study. Lab Invest. 1977 Mar;36(3):259–267. [PubMed] [Google Scholar]
- PREISIG R., COOPER H. L., WHEELER H. O. The relationship between taurocholate secretion rate and bile production in the unanesthetized dog during cholinergic blockade and during secretin administration. J Clin Invest. 1962 May;41:1152–1162. doi: 10.1172/JCI104568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichen J., Paumgartner G. Kinetics of taurocholate uptake by the perfused rat liver. Gastroenterology. 1975 Jan;68(1):132–136. [PubMed] [Google Scholar]
- SCHANKER L. S., HOGBEN C. A. Biliary excretion of inulin, sucrose, and mannitol: analysis of bile formation. Am J Physiol. 1961 May;200:1087–1090. doi: 10.1152/ajplegacy.1961.200.5.1087. [DOI] [PubMed] [Google Scholar]
- Schatzki P. F. Bile canaliculus and space of Disse. Electron microscopic relationships as delineated by lanthanum. Lab Invest. 1969 Jan;20(1):87–93. [PubMed] [Google Scholar]
- Schatzki P. F. The passage of radioactive lanthanum from the biliary to the vascular system. An electron microscopic and radioactive tracer study. Z Zellforsch Mikrosk Anat. 1971;119(4):451–459. doi: 10.1007/BF00455242. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. The role of paracellular pathways in isotonic fluid transport. Yale J Biol Med. 1977 Mar-Apr;50(2):99–113. [PMC free article] [PubMed] [Google Scholar]
- Shea S. M. Lanthanum staining of the surface coat of cells. Its enhancement by the use of fixatives containing Alcian blue or cetylpyridinium chloride. J Cell Biol. 1971 Dec;51(3):611–620. doi: 10.1083/jcb.51.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975 Dec;67(3):863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Tisher C. C., Yarger W. E. Lanthanum permeability of the tight junction (zonula occludens) in the renal tubule of the rat. Kidney Int. 1973 Apr;3(4):238–250. doi: 10.1038/ki.1973.37. [DOI] [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- Wade J. B., Karnovsky M. J. Fracture faces of osmotically disrupted zonulae occludentes. J Cell Biol. 1974 Aug;62(2):344–350. doi: 10.1083/jcb.62.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. B., Karnovsky M. J. The structure of the zonula occludens. A single fibril model based on freeze-fracture. J Cell Biol. 1974 Jan;60(1):168–180. doi: 10.1083/jcb.60.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. B., Revel J. P., DiScala V. A. Effect of osmotic gradients on intercellular junctions of the toad bladder. Am J Physiol. 1973 Feb;224(2):407–415. doi: 10.1152/ajplegacy.1973.224.2.407. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]