Abstract
Despite recent advances in biomaterial science, there is yet no culture system that supports long-term culture expansion of human adult hepatocytes, while preserving continued function. Previous studies suggested that acellular liver extracellular matrix (ECM), employed as a substrate, improved proliferation and function of liver cells. Here we investigated whether extracts prepared from acellular liver ECM (liver ECM extract, LEE), or from whole (fresh) liver tissue (liver tissue extract, LTE), could be combined with collagen Type I, hyaluronic acid (HA), or heparin-conjugated HA (HP) hydrogels to enhance survival and functional output of primary human hepatocytes. The liver-specific semi-synthetic ECMs (sECMs) were prepared by incorporating LEE or LTE into the gel matrices. Subsequently, primary human hepatocytes were maintained in sandwich-style hydrogel cultures for 4 weeks. Progressive increase in hepatocyte metabolism was observed in all HA and HP groups. Hepatocytes cultured in HA and HP hydrogels containing LEE or LTE synthesized and secreted steady levels of albumin and urea and sustained cytochrome p450-dependent drug metabolism of ethoxycoumarin. Collectively, these results indicate that customized HA hydrogels with liver-specific ECM components may be an efficient method for expansion human hepatocytes in vitro for cell therapy and drug and toxicology screening purposes.
Keywords: Hyaluronic acid, Heparin, Hepatocyte, Extracellular matrix, Growth factors
1. Introduction
There is a critical need for improved model systems to predict efficacy, bioavailability, and toxicology outcomes for candidate drugs. Currently, in vivo animal models serve as gold standards for testing prior to clinical trials, but the drawbacks associated with such models are major contributors to the costs and uncertainties in therapy development. in vitro systems that use human tissues would be preferable [1]; however, for these systems to serve as tools that reflect human biology, methods to expand human cells in culture, while preserving their functions, are key for future use in pharmacokinetic and toxicity testing. To that end, we have developed a 3-D culture system for preparing and maintaining human hepatocyte tissue constructs with potential to serve as effective drug and toxicology screening tools.
In vitro cultured primary hepatocytes are increasingly being used for screening in the pharmaceutical industry [2]. However, there is still a need for an optimal culture system that improves the long-term maintenance of liver cells with retention of liver function for in vitro drug screening. In recent years numerous biomaterials have been employed for in vitro hepatocyte culture [3]. They can be divided into two broad types: simpler non-surface modified biomaterials and more complex surface modified biomaterials. The non-surface modified scaffolds, for example, adding galactosylated hyaluronic acid to chitosan scaffolds [4] and the galactosylation of nanofibrous chitosan scaffolds [5] improved albumin and urea production and cytochrome p450 activity in primary rat hepatocytes in 15 and 7 day cultures, respectively. The surface modified biomaterials incorporate amendment of the primary scaffold components with bioactive molecules that support control over growth factor release, such as heparin or cell attachment, such as fibronectin or RGD peptides. For instance, polyethylene glycol (PEG)-heparin hydrogels supported consistent albumin and urea levels in rat hepatocytes for 3 weeks [6]. Similarly, micropatterned PEG-fibrinogen gels supported albumin and urea levels for 10 day, but only with crucial supplements such as fibronectin, epidermal growth factor (EGF), transforming growth factor-α (TGF-α), and hepatocyte growth factor (HGF) [7]. Poly(methylmethacrylate) chips containing microwells coated with RGD peptides were used to culture rat hepatocyte spheroids for 12 days. Multiple Cytochrome p450 assays were performed in which activity levels were greater in the chip wells than on collagen-coated dishes [8]. Rat hepatocytes were also maintained for 7 days on collagen-coated nanofibrous poly(l-lactic acid) scaffolds. Viability remained high, cells displayed good glycogen storage capability, and consistent albumin production, as well as several other markers. Cytochrome activity was inducible, but not necessarily greater than monolayer controls [9].
While undoubtedly useful, the studies discussed above only employ rat hepatocytes. Primary human hepatocytes are desirable, but are difficult to obtain and maintain in culture. Hepatoma cell lines, such as HEPG2, are often used as alternatives to primary hepatocytes due to their low cost and ease of culture. Unfortunately, the key drug metabolism enzymes necessary for drug screening are often absent or expressed only in low levels in HEPG2 cells [10]. Hepatocytes, on the other hand, are fragile when removed from liver tissue and cultured on plastic in vitro. The cells quickly lose liver-specific functions and tend to apoptosis due to lack of appropriate environmental support. However, our previous study demonstrated that decellularized liver ECM used as a substrate improved expansion and function of human primary hepatocytes over a 3 week time course [11]. Additionally, in recent years use of tissue-derived ECMs from different organs has been widely explored for culture of other cell types, including the concept of solubilizing ECM and reconstituting it at a later time. ECMs derived from porcine urinary bladder matrix were solubilized in pepsin, and subsequently self-assemble into a gel at physiological conditions, supporting expansion of rat aortic arch smooth muscle cells [12]. Moreover, alginate microspheres containing urinary bladder matrix and Sertoli cells that were implanted into nonobese diabetic mice resulted in diabetes prevention and reversion [13]. The beneficial effects of ECM-based supplements are likely a result of the ECM being a source of many specific factors including, but not limited to, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), EGF, transforming growth factor-β (TGF-β), keratinocyte growth factor (KGF), HGF, and platelet-derived growth factor (PDGF) [14]. The structural components in the ECM, such as collagens, glycosaminoglycans, fibronectins, laminins, and elastin, might improve bioactivity of materials through mechanical and biochemical cues. By incorporating liver-specific materials into hydrogels, we aimed to improve the in vitro viability, and support the long-term function of primary human hepatocytes. Our hypothesis in this study is that HA-heparin biomaterials can combine with growth factors and ECM components existing in liver tissue or liver ECM extracts so that these factors be slowly slow released, enhancing maintenance of functional liver cells. For the structural foundation of our constructs we chose Type I rat tail collagen as a control, as it has been used as a standard for cell culture, and an HA-based hydrogel, since HA-derivatives have been extensively implemented as a cell carrier for regenerative medicine applications in recent years [15]. Examples include wound healing of corneal lacerations [16], embryonic stem cell expansion [17], soft tissue engineering in vitro and in vivo [18–21], tumor xenograft models [22–24], and bioprinting of cellularized tubular structures[25,26]. The HA variety of semi-synthetic ECMs (sECMs) are commercially available as Extracel™ and HyStem™ for research use, and are in clinical use for veterinary medicine by Sentrx Surgical. Products for clinical use in humans are undergoing FDA review. Herein, we fabricated sECMs containing extracts from fresh or acellular liver tissues and investigated their support for survival, expansion, and function of primary human hepatocytes.
2. Materials and methods
2.1. Decellularization of liver tissues
Fresh porcine livers (Disher Packing, Inc., Yadkinville, NC) were pre-rinsed through the hepatic portal vein with chilled Dulbecco’s phosphate buffered saline (DPBS). The liver was cut into 7.6 cm by 10.2 cm blocks and flash frozen at −80C. Frozen liver blocks were sectioned into 3 mm slices. Slices (6–10) were transferred to 500 ml distilled water and shook on a rotary shaker at 200 rpm for 3 days at 4 °C, during which water was changed three times per day. The liver slices were treated with 2% Triton X-100 for 4 days followed by 2% TX-100 + 0.1% NH4OH for 24 h. During the TX-100 rinses, solutions were changed twice daily. The decellularized liver tissues were washed for 2 additional days in distilled water to remove any traces of TX-100, after which they were stored at 4 °C until further use.
2.2. Preparation of tissue and ECM digest
Fresh liver tissues and decellularized liver ECMs were lyophilized for 48 h. Following lyopholization, samples were ground into a powder with a freezer mill. One gram of liver tissue or liver ECM powder was mixed with 100 mg Pepsin (Porcine gastric mucosa, 3400 units of protein, Fisher Scientific, Fair Lawn, NJ) and sterilized by gamma irradiation (1 Mrad). All subsequent procedures following sterilization were carried out under sterile conditions. Hydrochloric acid (0.1 N, 100 μL) was added to the sterilized materials and incubated for 48 h at room temperature. The resulting mixture was transferred to a 50 ml conical tube and centrifuged at 3000 rpm for 15 min. The supernatant was removed and the pellet was discarded. This was repeated 3 times until the supernatant was clear. To ensure there was no more particulate matter remaining, the suspension was filtered through a 0.2 μm syringe filter (Fisher Scientific). The resulting liver decellularized ECM extract (LEE) and liver tissue extracts (LTE) were stored at −80 °C until further use.
2.3. Collagen and hyaluronic acid liver sECM and liver tissue gel preparation
Type I rat tail collagen (BD Biosciences, Bedford, MA) was diluted to 1 mg/mL in PBS and adjusted to pH 7.0 by 1 M NaOH. LEE and LTE solutions (1 mg/ml) were also adjusted to pH 7.0 by 1 M NaOH. Collagen only gels (COL) were formed by mixing the prepared collagen solution with William’s E Media (Invitrogen, Carlsbad, CA) 1:1 by volume. Collagen with LEE or LTE gels (COL + EG and COL + TG, respectively) were formed by mixing the collagen solution with LEE or LTE 1:1 by volume. Gels were allowed to crosslink for 1 h before use.
For HA and HP gels, Extracel and Extracel-HP (Glycosan Biosystems, Alameda, CA) components were dissolved in sterile water. Briefly, Glycosil (for HA gels), Heprasil (for HP gels), and Gelin-S were dissolved in water to make 2% w/v solutions. Extralink, the crosslinker, was dissolved in water to make a 4% w/v solution. For HA-only and HP-only gels, Glycosil (or Heprasil), Gelin-S, and Extralink were mixed 2:2:1 by volume. The resulting solution was mixed 1:1 with water and vortexed. For gels containing LEE or LTE (HA + EG, HA + TG, HP + EG, and HP + TG), LEE or LTE were substituted for the water in final mixing step. Solutions were allowed to crosslink for 30 min before use. Formulation of liver-specific collagen and hyaluronic acid gels are displayed in Table 1.
Table 1.
Combinations of all substrate groups implemented within the study. Nomenclature: P = plastic; Col = collagen; HA = Extracel (hyaluronic acid); HP = Extracel-HP (heparin-conjugated hyaluronic acid); ES = liver ECM extract delivered by media suspension; TS = whole liver tissue extract delivered by media suspension; EG = liver ECM extract incorporated into the gel. TG = whole liver tissue extract incorporated into the gel
| Abbreviation | Component | Modification | Concentration | Gel supplement | Gel Supp. concentration |
Media supplement | Media Supp. concentration |
|---|---|---|---|---|---|---|---|
| P | TC Plastic | – | – | – | – | – | – |
| P + ES | TC Plastic | – | – | – | – | ECM Extract | 1 mg/mL |
| P + TS | TC Plastic | – | – | – | – | Tissue Extract | 1 mg/mL |
| Col | Collagen 1 | – | 0.5 mg/mL | – | – | – | – |
| Col + EG | Collagen 1 | – | 0.5 mg/mL | ECM Extract | 0.5 mg/mL | – | – |
| Col + EG + TS | Collagen 1 | – | 0.5 mg/mL | ECM Extract | 0.5 mg/mL | Tissue Extract | 1 mg/mL |
| Col + TG | Collagen 1 | – | 0.5 mg/mL | Tissue Extract | 0.5 mg/mL | – | – |
| Col + TS | Collagen 1 | – | 0.5 mg/mL | – | – | Tissue Extract | 1 mg/mL |
| Col + ES | Collagen 1 | – | 0.5 mg/mL | – | – | ECM Extract | 1 mg/mL |
| HA | Hyaluronic Acid | – | 4 mg/mL | – | – | – | – |
| HA + EG | Hyaluronic Acid | – | 4 mg/mL | ECM Extract | 0.5 mg/mL | – | – |
| HA + EG + TS | Hyaluronic Acid | – | 4 mg/mL | ECM Extract | 0.5 mg/mL | Tissue Extract | 1 mg/mL |
| HA + TG | Hyaluronic Acid | – | 4 mg/mL | Tissue Extract | 0.5 mg/mL | – | – |
| HA + TS | Hyaluronic Acid | – | 4 mg/mL | – | – | Tissue Extract | 1 mg/mL |
| HA + ES | Hyaluronic Acid | – | 4 mg/mL | – | – | ECM Extract | 1 mg/mL |
| HP | Hyaluronic Acid | Heparin- Conjugation | 4 mg/mL | – | – | – | – |
| HP + EG | Hyaluronic Acid | Heparin- Conjugation | 4 mg/mL | ECM Extract | 0.5 mg/mL | – | – |
| HP + EG + TS | Hyaluronic Acid | Heparin- Conjugation | 4 mg/mL | ECM Extract | 0.5 mg/mL | Tissue Extract | 1 mg/mL |
| HP + TG | Hyaluronic Acid | Heparin- Conjugation | 4 mg/mL | Tissue Extract | 0.5 mg/mL | – | – |
| HP + TS | Hyaluronic Acid | Heparin- Conjugation | 4 mg/mL | – | – | Tissue Extract | 1 mg/mL |
| HP + ES | Hyaluronic Acid | Heparin- Conjugation | 4 mg/mL | – | – | ECM Extract | 1 mg/mL |
2.4. Extract extracellular matrix component and growth factor analysis
LEE and LTE solutions (1 mg/mL) were prepared as described above were analyzed for collagen, elastin and glycosaminoglycan (GAG) content. To measure each component, 25 mg of starting material was employed (n = 3). Following additional chemical digestion with HCL (collagen assay), papain (GAG assay) and oxalic acid (elastin assay), samples were analyzed with the Blyscan Collagen, GAG, and Elastin assay kits (Biocolor Life Sciences Assays, Carrickfergus, UK) according to the manufacturer’s instructions in order to quantify the amount of each component present.
Additionally, LEE and LTE solutions were analyzed for growth factor content using a Quantibody® Human Growth Factor Array (RayBiotech, Norcross, GA). LEE and LTE solutions (1 mL aliquots) were prepared as described above for analysis.
2.5. Primary human hepatocyte culture on liver-specific sECMs
Primary human hepatocytes were extracted from the liver of a Caucasian, 50yr old male (Invitrogen). Cells were suspended in 25 ml of William’s E Medium solution and counted on the hemocytometer with Trypan blue to assess viability (85%). Substrate groups (50 μL in 96-well plates) were prepared as described above and 60,000 cells were seeded on top of each substrate and incubated for 3 h (37 °C, 5% CO2). Following incubation substrates were washed 3 times with warm DPBS to remove any dead or unattached cells. An additional layer of the corresponding gel solutions were added to each well and allowed to crosslink for 1 h to create sandwich culture tissue constructs. After the gels were sufficiently crosslinked, 100 μl of fresh William’s E Medium or conditioned TS or ES media was added to the appropriate wells. The hepatocytes were maintained in culture for 4 weeks, with media changes every 2 days.
Every week, the cultures were analyzed for cellular morphology. Phase microscopy photos were taken with a Zeiss Axiovert (Carl Zeiss) at 10× and 20× magnification. Proliferation was evaluated by measuring mitochondrial metabolism, using the MTS assay at week 1, 2, 3, and 4, in the same manner as described above. On week 4, cell viability was assessed using by LIVE/DEAD© as described above, except due to the ÓsandwichÓ culture, the incubation period was extended to 45 min.
2.6. Human albumin ELISA and immunohistochemistry (IHC)
For functional characterization, cells were assessed for albumin secretion. Once each week, during normal media changes, spent media was extracted from triplicate wells of each group and transferred to microcentrifuge tubes. Samples were stored at −80 °C until the end of the study. Secreted levels of albumin were quantified using a Human Albumin ELISA Kit (Bethyl Laboratories, Montgomery, TX). Samples were quantified on the plate reader at 450 nm.
For immunohistochemistry (IHC), at the end of the study samples were prefixed in 4% paraformaldehyde for 30 min. Following fixation all samples were washed three times in PBS and stored at 4 °C. Prior to staining, all samples were permeabilized with 0.1% Triton X-100 (TX-100) in PBS. Cells were washed twice to remove any remaining TX-100. Treatments were incubated in 100 μml of Dako Antibody Protein Block, Serium-Free (Dako, Carpinteria, CA) for 30 m. Following blocking, the human albumin primary antibody (1:100, raised in mouse, Sigma) was prepared in Dako Antibody Diluent with background reduction (Dako) and added to each well and allowed to incubate at 4 °C overnight. Samples were washed three times for 15 min in PBS followed by application of the secondary Anti-mouse IgG FITC antibody (1:200, Sigma). Samples were incubated with the secondary for 3 h. Cells were washed three more times with PBS and viewed at 20× magnification with the Zeiss Axiovert.
2.7. Urea analysis
For analysis of urea secretion, as described above, spent media was extracted from triplicate wells of each group and transferred to microcentrifuge tubes once each week. Samples were stored at −80 °C until the end of the study. Urea levels in 75 μl media aliquots were analyzed using the QuantiChrom™ Urea Colorimetric Assay Kit (BioAssay Systems, Hayward, CA). Samples were quantified on the plate reader at 430 nm.
2.8. Cytochrome p450-dependent drug metabolism assay
On week 4 of culture a kinetic assay was performed to assess cytochrome p450 dependent drug metabolism activity. Solutions of 50 mM 3-cyano-7-ethoxycoumarin (100 μl) in William’s Media were added to triplicate groups after aspirating spent media. The multiwell dishes were immediately transferred to the plate reader where fluorescent readings (408 nm excitation/450 nm emission) were taken every 40 s for 15 min to measure the fluorescent metabolized drug product.
3. Results
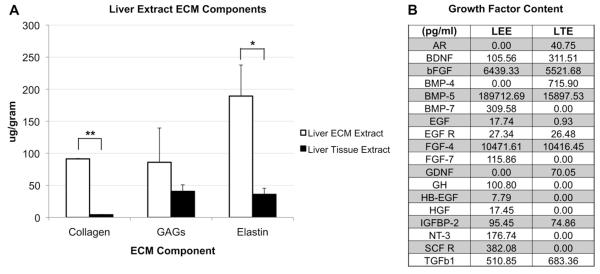
ECM component analysis was carried out using a series of colorimetric assays. The results revealed a clear trend, in which LEE solutions contained greater concentrations of collagen, glycosaminoglycans (GAGs), and elastin (Fig. 1A). Specifically, the total collagen content of LEE, 91.33 μg/mL, was significantly greater than that of LTE, which was 4.17 μg/mL (p < 0.001), the elastin content of LEE, 189.33 μg/mL, was significantly greater than that of LTE, which was 36.00 μg/mL (p < 0.05) and the GAG content of LEE, 86.00 μg/mL, was greater than that of LTE, which was 40.67 μg/mL, but not significantly (p > 0.05).
Fig. 1.
Concentrations of ECM components and growth factors in fresh liver and liver ECM extracts than. A) The concentrations of collagen, GAGs, and elastin in LEE and LTE. B) A panel displaying the growth factors and cytokines amounts (pg/ml) measured in LEE and LTE. Significance: *p < 0.05; **p < 0.001.
The Quantibody® Growth Factor array analysis revealed that, in general, LEE contained higher concentrations of growth factors and cytokines (shown in pg/mL, Fig. 1B). Of particular interest was that brain-derived neurotrophic factor (BDNF), bFGF, bone morphogenetic protein 5 (BMP-5), FGF-4, insulin-like growth factor binding protein 2 (IGFBP-2), and TGF-β1 were relatively conserved between both LEE and LTE. However, LEE also contained BMP-7, EGF, FGF-7, growth hormone (GH), heparin-binding EGF-like growth factor (HB-EGF), HGF and neurotrophin 3 (NT-3), which were not observed or were negligible in LTE. On the other hand, BMP-4, and glial-derived neurotrophic factor (GDNF) were present in LTE, but not in LEE.
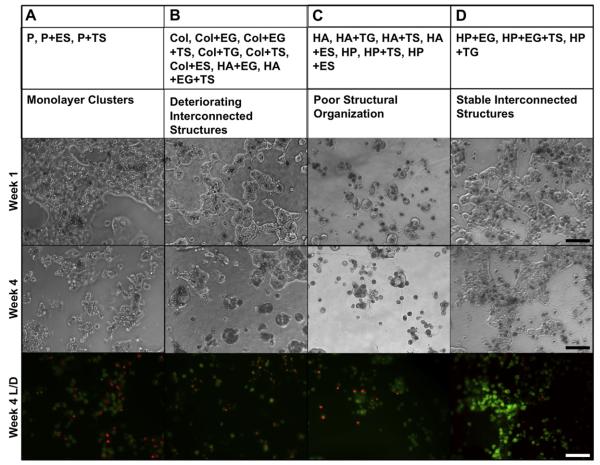
Primary human hepatocytes, cultured on different sECMs for 4 weeks, showed distinct differences in morphology. We were able to group the sECMs into 4 main categories, based on hepatocyte culture morphology (Fig. 2). Group A consisted of P, P + ES, and P + TS constructs; Group B consisted of COL, COL + EG, COL + EG + TS, COL + TG, COL + TS, COL + ES, HA + EG, and HA + EG + TS; Group C consisted of HA, HA + TG, HA +TS, HA + ES, HP, HP + TS, and HP + ES; and Group D consisted of HP + EG, HP + EG + TS, and HP + TG. In Group A, hepatocytes initially formed monolayer-like clusters. However, over time the monolayers deteriorated and cell density decreased. This was mirrored for the most part in the MTS data discussed below. In Group B, hepatocytes initially formed interconnecting structures, indicative of healthy hepatoctyes. However, like Group A, the interconnecting structures had greatly deteriorated and cell density had decreased by week 4. In Group C, there was no evidence of inter-cellular structural organization at any time point. Finally, in Category D, hepatocytes formed large interconnecting structures that remained healthy and stable throughout the study.
Fig. 2.
Morphological behavior of primary human hepatocyte cultures induced by sECMs and extract combinations. Representative images from week 1 and week 4 are shown: A) Monolayer clusters, B) interconnected structures that deteriorated, C) individual cells with no cell–cell organization, and D) stable interconnected structure. Constructs were LIVE/DEAD-stained to highlight viable and dead cells. Green fluorescence indicates calcein-AM-stained live cells and red fluorescence indicates ethidium homodimer-1-stained dead cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The effects of sECMs on primary hepatocyte viability was determined using LIVE/DEAD staining on week 4 of the culture. In general, Groups A and B displayed the highest incidence of ethidium homodimer-1-stained dead cells, followed by Group C, and lastly Group D, which had the most calcein-AM-stained viable cells and fewest dead cells (Fig. 2).
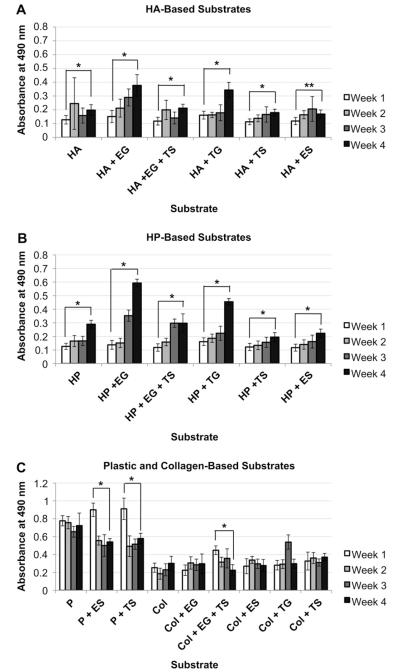
Hepatocyte proliferation on sECMs was determined using MTS assay. In general, the assay revealed that the mitochondrial metabolism of each primary human hepatocyte, culture on HA and HP liver sECMs, increased over the 4-week study (Fig. 3). Absorbance readings from week 1 vs week 4 for all groups were statistically significant (p < 0.01), with the exception of HA + ES, which was still significant, but at to a lesser extent (p < 0.05). The HP-EG constructs showed the greatest increase in absorbance levels from week 1 to week 4. Absorbance readings of heatocyted cultured on plastic (P) or collagen (COL) alone did not increase significantly over the duration of the study. In fact, the absorbance levels of the P + ES, P + TS, and COL + EG + TS sECMs decreased significantly from week 1 to week 4 (p < 0.01, + Fig. 3C).
Fig. 3.
Proliferation of primary human hepatocytes in different sECMs and extract combinations. Mitochondrial metabolism of primary human hepatocytes cultured in A) HA-based sECMs, B) HP-based sECMs, or C) on plastic or in collagen-based sECMs. MTS assays were performed on week 1, 2, 3, and 4. Significance: *p < 0.01; **p < 0.05.
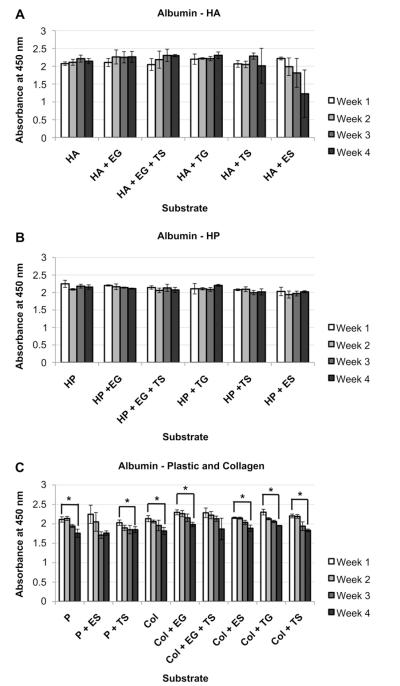
We determined albumin synthesis by hepatocytes cultured on different sECMs using ELISA. Albumin production was maintained in all HA and HP groups over the 4-week study, with the exception of one group, HA + ES, that showed a decreasing trend (Fig. 4A and B). In comparison, all plastic and collagen-based groups showed a week-to-week decreasing trend in albumin production for plastic and collagen groups (Fig. 4C). Albumin-dependent absorbance levels in P, P + TS, COL, COL + EG, COL + ES, COL + TG, and COL + TS groups all decreased significantly between week 1 and week 4 (p < 0.05).
Fig. 4.
Albumin secretion in primary human hepatocytes in different sECMs and extract combinations. Hepatocytes in A) HA, B) HP and C) Plastic and collagen, albumin secretion, as quantified by ELISA. Significance: *p < 0.05.
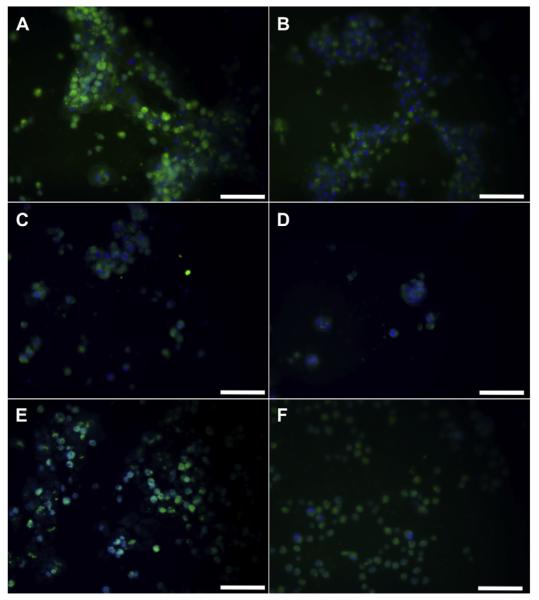
Immunofluorescent staining of albumin showed different degrees of staining intensity depending on the sECM combination. HP + EG groups stained the most strongly for albumin by far (Fig. 5A), followed by HP + EG + TS and HP + TG groups (Fig. 5B). These groups also featured a higher incidence of cytoplasmic staining than other groups in which albumin staining remained near the cell nuclei (Fig. 5C through 4D). This result supports the previous morphological observation (Fig. 2) and suggests that the HP + EG and HP + EG + TS groups show more spread out hepatocytes, in contrast to the smaller rounded cells of other groups.
Fig. 5.
Albumin synthesis in primary human hepatocytes in different sECMs and extract combinations. Albumin IHC reveal differences in intracellular albumin. Images are representative of the following groups: C) HP + EG; D)HP + EG + TS, HP + TG; E)HP, HA + EG, HA + EG + TS; F)HA, HA + TS, CG + EG, CG + EG + TS, CG + TG, CG + ES; G)P, P + ES, P + TS; and H)HP + ES, HP + TS, HA + ES, HA + TS. Scale bar – 50 μm.
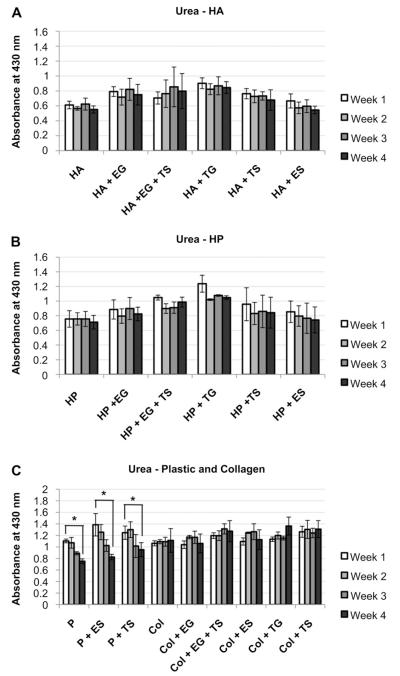
Urea secretion colorimetric assay revealed that in HA and HP groups, levels of secreted urea remained consistent for the duration of the study. In no HA and HP groups did urea secretion levels decrease significantly from week 1 to week 4 (Fig. 6). Similarly, collagen-based sECMs secreted consistent amounts of urea during the 4 weeks’ study. However, all 3 plastic groups secreted significantly less urea on week 4 than on week 1 (p < 0.05, Fig. 6C). Also, we observed that incorporation of LEE and LTE into HA and HP gels increased urea production trends, but not significantly.
Fig. 6.
Urea secretion in primary human hepatocytes in different sECMs and extract combinations. Hepatocytes in A) HA, B) HP and C) Plastic and collagen, urea secretion, as quantified by colorimetric assays. Significance: *p < 0.05.
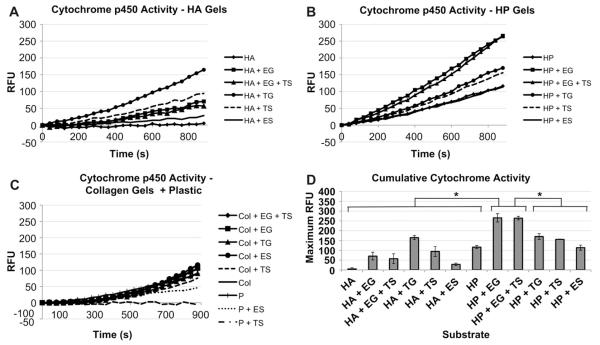
Drug metabolism was investigated using a kinetic assay for cytochrome p450-activity. Media containing 3-cyano-7-ethoxycoumarin was added to the constructs, and the fluorescence of the metabolized drug product was measured on the plate reader every 40 s over a 15 min time course. Of the HA groups, HA + TG performed best, reaching a maximum RFU of 164.68 ± 10.41 (Fig. 7A). Other HA-based groups did not exceed 100 RFU. Maximum RFU output of HP was generally higher (Fig. 7B). Some plastic and collagen-based groups showed increases in fluorescence during the kinetic assay (Fig. 7C), but in general performed poorly in comparison to HA and HP groups. In particular, RFU output of HP + EG and HP + EG + TS constructs were significantly higher than all other groups (p < 0.05, 7D), reaching maximum RFUs of 265.57 ± 21.28 and 264.20 ± 9.10, respectively, indicating the best cytochrome p450-dependent drug metabolism.
Fig. 7.
Cytochrome p450 activity secretion in primary human hepatocytes in different sECMs and extract combinations. Kinetic assay of Cytochrome p450-dependent metabolism of the drug ethoxycoumarin by hepatocytes cultured in A) HA, B) HP, and C) Plastic and collagen constructs. RFUs are proportional to the amount of drug metabolized. The assay was performed at week 4. D) Cumulative cytochrome p450 activity at the end of the 15 min kinetic assay. Significance: *p < 0.05.
4. Discussion
in vitro cultures of human hepatocytes are at the forefront of drug and toxicology screening systems. Our studies were successful in developing a biomaterial system in which the viability and function of primary human hepatocytes could be supported for at least 4 weeks. In contrast, prior studies, as discussed in the introduction, only 2 reached 3 weeks [6,11]. In fact, most of these prior studies maintained hepatocytes only for 1–2 weeks. Short culture time courses were likely due to over-simplified biomaterial applications. This illustrates the need for better in vitro mimicry of in vivo tissue environments, including composition and mechanics. Furthermore, other than one exception, primary rat hepatoctyes were used in every study. While useful in research settings, animalderived tissue-engineered constructs do not address they underlying problem; Tests employing primary human hepatoctyes are more likely to yield human-specific drug metabolism data that is more pertinent to the clinic.
In the current study we developed and evaluated a new hydrogel-based system that incorporates liver-derived extracts for maintenance of primary human hepatocyte viability and function in vitro. Of all the hydrogel system variations investigated, a heparin-conjugated hyaluronic acid hydrogel containing ECM components and growth factors derived from a decellularized liver most successfully supported hepatocyte proliferation and function during the 4 week study. These hepatocyte-sECM constructs gave rise to interconnected hepatocyte multi-cellular structures that remained viable and stable throughout the study, while secreting albumin and urea at consistent levels. IHC showed strong presence of cytoplasmic albumin, further illustrating active albumin production. Our best-performing conditions showed successful metabolism of ethoxycoumarin, a drug that metabolizes by CYP1A2, CYP2C9, and CYP2C19 isoforms of cytochrome p450. Ultimately, we would like to test the activity of other isoforms over longer time-courses to better verify the robust ability of our hepatocyte-liver sECM constructs to metabolize drugs. Additionally, thought should be given to analyzing secondary metabolites that are important for identifying downstream hepatocyte metabolic activity.
EG groups (gels containing LEE) generally outperformed TG groups (gels containing LTE). ECM component quantification of the extracts showed increased collagen, GAG, and elastin content across the board in LEE compared to LTE. It appears that adding these components into the cultures enhance hepatocyte function by providing a biomaterial environment closer to that of native liver tissue. Likewise, a growth factor array analysis of the extracts revealed that LEE generally contained greater concentrations of a series of growth factors and cytokines that likely play a major role in cell signaling. The differences in component concentrations could be a result of several factors. Initially, it was hypothesized that reactive cell death byproducts released during dissolution of the cellularized whole liver tissues may have further degraded the ECM components beyond that of the pepsin/HCl treatment. However, since the starting materials were equally weighted portions of decellularized liver ECM and whole liver, it is more likely that there were less collagens, GAGs, and elastins in the whole liver starting material in the first place, since cellular material was present and acted as a possible diluting factor.
The HA hydrogels employed, Extracel and Extracel-HP, are pure systems that allowed for more sensitive screening of the liverderived extracts. This is based on the assumption that collagen is one of the primary components of LEE and LTE. When the extracts are incorporated in collagen gels, the extract components, such as naturally occurring collagens, HA and other GAGs, elastins, fibronectins, and laminins, are largely overshadowed by the highly cell-adherent collagen in the gel. Extracel and Extracel-HP provide structural integrity, some cell adherence, and in the case of Extracel-HP, regulation of heparin-binding growth factor release, while allowing the bioactive components of the extracts to play integral parts in influencing hepatocyte viability and function.
The differences in quantitative albumin and urea data between corresponding HA and HP groups were minimal; suggesting all the hepatocytes cultured in these matrices retained some basic level of function. This is in contrast to the albumin IHC and drug metabolism data. HP + EG, HP + EG + TS, and HP + TG stained significantly stronger for albumin. Additionally, these hepatocytes fluoresced more intensely in the cytoplasm, indicating that the cells were more spread out. Indeed, this was mirrored by phase microscopy, in which only HP + EG, HP + EG+ TS, and HP + TG hepatocytes formed large interconnected multi-cell structures that remained healthy and stable over 4 weeks. In the cytochrome p450-dependent ethoxycoumarin drug metabolism assay, most groups showed some level of activity. However, HP + EG and HP + EG + TS constructs showed the steepest RFU curves during the 15 min kinetic assay, and cumulative cytochrome p450 activities that were significantly higher than those of all other groups indicating greater efficiency in metabolizing the drug.
Most likely, the factors primarily responsible for the success of the HP material combinations are, first, heparin-regulated growth factor release, and second, the incorporation of the extract components directly into the matrices. Extracel-HP-regulated extended growth factor release has been well investigated [27]. The hydrogel has been extensively used for release of angiogenic factors for increased blood vessel formation in several mouse models [28,29]. In our matrices, the conjugated heparin chains in the HP groups sequester and release the various growth factors contained within the extracts slowly, achieving a constant level of growth factor-cell signaling during the experiment. It also likely that the localization of the extract components was important. Hepatocytes within matrices in which extracts were supplemented as components of the gels functioned better than those in matrices that were only supplemented with extracts suspended in the media. This is significant considering extracts were added to gels in a final concentration of 0.5 μg/mL in comparison to over 10 applications of 1.0 μg/mL concentrations of extracts in media. When added as part of the HP gels, growth factors were more readily sequestered by the heparin chains and already localized to the nearby hepatocytes. Furthermore, ECM components such as the collagens, GAGs, elastins, and likely others, became part of matrices and interacted with the hepatocytes. In comparison, when added suspended in media, these ECM components are not readily available to the cells, as first they must diffuse through the upper layer of matrix, which is unlikely due to large molecular weights.
In future research, we wish to transition to fully 3-D liver constructs by encapsulating cells in liver sECM hydrogels, instead of employing sandwich cultures. It should then be possible to increase metabolism efficiency on a per-construct-level by increasing the number of hepatocytes per unit of gel volume. In fact, 3-D encapsulation would make the preparation/cell seeding step more simple and efficient. A single encapsulation step is quicker than sandwich culture preparation, which requires gel plating, crosslinking, cell seeding, waiting for attachment, washing, a second gel plating, crosslinking, and finally adding media. The only foreseeable problem is that direct encapsulation does not allow for washing off dead/non-adherent cells. However, this would likely not be a problem as the dead cells will break down and disappear eventually.
It would also be interesting to implement alternate sources for hepatocytes in this model, or a future iteration. Despite being the gold standard, human primary hepatocyte sources are scarce and expansion has mostly been difficult. To that end, research into alternate cell sources for human hepatocyte-like cells is ongoing. So far, hepatocyte-like cells have been derived from ES and iPS cells [1], and bone marrow-derived mesenchymal stem cells (MSCs) [30], but true functionality is yet limited. An alternative that might yield an effective solution is the implementation of this hydrogel system for expansion of liver stem and progenitor cells, which could later be used in drug screening and toxicology models akin to the system presented in this study without the need for biopsied human liver cells.
5. Conclusion
With an increasing need for functional human tissues to streamline the pharmaceutical pipeline, liver sECM tissue constructs could provide scientists with the tools necessary to perform drug and toxicology screening with increased efficiency. These studies suggest that this 3-D culture system, which combines HA hydrogels conjugated with heparin, and liver-derived extracts containing liver-specific growth factors and ECM components, could provide an alternative approach for liver tissue engineering and drug discovery research.
Acknowledgements
We thank Disher Packing, Inc. for providing porcine livers. We also thank Pedro Baptista for his contributions to parts of this project. This study was supported, in part, by the Telemedicine and Advanced Technology Research Center (TATRC) at the U.S. Army Medical Research and Material Command (USAMRMC) through award W81XWH-07-1-0718.
References
- [1].Greenhough S, Medine CN, Hay DC. Pluripotent stem cell derived hepatocyte like cells and their potential in toxicity screening. Toxicology. 2010;278:250–5. doi: 10.1016/j.tox.2010.07.012. [DOI] [PubMed] [Google Scholar]
- [2].Gomez-Lechon MJ, Castell JV, Donato MT. The use of hepatocytes to investigate drug toxicity. Methods Mol Biol. 2010;640:389–415. doi: 10.1007/978-1-60761-688-7_21. [DOI] [PubMed] [Google Scholar]
- [3].Ananthanarayanan A, Narmada BC, Mo X, McMillian M, Yu H. Purpose-driven biomaterials research in liver-tissue engineering. Trends Biotechnol. 2011;29:110–8. doi: 10.1016/j.tibtech.2010.10.006. [DOI] [PubMed] [Google Scholar]
- [4].Fan J, Shang Y, Yuan Y, Yang J. Preparation and characterization of chitosan/galactosylated hyaluronic acid scaffolds for primary hepatocytes culture. J Mater Sci Mater Med. 2010;21:319–27. doi: 10.1007/s10856-009-3833-y. [DOI] [PubMed] [Google Scholar]
- [5].Feng ZQ, Chu X, Huang NP, Wang T, Wang Y, Shi X, et al. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials. 2009;30:2753–63. doi: 10.1016/j.biomaterials.2009.01.053. [DOI] [PubMed] [Google Scholar]
- [6].Kim M, Lee JY, Jones CN, Revzin A, Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31:3596–603. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Williams CM, Mehta G, Peyton SR, Zeiger AS, Van Vliet KJ, Griffith LG. Auto-crine-controlled formation and function of tissue-like aggregates by primary hepatocytes in micropatterned hydrogel arrays. Tissue Eng Part A. 2011;17:1055–68. doi: 10.1089/ten.tea.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fukuda J, Nakazawa K. Hepatocyte spheroid arrays inside microwells connected with microchannels. Biomicrofluidics. 2011;5:22205. doi: 10.1063/1.3576905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bierwolf J, Lutgehetmann M, Feng K, Erbes J, Deichmann S, Toronyi E, et al. Primary rat hepatocyte culture on 3D nanofibrous polymer scaffolds for toxicology and pharmaceutical research. Biotechnol Bioeng. 2011;108:141–50. doi: 10.1002/bit.22924. [DOI] [PubMed] [Google Scholar]
- [10].Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, et al. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab Dispos. 2011;39:528–38. doi: 10.1124/dmd.110.035873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lang R, Stern MM, Smith L, Liu Y, Bharadwaj S, Liu G, et al. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials. 2011;32:7042–52. doi: 10.1016/j.biomaterials.2011.06.005. [DOI] [PubMed] [Google Scholar]
- [12].Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–7. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- [13].Mazzitelli S, Luca G, Mancuso F, Calvitti M, Calafiore R, Nastruzzi C, et al. Production and characterization of engineered alginate-based microparticles containing ECM powder for cell/tissue engineering applications. Acta Biomater. 2011;7:1050–62. doi: 10.1016/j.actbio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- [14].Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- [15].Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miki D, Dastgheib K, Kim T, Pfister-Serres A, Smeds KA, Inoue M, et al. A photopolymerized sealant for corneal lacerations. Cornea. 2002;21:393–9. doi: 10.1097/00003226-200205000-00012. [DOI] [PubMed] [Google Scholar]
- [17].Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leach JB, Bivens KA, Collins CN, Schmidt CE. Development of photocrosslinkable hyaluronic acid-polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J Biomed Mater Res A. 2004;70:74–82. doi: 10.1002/jbm.a.30063. [DOI] [PubMed] [Google Scholar]
- [19].Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041–52. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A. 2006;79:902–12. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- [21].Shu XZ, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–48. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- [22].Serban MA, Scott A, Prestwich GD. Use of hyaluronan-derived hydrogels for three-dimensional cell culture and tumor xenografts. Curr Protoc Cell Biol. 2008 doi: 10.1002/0471143030.cb1014s40. [Chapter 10]:Unit 10 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu Y, Shu XZ, Prestwich GD. Tumor engineering: orthotopic cancer models in mice using cell-loaded, injectable, cross-linked hyaluronan-derived hydrogels. Tissue Eng. 2007;13:1091–101. doi: 10.1089/ten.2006.0297. [DOI] [PubMed] [Google Scholar]
- [24].Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2008;41:139–48. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- [25].Skardal A, Zhang J, McCoard L, Oottamasathien S, Prestwich GD. Dynamically crosslinked gold nanoparticle - hyaluronan hydrogels. Adv Mater. 2010;22:4736–40. doi: 10.1002/adma.201001436. [DOI] [PubMed] [Google Scholar]
- [26].Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31:6173–81. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- [27].Liu Y, Cai S, Shu XZ, Shelby J, Prestwich GD. Release of basic fibroblast growth factor from a crosslinked glycosaminoglycan hydrogel promotes wound healing. Wound Repair Regen. 2007;15:245–51. doi: 10.1111/j.1524-475X.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- [28].Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–51. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- [29].Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials. 2010;31:4630–8. doi: 10.1016/j.biomaterials.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li J, Tao R, Wu W, Cao H, Xin J, Guo J, et al. 3D PLGA scaffolds improve differentiation and function of bone marrow mesenchymal stem cell-derived hepatocytes. Stem Cells Dev. 2010;19:1427–36. doi: 10.1089/scd.2009.0415. [DOI] [PubMed] [Google Scholar]