Abstract
Background: Approximately 72% of endometrial cancers are FIGO stage I at diagnosis and about 10% have lymph node metastases. An ideal diagnostic test for nodal disease would be able to prevent both overtreatment (i.e. unnecessary lymphadenectomy) and undertreatment (i.e. withholding lymphadenectomy or adjuvant postoperative treatment to patients with lymph node metastases). Objectives: In this review we compare the accuracy of preoperative tests (computed tomography, magnetic resonance imaging, positron emission tomography-computed tomography, CA-125 serum levels, and ultrasonography) for the detection of lymph node metastases in endometrial cancers with the final histopathologic diagnosis after complete pelvic and para-aortic lymphadenectomy as the gold standard. Method: A systematic search in MEDLINE (using PubMed), Embase and The Cochrane Library was performed up to 23 July 2012. Results: We found one article that met our inclusion criteria for computed tomography, none for magnetic resonance imaging, 2 for positron emission tomography/computed tomography), 2 for CA-125 and none for ultrasonography. Conclusions: Due to the lack of high-quality articles on a preoperative test for lymph node status in endometrial cancer, no proper comparison between these modalities can be made.
Keywords: Endometrial cancer, lymph nodes, diagnosis, staging, lymphadenectomy, test
Introduction
Endometrial cancer is the most common gynaecological cancer in North America and Europe and the incidence is still increasing due to prolonged life expectancy, changes in reproductive behaviour, prevalence of overweight, as well as (unopposed) use of hormone replacement therapy[1–6]. Approximately 72% of endometrial cancers are FIGO stage I at diagnosis, 12% are stage II, 13% are stage III, and 3% are stage IV[5,7]. Overall 5-year survival is around 80%[8,9]. Endometrial cancer is commonly classified into 2 types. Type I tumours (about 80%) are endometrioid carcinomas arising in a background of hyperplasia in obese women. These tumours are usually low grade, oestrogen related and follow a more favourable course. In contrast, type II tumours (about 20%) are non-endometrioid (predominantly serous and clear cell) carcinomas arising in endometrial polyps or from precancerous lesions in the vicinity of an atrophic endometrium. These tumours are high grade, not oestrogen related, often invade the myometrium and (lymph) vascular spaces, and have a high mortality rate[10].
At the time of operation, about 1 in every 10 clinical stage I endometrial cancers have lymph node metastases[11–15]. In this group, 6–8% have pelvic lymph node (PLN) metastases of which up to two-thirds have concomitant para-aortic lymph node (PAN) metastases. The remaining 2% have metastases to the PAN only[8,12,16–18]. These type II tumours are responsible for 50% of these lymph node metastases. The other 50% is present in clinically low-risk patients (type I). Lymph node involvement represents worse disease and in the case of PAN metastases, it is a separate predictor of poor outcome[18,19]. Therefore, complete surgical staging is recommended for most women with endometrial cancer by several (gynaecologic) societies and includes exploration of the abdomen and pelvis with biopsy of any suspicious lesions, total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO) and complete pelvic and para-aortic lymphadenectomy[8,16,20]. However, given the favourable outcome for most cases and the potential complications of an extended procedure, the benefits of a complete pelvic and para-aortic lymphadenectomy as part of the routine staging procedure remains controversial[1,9,11,12,19,21–23]. Despite increasing literature documenting that most patients with positive pelvic nodes also have metastatic para-aortic nodal involvement, lymphadenectomy is frequently limited to the pelvis[17,24–26]. Subsequently, in these cases often only pelvic radiotherapy is given, omitting the para-aortic region. As a result, in these patients and in patients with negative pelvic but positive para-aortic nodes, adequate postoperative treatment is withheld. Even in the recently published prospective randomized trials that aimed to test the therapeutic benefit of lymphadenectomy, the authors used incomplete staging procedures, thus failing to address the para-aortic area[14,18,27]. The goal of complete pelvic and para-aortic lymphadenectomy is to correctly stage all patients for adequate outcome comparison, stratification into prognostic categories, and guide optimal postoperative treatment in order to maximize survival. Moreover, as was again suggested recently, it may also have a therapeutic effect[28,29].
A preoperative test that could accurately recognize nodal disease would prevent both overtreatment (i.e. unnecessary pelvic and para-aortic lymphadenectomy) and undertreatment (i.e. withholding complete lymphadenectomy or adjuvant postoperative treatment to patients with lymph node metastases). For this reason, we conducted a review of the literature. We aimed to identify and compare all preoperative diagnostic tests that have been evaluated for the detection of lymph node metastases in endometrial cancer. We only included studies that used the final histopathologic diagnosis after complete pelvic and para-aortic lymphadenectomy as a reference standard.
Materials and methods
Search strategy and selection criteria
To identify all preoperative tests that are evaluated for prediction of lymph node metastases in endometrial cancer (Search I), we first performed a systematic search in MEDLINE (by using PubMed) Embase and The Cochrane Library up to 23 July 2012.
Search I: (“Endometrial Neoplasms”[MeSH] OR Endometrial Neoplasms OR Endometrial cancer OR Endometrial carcinoma) AND (“Lymph Nodes”[MeSH] OR “Lymphatic Metastasis”[MeSH] OR Lymphatic Metastasis OR Lymphatic Metastases OR Lymph node OR Lymph nodes) AND (“Diagnosis”[MeSH] OR “Tumor Markers, Biological”[MeSH] OR Tumor Markers, Biological OR Tumor Markers OR Tumor Marker OR Diagnosis OR Assessment OR Staging).
To find all original articles on a specific preoperative test for prediction of lymph node metastases by evaluation of lymph nodes in endometrial cancer we conducted Search II.
Search II: the preoperative tests identified by Search I were individually combined as MeSH and text words with the following terms to form Search II: (Preoperative test x) AND (“Endometrial Neoplasms”[MeSH] OR Endometrial Neoplasms OR Endometrial cancer OR Endometrial carcinoma) AND (“Lymph Nodes”[MeSH] OR “Lymphatic Metastasis”[MeSH] OR Lymphatic Metastasis OR Lymphatic Metastases OR Lymph node OR Lymph nodes).
No limits were set. Two investigators (HP and HT) extracted data independently when the following inclusion criteria were met:
Study population consisted of at least 10 patients. All patients underwent the index test as well as the reference test
Index test evaluated the PLNs and PANs before surgery
Reference test was the histopathologic examination of lymph nodes removed by complete pelvic and para-aortic lymphadenectomy
Data for 2 × 2 tables must be extractable
Quality assessment
Study quality was assessed using the QUADAS-list (Quality Assessment of Diagnostic Accuracy Studies), which is a generic tool developed specifically for use in reviews of diagnostic test accuracy[30]. The Cochrane Handbook for Diagnostic Test Accuracy Reviews recommends the use of 11 of the 14 original QUADAS items, because the 3 excluded items relate to the quality of reporting rather than methodology (http://srdta.cochrane.org/handbook-dta-reviews Chapter 9). In accordance with the Cochrane Handbook advice, we added one item that was relevant for this review: item 12 (see Table 1).
Table 1.
Quality assessment.
| 1 | Representative spectrum? | Positive, if the endometrial cancer patients were consecutively selected in a prospective way |
| 2 | Acceptable reference standard? | Positive, if a complete pelvic and para-aortic lymphadenectomy was performed and all removed lymph nodes were histopathologically examined |
| 3 | Acceptable delay between tests? | Positive, if the delay between the index test and the reference standard was less than 2 weeks |
| 4 | Partial verification avoided? | Positive, if all patients receiving the index test were verified by the reference standard |
| 5 | Differential verification avoided? | Positive, if the same reference standard was applied in all patients, irrespective of the index test |
| 6 | Incorporation avoided? | Positive, if the reference standard was independent of the index test and did not form a part of it |
| 7 | Index test results blinded? | Positive, if the reference standard results were interpreted without knowledge of the results of the index |
| 8 | Reference standard results blinded? | Positive, if the index test results were interpreted without knowledge of the results of the reference standard |
| 9 | Relevant clinical information? | Positive, if the same clinical data would be available when the test results would be interpreted if the test would be used in practice |
| 10 | Uninterpretable results reported? | Positive, if uninterpretable or intermediate test results were reported |
| 11 | Withdrawals explained? | Positive, if withdrawals from the study were explained |
| 12 | Cutoff values set before study start? | Positive, if cutoff values for the tests were established before the study start |
Items 1 to 11 are the QUADAS items recommended by the Cochrane Handbook for Diagnostic Test Accuracy Reviews. QUADAS and the Cochrane handbook advise adding quality items whenever relevant to a specific review topic. Item 12 was added in this review.
Results
Search results and study selection
The first selection, based on title and abstract, was done independently by 2 investigators (HP and HT). From all selected studies, the full-text articles were retrieved and evaluated to assess whether they met the inclusion criteria. Any disagreement was solved by involvement of a third investigator (RK). Reference lists in all selected articles were manually searched for additional eligible articles.
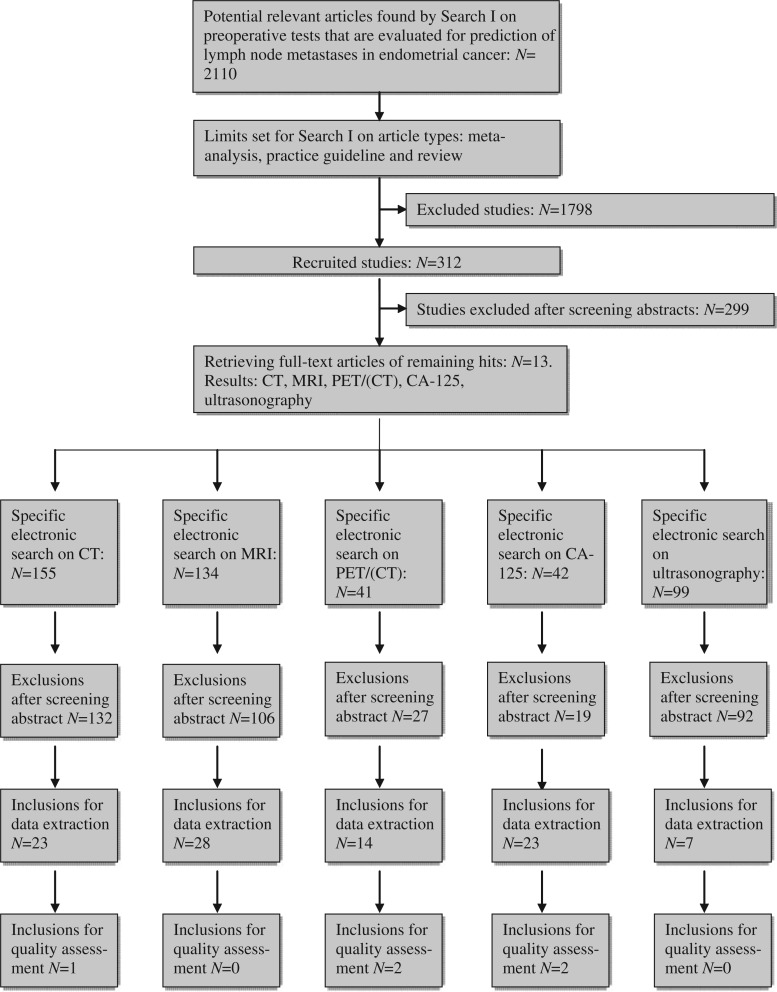
A total of 2110 articles were found by Search I. Subsequently, the search was limited to the following article types: meta-analysis, practice guideline and review. For the remaining articles (N = 312), the abstract was critically examined resulting in the exclusion of 299 studies. Thirteen full-text articles were retrieved for a thorough analysis and the following preoperative tests were identified: computed tomography (CT)[21,31–33], magnetic resonance imaging (MRI)[13,21,31–38], positron emission tomography (PET)[5,21,39], preoperative CA-125 serum levels[40], and ultrasonography[33] (Fig. 1).
Figure 1.
Results of Search I and Search II.
Search II yielded the following hits: 155 for CT, 134 for MRI, 41 for PET/(CT), 42 for CA-125 serum levels, and 99 for ultrasonography (Fig. 2). Most of the studies that were excluded focused on assessment of myometrial or cervical invasion rather than evaluation of the lymph nodes.
Figure 2.
Methodological quality summary of the studies included.
CT
The specific electronic search on CT yielded 155 articles of which 132 were excluded after reviewing the title and abstract (Fig. 1). Common reasons for exclusion were a focus on assessment of myometrial or cervical invasion rather than evaluation of the lymph nodes or therapeutic modalities other than a complete staging procedure. Of the remaining 23 studies, the full-text articles were retrieved for data extraction. Only 1 article met the inclusion criteria (Table 2).
Table 2.
Results of data extraction (number of publications)
| CT | MRI | PET/(CT) | CA-125 | Ultrasonography | |
|---|---|---|---|---|---|
| Inclusions based on title and abstract | 23 | 28 | 14 | 23 | 7 |
| Exclusions after data extraction | |||||
| Review article | 5 | 6 | 3 | – | 1 |
| No original data | 1 | 2 | – | – | – |
| Lymph nodes not assessed by index test | 1 | 1 | – | – | 4 |
| Complete pelvic and para-aortic lymphadenectomy not performed | 13 | 15 | 8 | 18 | 1 |
| 2 × 2 table not reproducible | 2 | 4 | 1 | 3 | – |
| Index test was not a preoperative test | 1 | ||||
| Inclusions for quality assessment | 1 | 0 | 2 | 2 | 0 |
In this article, Ryo et al.[41] evaluated the usefulness of CT, intraoperative ultrasonography and intrauterine pathologic findings in reducing the number of unnecessary para-aortic lymphadenectomies in women with endometrial cancer. The authors reported a sensitivity of 38.9% and a specificity of 100% for CT (Table 3) based on a population of 91 patients, 18 of whom had PAN metastases. The cutoff value for an abnormal lymph node on CT was set at a mean diameter of more than 5 mm. The high number of patients with PAN metastases (18/91) can be explained by the exclusion criteria for this prospective study: grade I endometrial carcinoma, no myometrial invasion, evident distant metastases, age older than 75 years and bad performance status, which was not further specified.
Table 3.
Clinical features of the studies included
| Author | Patients (n) | Index test | Reference test | PA LAD level | Statistical analysis | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ryo[48] | 91 | CT | P + PA LAD | RV | Per pat | 38·9 (7/18) | 0·18–0·64 | 100·0 (73/73) | 0·94–1·00 | 100·0 (7/7) | 86·9 (73/84) |
| Horowitz[49] | 18 | PET | P + PA LAD | IMA | Per pat (+LNA) | 50·0 (1/2) | 0·03–0·97 | 93·8 (15/16) | 0·68–1·00 | 50·0 (1/2) | 93·8 (15/16) |
| Nayot[50] | 12 | PET/CT | P + PA LAD | RV | Per pat (+LNA) | 100·0 (3/3) | 0·31–1·00 | 100·0 (9/9) | 0·63–1·00 | 100·0 (3/3) | 100·0 (9/9) |
| Todo[55] | 214 | CA-125 | P + PA LAD | RV | Per pat P | 72·4 (21/29) | 0·53–0·87 | 75·1 (139/185) | 0·68–0·81 | 31·3 (21/67) | 94·6 (139/147) |
| Per pat PA | 73·7 (14/19) | 0·49–0·90 | 78·3 (151/193) | 0·72–0·84 | 25·0 (14/56) | 96·8 (151/156) | |||||
| Yoon[56] | 131 | CA-125 | P + PA LAD | RV | Per pat | 83·3 (5/6) | 0·36–0·99 | 76·8 (96/125) | 0·68–0·84 | 14·7 (5/34) | 99·0 (96/97) |
IMA, inferior mesenteric artery; LNA, lymph node area (was not used in this table, but is possible based on information provided by the authors); P + PA LAD, pelvic and para-aortic lymphadenectomy; Per pat P, per patient concerning pelvic lymph node metastases; Per pat PA, per patient concerning para-aortic lymph node metastases; Per pat, per patient; RV, renal vessels.
MRI
After reviewing the title and abstract of 134 potential articles found by the specific search on MRI, we excluded 106 studies (Fig. 1), most of which concentrated on risk factors for lymph node metastases such as myometrial invasion by the endometrial cancer, rather than evaluating the lymph nodes themselves. None of the remaining 28 articles met the inclusion criteria (Table 2).
PET/(CT)
The specific electronic search on PET/(CT) yielded 41 articles of which 27 were excluded after reviewing the title and abstract (Fig. 1). Common reasons for exclusion were a focus on recurrent endometrial cancer or other types of cancer (e.g. cervix or breast). Of the remaining 14 studies, the full-text articles were retrieved for data extraction. Two articles met the inclusion criteria (Table 2).
Horowitz et al.[42] found a sensitivity of 66.7% and specificity of 93.8% for detection of metastatic lymph nodes by [18F]fluoro-2-deoxy-d-glucose (FDG)-PET when used without CT. This was a patient-based calculation. They evaluated 19 patients with uterine corpus cancer before surgical staging. In this population, 2 patients had positive lymph nodes confirmed by surgery. One patient had a positive supraclavicular lymph node (stage IV disease) and the abnormal FDG uptake in her right PLNs and PANs was considered to be a true-positive result despite the fact that surgery was not performed. We excluded this patient from further analysis and made a new 2 × 2 table, which was possible due to the detailed tables provided by the authors. At the patient level, sensitivity decreased to 50.0% and specificity remained 93.8% (Table 3).
The second article concerned a pilot study from Nayot et al.[43] in which 12 patients with endometrial cancer underwent a complete staging procedure up to the renal vessels. Although the surgeons were not blinded to the preoperative PET/CT results, the authors stated that all surgical procedures were performed systematically. Sensitivity and specificity at the patient level were 100% and 100%, respectively (Table 3).
CA-125
The specific electronic search on CA-125 yielded 42 articles of which 19 were excluded after reviewing the title and abstract (Fig. 1). Common reasons for exclusion were a focus on immunohistochemistry (IHC) or other treatment modalities (e.g. chemotherapy, radiotherapy). Of the remaining 23 studies, the full-text articles were retrieved for data extraction. Two articles met the inclusion criteria (Table 2).
Todo et al.[44] divided their patients with endometrial cancer into pre- and postmenopausal groups. In all but 2 patients, they performed a complete pelvic and para-aortic lymphadenectomy up to the level of the renal vessels. Because this was less than 1% of all patients, we decided not to exclude this study from further analysis. Of the 214 patients in their study, 29 had positive PLN and 19 had positive PAN. To calculate sensitivity and specificity, we took data from their tables in which they used a cutoff level of CA-125 for PLN and PAN metastases of 28 U/ml for patients aged >50 years and 70 U/ml for patients <50 years. The calculated sensitivity and specificity were 72.4% and 75.1% for PLN metastases and 73.7% and 78.3% for PAN metastases (Table 3).
Yoon et al.[45] staged 131 low-risk patients with endometrial cancer. All patients were preoperatively presumed to be grade 1, stage 1 and histologically confirmed as endometrioid carcinoma. After a complete staging procedure, 6 patients had lymph node metastases; 4 of these patients had metastasis in both the pelvic and para-aortic regions, and 2 only in the para-aortic region. In their study, 6.8% of patients were upgraded to grade 2 or 3 and 12.9% were upstaged. Using a CA-125 cutoff level of 31 U/ml, the calculated sensitivity and specificity were 83.3% and 76.8%, respectively, for PAN metastases (Table 3).
It was not possible to calculate a receiver operating curve for the included studies for 2 reasons. First, the articles did not provide the CA-125 levels of the individual patients and their lymph node status. Second, the first study examined the CA-125 level in comparison with PLN and PAN metastases separately and the second study only in comparison with PAN metastases.
Ultrasonography
The specific electronic search on ultrasonography yielded 99 articles of which 92 were excluded after reviewing the title and abstract (Fig. 1). Common reasons for exclusion were a focus on other endometrial cancer metastases, other types of cancer (e.g. cervix, ovary, breast) or myometrial invasion. Of the remaining 7 studies, the full-text articles were retrieved for data extraction. No article met the inclusion criteria. Ryo et al.[41] evaluated lymph nodes by ultrasonography; however this was done intraoperatively rather than preoperatively.
Quality assessment results
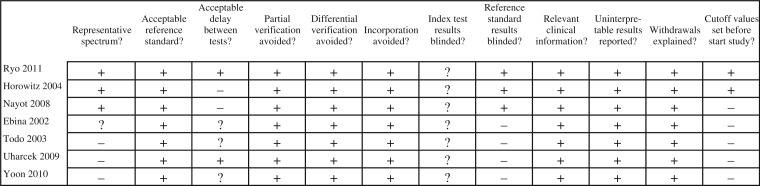
Items were scored independently by 2 investigators (HP and HT) as positive, negative or unclear and are summarized in Fig. 2. Any disagreement was solved by involvement of a third investigator (RK).
All selected articles scored positive on the following QUADAS items: acceptable reference standard, partial and differential verification avoided, relevant clinical information, uninterpretable results reported and withdrawals explained. The CA-125 studies scored negative or unclear on the representative spectrum item because the study design was retrospective or it was not clear if it was really prospective. The item acceptable delay between tests was scored negative in both PET/(CT) studies, because the delay was more than 2 weeks and, in 3 of 4 CA-125 studies, the delay between the tests was not described. No article described if the pathologist was blinded to the results of the index test or surgical outcome: item 7, index test results blinded. For obvious reasons, all the CA-125 articles scored negative on the items reference standard results blinded and cutoff values set before study start, because the CA-125 values were interpreted in conjunction with the histopathologic results.
Discussion
Accurate preoperative assessment of the lymph nodes could ideally identify those patients with advanced stage disease, who might benefit from more extensive surgical procedures and adjuvant therapies. Although most cases have negative lymph nodes, even in preoperative, supposedly extreme low-risk patients (stage I, grade I), the incidence of PAN metastases is still 4.6%[45].
Precise preoperative knowledge of the lymph node status can also prevent overtreatment in low-risk patients, who will not benefit from an extensive surgical procedure, but would be exposed unnecessarily to excess morbidity. After lymphadenectomy 2.4–10.5% of patients have lymphoedema[46,47]. Other common complications are small bowel obstruction (2.3–4.4%), lymphocysts (2.4–7.6%) and deep venous thrombus (2.6%)[11,45]. Comparing patients undergoing selective pelvic and aortic lymphadenectomy with those undergoing selective pelvic lymphadenectomy alone, the first group has longer anaesthesia time (median, 220 vs 204 min; P = 0.011), longer hospital stay (8 vs 5 days; P < 0.0001), and higher estimated blood loss (500 vs 300 ml; P < 0.0001). Because in the past blood transfusions were given more generously than today, a much higher frequency of blood transfusions can be observed in older studies (23% in pelvic and para-aortic lymphadenectomies vs 5% in pelvic lymphadenectomies alone) than in the more recent ones (1.5% in complete pelvic and para-aortic lymphadenectomies)[11,45].
After a thorough literature search, we identified 5 diagnostic tests for evaluation of lymph node status in patients with endometrial cancer. A separate search was performed for these 5 tests to compare their accuracy in terms of sensitivity and specificity, using the final histopathologic diagnosis as a reference. In general, several limitations were found. Most studies examined small patient numbers, which is reflected in the large confidence intervals calculated in Table 3 (http://faculty.vassar.edu/lowry/clin1.html). Most studies were retrospective analyses, with a mix of diverse endometrial cancer stages, comparing dissimilar end points (e.g. number of lymph nodes per patient or per lymph node area, or per region of interest), using different treatment strategies, or performing lymphadenectomy to different levels of extent. Furthermore, most studies did not describe how the histologic examination was performed and some studies used techniques such as IHC to identify micrometastases. All these limitations made it hard to compare this heterogeneous group of studies and draw solid conclusions.
The large variety in the extent of lymph node sampling and lymphadenectomy has an important effect on statistical values such as the number of false-negative cases and the sensitivity. Numerous studies compared preoperative tests with histopathologic results of lymph nodes yielded by lymph node sampling or pelvic lymphadenectomy only. The number of false-negative cases and the sensitivity would have been much lower if complete pelvic and para-aortic lymphadenectomy up to the level of the renal vessels had been performed[18]. For this reason, incomplete pelvic and para-aortic lymphadenectomy was one of the major reasons for exclusion. According to Mariani et al.[17], 67% of patients with lymphatic dissemination have para-aortic lymph metastases. Furthermore, 77% of these metastases are above the inferior mesenteric artery (IMA), whereas nodes in the ipsilateral para-aortic area below the IMA and ipsilateral common iliac basin were declared negative in 60% and 71%, respectively. In addition to the level to which the lymphadenectomy was performed, the number of resected lymph nodes differed greatly between the studies included.
Reviewing the results for the 4 radiologic modalities, we found 1 article that met our inclusion criteria for CT, none for MRI, 2 for PET/(CT) and none for ultrasonography. With its sensitivity of 38.9% and specificity of 100%, CT is a poor diagnostic tool for evaluating lymph node status in patients with endometrial cancer[41]. No conclusions can be drawn for MRI and ultrasonography and the position of PET/(CT) in the diagnostic arsenal is unclear. For PET/(CT), only 2 small studies were included, which showed a range in sensitivity from 50% to 100%[42,43].
A major restriction of all radiologic techniques is the minimal size a lymph node must be to be suspicious for metastasis. Most authors consider a lymph node to be enlarged when it exceeds 10 mm, although several reports suggest that metastatic lymph nodes may be 2 mm or less in diameter[48–50]. This also explains why lymph nodes may remain undiagnosed by sampling or selective lymphadenectomy, because they cannot be identified on direct vision or by palpation, which again strengthens the need for complete pelvic and para-aortic lymphadenectomy. The clinical significance of micrometastases and the use of IHC in endometrial cancer remain uncertain. The incidence of micrometastases among haematoxylin and eosin (H&E) negative lymph nodes varies widely. In a retrospective study of 51 patients with endometrial cancer, McCoy et al.[51] found 2 IHC-positive micrometastases in 151 H&E negative lymph nodes. The mean number of lymph nodes per patient was low (12.2). In another recent study, Erkanli et al.[52] retrospectively reviewed 47 patients with endometrial cancer with previously reported negative lymph nodes on routine histopathology. With IHC, 7 (14.9%) were found to have micrometastases. Six of these 7 patients (85.7%) had high-risk endometrial cancer. Among the patients with high-risk endometrial cancer, 50% had micrometastases. Future studies will have to determine the significance of micrometastases in endometrial cancer, the role they play in recurrent disease and the effect that adjuvant therapies have in these cases.
The CA-125 serum level is widely used as a preoperative test to estimate the risk of extrauterine spread and lymph node metastases in endometrial cancer. However, the optimal cutoff value has not been determined yet and correlations between the levels of CA-125 in serum and lymph node metastases remain inconsistent[53]. One of the possible explanations could be that different histologic tumour types cause different changes in serum levels of CA-125[54]. Another explanation could be that patients with lymph node metastases are at increased risk of other extrauterine metastases, which can also cause an increase in serum levels of CA-125, making differentiation of the origin of this increase impossible.
Conclusion
Due to the lack of high-quality articles on preoperative tests for lymph node status in endometrial cancer, no proper comparison between these modalities can be made. More prospective research must be done in this field of gynaecologic oncology with strict inclusion criteria and complete pelvic and para-aortic lymphadenectomies up to the renal vessels in all patients included as the first part of the reference test. The second part should not only include the classic histopathologic examination of the lymph nodes with H&E staining but also IHC to detect micrometastases. In this way, the prognostic value of micrometastases in endometrial cancer could also be determined.
Conflict of interest
The authors declare that there are no conflicts of interest
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. . PMid:16084259. [DOI] [PubMed] [Google Scholar]
- 2.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Treatment modalities in endometrial cancer. Curr Opin Oncol. 2007;19:479–485. doi: 10.1097/CCO.0b013e32827853c0. . PMid:17762575. [DOI] [PubMed] [Google Scholar]
- 3.Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121–127. doi: 10.1016/j.ygyno.2009.03.039. . PMid:19406460. [DOI] [PubMed] [Google Scholar]
- 4.Furness S, Roberts H, Marjoribanks J, Lethaby A, Hickey M, Farquhar C. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst Rev. 2009:CD000402. doi: 10.1002/14651858.CD000402.pub3. PMid:22895916. [DOI] [PubMed] [Google Scholar]
- 5.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436–447. doi: 10.1097/AOG.0b013e318162f690. . PMid:18238985. [DOI] [PubMed] [Google Scholar]
- 6.Uharcek P. Prognostic factors in endometrial carcinoma. J Obstet Gynaecol Res. 2008;34:776–783. doi: 10.1111/j.1447-0756.2008.00796.x. . PMid:18958927. [DOI] [PubMed] [Google Scholar]
- 7.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. Int J Gynaecol Obstet. 2003;83(Suppl 1):79–118. doi: 10.1016/S0020-7292(03)90116-0. . PMid:14763170. [DOI] [PubMed] [Google Scholar]
- 8.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. . PMid:17161155. [DOI] [PubMed] [Google Scholar]
- 9.McMeekin DS, Lashbrook D, Gold M, Johnson G, Walker JL, Mannel R. Analysis of FIGO Stage IIIc endometrial cancer patients. Gynecol Oncol. 2001;81:273–278. doi: 10.1006/gyno.2001.6157. . PMid:11330962. [DOI] [PubMed] [Google Scholar]
- 10.Prat J. Prognostic parameters of endometrial carcinoma. Hum Pathol. 2004;35:649–662. doi: 10.1016/j.humpath.2004.02.007. . PMid:15188130. [DOI] [PubMed] [Google Scholar]
- 11.Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–3675. doi: 10.1200/JCO.2005.04.144. . PMid:15738538. [DOI] [PubMed] [Google Scholar]
- 12.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::AID-CNCR2820601515>3.0.CO;2-8. . PMid:3652025. [DOI] [PubMed] [Google Scholar]
- 13.Kitchener H. Management of endometrial cancer. Eur J Surg Oncol. 2006;32:838–843. doi: 10.1016/j.ejso.2006.03.046. . PMid:16765558. [DOI] [PubMed] [Google Scholar]
- 14.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. PMid:19070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuurendonk LD, Smit RA, Mol BW, et al. Routine pelvic lymphadenectomy in apparently early stage endometrial cancer. Eur J Surg Oncol. 2006;32:450–454. doi: 10.1016/j.ejso.2006.02.008. . PMid:16546343. [DOI] [PubMed] [Google Scholar]
- 16.Boronow RC. Endometrial cancer and lymph node surgery: the spins continue–a case for reason. Gynecol Oncol. 2008;111:3–6. doi: 10.1016/j.ygyno.2008.07.004. . PMid:18692881. [DOI] [PubMed] [Google Scholar]
- 17.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariani A, Dowdy SC, Podratz KC. New surgical staging of endometrial cancer: 20 years later. Int J Gynaecol Obstet. 2009;105:110–111. doi: 10.1016/j.ijgo.2009.02.008. . PMid:19285672. [DOI] [PubMed] [Google Scholar]
- 19.Aalders JG, Thomas G. Endometrial cancer–revisiting the importance of pelvic and para aortic lymph nodes. Gynecol Oncol. 2007;104:222–231. doi: 10.1016/j.ygyno.2006.10.013. . PMid:17126892. [DOI] [PubMed] [Google Scholar]
- 20.ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005;106:413–425. doi: 10.1097/00006250-200508000-00050. . PMid:16055605. [DOI] [PubMed] [Google Scholar]
- 21.Chan JK, Kapp DS. Role of complete lymphadenectomy in endometrioid uterine cancer. Lancet Oncol. 2007;8:831–841. doi: 10.1016/S1470-2045(07)70275-9. . PMid:17765192. [DOI] [PubMed] [Google Scholar]
- 22.Frederick PJ, Straughn JM., Jr The role of comprehensive surgical staging in patients with endometrial cancer. Cancer Control. 2009;16:23–29. doi: 10.1177/107327480901600104. [DOI] [PubMed] [Google Scholar]
- 23.Orr JW, Jr, Holimon JL, Orr PF. Stage I corpus cancer: is teletherapy necessary? Am J Obstet Gynecol. 1997;176:777–788. doi: 10.1016/S0002-9378(97)70601-X. . PMid:9125601. [DOI] [PubMed] [Google Scholar]
- 24.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/S0140-6736(00)02139-5. . PMid:10791524. [DOI] [PubMed] [Google Scholar]
- 25.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. . PMid:14984936. [DOI] [PubMed] [Google Scholar]
- 26.Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol. 2009;27:3547–3556. doi: 10.1200/JCO.2008.20.2424. . PMid:19546404. [DOI] [PubMed] [Google Scholar]
- 27.Benedetti PP, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Kim TJ, Song T, et al. Patterns of recurrence in endometrial cancer patients at risk of lymph node metastasis or recurrence according to extent of lymphadenectomy. Int J Gynecol Cancer. 2012;22:611–616. doi: 10.1097/IGC.0b013e318249472d. . PMid:22398712. [DOI] [PubMed] [Google Scholar]
- 29.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–1172. doi: 10.1016/S0140-6736(09)62002-X. . PMid:20188410. [DOI] [PubMed] [Google Scholar]
- 30.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. . PMid:14606960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akin O, Mironov S, Pandit-Taskar N, Hann LE. Imaging of uterine cancer. Radiol Clin North Am. 2007;45:167–182. doi: 10.1016/j.rcl.2006.10.009. . PMid:17157628. [DOI] [PubMed] [Google Scholar]
- 32.Barakat RR, Hricak H. What do we expect from imaging? Radiol Clin North Am. 2002;40:521–6, vii. doi: 10.1016/S0033-8389(01)00002-1. . PMid:12117191. [DOI] [PubMed] [Google Scholar]
- 33.Selman TJ, Mann CH, Zamora J, Khan KS. A systematic review of tests for lymph node status in primary endometrial cancer. BMC Womens Health. 2008;8:8. doi: 10.1186/1472-6874-8-8. . PMid:18457596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascher SM, Reinhold C. Imaging of cancer of the endometrium. Radiol Clin North Am. 2002;40:563–576. doi: 10.1016/S0033-8389(01)00013-6. . PMid:12117193. [DOI] [PubMed] [Google Scholar]
- 35.Barwick TD, Rockall AG, Barton DP, Sohaib SA. Imaging of endometrial adenocarcinoma. Clin Radiol. 2006;61:545–555. doi: 10.1016/j.crad.2006.03.011. . PMid:16784939. [DOI] [PubMed] [Google Scholar]
- 36.Frei KA, Kinkel K. Staging endometrial cancer: role of magnetic resonance imaging. J Magn Reson Imaging. 2001;13:850–855. doi: 10.1002/jmri.1121. . PMid:11382943. [DOI] [PubMed] [Google Scholar]
- 37.Kinkel K. Pitfalls in staging uterine neoplasm with imaging: a review. Abdom Imaging. 2006;31:164–173. doi: 10.1007/s00261-005-0383-8. . PMid:16333697. [DOI] [PubMed] [Google Scholar]
- 38.Kinkel K, Forstner R, Danza FM, et al. Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. Eur Radiol. 2009;19:1565–1574. doi: 10.1007/s00330-009-1309-6. . PMid:19194709. [DOI] [PubMed] [Google Scholar]
- 39.Pandit-Taskar N. Oncologic imaging in gynecologic malignancies. J Nucl Med. 2005;46:1842–1850. PMid:16269598. [PubMed] [Google Scholar]
- 40.Gadducci A, Cosio S, Carpi A, Nicolini A, Genazzani AR. Serum tumor markers in the management of ovarian, endometrial and cervical cancer. Biomed Pharmacother. 2004;58:24–38. doi: 10.1016/j.biopha.2003.11.003. . PMid:14739059. [DOI] [PubMed] [Google Scholar]
- 41.Ryo E, Yasugi T, Mizutani K, Kita T, Takeshita S, Ayabe T. Diagnostic usefulness of intraoperative ultrasonography in avoiding unnecessary para-aortic lymphadenectomy in women with endometrial carcinoma. Int J Gynecol Cancer. 2011;21:859–863. doi: 10.1097/IGC.0b013e31821a35ef. . PMid:21666487. [DOI] [PubMed] [Google Scholar]
- 42.Horowitz NS, Dehdashti F, Herzog TJ, et al. Prospective evaluation of FDG-PET for detecting pelvic and para-aortic lymph node metastasis in uterine corpus cancer. Gynecol Oncol. 2004;95:546–551. doi: 10.1016/j.ygyno.2004.08.009. . PMid:15581961. [DOI] [PubMed] [Google Scholar]
- 43.Nayot D, Kwon JS, Carey MS, Driedger A. Does preoperative positron emission tomography with computed tomography predict nodal status in endometrial cancer? A pilot study. Curr Oncol. 2008;15:123–125. PMid:18596888. [PMC free article] [PubMed] [Google Scholar]
- 44.Todo Y, Sakuragi N, Nishida R, et al. Combined use of magnetic resonance imaging, CA 125 assay, histologic type, and histologic grade in the prediction of lymph node metastasis in endometrial carcinoma. Am J Obstet Gynecol. 2003;188:1265–1272. doi: 10.1067/mob.2003.318. . PMid:12748496. [DOI] [PubMed] [Google Scholar]
- 45.Yoon JH, Yoo SC, Kim WY, Chang SJ, Chang KH, Ryu HS. Para-aortic lymphadenectomy in the management of preoperative grade 1 endometrial cancer confined to the uterine corpus. Ann Surg Oncol. 2010;17:3234–3240. doi: 10.1245/s10434-010-1199-5. . PMid:20585865. [DOI] [PubMed] [Google Scholar]
- 46.Abu-Rustum NR, Alektiar K, Iasonos A, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol. 2006;103:714–718. doi: 10.1016/j.ygyno.2006.03.055. . PMid:16740298. [DOI] [PubMed] [Google Scholar]
- 47.Kodama J, Seki N, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Risk factors for early and late postoperative complications of patients with endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2006;124:222–226. doi: 10.1016/j.ejogrb.2005.06.027. . PMid:16051415. [DOI] [PubMed] [Google Scholar]
- 48.Ayhan A, Celik H, Dursun P. Lymphatic mapping and sentinel node biopsy in gynecological cancers: a critical review of the literature. World J Surg Oncol. 2008;6:53. doi: 10.1186/1477-7819-6-53. . PMid:18492253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boran N, Akdag D, Halici F, et al. A retrospective analysis of the diameter of metastatic lymph nodes in apparently early stage endometrial cancer. Tumori. 2008;94:681–685. doi: 10.1177/030089160809400506. PMid:19112940. [DOI] [PubMed] [Google Scholar]
- 50.Girardi F, Petru E, Heydarfadai M, Haas J, Winter R. Pelvic lymphadenectomy in the surgical treatment of endometrial cancer. Gynecol Oncol. 1993;49:177–180. doi: 10.1006/gyno.1993.1103. . PMid:8504985. [DOI] [PubMed] [Google Scholar]
- 51.McCoy A, Finan MA, Boudreaux FT, et al. The incidence and clinical significance of lymph node micrometastases determined by immunohistochemical staining in stage I - lymph node negative endometrial cancer. Histol Histopathol. 2012;27:181–185. doi: 10.14670/HH-27.181. PMid:22207552. [DOI] [PubMed] [Google Scholar]
- 52.Erkanli S, Bolat F, Seydaoglu G. Detection and importance of micrometastases in histologically negative lymph nodes in endometrial carcinoma. Eur J Gynaecol Oncol. 2011;32:619–625. PMid:22335022. [PubMed] [Google Scholar]
- 53.Dotters DJ. Preoperative CA 125 in endometrial cancer: is it useful? Am J Obstet Gynecol. 2000;182:1328–1334. doi: 10.1067/mob.2000.106251. . PMid:10871446. [DOI] [PubMed] [Google Scholar]
- 54.Koper NP, Massuger LF, Thomas CM, Kiemeney LA, Verbeek AL. Serum CA 125 measurements to identify patients with endometrial cancer who require lymphadenectomy. Anticancer Res. 1998;18:1897–1902. PMid:9677441. [PubMed] [Google Scholar]