Abstract
High-resolution (HR) magnetic resonance imaging (MRI) has become an indispensable tool for multidisciplinary teams (MDTs) addressing rectal cancer. It provides anatomic information for surgical planning and allows patients to be stratified into different groups according to the risk of local and distant recurrence. One of the objectives of the MDT is the preoperative identification of high-risk patients who will benefit from neoadjuvant treatment. For this reason, the correct evaluation of the circumferential resection margin (CRM), the depth of tumor spread beyond the muscularis propria, extramural vascular invasion and nodal status is of the utmost importance. Low rectal tumors represent a special challenge for the MDT, because decisions seek a balance between oncologic safety, in the pursuit of free resection margins, and the patient’s quality of life, in order to preserve sphincter function. At present, the exchange of information between the different specialties involved in dealing with patients with rectal cancer can rank the contribution of colleagues, auditing their work and incorporating knowledge that will lead to a better understanding of the pathology. Thus, beyond the anatomic description of the images, the radiologist’s role in the MDT makes it necessary to know the prognostic value of the findings that we describe, in terms of recurrence and survival, because these findings affect decision making and, therefore, the patients’ life. In this review, the usefulness of HR MRI in the initial staging of rectal cancer and in the evaluation of neoadjuvant treatment, with a focus on the prognostic value of the findings, is described as well as the contribution of HR MRI in assessing patients with suspected or confirmed recurrence of rectal cancer.
Keywords: Magnetic resonance imaging; rectal cancer; prognosis; staging; treatment, group meeting
Prognostic risk factors in rectal cancer: beyond the anatomic description
Histopathologic determination of the morphological characteristics of the tumor in the surgical specimen is the best method to stratify patients into different prognostic groups. Thus, the importance of high-resolution (HR) magnetic resonance imaging (MRI) is based on its high sensitivity and specificity in indentifying prognostic risk factors, either surgical or histopathologic, preoperatively, thereby allowing the selection of high-risk patients who will benefit from neoadjuvant treatment in order to improve the results[1,2]. In addition, HR MRI provides the information necessary to identify patients with a good prognosis whose indication is the primary surgery, thus avoiding overtreatment. Important characteristics in the stratification of patients include determination of (1) the depth of tumor spread beyond the muscularis propria, (2) nodal status, (3) extramural vascular invasion, (4) the circumferential resection margin (CRM) and (5) the presence of peritoneal perforation.
The information gathered from the staging, either clinical (c) or pathologic (p), is grouped according to the TNM classification into different prognostic groups/anatomic stages[3]. Each group and subgroup, according to the combination of T and N categories, has a certain impact on overall survival and disease-free survival, as well as on the risk of local recurrence and distant metastases. The latest edition of the TNM (seventh), in force since 2010, has made changes based on recurrence and survival data not available in the previous version.
New prognostic factors that are not part of the staging but are clinically relevant have been added to the data collected together with the pretreatment serum level of carcinoembryonic antigen (CEA). They are the so-called site-specific factors including tumor deposits, the tumor regression grade, which determines the degree of pathologic response to neoadjuvant treatment, the CRM, microsatellite instability, perineural invasion and KRAS mutation status[3]. HR MRI allows the status of the CRM[1,4,5] and the tumor regression grade after neoadjuvant treatment[6–9] to be assessed. Moreover, the following independent prognostic factors are also used in patient management: residual disease, histologic type, histologic grade, the level of CEA and cytokines, extramural venous invasion and submucosal vascular invasion by carcinomas arising in adenomas. HR MRI is the only imaging method that shows extramural vascular invasion[1,10,11].
Multidisciplinary activity involves the need to be familiar with the terms that are used by the different specialists, such as overall survival and disease-free survival. Both end points are used in most research protocols of adjuvant therapies in oncology. Each end point considers different events. The contribution of each event to the different end points has been defined by Punt et al.[12] in a consensus of experts on clinical research on colorectal cancer. Disease-free survival, which is being increasingly used as a primary end point, is defined as the time to any event, regardless of the cause. In its assessment, all events are considered, except loss to follow-up. Locoregional recurrence and distant metastases are among the events considered. On the other hand, overall survival is the time elapsed until death, regardless of the cause, and without clarifying whether the death was due to cancer. Locoregional recurrence and distant metastases are not taken into account.
HR MRI: stratification of patients with rectal cancer according to the prognosis, good or poor?
One of the central objectives of the multidisciplinary team (MDT) is the correct selection of patients who will benefit from neoadjuvant treatment. Approximately 40–50% of patients with rectal cancer may be successfully treated with primary surgery without significant risk of local recurrence or systemic disease[13]. The preoperative identification of patients with increased risk of local or distant recurrence allows their selection for presurgical treatment to reduce the size of the tumor, thus allowing a potentially unresectable tumor to become a tumor with free CRM and allowing patients with increased risk of systemic failure to receive combined intensive therapy to eliminate the presence of micrometastatic disease. The ability of HR MRI to detect the poor prognostic factors in rectal cancer is well documented[1,10,14]. Thus, HR MRI provides the information needed to stratify patients into 2 groups: good prognosis or poor prognosis. The standardized HR MRI report should include the following information for further discussion by the MDT: (1) the potential involvement of the CMR, (2) depth of tumor spread beyond the muscularis propria, (3) nodal status, (4) the presence of extramural vascular invasion, and (5) the commitment of the puborectalis sling muscle[13,15]. Patients with a good prognosis are selected for primary surgery, i.e. total mesorectal excision (TME), with a 5-year survival rate of 85–90%[13]. Tumors presenting the following features are considered to have a good prognosis: (1) potentially negative CRM (>1 mm), (2) T1–T2 or T3 tumors with extramural extension <5 mm, (3) absence of extramural vascular invasion, (4) N0/N1, (5) tumors located in the middle or upper third[13,15]. Patients with a poor prognosis include the following: patients with tumors with a low risk of local recurrence because of a free CRM, but increased risk of systemic failure. These tumors include (1) T3 tumors with extramural extension >5 mm, (2) or N2, (3) or the presence of extramural vascular invasion. On the other hand, those patients with tumors with potential involvement of the CRM, with an increased and more significant risk of local recurrence[13] are also included in this group. Patients with low rectal tumors, i.e. at the level of the puborectalis muscle, T2 or higher, are selected for preoperative treatment[15].
The Mercury Study Group[16] published a prospective and multicenter study showing that preoperative identification of tumors with a good prognosis by HR MRI allows appropriate stratification of patients, thus allowing the selection of those who will have a good outcome with primary surgery as the only treatment. This stratification also allows better selection of patients who should receive preoperative treatment. Thirty-three (33%) percent of patients (122/374) were considered to have a good prognosis, with stage I, II or III by HR MRI. Overall survival and disease-free survival in this group of patients were 68% and 85%, respectively; whereas the local recurrence rate was 3%. Thus, HR MRI identified a group of patients with stage II and III rectal cancer, with tumors with a good prognosis, and therefore preoperative treatment was avoided. Within the criteria of a good prognosis by HR MRI, this study took into account the following: (1) a potentially negative CRM (tumor >1 mm of the mesorectal fascia), (2) the absence of extramural vascular invasion, (3) T1–T2 tumors or T3a–b tumors (extramural extension <5 mm), (4) any N stage, (5) low rectal tumors, stages I and II by HR MRI, i.e. tumors that do not compromise the intersphincteric space or elevator muscles. The findings on HR MRI of the group with a poor prognosis included: (1) potentially positive CRM (<1 mm), (2) a committed intersphincteric space, (3) T3c–d tumors (extramural extension >5 mm) or T4 tumors, (4) presence of extramural vascular invasion, (5) any N stage. Primary surgery was the treatment of choice for the group with a good prognosis. Only 8 of the 122 patients with a good prognosis on HR MRI, stages I, II and III, (6.5%) showed involvement of 4 or more nodes (N2) in the histopathologic analysis. Postoperative treatment was given to patients with nodal involvement, based predominantly on administration of single-agent fluoropyridine chemotherapy. No patient received postoperative chemoradiotherapy. The centers participating in the study do not routinely indicate neoadjuvant treatment for patients with T3 tumors with good prognosis, regardless of nodal status. Therefore, the results validate this strategy, which, together with the proper selection of tumors with a good prognosis by HR MRI and good quality TME, prevented the delivery of neoadjuvant treatment in 30% of patients.
Regarding prognostic factors determined by HR MRI, Hunter et al.[17] have investigated a group of patients at increased risk of synchronous metastatic disease who would benefit from a more thorough preoperative staging and alternative neoadjuvant chemotherapy regimens, including induction chemotherapy. High-risk patients may harbor micrometastatic disease at initial presentation that may not be identifiable even with a proper staging strategy. To the authors’ knowledge, although previous studies have shown high rates of overall survival and disease-free survival without the administration of neoadjuvant chemoradiotherapy in low-risk patients identified by HR MRI, this is the only work comparing the incidence of synchronous metastases between high- and low-risk groups defined by HR MRI. The group of patients with increased risk of synchronous metastatic disease was determined by the presence of (1) extramural vascular invasion, (2) extramural extension >5 mm or T4, (3) positive CRM, (4) commitment of the intersphincteric space in low rectal tumors. Metastases were identified by fludeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) and contrast-enhanced multidetector computed tomography (ceMDCT). Two hundred thirty-six (236) patients were enrolled and the images from 230 patients were available (97.5%). The incidence of distant metastases, which was higher in the group of high-risk patients, was 20.7% (28/135). Low-risk patients had an incidence of synchronous metastatic disease of 4.2% (4/95).
Current recommendations for local staging of rectal cancer consider performing endorectal ultrasonography or MRI, combined with CT of the chest, abdomen and pelvis in search of distant metastases[18,19]. The preoperative identification of patients at increased risk of distant metastases enables a more aggressive initial staging strategy, facilitating the appropriate decision making regarding treatment options for this group of patients on early confirmation of metastatic disease. The therapeutic approach for these patients includes neoadjuvant chemotherapy, synchronous metastasectomy or resection of metastases before resection of the primary tumor[20–24]. In the search for metastatic disease in high-risk patients, recent studies have suggested that FDG-PET/CT may be more accurate than ceMDCT or PET alone[25]. Heriot et al.[26] demonstrated that FDG-PET/CT modified the management in 17% of cases with locally advanced primary rectal cancer; MRI with specific contrast agents has proved to be more accurate than ceMDCT and FDG-PET/CT[27] in hepatic lesions <1 cm. ceMDCT is the most accurate method for detection of small lung lesions[28]. Hunter et al.[17] proposed incorporating FDG-PET/CT, with or without liver MRI, in the initial evaluation of patients at high risk of metastatic disease, highlighting the need for future studies to determine the optimal preoperative staging strategy for patients with low- and high-risk rectal cancer.
Importance of multidisciplinary activity: more than a personal choice
The goal of teamwork is to treat each patient taking into account their individual characteristics and the specific characteristics of the tumor, i.e. to carry out so-called personalized medicine. The contribution of radiologists through the images is one of the keys to successful therapeutic decisions. Reports are changing from merely descriptive to accurate in staging and anatomic detail. In other words, the description of the images is based on determining the probable risk of local and distant recurrence, thus leading to selection of appropriate therapy for each patient according to the characteristics of the tumor. In addition, the type of tumor and genetic susceptibility are of the utmost importance when choosing a personalized treatment.
In 1995, a group of experts in the management of oncologic patients was created in the United Kingdom aiming to improve the operation and results of the oncology services of the National Health Service (NHS)[29]. This group was called an Expert Advisory Group. Decisions before the creation of this group were usually taken unilaterally; a multidisciplinary approach was only put into practice in large centers devoted to oncologic patients[30]. Since then, the concept and formation of teams dedicated to oncologic patients have evolved, currently providing a way of care with adequately documented benefits. Kesson et al.[31] have shown the impact of a multidisciplinary approach on breast cancer, comparing outcomes in patients treated simultaneously in 2 different regions of Scotland. Group decisions were made in one region but not in the other. The authors concluded that the introduction of a multidisciplinary approach in the care of oncologic patients improved survival and decreased the variation in survival rates between hospitals. Mortality was 18% lower in the region where the approach was multidisciplinary, demonstrating the impact of the multidisciplinary approach on the survival of patients for the first time.
Regarding rectal cancer, Burton et al.[32] audited CRM involvement in patients whose cases were discussed by an MDT, based on HR MRI findings, for the implementation of a preoperative treatment strategy and in those patients not addressed by a multidisciplinary team. The histopathologic determination of the CRM can be used to measure the success of rectal cancer treatment as well as the success of the MDT in proper decision making. The authors found positive CRMs in 26% of patients not discussed by an MDT and in 2% of patients with a multidisciplinary approach, concluding that the discussion of HR MRI findings by an MDT and the implementation of an appropriate preoperative treatment strategy allow a significant reduction of positive CRM in patients with rectal cancer. Reducing the number of patients with a positive CRM should be the goal of all MDTs specializing in rectal cancer; each specialist involved must contribute with their knowledge, experience and commitment to achieving this goal. Therefore, regarding optimal HR MRI, proper technique and standardized reporting, preoperative discussion of the different staging findings by an MDT, quality surgery, the availability of effective preoperative therapies and standardized histopathologic reports, seem to be the mainstay for reducing the rate of positive CRMs in patients with rectal cancer.
The multidisciplinary approach to rectal cancer allows patients to benefit from the contribution of different specialties, leading to an improvement in the results. Radiologists today face a major challenge. The role of HR MRI in treatment decisions has put radiologists in a place of maximum importance and responsibility when dealing with patients with rectal cancer. At present, best practice makes it essential to participate in an MDT, because sharing information enhances the work of the members of the various specialties, namely clinical oncologists, surgeons, radiation oncologists, pathologists and radiologists, among others. We can state that in the specific case of radiologists, the incorporation of this framework and patient care often allows useful and necessary information to be available before the beginning of the study. This availability of information results in correct indication of the method to be used and in an appropriate orientation of the examinations in order to find specific features of the disease. Proper training, based on knowledge of the patients and the disease, facilitates the orientation of the examinations to find the imaging manifestations of rectal cancer at the different stages: (1) diagnosis, staging and tumor characterization for appropriate therapeutic selection, (2) evaluation of tumor response to preoperative treatment and (3) subsequent monitoring. In addition, monitoring patients with this integrated approach allows the impact of participation at the different phases of the process to be evaluated.
Impact of prognostic factors on the choice of a preoperative treatment strategy by the MDT
As mentioned earlier, HR MRI allows patients to be stratified into different prognostic groups according to the risk of local and distant recurrence. The preoperative identification of the risk allows an adequate therapeutic strategy to be adopted and thereby improve the results.
TME implementation and neoadjuvant hypofractionated radiotherapy or chemoradiotherapy treatment have reduced local recurrence rates from 25–40% to less than 10%[33–36]. Despite multimodal management, survival has not improved, with only one clinical trial (Swedish Rectal Cancer Trial) showing benefits in terms of survival after hypofractionated radiotherapy, in the run up to TME[37,38].
To avoid the devastating impact of local recurrence of rectal cancer after surgery, neoadjuvant radiation therapy became the standard of care for those patients at increased risk in the initial assessment. The neoadjuvant treatment strategy provides certain advantages, such as preoperative downstaging of the primary tumor thus increasing the likelihood of R0 resection and/or surgery with preservation of sphincter function, enhancement of radiosensitivity by directing the radiation treatment to better oxygenated tissue, relief of early symptoms and reduction of the rates of acute and chronic toxicity compared with postoperative chemoradiotherapy[39]. Sauer et al.[40] have shown a reduction in the rate of local recurrence in patients receiving preoperative chemoradiotherapy compared with those who received postoperative chemoradiotherapy (5-year local recurrence of 6% versus 13%, respectively) and decreased acute toxicity, grade 3 and 4, in 27% of patients who received preoperative treatment versus 40% in the postoperative group. When comparing hypofractionated radiotherapy versus long-course chemoradiation, the superiority of one system over the other was not demonstrated[41,42]. However, long-course chemoradiation in high-risk patients is widely accepted as the treatment of choice[39].
In patients receiving neoadjuvant chemoradiation therapy alone, the distant recurrence rate is approximately 36%[40], contributing to the lack of survival benefit of this regimen, making it necessary to intensify the systemic component. In several studies, the adjuvant chemotherapy regimen has been shown to represent a benefit regarding survival[43–46]. In a pooled analysis, this regimen has significantly improved survival as part of a chemoradiation regimen or as maintenance combined with chemoradiotherapy when compared with treatment with surgery alone or surgery plus adjuvant radiotherapy[47]. Recent studies have shown promising initial results with induction neoadjuvant chemotherapy; the patients with increased risk of distant metastases benefit most from this scheme[17,48,49].
Glynne-Jones et al.[50] published a comparison of different strategies for the therapeutic management of patients with locally advanced rectal cancer. In this work, current trials suggest that for resectable tumors in which HR MRI suggests that the CRM is potentially free of disease, hypofractionated radiotherapy and chemoradiotherapy are equivalent in terms of outcomes such as local recurrence, disease-free survival and overall survival. In contrast, in patients with more advanced disease, with the CRM potentially threatened or infiltrated according to HR MRI, integration of more active chemotherapy and biological agents into chemoradiotherapy is an interesting strategy due to the high risk of metastases. However, in the last decade, there have been no published trials in which chemoradiotherapy has affected disease-free survival and overall survival.
As Hawkes et al.[39] concluded, 3 concepts are clear: (1) all locally advanced rectal tumors do not behave in the same manner, (2) multimodal treatment, especially neoadjuvant therapy, improves outcomes and (3) reduction of local recurrence does not confer any obvious survival benefit.
Tumor staging and depth of tumor spread beyond the muscularis propria
The relationship between survival and extramural tumor extension has been well established and is independent of other prognostic factors, including the CRM[51,52]. Thus, extramural extension has been validated as an important prognostic indicator[53–56]. Patients with tumors with an extramural extension of 5 mm or less, regardless of the nodal status, have a 5-year cancer-specific survival rate of 85%, whereas in tumors with extramural extension greater than 5 mm, the survival rate is 54%[53].
The Mercury group[14] demonstrated the accuracy of HR MRI in determining the depth of extramural tumor spread in patients with rectal cancer, considering histopathology as the standard of reference. The measurement of extramural extension, i.e. the distance between the outer edge of the longitudinal layer of the muscularis propria and the lateral border of the tumor by HR MRI is equivalent to the corresponding measurement in histopathologic analysis. The latest TNM classification takes into account the available evidence for the subclassification of T3 tumors into: T3a <1 mm, T3b 1–5 mm, T3c >5–15 mm and T3d >15 mm[14,57]. Pedersen et al.[58] evaluated the reproducibility of the measurement of the minimum distance of the tumor to the CRM for the prediction of extramural extension. Tumors were classified as early (≤5 mm of extramural extension) or advanced (>5 mm of extramural extension), and the status of the CRM was evaluated at the levels of 1 mm and 5 mm. The authors concluded that the measurement of extramural extension is more reproducible between different observers compared with the measurement of 5 mm to the CRM, as has been previously suggested by Beets-Tan et al.[59].
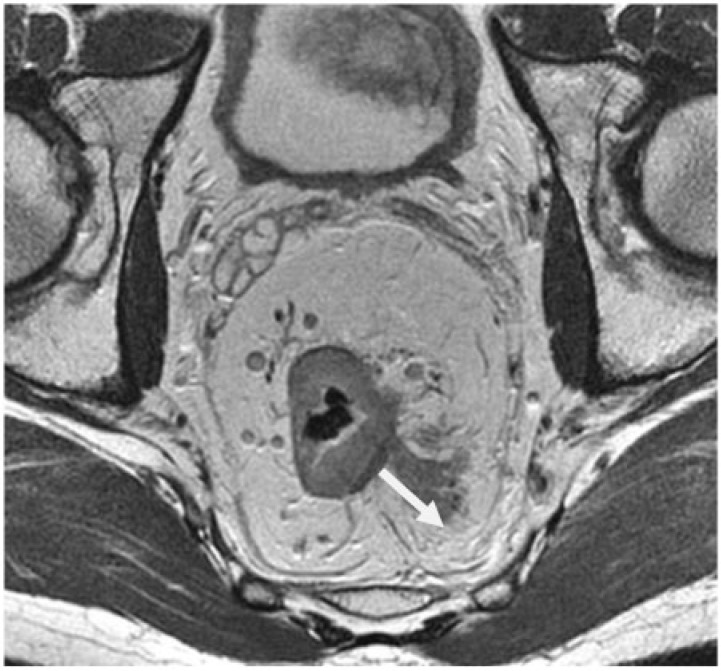
In clinical practice, about 80% of rectal tumors are represented by T3 tumors, within which there is a heterogeneous range of survival, requiring preoperative identification of the poor prognosis group[57]. The diagnosis of extramural tumor spread by HR MRI is based on the presence of tumor extending to the mesorectal fat, with a wide base protruding or with a nodular configuration, in continuity with the intramural portion of the tumor (Fig. 1). It is important to determine the continuity with the intramural component because discontinuity in the outer edge of the longitudinal layer of the muscularis propria can exist due to the presence of small penetrating vessels, but not necessarily meaning tumor invasion[57].
Figure 1.
Depth of tumor spread beyond the muscularis propria (arrow) in a 52-year-old man with rectal cancer. Axial T2-weighted HR MRI shows extramural spread of 21 mm.
For the correct choice of treatment, differentiation between T2 and T3 tumors is not what dominates decision making, but rather the identification of high-risk patients with T3 tumors with extramural extension greater than 5 mm. The greater the extramural extension into mesorectal fat is, the greater the nodal involvement is[60]. The same applies to extramural vascular invasion and, therefore, to the risk of distant metastases[58]. Clearly, in patients with disease-free CRM, with local recurrence rates of 5–10% and 5-year survival rates of 50–60%, distant metastases have become the leading cause of death[61]. Thus, the extramural extension of the tumor on HR MRI is a key factor in determining prognosis, therefore allowing proper selection of patients for preoperative treatment.
Peritoneal involvement
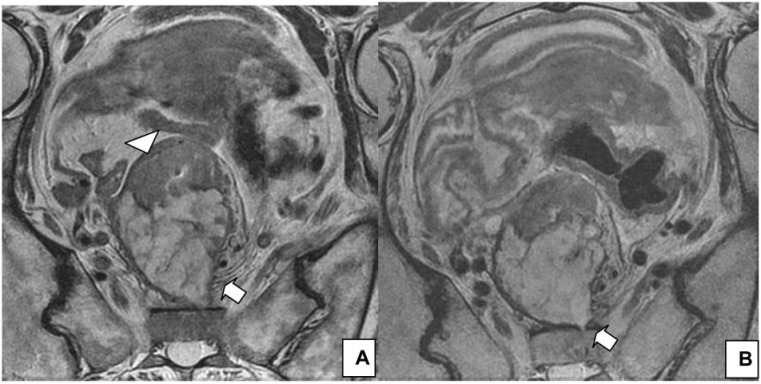
Upper rectal tumors can perforate the peritoneum. In HR MRI, the typical appearance is nodular extension through the peritoneal reflection at or above the level of the anterior fixation in the rectum, which is better demonstrated in the axial plane[13] (Fig. 2). In a prospective study of 412 patients with colon cancer, Shepherd et al.[62] showed that local peritoneal infiltration is an independent risk factor for intraperitoneal recurrence after surgery. Similarly, in patients with rectal cancer, peritoneal infiltration is a prognostic factor for local recurrence[63].
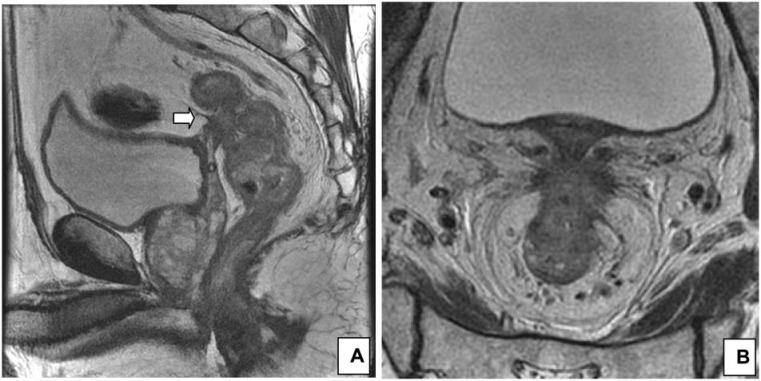
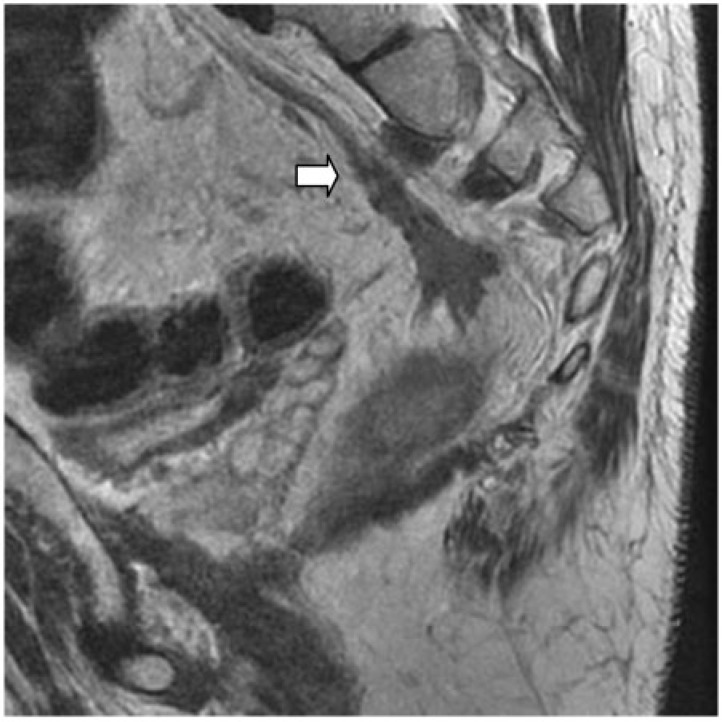
Figure 2.
Peritoneal involvement. (A) Sagittal T2-weighted HR MRI shows peritoneal involvement in a 65-year-old man with rectal cancer (arrow). (B) Axial T2-weighted image at the level of the peritoneal reflection best shows the involvement.
CRM
The status of the CRM is an important independent prognostic factor in the evaluation of rectal cancer. A free or negative margin reduces the risk of local recurrence and improves survival[60,64,65]. The significance of the CRM was shown by Quirke et al.[60] in 1986. The Mercury group showed that both the status of the mesorectal fascia, i.e. the potential CRM, and the extramural tumor extension can be predicted with great accuracy by HR MRI, with histopathologic analysis as the standard of reference[4,14]. A distance ≥1 mm to the mesorectal fascia determines a free margin, whereas a distance <1 mm is considered a positive margin. HR MRI showed a specificity of 92% in determining negative CRM.
In a national review of 686 patients in Norway, Wibe et al.[65] demonstrated that, despite TME, 9% of the patients had a positive CRM, with 22% of local recurrence in this group compared with 5% in patients with negative CRM. The infiltration of the CRM is itself a poor prognostic factor for survival, with 40% of patients developing distant metastases, compared with 12% in patients with free CRM[65–67]. As the distance to the CRM decreases, there is an exponential increase in the rates of local recurrence, metastasis and death[65].
As previously mentioned, a potentially positive CRM by HR MRI is defined as the presence of tumor at at least 1 mm from the mesorectal fascia[4] (Fig. 3). In the preoperative evaluation by HR MRI, the distance to the mesorectal fascia of the primary tumor, the presence of lymph nodes with suspicious criteria, extramural vascular invasion and tumor deposits are considered[57,68]. Shihan et al.[69] have shown that the involvement of the CRM exclusively by a metastatic lymph node is rare. Their study included 396 patients with rectal cancer. They analyzed mesorectal lymph nodes by HR MRI and the status of CRM in the histopathologic analysis together with the causes of commitment of the margin. Twelve percent (12%) of the patients (50/396) had positive CRM on histopathology, of which 10% (5/50) were due to a metastatic lymph node, i.e. 1.3% of all patients (5/396) and 3.1% of patients with metastatic lymph nodes on histopathology (5/163). Of the 5 malignant lymph nodes that compromised the CRM on the histopathologic analyses, 4 were not detected by HR MRI. In 31 patients, HR MRI reported suspicious lymph nodes located 1 mm or less from the CRM. None of these patients presented a CRM infiltrated by a positive lymph node in the histopathologic analysis. Thus, the authors conclude that it is necessary to be careful when recommending neoadjuvant treatment based solely on the presence of a suspicious lymph node close to the mesorectal fascia by HR MRI, because, regardless of the nodal status determined by HR MRI, it is uncommon that mesorectal lymph nodes are the only factor responsible for a positive CRM in the histopathologic analysis. Other prognostic factors determined by HR MRI, such as the infiltration of the CRM by direct extension of the primary tumor, extramural tumor extension and the presence of extramural vascular invasion, clinical evaluation and discussion by the MDT should determine the appropriate treatment for each patient.
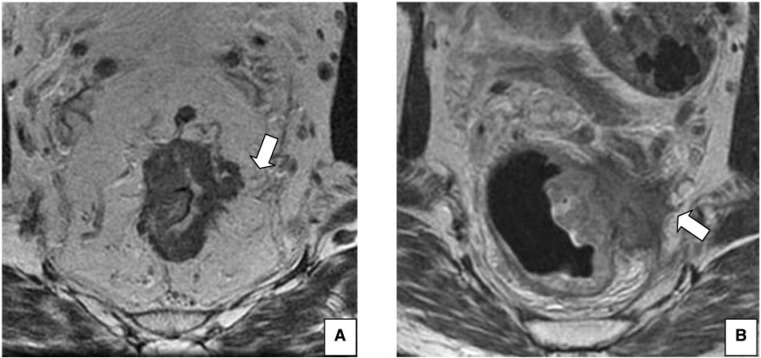
Figure 3.
CRM status. (A) Axial T2-weighted HR MRI shows negative CRM (>1 mm) in a 67-year-old man with rectal cancer (arrow). (B) Axial T2-weighted HR MRI shows infiltration of the left lateral portion of the CRM, with the tumor clearly extending beyond the mesorectal fascia (arrow) in a 56-year-old man with rectal cancer.
A recent meta-analysis published by Al-Sukhni et al.[5] showed that HR MRI has high specificity for determining the involvement of the CRM; its sensitivity for evaluating this prognostic factor is similar to that of tumor evaluation (T). Both parameters, assessment of the CRM and the T staging, are complementary in the indication of preoperative treatment and both should be considered. The result of this meta-analysis supports the use of HR MRI in the preoperative staging of rectal cancer.
The identification and aggressive preoperative treatment for tumors with a potentially positive CRM by HR MRI are crucial to prevent local recurrence[2]. Despite variation in the use of preoperative treatment, there is a broad consensus that patients with potentially infiltrated CRM by HR MRI should receive chemoradiotherapy, because discussion of the HR MRI by the MDT and implementation of this treatment regimen have reduced the rates of positive CRMs[32,68].
Extramural vascular invasion
Extramural vascular invasion is the presence of tumor cells within blood vessels located outside the muscular layer, in the vicinity of a primary tumor of the colon or rectum. Extramural vascular invasion is present in 17–52% of patients with colorectal cancer[10,11,70–73] and it is associated with more locally advanced tumors[70,71,73] (Fig. 4). The histologic finding of extramural vascular invasion is recognized as an independent prognostic factor for local recurrence[74,75], distant recurrence[73–77] and poor overall survival[78–81]. There is an association between the presence of extramural vascular invasion and the development of liver metastases[70,72,74,76].
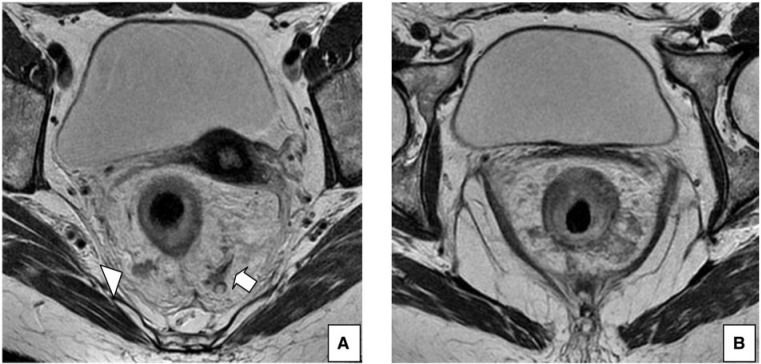
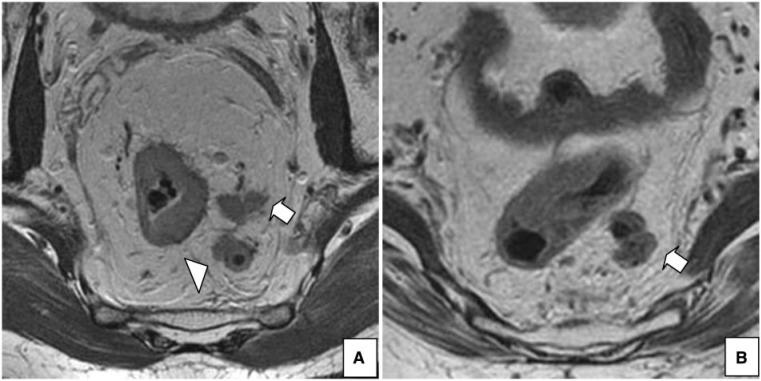
Figure 4.
A 55-year-old woman with rectal cancer. (A) Axial T2-weighted HR MRI shows positive extramural vascular invasion (arrow) and a lymph node with irregular borders (arrowhead). (B) Axial T2-weighted HR MRI, at a lower level, shows extramural tumor extension >5 mm and involvement of the CRM.
Smith et al.[11] analyzed the prognostic significance of extramural vascular invasion detected by HR MRI (Fig. 5). The sensitivity and specificity of HR MRI in detecting extramural vascular invasion in 94 patients after primary surgery were 62% and 88%, respectively. In a univariate analysis, relapse-free survival at 3 years was 35% in patients with positive findings of extramural vascular invasion (grade 3 and 4) on HR MRI and 74% in patients with negative findings (grade 0–2). The values reported were similar to those found in patients with positive and negative extramural vascular invasion on histologic analysis (34% and 73.7%, respectively). Therefore, the authors conclude that the detection of extramural vascular invasion by HR MRI provides information for predicting recurrence of the disease.
Figure 5.
Sagittal T2-weighted HR MRI shows extramural vascular invasion involving the superior rectal vein (arrow) in a 30-year-old man with rectal cancer.
The classification of extramural vascular invasion by HR MRI is important for decision making by the MDT. Hunter et al.[17] included patients with positive findings of extramural vascular invasion on HR MRI within the high risk group of synchronous metastatic disease, along with patients with extramural extension >5 mm or T4, a potentially positive CRM and commitment of the intersphincteric space in low rectal tumors. According to the authors, these patients would benefit from more thorough preoperative staging and alternative neoadjuvant chemotherapy regimens, including induction chemotherapy.
HR MRI probably provides additional information for staging, showing evidence of vascular invasion that cannot be recognized by histopathology due to destruction of the vessel walls, in these cases showing only an extramural tumor deposit without endothelial cells[10,11].
Thus, whereas nodal status has become less predictive of local recurrence in patients who have undergone careful excision of the rectum and mesorectum, extramural vascular invasion remains an important independent prognostic factor[57,82,83]. HR MRI is the only diagnostic modality that can demonstrate extramural vascular invasion in rectal cancer[1,57]. Therefore, the assessment of extramural vascular invasion by HR MRI is an important prognostic factor that can be identified and that contributes to the prediction of the results and to the selection of the appropriate preoperative treatment[11,57].
Nodal status
Nodal involvement is an independent poor prognostic factor; it is more significant when 4 or more nodes are involved in the histopathologic evaluation of the specimen after TME[84]. In the era before TME, nodal involvement, regardless of the number of nodes involved, predicted local recurrence. At present, the evidence suggests that when less than 4 metastatic nodes (N1) are involved after TME, there is no increased risk of local recurrence[84].
The risk of nodal involvement increases with the depth of tumor extension through and beyond the muscular layer. The close relationship between the 2 factors was noted in the original work of Dukes[85]. Yet nodal involvement can also be found in T1 tumors. The depth of involvement of the submucosa is important, because those tumors with deep submucosa involvement (SM3) have a higher rate of lymph node metastasis than a more superficial involvement (SM1 and SM2)[86–88]. The incidence of lymph node metastasis is 6–14% in T1 tumors, 17–23% in T2 tumors and 49–66% in T3 tumors[86–92].
Traditionally, the criterion used for lymph node evaluation on MRI was the size. However, several publications showed a lack of precision when using this feature[93,94]. Lymph node involvement may occur with only microscopic tumor foci, thus of normal size[13]. Brown et al.[93] evaluated the signal intensity and the edges of the lymph nodes on HR MRI and compared them with size in order to determine nodal status. The authors found that the size of benign and malignant lymph nodes was similar. Therefore, it can be concluded that size was a poor predictor for determining nodal status. In addition, 58% of positive lymph nodes found had a diameter <5 mm. When the irregular edges and mixed signal intensity of the lymph nodes were considered, a more accurate diagnosis was obtained with a sensitivity of 85% and a specificity of 97% (Fig. 6).
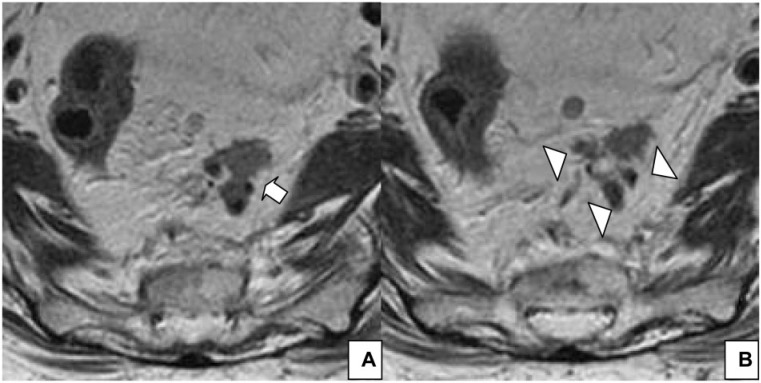
Figure 6.
(A) Axial T2-weighted HR MRI shows lymph nodes with irregular borders (arrow) and mixed signal intensity (arrowhead) in a 52-year-old man with rectal cancer. (B) Axial T2-weighted HR MRI shows a lymph node with mixed signal intensity (arrow) in a 64-year-old woman with rectal cancer.
Lymph nodes should only be evaluated with HR MRI techniques (in-plane minimum resolution of 0.6 mm × 0.6 mm, with 3-mm slice thickness)[57]. Promising data emerging from publications in which nodal status was evaluated with contrast agents would allow increasing MRI accuracy[95–98]. The prognostic significance of lymph nodes in patients with rectal cancer who have undergone TME is uncertain and most patients with 3 or fewer nodes involved have a good prognosis[57]. However, patients with involvement of 4 or more nodes have a worse prognosis and often, along with other poor prognostic factors, these patients present with extramural vascular invasion and increased extramural tumor extension, more easily detected on HR MRI[57].
Another goal of the preoperative evaluation of lymph nodes on HR MRI is the identification of those lymph nodes outside the mesorectal fascia, because those nodes that are not resected by extended lymphadenectomy[99,100] may be responsible for local recurrence despite the apparent free surgical resection margin[101] (Fig. 7).
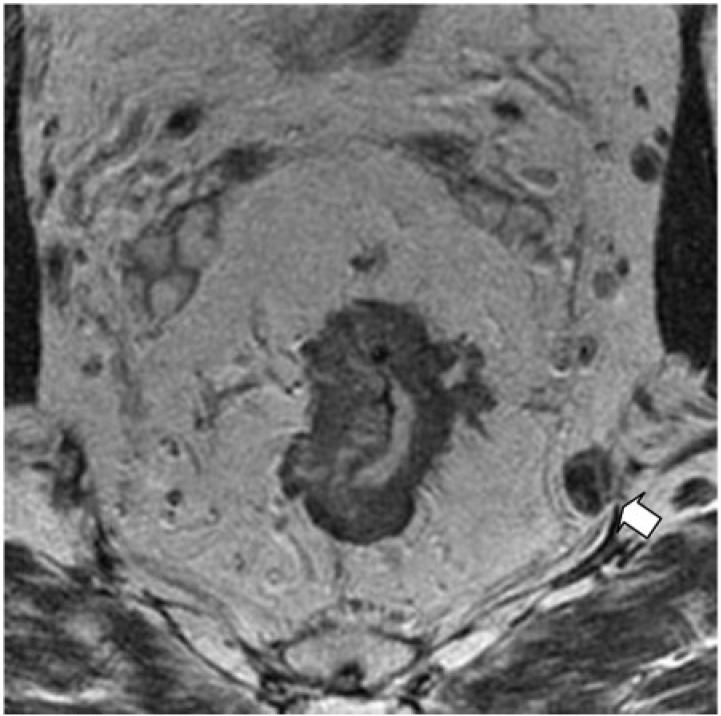
Figure 7.
Axial T2-weighted HR MRI shows an extramesorectal lymph node (arrow) in a 67-year-old man with rectal cancer.
Around 15–42% of small lymph nodes (<5 mm) located in the mesorectum may contain metastases[93,102,103]. In the work of Brown et al.[93], 23% of lymph nodes detected in the surgical specimens were not identified by HR MRI, but they all measured <3 mm and only 2/102 were metastatic. Furthermore, HR MRI correctly identifies many of the nodes between 2 and 5 mm and can correctly predict the presence of metastases in some, based on their irregular edges[13]. Because the ability of HR MRI to characterize small nodes is suboptimal, nodal evaluation on HR MRI using the signal intensity and the edges as diagnostic criteria would result in understaging of very few patients[13]. However, the inability to detect microscopic metastases in lymph nodes suggests that a negative HR MRI should not be used to select patients for local resection[13].
As previously mentioned, Shihan et al.[69] have shown that the involvement of the CRM exclusively by a metastatic lymph node is rare. Birbeck et al.[104] observed that the involvement of the CRM by the presence of a tumor within a lymph node is associated with a local recurrence rate significantly lower than expected when compared with the involvement by direct extension of the primary tumor, neural invasion or lymphovascular invasion. Some argue that if TME is correctly done, a malignant lymph node localized <1 mm from the CRM on histopathology (pCRM) rarely results in local recurrence[69]. This may reflect the protective effect exerted by the intact capsule of the node and an intact mesorectal fascia after optimal TME[69,105]. The presence of capsular invasion is most often associated with local recurrence, compared with microscopic deposits[106].
HR MRI cannot distinguish between a lymph node replaced by tumor and an extramural tumor deposit[13] (Fig. 8). However, knowing that patients with discontinuous tumor deposits have a worse prognosis, this is another advantage of HR MRI to detect such deposits in the preoperative assessment[107,108].
Figure 8.
A 64-year-old woman with rectal cancer. (A) Axial T2-weighted HR MRI shows a tumor deposit or replaced lymph node by tumor associated with extramural vascular invasion (arrow). (B) MRI is the only imaging modality that can demonstrate extramural vascular invasion[1,57] as discrete serpiginous projections of intermediate signal intensity into perirectal fat, following the course of a visible perirectal vein[57] (arrowhead).
The biggest challenge of imaging techniques lies in the ability to identify the nodal status before surgery, knowing that the nodes involved may only contain microscopic tumor foci that do not alter the size[13]. The inability to predict the nodal status in patients with rectal cancer is seen as a major limitation of preoperative staging techniques[13]. In a recent review and meta-analysis evaluating the accuracy of HR MRI to determine tumor stage, the presence of lymph node metastasis and the involvement of CRM, the authors concluded that HR MRI has good accuracy in the evaluation of T stage and CRM status. However, evaluation of nodal involvement is poor[5].
Low rectal tumors
Low rectal tumors, especially those treated with abdominoperineal excision (APE), are associated with poor outcomes in the short term (CRM involvement and perforation of the specimen) and the rates of local recurrence and 5-year survival[109–114]. Preoperative identification of a positive CRM is the key to the indication of preoperative treatment, as it is one of the most important factors for the development of local recurrence[115]. The Mercury group[4] showed that HR MRI may predict the status of the CRM with a high degree of accuracy.
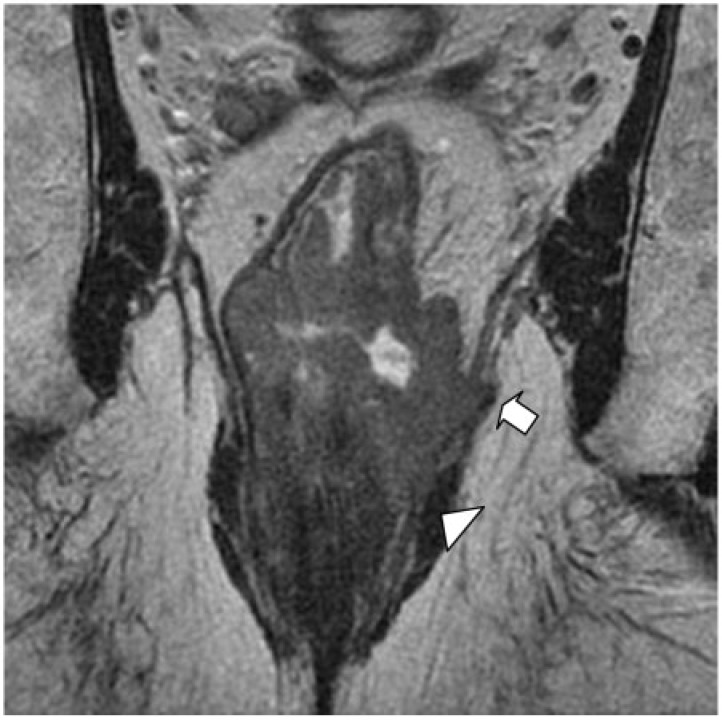
Anatomically, the beginning of the puborectalis sling marks the beginning of the thinner portion of the mesorectum, becoming a fine segment of perirectal fat at this level. Below the puborectalis sling lies the anal canal, formed by the mucosa, submucosa, internal sphincter, intersphincteric space of 1–2 mm and the external sphincter. At this level there is no mesorectum; therefore, there is no protective barrier effect to contain the tumor, such as in higher rectal tumors. Thus, any extension through the muscularis would result in exposure of the tumor to the CRM, whether an ultra low TME or a conventional APE is performed (Fig. 9). This is because, in conventional surgery, the muscularis propria rather than the mesorectal fascia determines the surgical margin[57]. Therefore, the surgical plane may be considered free if a considerable thickness of the muscularis propria is preserved in the deep position of the invasive edge of the tumor. If the muscle itself has full thickness compromised or has a minimal extension to the intersphincteric space, more radical surgery such as extralevator or extrasphincteric APE is needed, thus removing the entire sphincter complex, levator muscle and mesorectum en bloc[57].
Figure 9.
Coronal T2-weighted HR MRI shows a low rectal tumor in a 75-year-old man with involvement of the left levator muscle (arrow), intersphincteric space and external sphincter (arrowhead).
Several publications have focused on demonstrating the usefulness of HR MRI in patients with low rectal tumors, providing important information for the proper selection of preoperative treatment and surgical planes[1,112,114,116,117]. A staging system by HR MRI has been devised for the evaluation of low rectal tumors in order to identify patients at increased risk of positive CRM in traditional APE[68]. The classification consists of 4 stages: (1) the tumor is circumscribed to the rectal wall, without full thickness involvement, with the muscular outer layer preserved; (2) the tumor replaces the muscle layer without extension to the intersphincteric space; (3) the tumor invades the intersphincteric plane or is located within <1 mm of the levator muscle; (4) the tumor invades the external sphincter and is located at <1 mm and beyond the levator muscle with or without invasion of adjacent organs. As described above, the staging system for low rectal tumors by HR MRI takes into account the relevant anatomic detail of this location, providing more accurate information and enabling the surgeon to correctly select the surgical technique. Rates of CRM involvement after APE of 30% have been reported compared with 10% in patients with low anterior resection[68,109,118–120].
Salerno et al.[117] evaluated the usefulness of HR MRI in predicting margin involvement in low rectal tumors, concluding that tumors identified on or through the intersphincteric plane on HR MRI, as well as the degree of tumor regression determined by the method, may predict margin involvement. Therefore, stage 3 and 4 low rectal tumors on HR MRI and the tumor regression grade with absent or minimal response predict a positive margin for APE. The advantages of predicting a positive CRM for APE on HR MRI include the following: (1) the ability to properly plan neoadjuvant therapy and surgical technique; (2) according to the classification of low rectal tumors by HR MRI, stage 1 and 2 tumors would benefit from intersphincteric APE, whereas stages 3 and 4 would benefit from a broader plane outside the levator muscles (extralevator APE); (3) using HR MRI staging for low rectal tumors would reduce the rate of R1 in <10% of patients undergoing APE; (4) it allows evaluation of alternative surgical techniques, such as radical APE, and therapeutic strategies, such as intensive chemoradiotherapy, in order to prevent R1 disease in high-risk low rectal tumors identified by HR MRI, as candidates for APE.
Evaluation of response to neoadjuvant treatment
Staging after neoadjuvant treatment is a challenge for all diagnostic methods because of radiation-induced changes: edema, fibrosis, inflammation and necrosis[2]. Emerging data suggest the combination of HR MRI with diffusion images and PET/CT to provide information regarding prognosis before definitive surgery[2].
HR MRI has recently been validated as a method for staging after neoadjuvant treatment, as well as its correlation with survival[9]. The data were provided by the Mercury group through the Magnetic Resonance Imaging and Rectal Cancer European Equivalence. One hundred eleven patients with rectal cancer treated with neoadjuvant therapy were enrolled in a prospective study. Their response was evaluated by HR MRI and pathology. This was the first prospective study to demonstrate correlation between radiologic assessment of treatment and long-term results[6]. The determination of CRM after treatment by HR MRI (ymrCRM) and the degree of tumor regression predict survival in groups of good response and poor response[6]. The postoperative histopathologic evaluation of the tumor (ypT) and the CRM (ypCRM) were significant predictors of outcome; yet the assessment of nodal status after treatment (ypN) was not. The association between tumor regression grade determined by HR MRI and the long-term outcomes was subsequently confirmed[7]. Thus, a poor response to neoadjuvant treatment may be used to plan therapies before surgery in order to improve the outcome.
The parameters recommended for the assessment of neoadjuvant treatment by HR MRI are the determination of T stage (ymrT) and the degree of tumor regression, because both may predict the favorable and unfavorable findings of the histopathology[8]. Tumor response to treatment was assessed by HR MRI using 5 degrees: (1) complete radiologic response (no evidence of tumor treated); (2) good response (hypointense dense fibrosis, minimal residual tumor); (3) moderate response (about 50% fibrosis/mucin and intermediate signal representing residual tumor); (4) minor response (minimal fibrosis/mucinous degeneration, mainly tumor); (5) no response (the tumor has the same appearance as in the pretreatment)[6,8,68]. Thus, favorable response by HR MRI corresponds to tumor regression grade 1–3 and tumor stage ymrT0–T3a, whereas favorable response by histopathology corresponds to tumor regression grade 3–4 and tumor stage ypT0–T3a[8]. Moreover, the unfavorable response by HR MRI corresponds to tumor regression grade 4–5 and tumor stage ymrT3b–T4 (Fig. 10), which are represented by histopathology with tumor regression grade 0–2 and tumor stage ypT3b–T4[8].
Figure 10.
A 70-year-old woman with rectal cancer. (A) Axial T2-weighted HR MRI pretreatment image shows a tumor with a high signal, compatible with mucin (arrow), with intermediate signal intensity components (arrowhead). (B) Axial T2-weighted HR MRI posttreatment image shows the same appearance of the tumor indicating non-response[9]. An incipient infiltration of the anterior cortex of the sacrum is shown (arrow), not present at pretreatment MRI.
In the Mercury Study Group[9], the group of patients with a good response to treatment (tumor regression grade 1–3) had rates of overall survival, disease-free survival at 5 years and local recurrence of 72%, 64% and 14%, respectively. In patients with a poor response (tumor regression grade 4–5), the corresponding rates were 27%, 31% and 29%, respectively.
Posttreatment assessment has focused on the identification of a complete pathologic response in clinical trials[8]. Focusing only on the complete pathologic response may underestimate the benefit of treatment, because it is clear that patients with a good response but who fail to reach a complete response have equally good results[6,8]. Patel et al.[8] have focused their research on differentiating good responders from those with poor response, rather than on identifying the complete responders. Preoperative treatment benefits include local control and tumor downstaging[8,118]. Reassessment of the tumor after treatment is essential to optimize therapy in patients with rectal cancer. Thus, in clinical practice, the subgroup of patients with a poor response by HR MRI seem to be at higher risk of local and systemic failure after standard TME[8]. Surgeons should be alerted to this fact before surgery in order to plan the correct approach. In future studies, the group of patients with poor response may be considered for additional treatment, such as extended or intensified systemic chemotherapy, radiotherapy boost, extent of surgical resection or more intensive postoperative monitoring[8].
HR MRI cannot differentiate between complete pathologic response and residual microscopic disease in a single scan. Serial studies with clinical evaluation and CEA help confirm the complete response[2]. Kim et al.[121] demonstrated that HR MRI, along with the diffusion technique, allows greater precision with regard to diagnostic assessment compared with HR MRI alone in the determination of complete response to neoadjuvant therapy in patients with locally advanced rectal cancer.
Thus, the evaluation of response to neoadjuvant treatment determined by HR MRI provides important information to the MDT: (1) surgical planning, (2) the time of surgery, (3) sphincter preservation, (4) surgery deferral in responders, (5) treatment intensification in non-responders[9].
Evaluation of tumor response to treatment is not only based on reduction in tumor size. The reduction or absence of prognostic risk factors identified in the pretreatment HR MRI, i.e. extramural tumor extension, CRM, extramural vascular invasion, peritoneal perforation and nodal involvement, must be analyzed[2].
Therefore, the HR MRI report should be detailed and should consider the following: (1) tumor morphology, necrotic or mucinous component, (2) distance from the anal verge, (3) tumor size, (4) degree of tumor regression, (5) maximum extramural depth of tumor and fibrosis; (6) T stage, N stage, extramural vascular invasion, peritoneal reflection commitment; (7) distance to CRM[9].
HR MRI predicts response to neoadjuvant treatment by determining the degree of tumor regression and tumor stage. Its recent validation is an important aspect in MDT decision making.
HR MRI protocols and standardized report
For adequate rectal cancer staging by HR MRI, using the appropriate parameters is of paramount importance in order to obtain an optimum study. The technical characteristics together with the interpretation of the images have been widely reported[1,9,13,14,57,68,122–124]. Brown et al.[125] have described the relevant anatomic details on HR MRI for TME. In 1999, Blomvquist et al.[126] evaluated the HR MRI findings on the involvement of the lateral resection margin in 26 surgical specimens.
Suzuki et al.[127] demonstrated the importance of appropriate protocols for staging patients with rectal cancer; their study was the first to determine the importance of HR MRI imaging protocols for the evaluation of neighboring organs in locally advanced rectal cancer. The authors retrospectively evaluated the MRIs of 37 patients. They divided the MRIs into 2 groups according to the protocol used, i.e. optimal or suboptimal, with histopathology as the gold standard. Evaluation of images from other institutions was conducted by an MDT from a referral center for patients with advanced rectal cancer; a variation in the protocols used was identified. MRI performed with an optimal protocol showed a better correlation with histopathology in relation to the involvement of an anterior organ, with a sensitivity of 86% and a specificity of 94% versus 50% and 33%, respectively, in patients studied with a suboptimal protocol. The authors concluded that suitable MRI protocols allow more accurate staging of patients with locally advanced rectal cancer, with fewer sequences and without the administration of contrast agents. The causes of this discrepancy were attributed to technical factors and to the degree of training in the evaluation of MR images. Thus, the authors considered that constant training of radiologists and radiology technicians, including workshops and seminars, seems to be the right way to improve the accuracy of MRI in the evaluation of patients with rectal cancer.
Apart from the technical parameters, the characteristics of the standardized HR MRI report in staging patients with rectal cancer are also key to proper evaluation[13,14,57]. Taylor et al.[57] have published a meticulous description of the standardized report, with the corresponding proforma. The authors emphasize the importance of using a proforma to prevent loss of information. Free text reports carry the risk of omitting important information on the staging, therefore preventing a correct approach to patients with rectal cancer. The same used to happen with histopathology reports. Regarding histopathologic evaluation of the CRM, a crucial risk prognostic factor in predicting local recurrence and postoperative treatment indicator was only reported in one-third of patients[57,128] in an audit carried out in 1997. After the introduction of a minimum amount of information necessary to the reports, the rate improved nearly 100%, resulting in better selection of patients who had to receive postoperative adjuvant treatment. As noted in the audit, lack of description of the different prognostic risk factors can result in a false assumption leading to understaging and undertreatment[57,129]. Similarly, the authors mention a radiology audit that compared the free text reports versus the use of a proforma. Data relevant to the staging were omitted in 97% of the free text reports, whereas the omission was reduced to 3%[57] with the use of the proforma. Assessing the potential involvement of CRM by HR MRI, i.e. the main factor in determining the use of preoperative chemoradiotherapy, was not reported in 74% of free text reports. However, with the introduction of proformas, this omission was reduced to 4%[57].
Therefore, the use of standardized reports through a proforma and the optimum HR MRI protocol are key to the correct staging of patients with rectal cancer.
Usefulness of HR MRI in the evaluation of patients with suspected or confirmed recurrence
As previously mentioned, the implementation of TME and neoadjuvant hypofractionated radiotherapy or chemoradiotherapy treatments have reduced local recurrence rates from 25–40% to less than 10%[33–36]. It is evident that in patients with a negative CRM, with local recurrence rates of 5–10% and 5-year survival of 50–60%, distant metastases have become the leading cause of death[61]. In patients receiving neoadjuvant chemoradiation therapy alone, the distant recurrence rate is approximately 36%[40], contributing to the lack of survival benefit of this regimen and making it necessary to intensify the systemic component. In several studies, the adjuvant chemotherapy regimen has proved to be a benefit in terms of survival[43–46].
Local and distant recurrence of rectal cancer is usually diagnosed within the first 3 years after surgery, during routine follow-up[130–135]. There is growing evidence supporting that early detection of recurrence decreases deaths from colorectal cancer by 30–40%, with an absolute reduction in mortality of 7%[135–138]. Advances in surgical techniques along with a better selection of patients before surgery have improved survival results after resection of liver and lung metastases[135]. Therefore, early detection of recurrence, when potentially treatable, is the target of strict monitoring imaging. Patients with early asymptomatic recurrence, detected on time, are more likely to have a successful resection because as they become symptomatic, the probability of complete resection is lower[130,139]. In addition, knowledge of the predictable patterns of recurrence allows more careful evaluation of the images. These patterns are tumor spread, CRM involvement, peritoneal perforation, distal margin involvement and residual disease[139].
In a meta-analysis by Renehan et al.[138], the best results for monitoring that improved survival were obtained using CEA, clinical examination and annual chest, abdomen and pelvis CT. Intensive monitoring allowed earlier detection of all recurrences, with an average of 8.5 months (95% confidence interval 7.6–9.4 months), and a higher detection rate of isolated local recurrences. The absolute reduction in mortality was 9–13%. The American Society of Clinical Oncologists published guidance on monitoring after curative treatment of these patients in order to identify potentially treatable early recurrences, recommending annual CT in patients at risk of developing recurrent disease[140].
Potter et al.[135] compared the accuracy of serial CT/MRI review versus the FDG-PET/CT in patients with suspected or known recurrence. In patients with suspected recurrence, the authors recommended reviewing the serial images in order to correlate suspicious findings with previous studies, indicating FDG-PET/CT when findings are equivocal after review. The sensitivity, specificity and accuracy to detect recurrence showed no significant differences between serial review of images and FDG-PET/CT. In this series, FDG-PET/CT was indicated when the findings described in the report of the conventional studies were equivocal for the diagnosis of recurrence, when there was an unexplained increase in CEA and for excluding other sites of disease before planning curative surgery in patients with potentially resectable recurrent disease. These guidelines were based on previous studies, including a meta-analysis, which showed that after FDG-PET/CT, management of patients evaluated for recurrent rectal cancer changed in 29% of cases[135,141].
HR MRI provides important anatomic information when recurrence is detected by CT or is clinically suspected, because it has a better contrast of the soft tissues, allowing more accurate delineation of the tumor[135]. Thus, early detection of recurrence after a strict follow-up by imaging and anatomic characterization by HR MRI provide the necessary information for the discussion of the best diagnostic and treatment options by the MDT.
Radical resection is the only potential cure for patients with locally advanced primary rectal cancer and for recurrence; it is considered curative only when histologic margins are tumor free (R0: 10–67% of cases)[130,142]. Complete tumor resection (R0) can result in a survival rate at 5 years of over 35%[130,143]. Microscopic or macroscopic residual disease (R1 and R2, respectively) in the resection margins may result in poorer survival[130]. However, curative resection is only possible when there is no spread of the disease and tumor invasion to adjacent structures is within the limits of resectability[130].
The ability to provide detailed anatomic information from HR MRI allows the possibility of resection to be discussed by the MDT (Fig. 11). Compared with CT, MRI can more accurately differentiate recurrence within a presacral scar, based on the difference in signal intensity between tumor and fibrosis on T2-weighted images or by administering intravenous contrast[130,144]. PET is an accurate diagnostic tool, showing advantages over CT and MRI in the differential diagnosis between recurrence and scar[141]. In a meta-analysis, PET showed a sensitivity and specificity of 94% in detecting local recurrence[145], with a high accuracy in the detection of pelvic recurrence in patients who had previously received radiation therapy[146]. However, PET has limitations in detecting small volumes of disease and a relatively low sensitivity for the detection of lymph node metastasis[147]. Mucinous adenocarcinoma has poor FDG uptake and hence cannot be easily detected on PET[148]. HR MRI is essential to differentiate unifocal from multifocal disease, as in the detection of small and metabolically inactive lesions[139].
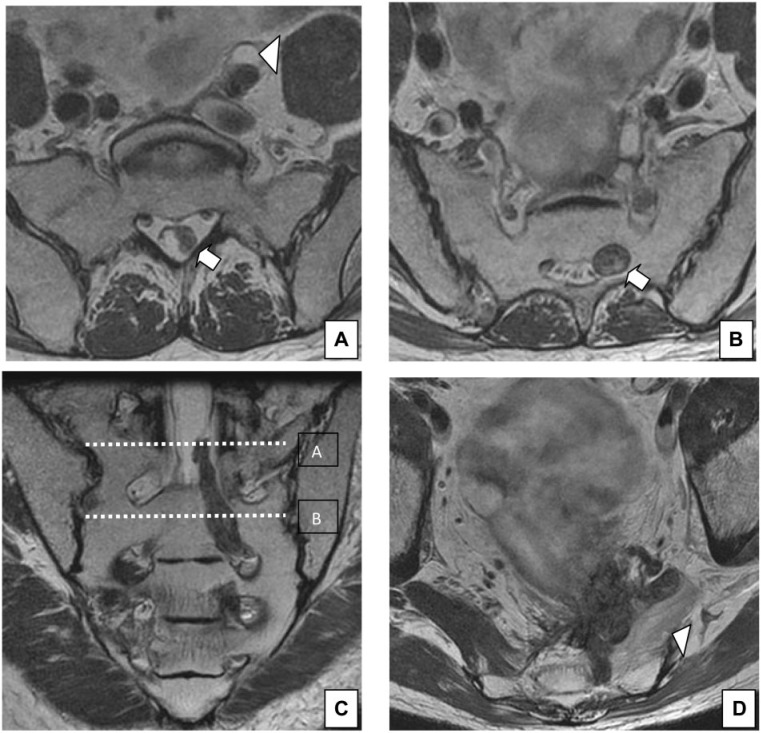
Figure 11.
A 60-year-old man with rectal cancer recurrence. The recurrence involves the left sacral roots S2, S3 and S4. (A) Axial T2-weighted HR MRI shows extension of the tumor to the dural sac (arrow) at the level shown in (C). Ureteral dilatation is also seen (arrowhead). (B) Axial T2-weighted HR MRI shows extension of the tumor through the sacral root S3 (arrow) at the level shown in (C). (C) Coronal T2-weighted HR MRI shows the extension up to the dural sac through the sacral root S3 (arrow). (D) Rectal cancer recurrence involving the posterior and left lateral compartment. Fatty infiltration of the left piriformis muscle is seen (arrowhead).
It is important to know the pattern of pelvic recurrences for proper interpretation and therapeutic decision making by the MDT. In an effort to establish a criterion for better prediction of tumor resectability and outcome, different classifications have been proposed[130]. Georgiou et al.[130] proposed a new classification based on the limits defined by the fascia and the anatomic planes of dissection between intrapelvic organs. Thus, the recurrence is classified according to the HR MRI findings of tumor invasion within 7 intrapelvic compartments: (1) central (rectum or neorectum, intraluminal recurrence, mesorectum or perirectal fat, extraluminal recurrence), (2) reflection peritoneal (rectovesical pouch or rectouterine pouch of Douglas), (3) anterior above the peritoneal reflection (ureters and iliac vessels above the peritoneal reflection, sigmoid colon, small bowel and lateral parietal fascia), 4) anterior below the peritoneal reflection (genitourinary system), (5) lateral (ureters, internal and external iliac vessels, lateral pelvic lymph nodes, sciatic nerve, sciatic notch, nerve roots S1 and S2, internal obturator or piriformis muscles), (6) posterior (coccyx, presacral fascia, retrosacral space, sacrum to the upper level of S1) and (7) inferior (levator ani, external sphincter complex, perineal scar (APE), ischioanal fossa). Diagnosis of tumor invasion by HR MRI into the lateral compartment, posterior compartment or into more than 2 compartments was associated with reduced disease-free survival[130]. In a recent publication, Georgiou et al.[149] analyzed the accuracy of HR MRI to detect invasion of adjacent structures at the expense of colorectal tumors, according to 7 intrapelvic compartments. Information was used for planning pelvic exenteration, defined as surgical excision beyond the conventional mesenteric plane in patients with locally advanced colorectal cancer (n=23) and patients with recurrent colorectal cancer (n=41). The sensitivity of HR MRI was ≥93.3% for all the compartments, except the lateral (89.3%). Specificity for the posterior compartment (82.2%) and specificity for the anterior compartment below the peritoneal reflection (86.4%) were lower compared with the other compartments. The diagnostic invasion by HR MRI of the anterior compartment above the peritoneal reflection was associated with poorer survival. The authors conclude that HR MRI is accurate to determine the extent of colorectal tumors in the pelvis and can therefore be used to determine the type of surgery required for curative resection, emphasizing that HR MRI should always be used in the staging of patients with locally advanced pelvic colorectal cancer.
Thus, the recurrences identified on HR MRI in the central compartment, in the anterior compartment below the peritoneal reflection, in the posterior compartment below S2 or a perineal location are potentially resectable[139]. The following recurrences are probably not resectable: those localized in the lateral compartment, those infiltrating the sciatic nerve, those compromising S1–S2 and those with peritoneal perforation[139].
In patients with increased CEA levels and a negative serial review of previous CT exams, HR MRI would allow ruling out local recurrence of rectal cancer. HR MRI along with additional information from FDG-PET/CT and liver MRI would therefore contribute to MDT decision making[135,139]. When recurrence is suspected by a positive serial examination of previous CT scans, because of a suspicious mass or an increase in size of the mass, HR MRI can delineate pelvic recurrence. Positive findings on HR MRI and an increase in CEA lead to the diagnosis of recurrence[13,139]. In these cases, the next question to be answered by HR MRI is related to the possibility of resection of the lesion. In patients with positive findings on HR MRI and normal CEA level, FDG-PET/CT provides additional information[135,139].
Radiologic findings are what determine tumor resectability[130]. Therefore, accurate preoperative staging can help to establish the extent of local disease and the presence or absence of distant metastases. The identification or absence of extrapelvic disease is the key to MDT decision making when considering exenterative surgery in patients with locally advanced primary rectal cancer or recurrence of rectal cancer[130].
Diffusion-weighted imaging can provide additional information regarding the extent of disease. However, its role remains unclear because it has not been validated due to the absence of substantial evidence in connection with diagnostic accuracy[130]. The role of HR MRI and the role of diffusion-weighted imaging were evaluated in a retrospective study that reported a high accuracy of MRI in the diagnosis of local recurrence of rectal cancer when this recurrence is suspected[150]. Forty-two patients with suspected recurrent rectal cancer, including 19 recurrences, showed that diffusion-weighted imaging did not significantly improve the diagnostic yield. However, a trend was noted toward additional value of diffusion-weighted imaging by improving the specificity in the diagnosis of recurrence. The limitation of HR MRI is generally related to overestimation of the presence of tumor within fibrotic scar tissue after surgery[151–153]. Diffusion-weighted imaging clearly discriminates abnormal signal intensity of the tumor from adjacent organs such as the intestine and fibrosis[154]. Moreover, the benefit of diffusion-weighted imaging would be more obvious in the detection of small anastomotic recurrences[150].
In summary, regarding the evaluation of recurrence of rectal cancer, HR MRI allows the lesion to be characterized, providing anatomic detail, detecting small lesions and metabolically inactive disease[139]. MDCT provides information concerning the pulmonary nodules and small peritoneal nodules, whereas FDG-PET/CT detects other sites such as remote nodes or peritoneal deposits and makes other pathologies evident[139].
Conclusion
The complexity of the management of rectal cancer is currently a challenge for all disciplines involved. HR MRI plays a crucial role in the dynamics of the MDT, namely initial staging, subsequent evaluation of response to neoadjuvant treatment and monitoring of patients for early detection of probable recurrence. Knowledge of the disease and the prognostic impact of the information provided by HR MRI improve the quality of the contribution of radiologists to the MDT. Proper selection of the technical parameters of HR MRI and the use of standardized reports make it possible to achieve better results. The same applies to the MDT discussion of the HR MRI findings. Because the target of teamwork is to treat each patient taking into account the characteristics of the individual and the cancer the patient has, i.e. so-called personalized medicine, the radiologist’s role in the MDT entails the need to manage information and incorporate knowledge beyond anatomic description of the findings.
Conflict of interest
The author has no conflicts of interest to declare.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355–364. doi: 10.1002/bjs.4034. . PMid:12594673. [DOI] [PubMed] [Google Scholar]
- 2.Evans J, Patel U, Brown G. Rectal cancer: primary staging and assessment after chemoradiotherapy. Semin Radiat Oncol. 2011;21:169–177. doi: 10.1016/j.semradonc.2011.02.002. . PMid:21645861. [DOI] [PubMed] [Google Scholar]
- 3.Edge BE, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC cancer staging manual. 7th edition. Colon and rectum. New York: Springer-Verlag; 2010. pp. p. 173–206. [Google Scholar]
- 4.Mercury Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Sukhni E, Milot L, Fruitman M, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systemic review and meta-analysis. Ann Surg Oncol. 2012;19:2212–2223. doi: 10.1245/s10434-011-2210-5. . PMid:22271205. [DOI] [PubMed] [Google Scholar]
- 6.Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–3760. doi: 10.1200/JCO.2011.34.9068. . PMid:21876084. [DOI] [PubMed] [Google Scholar]
- 7.Shihab OC, Taylor F, Salerno G, et al. MRI predictive factors for long-term outcomes of low rectal tumours. Ann Surg Oncol. 2011;18:3278–3284. doi: 10.1245/s10434-011-1776-2. . PMid:21590453. [DOI] [PubMed] [Google Scholar]
- 8.Patel UB, Brown G, Rutten H, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012;19:2842–2852. doi: 10.1245/s10434-012-2309-3. . PMid:22526897. [DOI] [PubMed] [Google Scholar]
- 9.Patel UB, Blomqvist LK, Taylor F, et al. MRI after treatment of locally advanced rectal cancer: how to report tumor response - the MERCURY experience. AJR. 2012;199:486–495. doi: 10.2214/AJR.11.8210. [DOI] [PubMed] [Google Scholar]
- 10.Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR. 2008;191:1517–1522. doi: 10.2214/AJR.08.1298. . PMid:18941094. [DOI] [PubMed] [Google Scholar]
- 11.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. . PMid:17932879. [DOI] [PubMed] [Google Scholar]
- 12.Punt CJA, Buyse M, Kohne CH, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007;99:998–1003. doi: 10.1093/jnci/djm024. . PMid:17596575. [DOI] [PubMed] [Google Scholar]
- 13.Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20–31. doi: 10.1080/02841860701697720. . PMid:17957502. [DOI] [PubMed] [Google Scholar]
- 14.Mercury Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the Mercury Study. Radiology. 2007;243:132–139. doi: 10.1148/radiol.2431051825. [DOI] [PubMed] [Google Scholar]
- 15.Cervantes A, Roselló S, Rodríguez-Braun E, et al. Progress in the multidisciplinary treatment of gastrointestinal cancer and the impact on clinical practice: perioperative management of rectal cancer. Ann Oncol. 2008;19(Suppl 7):266–272. doi: 10.1093/annonc/mdn438. [DOI] [PubMed] [Google Scholar]
- 16.Taylor F, Quirke P, Heald R, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II and III rectal cancer best managed by surgery alone. Ann Surg. 2011;253:711–719. doi: 10.1097/SLA.0b013e31820b8d52. . PMid:21475011. [DOI] [PubMed] [Google Scholar]
- 17.Hunter C, Garant A, Vuong T, et al. Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol. 2012;19:1199–1205. doi: 10.1245/s10434-011-2036-1. . PMid:21913017. [DOI] [PubMed] [Google Scholar]
- 18.Scholefield JH. Guidelines for the management of colorectal cancer. 3rd edition. London: Association of Coloproctology of Great Britain and Ireland; 2007. [DOI] [PubMed] [Google Scholar]
- 19.Valentini V, Aristei C, Grimelius B, et al. Multidisciplinary rectal cancer management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2) Radiother Oncol. 2009;92:148–163. doi: 10.1016/j.radonc.2009.06.027. . PMid:19595467. [DOI] [PubMed] [Google Scholar]
- 20.Aloia TA, Fahy BN. A decision analysis model predicts the optimal treatment pathway for patients with colorectal cancer and resectable synchronous liver metastases. Clin Colorectal Cancer. 2008;7:197–201. doi: 10.3816/CCC.2008.n.026. . PMid:18621638. [DOI] [PubMed] [Google Scholar]
- 21.Slupski M, Wlodarczyk Z, Jasinski M, Masztalerz M, Tujakowski J. Outcomes of simultaneous and delayed resections of synchronous colorectal liver metastases. Can J Surg. 2009;52:241–244. [PMC free article] [PubMed] [Google Scholar]
- 22.Hillingso JG, Wille-Jorgensen P. Staged or simultaneous resection of synchronous liver metastases from colorectal cancer-a systematic review. Colorectal Dis. 2009;11:3–10. doi: 10.1111/j.1463-1318.2008.01625.x. Erratum in: Colorectal Dis 2009; 11: 540. [DOI] [PubMed] [Google Scholar]
- 23.Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872–878. doi: 10.1002/bjs.5346. . PMid:16671066. [DOI] [PubMed] [Google Scholar]
- 24.Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934–941. doi: 10.1016/j.jamcollsurg.2010.02.039. . PMid:20510802. [DOI] [PubMed] [Google Scholar]
- 25.Orlacchio A, Schillaci O, Fusco N, et al. Role of PET/CT in the detection of liver metastases from colorectal cancer. Radiol Med. 2009;114:571–585. doi: 10.1007/s11547-009-0393-7. . PMid:19444590. [DOI] [PubMed] [Google Scholar]
- 26.Heriot AG, Hicks RJ, Drummond EG, et al. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum. 2004;47:451–458. doi: 10.1007/s10350-003-0089-3. . PMid:14978612. [DOI] [PubMed] [Google Scholar]
- 27.Kong G, Jackson C, Koh DM, et al. The use of 18F-FDG PET/CT in colorectal liver metastases - comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging. 2008;35:1323–1329. doi: 10.1007/s00259-008-0743-z. . PMid:18347794. [DOI] [PubMed] [Google Scholar]
- 28.Reinhardt MJ, Wiethoelter N, Matthies A, et al. PET recognition of pulmonary metastases on PET/CT imaging: impact of attenuation-corrected and non-attenuation-corrected PET images. Eur J Nucl Med Mol Imaging. 2006;33:134–139. doi: 10.1007/s00259-005-1901-1. . PMid:16193313. [DOI] [PubMed] [Google Scholar]
- 29.Expert Advisory Group on Cancer. A policy framework for commissioning cancer services: a report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales 1995. Available at http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4071083.
- 30.Brown G. Specialist multidisciplinary team working in the treatment of cancer. BMJ. 2012;344:2780. doi: 10.1136/bmj.e2780. [DOI] [PubMed] [Google Scholar]
- 31.Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: results from a retrospective, comparative, interventional cohort study of 13722 women. BMJ. 2012;344:2718. doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton S, Brown G, Daniels IR, Norman AR, Mason B, Cunningham D. MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer. 2006;94:351–357. doi: 10.1038/sj.bjc.6602947. . PMid:16465171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havenga K, Enker WE, Norstein J, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999;25:368–374. doi: 10.1053/ejso.1999.0659. . PMid:10419706. [DOI] [PubMed] [Google Scholar]
- 34.Nesbakken A, Nygaard K, Westerheim O, Mala T, Lunde OC. Local recurrence after mesorectal excision for rectal cancer. Eur J Surg Oncol. 2002;28:126–134. doi: 10.1053/ejso.2001.1231. . PMid:11884047. [DOI] [PubMed] [Google Scholar]
- 35.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. . PMid:9711965. [DOI] [PubMed] [Google Scholar]
- 36.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. . PMid:11547717. [DOI] [PubMed] [Google Scholar]
- 37.Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. . PMid:17968156. [DOI] [PubMed] [Google Scholar]
- 38.Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 39.Hawkes EA, Cunningham D, Tait D, Brown G, Chau I. Neoadjuvant chemotherapy alone for early-stage rectal cancer: an evolving paradigm? Semin Radiat Oncol. 2011;21:196–202. doi: 10.1016/j.semradonc.2011.02.005. . PMid:21645864. [DOI] [PubMed] [Google Scholar]
- 40.Sauer R, Becker H, Hohenberger W, et al. German Rectal Cancer Study. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. . PMid:15496622. [DOI] [PubMed] [Google Scholar]
- 41.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. . PMid:16983741. [DOI] [PubMed] [Google Scholar]
- 42.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. . PMid:23008301. [DOI] [PubMed] [Google Scholar]
- 43.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. . PMid:3276900. [DOI] [PubMed] [Google Scholar]
- 44.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. . PMid:1997835. [DOI] [PubMed] [Google Scholar]
- 45.Tepper JE, O’Connell M, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control—final report of Intergroup 0114. J Clin Oncol. 2002;20:1744–1750. doi: 10.1200/JCO.2002.07.132. . PMid:11919230. [DOI] [PubMed] [Google Scholar]
- 46.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. PMid:8410113. [DOI] [PubMed] [Google Scholar]
- 47.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. . PMid:15067027. [DOI] [PubMed] [Google Scholar]
- 48.Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668–674. doi: 10.1200/JCO.2005.04.4875. . PMid:16446339. [DOI] [PubMed] [Google Scholar]
- 49.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. . PMid:22473163. [DOI] [PubMed] [Google Scholar]
- 50.Glynne-Jones R, Kronfli M. Locally advanced rectal cancer: a comparison of management strategies. Drugs. 2011;71:1153–1177. doi: 10.2165/11591330-000000000-00000. . PMid:21711061. [DOI] [PubMed] [Google Scholar]
- 51.Cawthorn SJ, Parums DV, Gibbs NM, et al. Extent of mesorectal spread and involvement of lateral resection margin as prognostic factors after surgery for rectal cancer [see comments] Lancet. 1990;335:1055–1059. doi: 10.1016/0140-6736(90)92631-Q. . PMid:1691810. [DOI] [PubMed] [Google Scholar]
- 52.Willett CG. Technical advances in the treatment of patients with rectal cancer [editorial comment] Int J Radiat Oncol Biol Phys. 1999;45:1107–1108. doi: 10.1016/S0360-3016(99)00329-6. . PMid:10613301. [DOI] [PubMed] [Google Scholar]
- 53.Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16:298–304. doi: 10.1007/s003840100309. PMid:11686527. [DOI] [PubMed] [Google Scholar]
- 54.Willett CG, Badizadegan K, Ancukiewicz M, Shellito PC. Prognostic factors in stage T3N0 rectal cancer: do all patients require postoperative pelvic irradiation and chemotherapy? Dis Colon Rectum. 1999;42:167–173. doi: 10.1007/BF02237122. . PMid:10211491. [DOI] [PubMed] [Google Scholar]
- 55.Steel MC, Woods R, Mackay JM, Chen F. Extent of mesorectal invasion is a prognostic indicator in T3 rectal carcinoma. ANZ J Surg. 2002;72:483–487. doi: 10.1046/j.1445-2197.2002.02457.x. . PMid:12123507. [DOI] [PubMed] [Google Scholar]
- 56.Miyoshi M, Ueno H, Hashiguchi Y, Mochizuki H, Talbot IC. Extent of mesorectal tumor invasion as a prognostic factor after curative surgery for T3 rectal cancer patients. Ann Surg. 2006;243:492–498. doi: 10.1097/01.sla.0000205627.05769.08. . PMid:16552200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor F, Mangat N, Swift LR, Brown G. Proforma-based reporting in rectal cancer. Cancer Imaging. 2010;10:142–150. doi: 10.1102/1470-7330.2010.9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedersen BG, Moran B, Brown G, Blomqvist L, Fenger-Grøn M, Laurberg S. Reproducibility of depth of extramural tumor spread and distance to circumferential resection margin at rectal MRI: enhancement of clinical guidelines for neoadjuvant therapy. AJR Am J Roentgenol. 2011;197:1360–1366. doi: 10.2214/AJR.11.6508. . PMid:22109290. [DOI] [PubMed] [Google Scholar]
- 59.Beets-Tan RG, Beets GL, Vliegen RF, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497–504. doi: 10.1016/S0140-6736(00)04040-X. . PMid:11229667. [DOI] [PubMed] [Google Scholar]
- 60.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;1:996–999. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 61.Birgisson H, Talback M, Gunnarsson U, Pahlman L, Glimelius B. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol. 2005;31:845–853. doi: 10.1016/j.ejso.2005.05.002. . PMid:15979269. [DOI] [PubMed] [Google Scholar]
- 62.Shepherd NA, Baxter KJ, Love SB. The prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology. 1997;112:1096–1102. doi: 10.1016/S0016-5085(97)70119-7. . PMid:9097991. [DOI] [PubMed] [Google Scholar]
- 63.Shepherd NA, Baxter KJ, Love SB. Influence of local peritoneal involvement on pelvic recurrence and prognosis in rectal cancer. J Clin Pathol. 1995;48:849–855. doi: 10.1136/jcp.48.9.849. . PMid:7490320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449–457. doi: 10.1097/00000658-200204000-00001. . PMid:11923599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wibe A, Rendedal PR, Svensson E, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89:327–334. doi: 10.1046/j.0007-1323.2001.02024.x. . PMid:11872058. [DOI] [PubMed] [Google Scholar]
- 66.Hall NR, Finan T, Al-Jaberi T, Tsang CS, Brown SR, Dixon MF, et al. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent. Predictor of survival but not local recurrence? Dis Colon Rectum. 1998;41:979–983. doi: 10.1007/BF02237384. . PMid:9715152. [DOI] [PubMed] [Google Scholar]
- 67.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/S0140-6736(94)92206-3. . PMid:7915774. [DOI] [PubMed] [Google Scholar]
- 68.Taylor F, Swift R, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR. 2008;191:1827–1835. doi: 10.2214/AJR.08.1004. . PMid:19020255. [DOI] [PubMed] [Google Scholar]
- 69.Shihab O, Quirke P, Heald R, Moran B, Brown G. Magnetic resonance imaging-detected lymph nodes close to the mesorectal fascia are rarely a cause of margin involvement after total mesorectal excision. Br J Surg. 2010;97:1431–1436. doi: 10.1002/bjs.7116. . PMid:20603854. [DOI] [PubMed] [Google Scholar]
- 70.Sunderland D. The significance of vein invasion by cancer of the rectum and sigmoid: a microscopic study of 210 cases. Cancer. 1949;2:429–437. doi: 10.1002/1097-0142(194905)2:3<429::AID-CNCR2820020307>3.0.CO;2-G. . PMid:18131402. [DOI] [PubMed] [Google Scholar]
- 71.Dukes C, Bussey HJ. Venous spread in rectal cancer. Proc R Soc Med. 1941;34:571–573. PMid:19992363. [PMC free article] [PubMed] [Google Scholar]
- 72.Talbot IC, Ritchie S, Leighton M, Hughes AO, Bussey HJ, Morson BC. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5:141–163. doi: 10.1111/j.1365-2559.1981.tb01774.x. . PMid:7216178. [DOI] [PubMed] [Google Scholar]
- 73.Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer. 1988;61:1018–1023. doi: 10.1002/1097-0142(19880301)61:5<1018::AID-CNCR2820610527>3.0.CO;2-H. . PMid:3338045. [DOI] [PubMed] [Google Scholar]
- 74.Shirouzu K, Isomoto H, Kakegawa T, Morimatsu M. A prospective clinicopathologic study of venous invasion in colorectal cancer. Am J Surg. 1991;162:216–222. doi: 10.1016/0002-9610(91)90073-M. . PMid:1928581. [DOI] [PubMed] [Google Scholar]
- 75.Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–1329. doi: 10.1002/1097-0142(19831001)52:7<1317::AID-CNCR2820520731>3.0.CO;2-6. . PMid:6192900. [DOI] [PubMed] [Google Scholar]
- 76.Horn A, Dahl O, Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum. 1991;34:798–804. doi: 10.1007/BF02051074. . PMid:1914747. [DOI] [PubMed] [Google Scholar]
- 77.Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439–442. doi: 10.1002/bjs.1800670619. . PMid:7388345. [DOI] [PubMed] [Google Scholar]
- 78.Bokey EL, Chapuis PH, Dent OF, et al. Factors affecting survival after excision of the rectum for cancer: a multivariate analysis. Dis Colon Rectum. 1997;40:3–10. doi: 10.1007/BF02055674. . PMid:9102257. [DOI] [PubMed] [Google Scholar]
- 79.Harrison JC, Dean PJ, el-Zeky F, Vander Zwaag R. From Dukes through Jass: pathological prognostic indicators in rectal cancer. Hum Pathol. 1994;25:498–505. doi: 10.1016/0046-8177(94)90122-8. . PMid:8200644. [DOI] [PubMed] [Google Scholar]
- 80.Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. Spread of rectal cancer within veins. Histologic features and clinical significance. Am J Surg. 1981;14:15–17. doi: 10.1016/0002-9610(81)90004-0. [DOI] [PubMed] [Google Scholar]
- 81.Freedman LS, Macaskill P, Smith AN. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet. 1984;2:733–736. doi: 10.1016/s0140-6736(84)92636-9. PMid:6148482. [DOI] [PubMed] [Google Scholar]
- 82.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. PMid:2425199. [DOI] [PubMed] [Google Scholar]
- 83.Bokey EL, Ojerskog B, Chapuis P, Dent O, Newland RC, Sinclair G. Local recurrence after curative excision of the rectum for cancer without adjuvant therapy: role of total anatomical dissection. Br J Surg. 1999;86:1164–1170. doi: 10.1046/j.1365-2168.1999.01216.x. . PMid:10504371. [DOI] [PubMed] [Google Scholar]
- 84.Hermanek P, Merkel S, Fietkau R, Rödel C, Hohenberger W. Regional lymph node metastasis and locoregional recurrence of rectal cancer in the era of TNM surgery. Implications for treatment decisions. Int J Colorectal Dis. 2010;25:359–368. doi: 10.1007/s00384-009-0864-2. . PMid:20012295. [DOI] [PubMed] [Google Scholar]
- 85.Dukes CE. The classification of cancer of the rectum. J Pathol Bacteriol. 1932;35:323–332. doi: 10.1002/path.1700350303. [DOI] [Google Scholar]
- 86.Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–206. doi: 10.1007/s10350-004-6147-7. . PMid:11852333. [DOI] [PubMed] [Google Scholar]
- 87.Sitzler PJ, Seow-Choen F, Ho YH, Leong AP. Lymph node involvement and tumor depth in rectal cancers: An analysis of 805 patients. Dis Colon Rectum. 1997;40:1472–1476. doi: 10.1007/BF02070714. . PMid:9407987. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto S, Watanabe M, Hasegawa H, et al. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51:998–1000. PMid:15239233. [PubMed] [Google Scholar]
- 89.Ricciardi R, Madoff RD, Rothenberger DA, Baxter NN. Population-based analyses of lymph node metastases in colorectal cancer. Clin Gastroenterol Hepatol. 2006;4:1522–1527. doi: 10.1016/j.cgh.2006.07.016. . PMid:16979956. [DOI] [PubMed] [Google Scholar]
- 90.Blumberg D, Paty PB, Guillem JG, et al. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum. 1999;42:881–885. doi: 10.1007/BF02237095. . PMid:10411434. [DOI] [PubMed] [Google Scholar]
- 91.Fang WL, Chang SC, Lin JK, et al. Metastatic potential in T1 and T2 colorectal cancer. Hepatogastroenterology. 2005;52:1688–1691. PMid:16334758. [PubMed] [Google Scholar]
- 92.Baxter N, García-Aguilar J. Organ preservation for rectal cancer. J Clin Oncol. 2007;25:1014–1020. doi: 10.1200/JCO.2006.09.7840. . PMid:17350952. [DOI] [PubMed] [Google Scholar]
- 93.Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–377. doi: 10.1148/radiol.2272011747. . PMid:12732695. [DOI] [PubMed] [Google Scholar]
- 94.Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52:78–83. doi: 10.1016/j.ejrad.2003.12.005. . PMid:15380850. [DOI] [PubMed] [Google Scholar]
- 95.Koh DM, Brown G, Temple L, et al. Rectal cancer: mesorrectal lymph nodes at MR imaging with USPIO versus histopathologic findings - initial observations. Radiology. 2004;231:91–99. doi: 10.1148/radiol.2311030142. . PMid:14976266. [DOI] [PubMed] [Google Scholar]
- 96.Koh DM, George C, Temple L, et al. Diagnostic accuracy of nodal enhancement pattern of rectal cancer at MRI enhanced with ultrasmall superparamagnetic iron oxide: findings in pathologically matched mesorectal lymph nodes. AJR Am J Roentgenol. 2010;194:505–513. doi: 10.2214/AJR.08.1819. [DOI] [PubMed] [Google Scholar]
- 97.Lambregts DM, Beets GL, Maas M, et al. Accuracy of gadofosveset-enhanced MRI for nodal staging and restaging in rectal cancer. Ann Surg. 2011;253:539–545. doi: 10.1097/SLA.0b013e31820b01f1. . PMid:21239980. [DOI] [PubMed] [Google Scholar]
- 98.Lambregts DM, Heijnen LA, Maas M, et al. Gadofosveset-enhanced MRI for the assessment of rectal cancer lymph nodes: predictive criteria. Abdom Imaging. 2012 doi: 10.1007/s00261-012-9957-4. doi:10.1007/s00261-012-9957-4. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki K, Muto T, Sawada T. Prevention of local recurrence by extended lymphadenectomy for rectal cancer. Surg Today. 1995;25:795–801. doi: 10.1007/BF00311455. . PMid:8555697. [DOI] [PubMed] [Google Scholar]
- 100.Billingham RP. Extended lymphadenectomy for rectal cancer: cure vs quality of life. Int Surg. 1994;79:11–22. PMid:8063549. [PubMed] [Google Scholar]
- 101.Moreira LF, Kenmotsu M, Gochi A, Tanaka N, Orita K. Lymphovascular and neural invasion in low-lying rectal carcinoma. Cancer Detect Prevent. 1999;23:123–128. doi: 10.1046/j.1525-1500.1999.09908.x. . PMid:10101593. [DOI] [PubMed] [Google Scholar]
- 102.Gunther K, Dworak O, Remke S, et al. Prediction of distant metastases after curative surgery for rectal cancer. J Surg Res. 2002;103:68–78. doi: 10.1006/jsre.2001.6312. . PMid:11855920. [DOI] [PubMed] [Google Scholar]
- 103.Hildebrandt U, Klein T, Feifel G, Schwarz HP, Koch B, Schmitt RM. Endosonography of pararectal lymph nodes. In vitro and in vivo evaluation. Dis Colon Rectum. 1990;33:863–868. doi: 10.1007/BF02051923. . PMid:2209276. [DOI] [PubMed] [Google Scholar]
- 104.Birbeck K, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449–457. doi: 10.1097/00000658-200204000-00001. . PMid:11923599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cecil TD, Sexton R, Moran BJ, Heald RJ. Total mesorrectal excision results in low local recurrence rates in lymph node-positive rectal cancer. Dis Colon Rectum. 2004;47:1145–1149. doi: 10.1007/s10350-004-0086-6. . PMid:15164243. [DOI] [PubMed] [Google Scholar]
- 106.Yano H, Saito Y, Kirihara Y, Takashima J. Tumor invasion of lymph node capsules in patients with Dukes C colorectal adenocarcinoma. Dis Colon Rectum. 2006;49:1867–1877. doi: 10.1007/s10350-006-0733-9. . PMid:17080279. [DOI] [PubMed] [Google Scholar]
- 107.Ueno H, Mochizuki H, Tamakuma S. Prognostic significance of extranodal microscopic foci discontinuous with primary lesion in rectal cancer. Dis Colon Rectum. 1998;41:55–61. doi: 10.1007/BF02236896. . PMid:9510311. [DOI] [PubMed] [Google Scholar]
- 108.Singh AK, Myerson RJ, Birnbaum EH, et al. Outcome of patients with rectal adenocarcinoma and localized pelvic non-nodal metastatic foci. Dis Colon Rectum. 2000;43:1217–1221. doi: 10.1007/BF02237424. . PMid:11005486. [DOI] [PubMed] [Google Scholar]
- 109.Marr R, Birbeck K, Garvican J, et al. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg. 2005;242:74–82. doi: 10.1097/01.sla.0000167926.60908.15. . PMid:15973104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nagtegaal ID, van de Velde CJH, Marijnen CAM, van Krieken JHJM, Quirke P. Dutch Colorectal Cancer Group; Pathology Review Committee. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257–9264. doi: 10.1200/JCO.2005.02.9231. . PMid:16361623. [DOI] [PubMed] [Google Scholar]
- 111.Wibe A, Syse A, Andersen E, Tretli S, Myrvold HE, Soreide O. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum. 2004;47:48–58. doi: 10.1007/s10350-003-0012-y. . PMid:14719151. [DOI] [PubMed] [Google Scholar]
- 112.Shihab OC, Taylor F, Salerno G, et al. MRI predictive factors for long-term outcomes of low rectal tumours. Ann Surg Oncol. 2011;18:3278–3284. doi: 10.1245/s10434-011-1776-2. . PMid:21590453. [DOI] [PubMed] [Google Scholar]
- 113.Salerno G, Chandler I, Wotherspoon A, Thomas K, Moran B, Brown G. Sites of surgical wasting in the abdominoperineal specimen. Br J Surg. 2008;95:1147–1154. doi: 10.1002/bjs.6231. . PMid:18690619. [DOI] [PubMed] [Google Scholar]
- 114.Shihab OC, Moran BJ, Heald RJ, Quirke P, Brown G. MRI staging of low rectal cancer. Eur Radiol. 2009;19:643–650. doi: 10.1007/s00330-008-1184-6. . PMid:18810451. [DOI] [PubMed] [Google Scholar]
- 115.Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis. 1988;3:127–131. doi: 10.1007/BF01645318. . PMid:3045231. [DOI] [PubMed] [Google Scholar]
- 116.Shihab OC, Heald R, Rullier E, Brown G, Holm T, Quirke P. Defining the surgical planes on MRI improves surgery for cancer of the low rectum. Lancet Oncol. 2009;10:1207–1211. doi: 10.1016/S1470-2045(09)70084-1. . PMid:19959077. [DOI] [PubMed] [Google Scholar]
- 117.Salerno G, Daniels I, Moran B, Heald R, Thomas K, Brown G. Magnetic resonance imaging prediction of an involved surgical resection margin in low rectal cancer. Dis Colon Rectum. 2009;52:632–639. doi: 10.1007/DCR.0b013e3181a0a37e. . PMid:19404067. [DOI] [PubMed] [Google Scholar]
- 118.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. . PMid:11547717. [DOI] [PubMed] [Google Scholar]
- 119.Dehni N, McFadden N, McNamara DA, Guiguet M, Tiret E, Parc R. Oncologic results following abdominoperineal resection for adenocarcinoma of the low rectum. Dis Colon Rectum. 2003;46:867–874; discussion 874. doi: 10.1007/s10350-004-6675-1. . PMid:12847358. [DOI] [PubMed] [Google Scholar]
- 120.Heald RJ, Smedh RK, Kald A, Sexton R, Moran BJ. Abdominoperineal excision of the rectum: an endangered operation. Norman Nigro Lectureship. Dis Colon Rectum. 1997;40:747–751. doi: 10.1007/BF02055425. . PMid:9221846. [DOI] [PubMed] [Google Scholar]
- 121.Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo and radiation therapy. Radiology. 2009;253:116–125. doi: 10.1148/radiol.2532090027. . PMid:19789256. [DOI] [PubMed] [Google Scholar]
- 122.Brown G, Richards CJ, Newcombe RG, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211:215–222. doi: 10.1148/radiology.211.1.r99ap35215. PMid:10189474. [DOI] [PubMed] [Google Scholar]
- 123.Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology. 2004;232:335–436. doi: 10.1148/radiol.2322021326. . PMid:15286305. [DOI] [PubMed] [Google Scholar]
- 124.Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol. 2005;78:245–251. doi: 10.1259/bjr/33540239. . PMid:15730990. [DOI] [PubMed] [Google Scholar]
- 125.Brown G, Kirkham A, Williams GT, et al. High resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR. 2004;182:431–439. doi: 10.2214/ajr.182.2.1820431. . PMid:14736677. [DOI] [PubMed] [Google Scholar]
- 126.Blomqvist L, Rubio C, Holm T, Machado M, Hindmarsh T. Rectal adenocarcinoma: assessment of tumour involvement of the lateral resection margin by MRI of resected specimen. Br J Radiol. 1999;72:18–23. doi: 10.1259/bjr.72.853.10341684. PMid:10341684. [DOI] [PubMed] [Google Scholar]
- 127.Suzuki C, Torkzad MR, Tanaka S, et al. The importance of rectal cancer MRI protocols on interpretation accuracy. World J Surg Oncol. 2008;6:89. doi: 10.1186/1477-7819-6-89. . PMid:18715510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bull AD, Biffin AH, Mella J, et al. Colorectal cancer pathology reporting: a regional audit. J Clin Pathol. 1997;50:138–142. doi: 10.1136/jcp.50.2.138. . PMid:9155695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morris EJ, Maughan NJ, Forman D, Quirke P. Who to treat with adjuvant therapy in Dukes B/stage II colorectal cancer? The need for high quality pathology. Gut. 2007;56:1419–1425. doi: 10.1136/gut.2006.116830. . PMid:17494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Georgiou PA, Tekkis PP, Brown G. Pelvic colorectal recurrence: crucial role of radiologists in oncologic and surgical treatment options. Cancer Imaging. 2011;11:103–111. doi: 10.1102/1470-7330.2011.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rao AR, Kagan AR, Chan PM, Gilbert HA, Nussbaum H, Hintz BL. Patterns of recurrence following curative resection alone for adenocarcinoma of the rectum and sigmoid colon. Cancer. 1981;48:1492–1495. doi: 10.1002/1097-0142(19810915)48:6<1492::AID-CNCR2820480636>3.0.CO;2-K. . PMid:7272970. [DOI] [PubMed] [Google Scholar]
- 132.Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following curative surgery for large bowel cancer: II. The rectum and rectosigmoid. Br J Surg. 1984;71:17–20. doi: 10.1002/bjs.1800710105. . PMid:6689963. [DOI] [PubMed] [Google Scholar]
- 133.Cass AW, Million RR, Pfaff WW. Patterns of recurrence following surgery alone for adenocarcinoma of the colon and rectum. Cancer. 1976;37:2861–2865. doi: 10.1002/1097-0142(197606)37:6<2861::AID-CNCR2820370643>3.0.CO;2-3. . PMid:949706. [DOI] [PubMed] [Google Scholar]
- 134.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. . PMid:16260700. [DOI] [PubMed] [Google Scholar]
- 135.Potter KC, Husband JE, Houghton SL, Thomas K, Brown G. Diagnostic accuracy of serial CT/magnetic resonance imaging review vs. positron emission tomography/CT in colorectal cancer patients with suspected and known recurrence. Dis Colon Rectum. 2009;52:253–259. doi: 10.1007/DCR.0b013e31819d11e6. . PMid:19279420. [DOI] [PubMed] [Google Scholar]
- 136.Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer. 2003;3:26. doi: 10.1186/1471-2407-3-26. . PMid:14529575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jeffery GM, Hickey BE, Hider P. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2002:CD002200. doi: 10.1002/14651858.CD002200. PMid:17253476. [DOI] [PubMed] [Google Scholar]
- 138.Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. doi: 10.1136/bmj.324.7341.813. . PMid:11934773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brown G. Imaging for colorectal recurrence. Proceedings of the European Congress of Radiology, Vienna, 2010. [Google Scholar]
- 140.Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. Erratum in J Clin Oncol 2006; 24: 1224. [DOI] [PubMed] [Google Scholar]
- 141.Huebner RH, Park KC, Shepherd JE, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41:1177–1189. PMid:10914907. [PubMed] [Google Scholar]
- 142.Asoglu O, Karanlik H, Muslumanoglu M, et al. Prognostic and predictive factors after surgical treatment for locally recurrent rectal cancer: a single institute experience. Eur J Surg Oncol. 2007;33:1199–1206. doi: 10.1016/j.ejso.2007.02.026. . PMid:17400423. [DOI] [PubMed] [Google Scholar]
- 143.Heriot AG, Byrne CM, Lee P, et al. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51:284–291. doi: 10.1007/s10350-007-9152-9. . PMid:18204879. [DOI] [PubMed] [Google Scholar]
- 144.Dicle O, Obuz F, Cakmakci H. Differentiation of recurrent rectal cancer and scarring with dynamic MR imaging. Br J Radiol. 1999;72:1155–1159. doi: 10.1259/bjr.72.864.10703471. PMid:10703471. [DOI] [PubMed] [Google Scholar]
- 145.Zhang C, Chen Y, Xue H, et al. Diagnostic value of FDG-PET in recurrent colorectal carcinoma: a meta-analysis. Int J Cancer. 2009;124:167–173. doi: 10.1002/ijc.23926. . PMid:18844237. [DOI] [PubMed] [Google Scholar]
- 146.Moore HG, Akhurst T, Larson SM, Minsky BD, Mazumdar M, Guillem JG. A case-controlled study of 18-fluorodeoxyglucose positron emission tomography in the detection of pelvic recurrence in previously irradiated rectal cancer patients. J Am Coll Surg. 2003;197:22–28. doi: 10.1016/S1072-7515(03)00337-5. . PMid:12831920. [DOI] [PubMed] [Google Scholar]
- 147.von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology. 2006;238:405–422. doi: 10.1148/radiol.2382041977. . PMid:16436809. [DOI] [PubMed] [Google Scholar]
- 148.Kamel IR, Cohade C, Neyman E, Fishman EK, Wahl RL. Incremental value of CT in PET/CT of patients with colorectal carcinoma. Abdom Imaging. 2004;29:663–668. doi: 10.1007/s00261-003-0163-2. . PMid:15162236. [DOI] [PubMed] [Google Scholar]
- 149.Georgiou PA, Tekkis PP, Constantinides VA, et al. Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. Eur J Cancer. 2013;49:72–81. doi: 10.1016/j.ejca.2012.06.025. . PMid:23036847. [DOI] [PubMed] [Google Scholar]
- 150.Lambregts DM, Cappendijk VC, Maas M, Beets GL, Beets-Tan RG. Value of MRI and diffusion-weighted MRI for the diagnosis of locally recurrent rectal cancer. Eur Radiol. 2011;21:1250–1258. doi: 10.1007/s00330-010-2052-8. . PMid:21240647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim DJ, Kim JH, Lim JS, et al. Restaging of rectal cancer with MR imaging after concurrent chemotherapy and radiation therapy. Radiographics. 2010;30:503–516. doi: 10.1148/rg.302095046. . PMid:20228331. [DOI] [PubMed] [Google Scholar]
- 152.Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253:116–125. doi: 10.1148/radiol.2532090027. . PMid:19789256. [DOI] [PubMed] [Google Scholar]
- 153.Barbaro B, Vitale R, Leccisotti L, et al. Restaging locally advanced rectal cancer with MR imaging after chemoradiation therapy. Radiographics. 2010;30:699–716. doi: 10.1148/rg.303095085. . PMid:20462989. [DOI] [PubMed] [Google Scholar]
- 154.Colosio A, Fornès P, Soyer P, Lewin M, Loock M, Hoeffel C. Local colorectal cancer recurrence: pelvic MRI evaluation. Abdom Imaging. 2013;38:72–81. doi: 10.1007/s00261-012-9891-5. . PMid:22484342. [DOI] [PubMed] [Google Scholar]